Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Model P., Dixon G. H., Wosnick N. A. An E. coli gene coding for a protamine-like protein. Cell. 1981 Nov;26(3 Pt 1):299–304. doi: 10.1016/0092-8674(81)90198-7. [DOI] [PubMed] [Google Scholar]
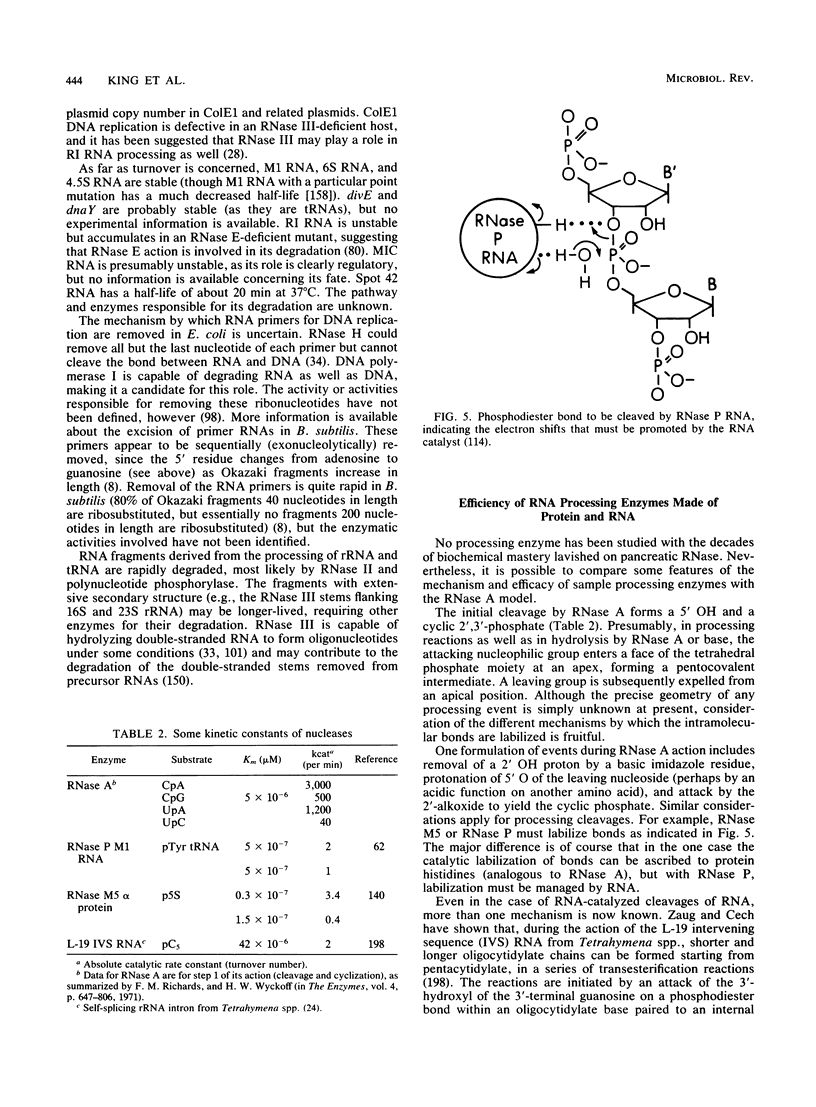
- Asha P. K., Blouin R. T., Zaniewski R., Deutscher M. P. Ribonuclease BN: identification and partial characterization of a new tRNA processing enzyme. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3301–3304. doi: 10.1073/pnas.80.11.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer M., Altman S. A catalytic RNA and its gene from Salmonella typhimurium. Science. 1985 May 24;228(4702):999–1002. doi: 10.1126/science.2408335. [DOI] [PubMed] [Google Scholar]
- Banfalvi G., Sarkar N. Origin and degradation of the RNA primers at the 5' termini of nascent DNA chains in Bacillus subtilis. J Mol Biol. 1985 Nov 20;186(2):275–282. doi: 10.1016/0022-2836(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett B. O., Scott Russell R., Jenkins W. Improved relationship between the deposition of strontium-90 and the contamination of milk in the United Kingdom. Nature. 1972 Jul 7;238(5358):46–48. doi: 10.1038/238046a0. [DOI] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Belfort M., Pedersen-Lane J., West D., Ehrenman K., Maley G., Chu F., Maley F. Processing of the intron-containing thymidylate synthase (td) gene of phage T4 is at the RNA level. Cell. 1985 Jun;41(2):375–382. doi: 10.1016/s0092-8674(85)80010-6. [DOI] [PubMed] [Google Scholar]
- Ben-Hamida F., Schlessinger D. Synthesis and breakdown of ribonucleic acid in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):183–191. doi: 10.1016/0005-2787(66)90049-9. [DOI] [PubMed] [Google Scholar]
- Birenbaum M., Schlessinger D., Hashimoto S. RNase III cleavage of Escherichia coli rRNA precursors: fragment release and dependence on salt concentration. Biochemistry. 1978 Jan 24;17(2):298–307. doi: 10.1021/bi00595a017. [DOI] [PubMed] [Google Scholar]
- Blanchetot A., Hajnsdorf E., Favre A. Metabolism of tRNA in near-ultraviolet-illuminated Escherichia coli. The tRNA repair hypothesis. Eur J Biochem. 1984 Mar 15;139(3):547–552. doi: 10.1111/j.1432-1033.1984.tb08040.x. [DOI] [PubMed] [Google Scholar]
- Blouin R. T., Zaniewski R., Deutscher M. P. Ribonuclease D is not essential for the normal growth of Escherichia coli or bacteriophage T4 or for the biosynthesis of a T4 suppressor tRNA. J Biol Chem. 1983 Feb 10;258(3):1423–1426. [PubMed] [Google Scholar]
- Blumer K. J., Steege D. A. mRNA processing in Escherichia coli: an activity encoded by the host processes bacteriophage f1 mRNAs. Nucleic Acids Res. 1984 Feb 24;12(4):1847–1861. doi: 10.1093/nar/12.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Garber R. L., Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J Biol Chem. 1976 Dec 10;251(23):7709–7716. [PubMed] [Google Scholar]
- Bram R. J., Young R. A., Steitz J. A. The ribonuclease III site flanking 23S sequences in the 30S ribosomal precursor RNA of E. coli. Cell. 1980 Feb;19(2):393–401. doi: 10.1016/0092-8674(80)90513-9. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brown S., Fournier M. J. The 4.5 S RNA gene of Escherichia coli is essential for cell growth. J Mol Biol. 1984 Sep 25;178(3):533–550. doi: 10.1016/0022-2836(84)90237-7. [DOI] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. The 5' ends of Escherichia coli lac mRNA. J Mol Biol. 1985 Mar 20;182(2):241–248. doi: 10.1016/0022-2836(85)90342-0. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A., Dotto G. P., Altruda F., Perlo C., Silengo L., Turco E., Mangiarotti G. Immature 50 S subunits in Escherichia coli polyribosomes. FEBS Lett. 1978 Sep 15;93(2):348–350. doi: 10.1016/0014-5793(78)81137-5. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Chanda P. K., Ono M., Kuwano M., Kung H. Cloning, sequence analysis, and expression of alteration of the mRNA stability gene (ams+) of Escherichia coli. J Bacteriol. 1985 Jan;161(1):446–449. doi: 10.1128/jb.161.1.446-449.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. W., Lake J. A. Unusual rRNA-linked complex of 50S ribosomal subunits isolated from an Escherichia coli RNase III mutant. J Bacteriol. 1984 Mar;157(3):971–974. doi: 10.1128/jb.157.3.971-974.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Campbell J. L. Role of plasmid-coded RNA and ribonuclease III in plasmid DNA replication. Cell. 1979 Sep;18(1):61–71. doi: 10.1016/0092-8674(79)90354-4. [DOI] [PubMed] [Google Scholar]
