Abstract
Staphylococcus aureus is a notorious pathogen highly successful at developing resistance to virtually all antibiotics to which it is exposed. Staphylococcal phage 2638A endolysin is a peptidoglycan hydrolase that is lytic for S. aureus when exposed externally, making it a new candidate antimicrobial. It shares a common protein organization with more than 40 other reported staphylococcal peptidoglycan hydrolases. There is an N-terminal M23 peptidase domain, a mid-protein amidase 2 domain (N-acetylmuramoyl-L-alanine amidase), and a C-terminal SH3b cell wall-binding domain. It is the first phage endolysin reported with a secondary translational start site in the inter-lytic-domain region between the peptidase and amidase domains. Deletion analysis indicates that the amidase domain confers most of the lytic activity and requires the full SH3b domain for maximal activity. Although it is common for one domain to demonstrate a dominant activity over the other, the 2638A endolysin is the first in this class of proteins to have a high-activity amidase domain (dominant over the N-terminal peptidase domain). The high activity amidase domain is an important finding in the quest for high-activity staphylolytic domains targeting novel peptidoglycan bonds.
Keywords: Bacteriophage, Endolysin, M23 peptidase domain, SH3b cell wall-binding domain, Staphylococcus aureus
Introduction
Staphylococcus aureus is a notorious pathogen with a high capacity for resistance development. Resistant S. aureus strains exist to virtually every known antibiotic. Endolysins help nascent phage escape their host by degrading peptidoglycan, the major structural component of bacterial cell walls. Phage and host have co-evolved such that, for those species examined, no endolysin-resistant host strains have been identified despite efforts to find them (Fischetti 2005), putting phage endolysin antimicrobials in a class that is highly refractory to resistance development. Our lab is interested in identifying staphylococcal endolysins that might serve to stem the tide of S. aureus-resistant strain development. To ensure our antimicrobials are refractory to resistance development, we have created fusion antimicrobials with three active lytic domains (Donovan et al. 2009), in the belief that rarely can a bacterium evade three unique, simultaneous, synergistic lytic activities. To identify novel domains, we recently collated the SH3b cell wall-binding domain containing staphylococcal peptidoglycan hydrolases (Becker et al. 2009b) from public datasets, including many with dual lytic domains. In as far as amino acid sequence can alter protein properties or affinities, the 2638A endolysin was of interest as a poorly conserved member of the SH3b-containing endolysins (<50 % identity) and thus potentially harboring novel sequences that might convey antimicrobial activity in diverse environments.
Phage endolysins are known to be modular in structure (Diaz et al. 1990;Garcia et al. 1990;Donovan et al. 2006a), and there are numerous examples where single domains are functional without the need for the second lytic domain or the cell wall-binding domain (Becker et al. 2009a;Donovan et al. 2006c;Donovan et al. 2006b). It is important to demonstrate “lysis from without” for each lytic domain when considering them as potential antimicrobials. Toward this end, we isolated the 2638A gene, created deletion constructs to isolate each lytic domain, expressed the constructs in Escherichia coli, and analyzed the purified proteins for relative antimicrobial potential.
Materials and methods
Plasmids and mutagenesis
The phage 2638A endolysin gene was isolated from S. aureus 2854 (HER 1283; University Laval, Quebec, Canada) genomic DNA using PCR (primers described in Table 1). The resultant fragment was subcloned into pET21a (Novagen) E. coli expression vector on NdeI and XhoI restriction enzyme sites (construct 2638A 1–486; Fig. 1), expressed, and purified from strain BL21 (DE3) essentially as described previously (Becker et al. 2009a). Seven deletion constructs were created via PCR cloning technology in pET21a (conferring a C-terminal 6 X His tag) as described previously (Becker et al. 2009a) using primers in Table 1 (2638A 1–196; 2638A 1–220; 2638A 1–244; 2638A 1–220::355–486; 2638A 1–411; 2638A 139–411; 2638A 139–486). A four-primer PCR site-directed mutagenesis protocol utilizing the primers 2638a CTC 180 mutant F, 2638a CTC 180 mutant R, 2638 NdeI-1 F, and 2638 XhoI-486R (Table 1) was performed as described previously (http://www.csun.edu/~hcbio027/biotechnology/lec5/lec5.html). The PCR fragment harboring the mutation was subcloned into pET21a. All constructs were sequence-verified.
Table 1.