- Court D., Huang T. F., Oppenheim A. B. Deletion analysis of the retroregulatory site for the lambda int gene. J Mol Biol. 1983 May 15;166(2):233–240. doi: 10.1016/s0022-2836(83)80010-2. [DOI] [PubMed] [Google Scholar]
- Cremer K., Schlessinger D. Ca2+ ions inhibit messenger ribonucleic acid degradation, but permit messenger ribonucleic acid transcription and translation in deoxyribonucleic acid-coupled systems from Escherichia coli. J Biol Chem. 1974 Aug 10;249(15):4730–4736. [PubMed] [Google Scholar]
- Cremer K., Silengo L., Schlessinger D. Polypeptide formation and polyribosomes in Escherichia coli treated with chloramphenicol. J Bacteriol. 1974 May;118(2):582–589. doi: 10.1128/jb.118.2.582-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Cudny H., Deutscher M. P. Apparent involvement of ribonuclease D in the 3' processing of tRNA precursors. Proc Natl Acad Sci U S A. 1980 Feb;77(2):837–841. doi: 10.1073/pnas.77.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E., Lund E., Tokimatsu H., Rabson A. B., Calvert P. C., Reynolds F., Zahalak M. Processing of the 5' end of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3598–3602. doi: 10.1073/pnas.75.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. J., Gupta R., Doolittle W. F. Transcription and excision of a large intron in the tRNATrp gene of an archaebacterium, Halobacterium volcanii. J Biol Chem. 1985 Mar 10;260(5):3132–3134. [PubMed] [Google Scholar]
- Deutscher M. P., Lin J. J., Evans J. A. Transfer RNA metabolism in Escherichia coli cells deficient in tRNA nucleotidyltransferase. J Mol Biol. 1977 Dec 25;117(4):1081–1094. doi: 10.1016/s0022-2836(77)80014-4. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Marlor C. W., Zaniewski R. RNase T is responsible for the end-turnover of tRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6427–6430. doi: 10.1073/pnas.82.19.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Marlor C. W., Zaniewski R. Ribonuclease T: new exoribonuclease possibly involved in end-turnover of tRNA. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4290–4293. doi: 10.1073/pnas.81.14.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. Processing of tRNA in prokaryotes and eukaryotes. CRC Crit Rev Biochem. 1984;17(1):45–71. doi: 10.3109/10409238409110269. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Jan;83(1):120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Jordan B. R., Monier R. Study of the maturation of 5 s RNA precursors in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):465–474. doi: 10.1016/0022-2836(72)90553-0. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Friesen J. D., Downing W. L., Dennis P. P. Post-transcriptional regulatory mutants in a ribosomal protein-RNA polymerase operon of E. coli. Cell. 1980 Apr;19(4):837–844. doi: 10.1016/0092-8674(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Jackson E. N., Yanofsky C. Different half-lives of messenger RNA corresponding to different segments of the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):687–699. doi: 10.1016/s0022-2836(72)80032-9. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Ozeki H. Structure and organization of the transfer ribonucleic acid genes of Escherichia coli K-12. Microbiol Rev. 1985 Dec;49(4):379–397. doi: 10.1128/mr.49.4.379-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada K., Abelson J. DNA sequence of a T4 transfer RNA gene cluster. J Mol Biol. 1980 May 25;139(3):377–391. doi: 10.1016/0022-2836(80)90136-9. [DOI] [PubMed] [Google Scholar]
- Furdon P. J., Guerrier-Takada C., Altman S. A G43 to U43 mutation in E. coli tRNAtyrsu3+ which affects processing by RNase P. Nucleic Acids Res. 1983 Mar 11;11(5):1491–1505. doi: 10.1093/nar/11.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K. J., Marsh T. L., Pace N. R. Ion dependence of the Bacillus subtilis RNase P reaction. J Biol Chem. 1985 May 10;260(9):5415–5419. [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981 Dec;45(4):502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman D. R., Apirion D. The synthesis of some proteins is affected in RNA processing mutants of Escherichia coli. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1063–1070. doi: 10.1016/0006-291x(80)90060-1. [DOI] [PubMed] [Google Scholar]