Primers used in making 2638A constructs
| Primers | Sequences | Construct |
|---|---|---|
| 2638 NdeI-1 F | 5′-TAA GAA GGA GAT ATA CAT ATG CTA ACTGCT | 2638A 1–486, 2638A 1–196, 2638A 1–220, 2638A 1–244, 2638A 1–411, 2638A 1–220::355–486 |
| 2638 XhoI-196R | 5′-CCTTGAATACTCTC GAG TGG TGC T | 2638A 1–196 |
| 2638 XhoI-220R | 5′-T CTC ACG TGC CTC GAG CCA TGG TAA G | 2638A 1–220, 2638A 1–220::355–486 |
| 2638 XhoI-244R | 5′-C TGT CGG ATG ATA CTC GAG CAC TTC | 2638A 1–244 |
| 2638 NdeI-139 F | 5′-TTA CAA TTA CGC CAT ATG GAC GCA A | 2638A 139–411, 2638A 139–486 |
| 2638 XhoI-355 F | 5′-ATC AAA CAT CTC GAG GAC GGT GGA | 2638A 1–220::355–486 |
| 2638 XhoI-411R | 5′-TCC CTC TGG CTC GAG CAC TGT GAA C | 2638A 1–411 |
| 2638 XhoI-486R | 5′-GTG GTG GTG GTG CTC GAG TTT AAT TTC G | 2638A 1–486, 2638A 1–220::355–486, 2638A 139– 486 |
| 2638A Nde F | 5′-ATC GAC ATA TGC TAA CTG | 2638A 1–180Mut-486, 2638A 180–486 |
| 2638A Xho R | 5′-GTG GTG CTC GAG TTT AAT TTC GC | 2638A 1–180Mut-486 |
| 2638a CTC 180 mutant F | 5′-GTG AAA GAG CTC AAA CAT ATC TAT TC | 2638A 1–180Mut-486 |
| 2638a CTC 180 mutant R | 5′-GAT ATG TTT GAG CTC TTT CAC GCT CC | 2638A 1–180Mut-486 |
| pET21a Bgl II F | 5′-GAG GAT CGA GAT CTC GAT CCC GCG AAA | 2638A 1–180Mut-486 |
| pET21a Sty I R | 5′-CGT TTA GAG GCC CCA AGG GGT TAT G | 2638A 1–180Mut-486 |
| 2638A NdeI-180 F | 5′-CGC GCG CGC ATATGA AAC ATATCTATT CAA ACC | 2638A 180–486 |
Underlined nucleotides indicate either mutagenized bases or XhoI or NdeI restriction enzyme sites used for cloning the PCR-generated DNA fragment into pET21a
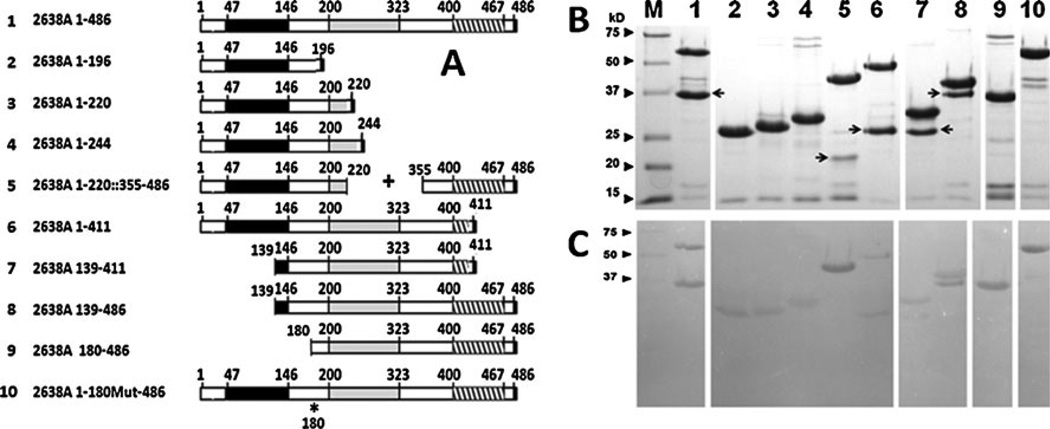
Fig 1.
Schematic, SDS-PAGE, and zymogram analysis of endolysin 2638A constructs. a Schematic of constructs. Black boxes indicate M23 peptidase domain; Gray boxes indicate amidase domain; Striped boxes indicate SH3b domain. Amino acid positions are numbered. Asterisk indicates mutated amino acid 180. b SDS-PAGE analysis (shadow bands are indicated with arrows) and c Zymogram analysis. Gels were loaded (5 µg) and electrophoresed identically. Zymograms contained S. aureus strain Newman, were rinsed in water to remove SDS, and soaked in 50 mM Phosphate, 150 mM NaCl, pH 7.5 for 2.5 h. Lane and predicted molecular weights of each construct: Lane M, Kaleidoscope Molecular Weight Markers (Bio-Rad); Lane 1, 2638A 1–486, 56.6 kD; Lane 2, 2638A 1–196, 23.4 kD; Lane 3, 2638A 1–220, 25.9 kD; Lane 4, 2638A 1–244, 28.6 kD; Lane 5, 2638A 1–220::355–486, 41.25 kD; Lane 6, 2638A 1–411, 48 kD; Lane 7, 2638A 139–411, 32.4 kD; Lane 8, 2638A 139– 486, 40.9 kD; Lane 9, 2638A 180–486, 36.3 kD; Lane 10, 2638A 1– 180Mut-486, 56.6 kD. Arrows shadow band
Strains
Staphylococcal strains used included methicillin-resistant S. aureus (MRSA) strain (CSA #175, SRCAMB collection), S. epidermidis (ATCC 14990), S. aureus Strain Newman (ATCC 25904), a gift from Jean Lee, Channing Lab, Brigham and Women’s Hospital, Boston, MA), and S. aureus strain NRS119 (NARSA; Network on Antimicrobial Resistance in S. aureus).
Recombinant protein expression and purification
For protein expression, E. coli B21 (DE3) (EMDBiosciences) cultures harboring pET21a expression vectors were grown at 37 °C in Luria-Bertani broth with ampicillin (100 µg/ml) to an OD600 nm of 0.4–0.6, on ice for 30 min., induced with 1 mM isopropyl-b-D-thiogalactopyranoside and grown at 10 °C for 18 h. Harvested E. coli were lysed via sonication and 6×His-tagged proteins isolated via nickel-chromatography Ni-NTA (Qiagen, Valencia, CA) as described previously (Donovan and Foster-Frey 2008; Becker et al. 2009).Wash and elution profiles were 10 ml of 10mMimidazole, 20 ml of 20 mM imidazole, and elution with 0.5 ml of 250 mM imidazole in the same phosphate-buffered saline (PBS) (50 mM NaH2PO3, 300 mM NaCl, pH 8.0) with 30 % glycerol and filter sterilized. Sterilized protein preparation was stored at 4 °C until the time of assay.
SDS-PAGE and zymogram analysis
Using 15 % SDS-PAGE, 5 µg of each purified proteins and Kaleidoscope protein standards (Bio-Rad) were analyzed with or without 300 ml equivalents of mid-log phase S. aureus cells (OD600 nm of 0.4–0.6). The SDS-PAGE and zymogram were run simultaneously. SDS gels were Coomassie stained. Zymograms were soaked in excess water for 1 h and incubated at room temperature in PBS, resulting in areas of clearing in the turbid gel identifying a lytic protein.
Plate lysis assay
Plate lysis assays were described previously (Donovan and Foster-Frey, 2008; Becker et al. 2009). In brief, purified proteins for each construct were filter sterilized and diluted in sterile PBS buffer with 30 % glycerol. Ten microliters containing 10, 1, or 0.1 µg of test protein were spotted onto a freshly spread lawn of mid-log phase (OD600 nm~0.4–0.6) of either S. aureus cells that had air-dried for 10 min on tryptic soy agar plates. Spotted plates were air-dried in a laminar flow hood (10 min), and incubated overnight at 37 °C. Cleared spots were scored within 20 h of plating.
Turbidity reduction assay
Turbidity reduction assays were performed as described previously in a 96-well dish format using frozen viable S. aureus strain Newman cells as substrate (Becker et al. 2009b). In brief, substrate cells were added to enzyme dilutions in buffer so that the initial OD600 nm of the suspension in a total volume of 0.2 ml equaled 1.0, and OD600 nm was determined every 20 s for 5 min by a 96-well plate reader. Peaks in ΔOD (i.e., the steepest slope of the resulting lysis curve) were determined by a sliding 40-s window, and reported results are within the linear range of the assay. Results are presented as specific activities (ΔOD600 nm/µM/min).
Results
The 486-amino acid phage 2638A endolysin (Genbank Accession number AAX90995) harbors an N-terminal M23 peptidase domain (http://pfam.sanger.ac.uk/family?acc=PF01551), a mid-protein amidase 2 domain (N-acetylmuramoyl-Lalanine amidase; http://www.ebi.ac.uk/QuickGO/GTerm?id=GO:0008745), and a C-terminal SH3b_5 (SH3b) (http://pfam.sanger.ac.uk/family/PF08239) cell wall-binding domain (Fig. 1 construct 2638A 1–486).
Deletion analysis reveals a co-purifying shadow band
SDS-PAGE analysis revealed >90 % purity of the resultant purified proteins, except for five of the constructs that extended across the inter-domain region between the peptidase and amidase domains (2638A 1–486; 2638A 1–220::355– 486; 2638A 1–411; 2638A 139–411; 2638A 139–486). In these five constructs, there was a consistent second “shadow” band that was co-isolated at high concentration and purity (Fig. 1). The predicted size of the shadow band protein was consistent between those constructs that terminated at the same residue (e.g., 2638A 1–486 and 2638A 139–486 vs. 2638A 1–411 and 2638A 139–411), suggesting either a favored protein degradation site or a secondary translational start site. All full-length constructs and the shadow bands (except for 2638A 1–220::355–486) showed staphylolytic activity (zones of clearing) in the zymogram (Fig. 1), indicating that (1) the N-terminal M23 peptidase domain was enzymatically active with or without the SH3b cell wall-binding domain (2638A 1–196; 2638A 1–220; 2638A 1–244; 2638A 1–220::355–486), and (2) the amidase domain was active with or without the full length SH3b domain (2638A 139–411; 2638A 139–486). Zymograms are not usually interpreted quantitatively (especially since these were loaded with µg equivalents and not molar equivalents of enzyme) but rather are used to indicate that the minor contaminating bands in the preparations are not contributing to the activity of the preparation. There was virtually no activity from any of the minor (non-shadow) bands in the zymogram assay after extended periods. However, the presence of nearly equal amounts of the active shadow band (from stained SDS gel) in many preparations negated the ability to quantify the activity from either the isolated amidase domain (2638A 139–411; 2638A 139–486) or the full-length construct.