- Goldfarb A., Daniel V. An Escherichia coli endonuclease responsible for primary cleavage of in vitro transcripts of bacteriophage T4 tRNA gene cluster. Nucleic Acids Res. 1980 Oct 10;8(19):4501–4516. doi: 10.1093/nar/8.19.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Roch J. M., Prentki P., Krisch H. M. The stability of bacteriophage T4 gene 32 mRNA: a 5' leader sequence that can stabilize mRNA transcripts. Cell. 1985 Dec;43(2 Pt 1):461–469. doi: 10.1016/0092-8674(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., LaFauci G., Rudner R. Alterations in the number of rRNA operons within the Bacillus subtilis genome. Gene. 1985;33(3):259–268. doi: 10.1016/0378-1119(85)90233-1. [DOI] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Sequence analysis of a cluster of twenty-one tRNA genes in Bacillus subtilis. Nucleic Acids Res. 1983 Aug 25;11(16):5763–5774. doi: 10.1093/nar/11.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G., Montañez C., Hernandez T., Court D. Posttranscriptional control of bacteriophage lambda gene expression from a site distal to the gene. Proc Natl Acad Sci U S A. 1982 Jan;79(2):238–242. doi: 10.1073/pnas.79.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Altman S. Catalytic activity of an RNA molecule prepared by transcription in vitro. Science. 1984 Jan 20;223(4633):285–286. doi: 10.1126/science.6199841. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., McClain W. H., Altman S. Cleavage of tRNA precursors by the RNA subunit of E. coli ribonuclease P (M1 RNA) is influenced by 3'-proximal CCA in the substrates. Cell. 1984 Aug;38(1):219–224. doi: 10.1016/0092-8674(84)90543-9. [DOI] [PubMed] [Google Scholar]
- Gurevitz M., Jain S. K., Apirion D. Identification of a precursor molecular for the RNA moiety of the processing enzyme RNase P. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4450–4454. doi: 10.1073/pnas.80.14.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J. Association of nascent ribosomal ribonucleic acid with polyribosomes in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1330–1343. doi: 10.1128/jb.124.3.1330-1343.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M. N., Hayashi M. Cloned DNA sequences that determine mRNA stability of bacteriophage phi X174 in vivo are functional. Nucleic Acids Res. 1985 Aug 26;13(16):5937–5948. doi: 10.1093/nar/13.16.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes F., Vasseur M. Processing of the 17-S Escherichia coli precursor RNA in the 27-S pre-ribosomal particle. Eur J Biochem. 1976 Jan 15;61(2):433–442. doi: 10.1111/j.1432-1033.1976.tb10037.x. [DOI] [PubMed] [Google Scholar]
- Hercules K., Schweiger M., Sauerbier W. Cleavage by RNase 3 converts T3 and T7 early precursor RNA into translatable message. Proc Natl Acad Sci U S A. 1974 Mar;71(3):840–844. doi: 10.1073/pnas.71.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Hofman J. D., Lau R. H., Doolittle W. F. The number, physical organization and transcription of ribosomal RNA cistrons in an archaebacterium: Halobacterium halobium. Nucleic Acids Res. 1979 Nov 10;7(5):1321–1333. doi: 10.1093/nar/7.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. M., Zagorski J., Fournier M. J. Cloning and sequence analysis of the Escherichia coli 4.5 S RNA gene. J Mol Biol. 1984 Sep 25;178(3):509–531. doi: 10.1016/0022-2836(84)90236-5. [DOI] [PubMed] [Google Scholar]
- Hsu L. M., Zagorski J., Wang Z., Fournier M. J. Escherichia coli 6S RNA gene is part of a dual-function transcription unit. J Bacteriol. 1985 Mar;161(3):1162–1170. doi: 10.1128/jb.161.3.1162-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L., Rossi J., Landy A. Dual function transcripts specifying tRNA and mRNA. Nature. 1981 Dec 3;294(5840):422–427. doi: 10.1038/294422a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Ozeki H. Gross map location of Escherichia coli transfer RNA genes. J Mol Biol. 1977 Dec 5;117(2):419–446. doi: 10.1016/0022-2836(77)90136-x. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Ishii S., Kuroki K., Imamoto F. tRNAMetf2 gene in the leader region of the nusA operon in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):409–413. doi: 10.1073/pnas.81.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R., Ohnishi Y. The roles of RNA polymerase and RNAase I in stable RNA degradation in Escherichia coli carrying the srnB+ gene. Biochim Biophys Acta. 1983 Jan 20;739(1):27–34. doi: 10.1016/0167-4781(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Jakubowski H., Goldman E. Quantities of individual aminoacyl-tRNA families and their turnover in Escherichia coli. J Bacteriol. 1984 Jun;158(3):769–776. doi: 10.1128/jb.158.3.769-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo D. K., Zwieb C., Dahlberg A. E. Point mutations in the 3' minor domain of 16S rRNA of E.coli. Nucleic Acids Res. 1985 Dec 9;13(23):8631–8643. doi: 10.1093/nar/13.23.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R., Feuteun J., Monier R. Identification of a 5 s RNA precursor in exponentially growing Escherichia coli cells. J Mol Biol. 1970 Jun 28;50(3):605–615. doi: 10.1016/0022-2836(70)90088-4. [DOI] [PubMed] [Google Scholar]