Protein sequencing identifies the N-terminus of the shadow band
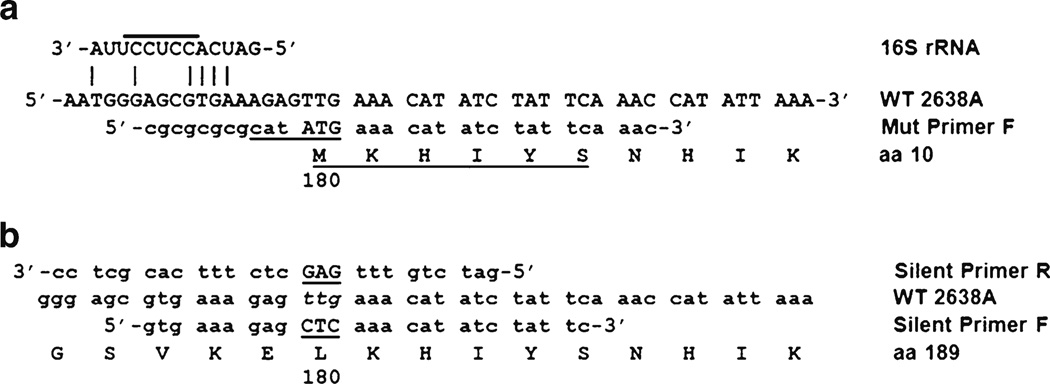
In order to identify the source of the shadow band, it was extracted from the SDS gel (from the full-length construct 2638A 1–486 sample) using standard methods and commercially subjected to six cycles of Edman degradation N-terminal protein sequencing (M-SCAN, West Chester, PA). The amino acid sequence obtained was MKHIYS. The last five residues of KHIYS matched perfectly the residues at position 181–184 of the full-length 2638A endolysin protein (Fig. 2), which was consistent with the predicted size of the shadow band from the SDS-PAGE (~37 kD). The N-terminal methionine residue matched the predicted amino acid sequence of a protein expressed from a secondary translational start site (TTG) at residue 180 thru 486 (36.3 kD), of the published DNA sequence. Codon 180, TTG, is a known translational start codon in E. coli (Blattner et al. 1997) that is present in 2 % of E. coli genes (Starmer et al. 2006). There was not a canonical E. coli Shine–Dalgarno (SD) ribosome binding site (UAAGGAGGU) in the 2638A gene sequences immediate upstream of codon 180, but there is a region of similarity to the 3′ end of the E. coli 16 S ribosomal RNA sequence (Fig. 2a) located within the 5–13 nt pre-cistronic spacing between the SD and translational start codon considered optimal for expression in E. coli (Chen et al. 1994). These lines of evidence suggested that the TTG codon 180 encoded a translational start site. To test this secondary translational start site hypothesis, a construct with two silent mutations was created where the TTG codon 180 was altered through site-directed mutagenesis to an alternative (CTC) codon that still codes for leucine but did not resemble a translational start site (construct 2638A 1– 180Mut-486; Fig. 1; illustrated in Fig. 2b). The resultant construct (2638A 1–180Mut-486; Fig. 1) does not have a shadow band in either the SDS or zymogram gels, making it very likely that our alternative translational start-site hypothesis was correct. The single lytic protein product from this construct allowed us to quantify the activity of the full-length 2638A endolysin.
Fig 2.
DNA and protein sequences near codon 180 of 2638A endolysin, shadow protein, and 2638A 180–486 construct. a Edman degradation analysis of the shadow band in SDS-PAGE analysis yielded underlined protein sequences. Nde I restriction enzyme recognition sequence (underlined) for cloning of the 2638A 180–486 PCR fragment into the pET21a multi-cloning site. Nde I site includes the ATG start of translation for the 2638A 180–486 truncation construct. A potential SD binding site for the 3′ end of the E. coli 16 S rRNA sequence (UCCUCC) is overlined. Identity is indicated with vertical bars. b Silent Primer R and Silent Primer F were used in the site-directed mutagenesis PCR strategy to create the 2638A 1–180Mut-486
In order to test the activity of the amidase domain (+ SH3b) construct in the absence of the contaminating shadow band protein, we created a construct via PCR cloning that initiated at codon 180 (2638A 180–486; Fig. 1 and described in Fig. 2a). In the SDS-PAGE (Fig. 1), this construct expressed a single major protein band as predicted, and none of the minor contaminating bands contributed to any activity in the zymogram analysis.