- Joseph E., Danchin A., Ullmann A. Modulation of the lactose operon mRNA turnover by inhibitors of dihydrofolate reductase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):769–776. doi: 10.1016/0006-291x(78)90771-4. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya S., Crouch R. J. The rnh gene is essential for growth of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3447–3451. doi: 10.1073/pnas.81.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Imamoto F. Evidence for endonucleolytic cleavage at the 5'-proximal segment of the trp messenger RNA in Escherichia coli. Mol Gen Genet. 1979 Apr 17;172(1):25–30. doi: 10.1007/BF00276211. [DOI] [PubMed] [Google Scholar]
- Karam J., Gold L., Singer B. S., Dawson M. Translational regulation: identification of the site on bacteriophage T4 rIIB mRNA recognized by the regA gene function. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4669–4673. doi: 10.1073/pnas.78.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J., McCulley C., Leach M. Genetic control of mRNA decay in T4 phage-infected Escherichia coli. Virology. 1977 Feb;76(2):685–700. doi: 10.1016/0042-6822(77)90251-3. [DOI] [PubMed] [Google Scholar]
- Kasai T., Gupta R. S., Schlessinger D. Exoribonucleases in wild type Escherichia coli and RNase II-deficient mutants. J Biol Chem. 1977 Dec 25;252(24):8950–8956. [PubMed] [Google Scholar]
- Kennell D., Riezman H. Transcription and translation initiation frequencies of the Escherichia coli lac operon. J Mol Biol. 1977 Jul;114(1):1–21. doi: 10.1016/0022-2836(77)90279-0. [DOI] [PubMed] [Google Scholar]
- Kepes A. Sequential transcription and translation in the lactose operon of Escherichia coli. Biochim Biophys Acta. 1967 Mar 29;138(1):107–123. doi: 10.1016/0005-2787(67)90591-6. [DOI] [PubMed] [Google Scholar]
- King T. C., Schlessinger D. S1 nuclease mapping analysis of ribosomal RNA processing in wild type and processing deficient Escherichia coli. J Biol Chem. 1983 Oct 10;258(19):12034–12042. [PubMed] [Google Scholar]
- King T. C., Sirdeshmukh R., Schlessinger D. RNase III cleavage is obligate for maturation but not for function of Escherichia coli pre-23S rRNA. Proc Natl Acad Sci U S A. 1984 Jan;81(1):185–188. doi: 10.1073/pnas.81.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinscherf T. G., Apirion D. Polynucleotide phosphorylase can participate in decay of mRNA in Escherichia coli in the absence of ribonuclease II. Mol Gen Genet. 1975 Sep 8;139(4):357–362. doi: 10.1007/BF00267975. [DOI] [PubMed] [Google Scholar]
- Klein B. K., Staden A., Schlessinger D. Alternative conformations in Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3539–3542. doi: 10.1073/pnas.82.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. K., Staden A., Schlessinger D. Electron microscopy of secondary structure in partially denatured precursor and mature Escherichia coli 16 S and 23 S rRNA. J Biol Chem. 1985 Jul 5;260(13):8114–8120. [PubMed] [Google Scholar]
- LaFauci G., Widom R. L., Eisner R. L., Jarvis E. D., Rudner R. Mapping of rRNA genes with integrable plasmids in Bacillus subtilis. J Bacteriol. 1986 Jan;165(1):204–214. doi: 10.1128/jb.165.1.204-214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Fournier M. J., Beckwith J. Escherichia coli 6S RNA is not essential for growth or protein secretion. J Bacteriol. 1985 Mar;161(3):1156–1161. doi: 10.1128/jb.161.3.1156-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libonati M., Carsana A., Furia A. Double-stranded RNA. Mol Cell Biochem. 1980 Aug 16;31(3):147–164. doi: 10.1007/BF00225848. [DOI] [PubMed] [Google Scholar]
- Lim L. W., Kennell D. Evidence for random endonucleolytic cleavages between messages in decay of Escherichia coli trp mRNA. J Mol Biol. 1980 Aug 5;141(2):227–233. doi: 10.1016/0022-2836(80)90388-5. [DOI] [PubMed] [Google Scholar]
- Lim L. W., Kennell D. Models for decay of Escherichia coli lac messenger RNA and evidence for inactivating cleavages between its messages. J Mol Biol. 1979 Dec 5;135(2):369–390. doi: 10.1016/0022-2836(79)90442-x. [DOI] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. Ribosomal RNA precursors of Bacillus subtilis. Nucleic Acids Res. 1983 Oct 11;11(19):6709–6721. doi: 10.1093/nar/11.19.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. tRNA genes are found between 16S and 23S rRNA genes in Bacillus subtilis. Nucleic Acids Res. 1982 Mar 11;10(5):1607–1624. doi: 10.1093/nar/10.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen K. R., Nicholson D. E., Eubanks D. C., Fox G. E. An archaebacterial 5S rRNA contains a long insertion sequence. Nature. 1981 Oct 29;293(5835):755–756. doi: 10.1038/293755a0. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Chang F. N. Processing of precursor ribosomal RNA and the presence of a modified ribosome assembly scheme in Escherichia coli relaxed strain. FEBS Lett. 1985 Mar 25;182(2):407–412. doi: 10.1016/0014-5793(85)80343-4. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Turco E., Ponzetto A., Altruda F. Precursor 16S RNA in active 30S ribosomes. Nature. 1974 Jan 18;247(5437):147–148. doi: 10.1038/247147a0. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Kagramanova V. K., Teterina N. L., Rubtsov P. M., Belova E. N., Kopylov A. M., Baratova L. A., Bogdanov A. A. The nucleotide sequence of the gene coding for the 16S rRNA from the archaebacterium Halobacterium halobium. Gene. 1985;37(1-3):181–189. doi: 10.1016/0378-1119(85)90271-9. [DOI] [PubMed] [Google Scholar]
- Mankin A. S., Teterina N. L., Rubtsov P. M., Baratova L. A., Kagramanova V. K. Putative promoter region of rRNA operon from archaebacterium Halobacterium halobium. Nucleic Acids Res. 1984 Aug 24;12(16):6537–6546. doi: 10.1093/nar/12.16.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L. Synthesis and degradation of termination and premature-termination fragments of beta-galactosidase in vitro and in vivo. J Mol Biol. 1978 Nov 15;125(4):407–432. doi: 10.1016/0022-2836(78)90308-x. [DOI] [PubMed] [Google Scholar]
- March P. E., Ahnn J., Inouye M. The DNA sequence of the gene (rnc) encoding ribonuclease III of Escherichia coli. Nucleic Acids Res. 1985 Jul 11;13(13):4677–4685. doi: 10.1093/nar/13.13.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh T. L., Pace N. R. Ribonuclease P catalysis differs from ribosomal RNA self-splicing. Science. 1985 Jul 5;229(4708):79–81. doi: 10.1126/science.4012313. [DOI] [PubMed] [Google Scholar]
- Menninger J. R., Otto D. P. Erythromycin, carbomycin, and spiramycin inhibit protein synthesis by stimulating the dissociation of peptidyl-tRNA from ribosomes. Antimicrob Agents Chemother. 1982 May;21(5):811–818. doi: 10.1128/aac.21.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R. Peptidyl transfer RNA dissociates during protein synthesis from ribosomes of Escherichia coli. J Biol Chem. 1976 Jun 10;251(11):3392–3398. [PubMed] [Google Scholar]
- Menninger J. R. The accumulation as peptidyl-transfer RNA of isoaccepting transfer RNA families in Escherichia coli with temperature-sensitive peptidyl-transfer RNA hydrolase. J Biol Chem. 1978 Oct 10;253(19):6808–6813. [PubMed] [Google Scholar]
- Meyhack B., Pace B., Pace N. R. Involvement of precursor-specific segments in the in vitro maturation of Bacillus subtilis precursor 5S ribosomal RNA. Biochemistry. 1977 Nov 15;16(23):5009–5015. doi: 10.1021/bi00642a011. [DOI] [PubMed] [Google Scholar]
- Meyhack B., Pace B., Uhlenbeck O. C., Pace N. R. Use of T4 RNA ligase to construct model substrates for a ribosomal RNA maturation endonuclease. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3045–3049. doi: 10.1073/pnas.75.7.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyhack B., Pace N. R. Involvement of the mature domain in the in vitro maturation of Bacillus subtilis precursor 5S ribosomal RNA. Biochemistry. 1978 Dec 26;17(26):5804–5810. doi: 10.1021/bi00619a030. [DOI] [PubMed] [Google Scholar]
- Miller E. S., Winter R. B., Campbell K. M., Power S. D., Gold L. Bacteriophage T4 regA protein. Purification of a translational repressor. J Biol Chem. 1985 Oct 25;260(24):13053–13059. [PubMed] [Google Scholar]
- Misra T. K., Apirion D. Characterization of an endoribonuclease, RNase N, from Escherichia coli. J Biol Chem. 1978 Aug 25;253(16):5594–5599. [PubMed] [Google Scholar]
- Misra T. K., Apirion D. RNase E, an RNA processing enzyme from Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):11154–11159. [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa N., Imamoto F. Degradation of tryptophan messenger. On the degradation of messenger RNA for the tryptophan operon in Escherichia coli. Nature. 1969 Jul 5;223(5201):37–40. doi: 10.1038/223037a0. [DOI] [PubMed] [Google Scholar]