Lytic activity of the 2638A endolysin on live staphylococci
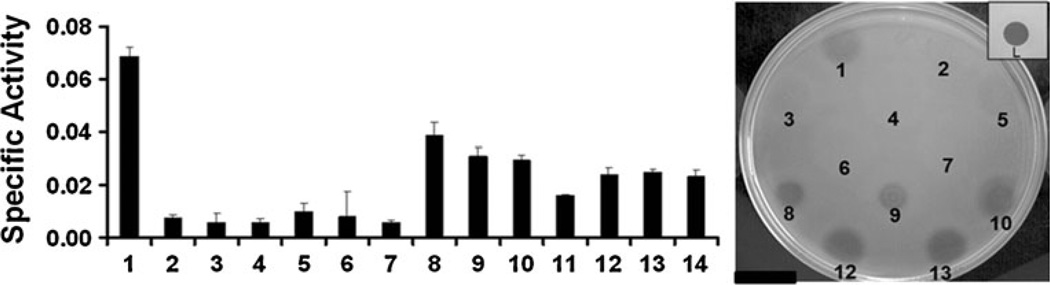
Staphylolytic activity was further characterized with two quantitative peptidoglycan hydrolase assays, turbidity reduction, and plate lysis. It is apparent from the zymogram analysis (Fig. 1) that both the M23 peptidase and the amidase domains are enzymatically active on SDS treated S. aureus cells. However, in turbidity reduction assays (Fig. 3), the M23 peptidase domain isolating constructs show minimal activity (2638A 1–196, 2638A 1–220, 2638A 1–244) on live, non-SDS-treated S. aureus cells. The full-length SH3b domain does not seem to enhance the activity of the M23 peptidase domain (2638A 1–220::355–486), but it appears essential to the activity of the amidase domain, as indicated by the low activity of the 2638A 139–411 construct with a full amidase but truncated SH3b domain. Lytic activity is also minimal for the 2638A 1–411 construct (M23 peptidase + amidase) dual domain construct lacking the full length SH3b domain. These results were verified in a second strain of S. aureus BAC170190 (data not shown). Only in those constructs where there is a full-length SH3b and amidase domain is there appreciable activity (2638A 139–486, 2638A 180–486, 2638A 1–180Mut-486). The amidase domain appears to be contributing the majority of the lytic activity. However, the turbidity reduction assay results indicate that the parent full-length construct (2638A 1–486), including its shadow band, shows the highest activity in the turbidity reduction assay (Fig. 3) of all constructs. The exact ratio of shadow band/ full-length construct is unknown as they are produced and purified simultaneously in the nickel column purified preparation. Protein sequence analysis described above indicates that the shadow band produced by the full-length construct (2638A 1–486) is the same protein as produced by construct 2638A 180–486. It was reasoned that the protein mixture might be the source of the enhanced activity. To test this hypothesis, a series of mixing experiments were performed where defined molar ratios of both the repaired full-length construct (2638A 1–180Mut-486) and the 2638A 180–486 amidase construct were added in the turbidity reduction assay in an effort to mimic the ratio of full length to shadow band produced by the parent construct (2638A 1–486). Although it is impossible to know the exact concentration of the full length and shadow band in the 2638A 1–486 construct, 0.5 µMof the full length repaired construct corresponds to 5.7 µg of protein in a volume of 200 µl. Thus 5.7 µg of the full length + shadow band was used in the turbidity reduction assay for comparison. Molar ratios of 1:1, 1:3, and 3:1 (2638A 1– 180Mut-486 repaired : 2638A 180–486 amidase), performed at room temperature (Fig. 3) and even after heating the mixtures to 42 °C for 1 h (to potentially allow heterodimer formation; data not shown) did not yield activity levels that approached the naturally occurring double band product produced by 2638A 1–486.
Fig 3.