- Moritz A., Goebel W. Characterization of the 7S RNA and its gene from halobacteria. Nucleic Acids Res. 1985 Oct 11;13(19):6969–6979. doi: 10.1093/nar/13.19.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A., Lankat-Buttgereit B., Gross H. J., Goebel W. Common structural features of the genes for two stable RNAs from Halobacterium halobium. Nucleic Acids Res. 1985 Jan 11;13(1):31–43. doi: 10.1093/nar/13.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- Mott J. E., Galloway J. L., Platt T. Maturation of Escherichia coli tryptophan operon mRNA: evidence for 3' exonucleolytic processing after rho-dependent termination. EMBO J. 1985 Jul;4(7):1887–1891. doi: 10.1002/j.1460-2075.1985.tb03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin D. A., Garcia G. M., Walker J. R. An E. coli DNA fragment 118 base pairs in length provides dnaY+ complementing activity. Cell. 1984 Jun;37(2):669–674. doi: 10.1016/0092-8674(84)90399-4. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. SOME OBSERVATIONS ON THE "LATENT" RIBONUCLEASE OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Jun;51:1267–1274. doi: 10.1073/pnas.51.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Glazier D., Schlessinger D. Cleavage by ribonuclease III of the complex of 30 S pre-ribosomal RNA and ribosomal proteins of Escherichia coli. J Mol Biol. 1975 May 15;94(2):301–304. doi: 10.1016/0022-2836(75)90085-6. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Seiki M., Yoshikawa H. Replication origin region of Bacillus subtilis chromosome contains two rRNA operons. J Bacteriol. 1983 Apr;154(1):50–57. doi: 10.1128/jb.154.1.50-57.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kuwano M. A conditional lethal mutation in an Escherichia coli strain with a longer chemical lifetime of messenger RNA. J Mol Biol. 1979 Apr 15;129(3):343–357. doi: 10.1016/0022-2836(79)90500-x. [DOI] [PubMed] [Google Scholar]
- Ono T., Ohnishi Y. Degradation of ribosomal RNA in bacteriophage lambda lysogens after thermal induction. Microbiol Immunol. 1981;25(5):433–444. doi: 10.1111/j.1348-0421.1981.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Pace B., Stahl D. A., Pace N. R. The catalytic element of a ribosomal RNA-processing complex. J Biol Chem. 1984 Sep 25;259(18):11454–11458. [PubMed] [Google Scholar]
- Panayotatos N., Truong K. Cleavage within an RNase III site can control mRNA stability and protein synthesis in vivo. Nucleic Acids Res. 1985 Apr 11;13(7):2227–2240. doi: 10.1093/nar/13.7.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T., Whiteley H. R. Purification and properties of a new bacillus subtilis RNA processing enzyme. Cleavage of phage SP82 mRNA and Bacillus subtilis precursor rRNA. J Biol Chem. 1983 Oct 25;258(20):12487–12493. [PubMed] [Google Scholar]
- Pastushok C., Kennell D. Residual polarity and transcription-translation coupling during recovery from chloramphenicol or fusidic acid. J Bacteriol. 1974 Feb;117(2):631–640. doi: 10.1128/jb.117.2.631-640.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. V., Cech T. R. Coupling of Tetrahymena ribosomal RNA splicing to beta-galactosidase expression in Escherichia coli. Science. 1985 May 10;228(4700):719–722. doi: 10.1126/science.2986286. [DOI] [PubMed] [Google Scholar]
- Raziuddin, Chatterji D., Ghosh S., Burma D. P. Site of action of RNase I on the 50 S ribosome of Escherichia coli and the association of the enzyme with the partially degraded subunit. J Biol Chem. 1979 Nov 10;254(21):10575–10578. [PubMed] [Google Scholar]
- Reed R. E., Altman S. Repeated sequences and open reading frames in the 3' flanking region of the gene for the RNA subunit of Escherichia coli ribonuclease P. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5359–5363. doi: 10.1073/pnas.80.17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. E., Baer M. F., Guerrier-Takada C., Donis-Keller H., Altman S. Nucleotide sequence of the gene encoding the RNA subunit (M1 RNA) of ribonuclease P from Escherichia coli. Cell. 1982 Sep;30(2):627–636. doi: 10.1016/0092-8674(82)90259-8. [DOI] [PubMed] [Google Scholar]
- Rice P. W., Dahlberg J. E. A gene between polA and glnA retards growth of Escherichia coli when present in multiple copies: physiological effects of the gene for spot 42 RNA. J Bacteriol. 1982 Dec;152(3):1196–1210. doi: 10.1128/jb.152.3.1196-1210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D. Escherichia coli ribonuclease III cleavage sites. Cell. 1982 Oct;30(3):669–672. doi: 10.1016/0092-8674(82)90270-7. [DOI] [PubMed] [Google Scholar]
- Rossi J., Egan J., Hudson L., Landy A. The tyrT locus: termination and processing of a complex transcript. Cell. 1981 Nov;26(3 Pt 1):305–314. doi: 10.1016/0092-8674(81)90199-9. [DOI] [PubMed] [Google Scholar]
- Roy M. K., Apirion D. Purification and properties of ribonuclease E, an RNA-processing enzyme from Escherichia coli. Biochim Biophys Acta. 1983 Sep 28;747(3):200–208. doi: 10.1016/0167-4838(83)90098-5. [DOI] [PubMed] [Google Scholar]
- Roy M. K., Singh B., Ray B. K., Apirion D. Maturation of 5-S rRNA: ribonuclease E cleavages and their dependence on precursor sequences. Eur J Biochem. 1983 Mar 1;131(1):119–127. doi: 10.1111/j.1432-1033.1983.tb07238.x. [DOI] [PubMed] [Google Scholar]
- Sahagan B. G., Dahlberg J. E. A small, unstable RNA molecule of Escherichia coli: spot 42 RNA. I. Nucleotide sequence analysis. J Mol Biol. 1979 Jul 5;131(3):573–592. doi: 10.1016/0022-2836(79)90008-1. [DOI] [PubMed] [Google Scholar]
- Sahagan B. G., Dahlberg J. E. A small, unstable RNA molecule of Escherichia coli: spot 42 RNA. II. Accumulation and distribution. J Mol Biol. 1979 Jul 5;131(3):593–605. doi: 10.1016/0022-2836(79)90009-3. [DOI] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Processing of mRNA by ribonuclease III regulates expression of gene 1.2 of bacteriophage T7. Cell. 1981 Dec;27(3 Pt 2):533–542. doi: 10.1016/0092-8674(81)90395-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Kimura N., Nagawa F., Shimura Y. Nucleotide sequence and stability of the RNA component of RNase P from a temperature-sensitive mutant of E. coli. Nucleic Acids Res. 1983 Dec 10;11(23):8237–8251. doi: 10.1093/nar/11.23.8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Shimura Y. Sequential processing of precursor tRNA molecules in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3369–3373. doi: 10.1073/pnas.72.9.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D., Jacobs K. A., Gupta R. S., Kano Y., Imamoto F. Decay of individual Escherichia coli trp messenger RNA molecules is sequentially ordered. J Mol Biol. 1977 Mar 5;110(3):421–439. doi: 10.1016/s0022-2836(77)80107-1. [DOI] [PubMed] [Google Scholar]
- Schmidt F. J., McClain W. H. Transfer RNA biosynthesis. Alternate orders of ribonuclease P cleavage occur in vitro but not in vivo. J Biol Chem. 1978 Jul 10;253(13):4730–4738. [PubMed] [Google Scholar]
- Shen V., Cynamon M., Daugherty B., Kung H., Schlessinger D. Functional inactivation of lac alpha-peptide mRNA by a factor that purifies that Escherichia coli RNase III. J Biol Chem. 1981 Feb 25;256(4):1896–1902. [PubMed] [Google Scholar]
- Shen V., Imamoto F., Schlessinger D. RNase III cleavage of Escherichia coli beta-galactosidase and tryptophan operon mRNA. J Bacteriol. 1982 Jun;150(3):1489–1494. doi: 10.1128/jb.150.3.1489-1494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirdeshmukh R., Krych M., Schlessinger D. Escherichia coli 23S ribosomal RNA truncated at its 5' terminus. Nucleic Acids Res. 1985 Feb 25;13(4):1185–1192. doi: 10.1093/nar/13.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirdeshmukh R., Schlessinger D. Why is processing of 23 S ribosomal RNA in Escherichia coli not obligate for its function? J Mol Biol. 1985 Dec 5;186(3):669–672. doi: 10.1016/0022-2836(85)90139-1. [DOI] [PubMed] [Google Scholar]
- Skinner R. H., Stark M. J., Dahlberg A. E. Mutations within the 23S rRNA coding sequence of E. coli which block ribosome assembly. EMBO J. 1985 Jun;4(6):1605–1608. doi: 10.1002/j.1460-2075.1985.tb03824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Meyhack B., Pace N. R. Recognition of local nucleotide conformation in contrast to sequence by a rRNA processing endonuclease. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5644–5648. doi: 10.1073/pnas.77.10.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Pace B., Marsh T., Pace N. R. The ribonucleoprotein substrate for a ribosomal RNA-processing nuclease. J Biol Chem. 1984 Sep 25;259(18):11448–11453. [PubMed] [Google Scholar]