Turbidity reduction and plate lysis assay results of 2638A constructs. Sample numbering is the same between the turbidity reduction and plate lysis spots. Turbidity reduction assay (S. aureus strain Newman) contained 0.5 µM protein (5.7 µg of full length repaired construct: 2638A 1–180Mut-486 in 200-µl assay) unless otherwise noted. Lane 1, 2638A 1– 486 (5.7 µg total protein in 200-µl assay); Lane 2, 2638A 1–196; Lane 3, 2638A 1–220; Lane 4, 2638A 1–244; Lane 5, 2638A 1–220::355–486; Lane 6, 2638A 1–411; Lane 7, 2638A 139–411; Lane 8, 2638A 139–486; Lane 9, 2638A 180–486; Lane 10, 2638A 1–180Mut-486. Mixing reactions between the repaired full-length construct 2638A 1–180Mut- 486 (R) and the engineered amidase-SH3b construct 2638A 180–486 (A) were performed in the following molar ratios: Lane 11, R/A=1:1, 1 µM total protein concentration; Lane 12, R/A=1:1, 0.5 µM; Lane 13, R/A= 1:3, 0.5 µM; and Lane 14, R/A=3:1, 0.5 µM. Specific activity=ΔOD600 nm/µM/min. Plate lysis assay: S. aureus strain NRS119 (SA LinR #12), linezolid resistant. L=1 µg Lysostaphin (Sigma); Spot 1=11 µg; all other constructs are 0.2 nmol (~11 µg for the construct 2638A 1–180Mut-486) spotted in 10 µl
There was weak turbidity reduction activity from the 2638A 1–486 parental construct on MRSA strain (CSA #175, SRCAMB collection) and no activity on S. epidermidis (ATCC 14990) (data not shown).
The plate lysis assay results on live cells (Fig. 3) agree with the turbidity reduction assay. Each of the M23 peptidase-dependent or SH3b-truncating constructs show weak activity on S. aureus strain NRS119 (Network on Antimicrobial Resistance in S. aureus). In addition to the plate lysis results in Fig. 3, we have also examined 22 other staphylococcal strains that show susceptibility to the 2638A endolysin lytic activity (data not shown). However, the plate lysis results with the 2638A endolysin constructs are extremely novel in appearance. Plate lysis results are routinely visualized as a discrete cleared spot on a lawn of bacteria that remains that way for days or weeks, as seen for Lysostaphin in Fig. 3. In contrast, the 2638A results never show a discrete spot, rather there is a very broad, ill-defined region of clearing that grows with time, up to 4 days, suggesting that the enzyme is still active for 4 days and has a heightened diffusion in the media compared to other peptidoglycan hydrolases.
Discussion
The staphylococcal phage 2638a endolysin is one of many staphylococcal endolysins with a peptidase–amidase–SH3b protein architecture (Becker et al. 2009b). It is highly lytic for S. aureus in turbidity reduction, zymogram, and plate lysis assays. It is the only staphylococcal endolysin reported with a secondary translational start site located in the interlytic- domain region.
The finding that the 2638A amidase domain is highly active and the M23 peptidase domain appears nearly inactive is unexpected and in direct opposition to the results of studies with similar proteins, e.g., the staphylococcal LysK (phage K endolysin) and phage phi11 endolysin. Despite a nearly identical protein organization in all three proteins (peptidase–amidase–SH3b), the LysK amidase domain was virtually inactive in constructs where it was isolated, although it was shown to be active in the context of the whole protein (Becker et al. 2009a). Similarly, the phi11 endolysin amidase domain was virtually inactive when isolated in a deletion construct (Sass and Bierbaum, 2007). In contrast, the cysteine, histidine-dependent amidohydrolases/peptidases (CHAP) endopeptidase (Bateman and Rawlings, 2003;Rigden et al. 2003) domain isolating constructs from both the phi11 endolysin (Sass and Bierbaum, 2007; Donovan et al. 2006c) and LysK (Horgan et al. 2009; Becker et al. 2009a) demonstrate strong lytic activity. Despite readily observed zones of clearing in the zymogram (Fig. 1), the 2638A M23 peptidase domain constructs show very little to no activity in the turbidity reduction or plate lysis assays (Fig. 3). Although confounding, it has been reported that the same enzyme when tested in multiple staphylococcal peptidoglycan hydrolase assays can yield very different quantitative results (Kusuma and Kokai-Kun, 2005). There are numerous unique constructs of the 2638A M23 peptidase domain that extend into the amidase domain, reducing the likelihood that the low activity of the peptidase domain is due to cutting the domain sequences too short and inadvertently clipping off essential domain sequences required for activity. In fact, it is often the shorter deletion constructs that favor endolysin domain lytic activity as has been shown for staphylococcal LysK constructs (Horgan et al. 2009;Becker et al. 2009a) and streptococcal PlyGBS vs. B30 endolysin constructs (Cheng and Fischetti, 2007;Donovan et al. 2006b) where specific (shorter) CHAP harboring constructs show higher lytic activity than slightly longer constructs.
There are similar levels of expression in the SDS-PAGE (Fig. 1) for the predicted full-length construct and associated shadow bands for four of the constructs (2638A 1–486, 2638A 1–411, 2638A 139–411, and 2638A 139–486) where the inter-domain sequences included codon 180 (the secondary TTG translational start site). It was unexpected that expression from the parental pET21a ATG translation start site (commercially optimized for expression with a near consensus E. coli SD sequence AGGGAG), would be at a level similar to that of the codon 180 (with a poorly used TTG translational start site and poorly conserved SD sequence (Fig. 2)). However, it should be remembered that this expression is from a high copy (~40/cell) plasmid and thus expression levels might be near the upper limit of expression possible, such that the expected differences are masked. The construct where the inter-domain region did not yield high shadow band expression levels (2638A 1– 220::3256–486 (Fig. 1)) might have problems achieving a stable tertiary structure in the shadow band resulting in the shadow protein being unstable, or sequestered in inclusion bodies and unavailable via our native protein isolation procedures.
Another confounding result in this study is that in the turbidity reduction assay, the parent construct 2638A 1–486 (contaminated with the shadow band) shows almost twice the activity of either the repaired full-length construct 2638A 1– 180Mut-486 or the engineered amidase + SH3b construct 2638A 180–486. It was hypothesized that there might be an interaction (e.g., dimerization) between the full-length enzyme and the naturally occurring shadow band 2638A 180– 486 that yields this higher activity. The results of the mixing reactions where the repaired full length was mixed with the engineered amidase + SH3b construct (Fig. 3) indicate no higher activity level in our spiked assays, although this does not exclude dimerization that might occur during co-synthesis from the same mRNA. There was no apparent classical Pribnow-Gilbert −35 or −10 promoter consensus sequence (TTGACA -17- TATAAT) in the upstream sequences, suggesting a bicistronic mRNA, rather than a secondary interlytic domain promoter element.
The presence and use of the codon 180 TTG secondary translational start site in this E. coli system begs the question of whether or not this codon 180 translational start site is functional in S. aureus. Our results do not address this question, but one study suggests that TTG translational start codons are used in 8 % of the S. aureus genes examined, a much higher frequency than the 2 % of E. coli genes cited in the same work (Starmer et al. 2006). A search for S. aureus SD sequences has identified several: AGAGAG, AGAAAG (Strommenger et al. 2004), GGAGGG (East and Dyke 1989), AAAGGAG (Jones and Khan 1986), and AAAG GAAGGAATTA (Cuny and Witte 1996). A cursory comparison of these published sequences to the DNA sequences shown in Fig. 2, immediately 5′ to codon 180 (5′-AAA GAATGGGAGCGTGAAAGAGTTG-3′) (codon 180 is underlined) reveals that there are numerous potential/partial binding sites for these staphylococcal SD sequences, suggesting that the use of the codon 180 as a translational start site in S. aureus is likely. We realize that this comparison is superficial, as we do not know if these sequences reflect the 3′ 16 S rRNA sequences from a S. aureus host strain of the 2638A phage.
If the 180 codon TTG translational start site is functional in S. aureus, then the location of the TTG secondary translational start site in the inter-lytic-domain region supports the hypothesis that gene fusions are in part responsible for the evolution of the dual lytic domain endolysin genes as has been hypothesized for the evolution of dual lytic domain lysin genes (Donovan et al. 2006b). This hypothesis is further supported by a study of SH3b-containing staphylococcal lysin genes where among 26 dual lytic domain lysins genes that encode proteins with >90 % amino acid identity, there are two genes that contain inter-lytic-domain introns (Becker et al. 2009b). The location of these introns suggests a role for exon hopping in the formation of dual lytic domain genes. Here, the presence of a functional translational start site and ribosome binding site in the inter-lytic-domain region of a gene encoding a similarly organized protein (peptidase–amidase– SH3b) further supports the notion that a gene fusion event occurred at some point.
An alternative consideration: If the codon 180 TTG translational start site did derive from a gene fusion event but is not required for protein function, then why has it been maintained through the millennia of evolution? One explanation might lie in the heightened endolysin activity derived from the bicistronic gene in E. coli. Turbidity reduction assays suggest that when both the full length and the shadow band proteins are putatively produced from the same transcript, there is a ~2× heightened staphylolytic activity. If there is a selective advantage to this heightened activity, this might explain evolutionary maintenance of this site.
Regardless of advantages that might be conferred by the dual translational start sites, the 2638A endolysin is a potent antimicrobial with a uniquely active amidase domain that we predict will be a good addition to future antimicrobial constructs. The plate lysis pattern of a broad, ill-defined region of clearing observed with each of the 2638A constructs that either harbor or lack the SH3b cell wall-binding domain suggests that the 2638A lysin might have properties that will make it a very unique antimicrobial with unusual staphylolytic properties in highly ordered or structured settings such as mucosal membranes, e.g., nasal decolonization. Certainly, these diffusion results indicate that the enzyme is likely active for several days in the plate assay, or at least as long as required for the diffusion to occur. We are eager to test the 2638A endolysin constructs in novel environments to determine if the unique plate lysis phenotype is predictive of novel environments where this endolysin will find special application.
Acknowledgments
Thanks to Sylvain Moineau and Genevieve Rousseau for the strain S. aureus 2854. This work was supported in part by National Institutes of Health, grant 1RO1AI075077-01A1 (DMD); National Research Initiative grant 2007-35204-18395 (DMD) and US State Dept funds supporting a US–Pakistani (DMD) and US–Russian collaboration (IA, DMD). Mentioning of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual's income is derived from any public assistance program. USDA is an equal opportunity provider and employer.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Igor Abaev, State Research Center for Applied Microbiology and Biotechnology (SRCAMB), Obolensk, Moscow Region 142279, Russia.