- Stahl D. A., Walker T. A., Meyhack B., Pace N. R. Precursor-specific nucleotide sequences can govern RNA folding. Cell. 1979 Dec;18(4):1133–1143. doi: 10.1016/0092-8674(79)90226-5. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Gourse R. L., Dahlberg A. E. Site-directed mutagenesis of ribosomal RNA. Analysis of ribosomal RNA deletion mutants using maxicells. J Mol Biol. 1982 Aug 15;159(3):417–439. doi: 10.1016/0022-2836(82)90292-3. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Gourse R. L., Jemiolo D. K., Dahlberg A. E. A mutation in an Escherichia coli ribosomal RNA operon that blocks the production of precursor 23 S ribosomal RNA by RNase III in vivo and in vitro. J Mol Biol. 1985 Mar 20;182(2):205–216. doi: 10.1016/0022-2836(85)90339-0. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Gregory R. J., Gourse R. L., Thurlow D. L., Zwieb C., Zimmermann R. A., Dahlberg A. E. Effects of site-directed mutations in the central domain of 16 S ribosomal RNA upon ribosomal protein binding, RNA processing and 30 S subunit assembly. J Mol Biol. 1984 Sep 15;178(2):303–322. doi: 10.1016/0022-2836(84)90146-3. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Suryanarayana T., Burma D. P. Substrate specificity of Salmonella typhimurium RNAase III and the nature of products formed. Biochim Biophys Acta. 1975 Nov 4;407(4):459–468. doi: 10.1016/0005-2787(75)90299-3. [DOI] [PubMed] [Google Scholar]
- Szeberényi J., Roy M. K., Vaidya H. C., Apirion D. 7S RNA, containing 5S ribosomal RNA and the termination stem, is a specific substrate for the two RNA processing enzymes RNase III and RNase E. Biochemistry. 1984 Jun 19;23(13):2952–2957. doi: 10.1021/bi00308a016. [DOI] [PubMed] [Google Scholar]
- Takata R., Mukai T., Hori K. Attenuation and processing of RNA from the rpsO-pnp transcription unit of Escherichia coli. Nucleic Acids Res. 1985 Oct 25;13(20):7289–7297. doi: 10.1093/nar/13.20.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura F., Nishimura S., Ohki M. The E. coli divE mutation, which differentially inhibits synthesis of certain proteins, is in tRNASer1. EMBO J. 1984 May;3(5):1103–1107. doi: 10.1002/j.1460-2075.1984.tb01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomcsányi T., Apirion D. Processing enzyme ribonuclease E specifically cleaves RNA I. An inhibitor of primer formation in plasmid DNA synthesis. J Mol Biol. 1985 Oct 20;185(4):713–720. doi: 10.1016/0022-2836(85)90056-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. Control of ColE1 plasmid replication: the process of binding of RNA I to the primer transcript. Cell. 1984 Oct;38(3):861–870. doi: 10.1016/0092-8674(84)90281-2. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30 s ribosomes studied in vitro. J Mol Biol. 1969 Mar 28;40(3):391–413. doi: 10.1016/0022-2836(69)90161-2. [DOI] [PubMed] [Google Scholar]
- Varenne S., Buc J., Lloubes R., Lazdunski C. Translation is a non-uniform process. Effect of tRNA availability on the rate of elongation of nascent polypeptide chains. J Mol Biol. 1984 Dec 15;180(3):549–576. doi: 10.1016/0022-2836(84)90027-5. [DOI] [PubMed] [Google Scholar]
- Watson N., Apirion D. Molecular cloning of the gene for the RNA-processing enzyme RNase III of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Feb;82(3):849–853. doi: 10.1073/pnas.82.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Wilder D. A., Lozeron H. A. Differential modes of processing and decay for the major N-dependent RNA transcript of coliphage lambda. Virology. 1979 Dec;99(2):241–256. doi: 10.1016/0042-6822(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Wireman J. W., Sypherd P. S. In vitro assembly of 30S ribosomal particles from precursor 16S RNA of Escherichia coli. Nature. 1974 Feb 22;247(5442):552–554. doi: 10.1038/247552a0. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakada D. Translation of T7 RNA in vitro without cleavage by RNase III. J Virol. 1976 Jun;18(3):1155–1159. doi: 10.1128/jvi.18.3.1155-1159.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C., Green L., Miller C. G. Peptide accumulation during growth of peptidase deficient mutants. J Mol Biol. 1980 Oct 15;143(1):35–48. doi: 10.1016/0022-2836(80)90123-0. [DOI] [PubMed] [Google Scholar]
- Yuki A. Detection of precursor 16 s ribosomal RNA in the polysome fraction of Escherichia coli. J Mol Biol. 1971 Nov 14;61(3):739–744. doi: 10.1016/0022-2836(71)90077-5. [DOI] [PubMed] [Google Scholar]
- Zaniewski R., Petkaitis E., Deutscher M. P. A multiple mutant of Escherichia coli lacking the exoribonucleases RNase II, RNase D, and RNase BN. J Biol Chem. 1984 Oct 10;259(19):11651–11653. [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. The intervening sequence RNA of Tetrahymena is an enzyme. Science. 1986 Jan 31;231(4737):470–475. doi: 10.1126/science.3941911. [DOI] [PubMed] [Google Scholar]
- Zweib C., Dahlberg A. E. Structural and functional analysis of Escherichia coli ribosomes containing small deletions around position 1760 in the 23S ribosomal RNA. Nucleic Acids Res. 1984 Sep 25;12(18):7135–7152. doi: 10.1093/nar/12.18.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]