Juli Foster-Frey, Animal Biosciences and Biotechnology Lab, ANRI, ARS, USDA, Bldg 230, Room 104, BARC-East, 10300 Baltimore Ave., Beltsville, MD 20705-2350, USA.
Olga Korobova, State Research Center for Applied Microbiology and Biotechnology (SRCAMB), Obolensk, Moscow Region 142279, Russia.
Nina Shishkova, State Research Center for Applied Microbiology and Biotechnology (SRCAMB), Obolensk, Moscow Region 142279, Russia.
Natalia Kiseleva, State Research Center for Applied Microbiology and Biotechnology (SRCAMB), Obolensk, Moscow Region 142279, Russia.
Pavel Kopylov, State Research Center for Applied Microbiology and Biotechnology (SRCAMB), Obolensk, Moscow Region 142279, Russia.
Sergey Pryamchuk, State Research Center for Applied Microbiology and Biotechnology (SRCAMB), Obolensk, Moscow Region 142279, Russia.
Mathias Schmelcher, Animal Biosciences and Biotechnology Lab, ANRI, ARS, USDA, Bldg 230, Room 104, BARC-East, 10300 Baltimore Ave., Beltsville, MD 20705-2350, USA.
Stephen C. Becker, Animal Biosciences and Biotechnology Lab, ANRI, ARS, USDA, Bldg 230, Room 104, BARC-East, 10300 Baltimore Ave., Beltsville, MD 20705-2350, USA
David M. Donovan, Email: david.donovan@ars.usda.gov, Animal Biosciences and Biotechnology Lab, ANRI, ARS, USDA, Bldg 230, Room 104, BARC-East, 10300 Baltimore Ave., Beltsville, MD 20705-2350, USA.
References
- Bateman A, Rawlings ND. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem Sci. 2003;28:234–237. doi: 10.1016/S0968-0004(03)00061-6. [DOI] [PubMed] [Google Scholar]
- Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG, Donovan DM. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol Lett. 2009a;294:52–60. doi: 10.1111/j.1574-6968.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene. 2009b;443:32–41. doi: 10.1016/j.gene.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K- 12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Chen H, Bjerknes M, Kumar R, Jay E. Determination of the optimal aligned spacing between the Shine–Dalgarno sequence and the translation initiation codon of Escherichia coli mRNAs. Nucleic Acids Res. 1994;22:4953–4957. doi: 10.1093/nar/22.23.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Fischetti VA. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbiol Biotechnol. 2007;74:1284–1291. doi: 10.1007/s00253-006-0771-1. [DOI] [PubMed] [Google Scholar]
- Cuny C, Witte W. Typing of Staphylococcus aureus by PCR for DNA sequences flanked by transposon Tn916 target region and ribosomal binding site. J Clin Microbiol. 1996;34:1502–1505. doi: 10.1128/jcm.34.6.1502-1505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E, Lopez R, Garcia JL. Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc Natl Acad Sci USA. 1990;87:8125–8129. doi: 10.1073/pnas.87.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Foster-Frey J. LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol Lett. 2008;287:22–33. doi: 10.1111/j.1574-6968.2008.01287.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Dong S, Garrett W, Rousseau GM, Moineau S, Pritchard DG. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl Environ Microbiol. 2006a;72:2988–2996. doi: 10.1128/AEM.72.4.2988-2996.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Foster-Frey J, Dong S, Rousseau GM, Moineau S, Pritchard DG. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl Environ Microbiol. 2006b;72:5108–5112. doi: 10.1128/AEM.03065-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Lardeo M, Foster-Frey J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett. 2006c;265:133–139. doi: 10.1111/j.1574-6968.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Becker SC, Dong S, Baker JR, Foster-Frey J, Pritchard DG. Peptidoglycan hydrolase enzyme fusions for treating multi-drug resistant pathogens. Biotech International. 2009;21:6–10. [Google Scholar]
- East AK, Dyke KG. Cloning and sequence determination of six Staphylococcus aureus beta-lactamases and their expression in Escherichia coli and Staphylococcus aureus. J Gen Microbiol. 1989;135:1001–1015. doi: 10.1099/00221287-135-4-1001. [DOI] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 2005;13:491–496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Garcia P, Garcia JL, Garcia E, Sanchez-Puelles JM, Lopez R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- Horgan M, O'Flynn G, Garry J, Cooney J, Coffey A, Fitzgerald GF, Ross RP, McAuliffe O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibioticresistant staphylococci. Appl Environ Microbiol. 2009;75:872–874. doi: 10.1128/AEM.01831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Khan S. Nucleotide sequence of the enterotoxin B genes from Staphylococcus aureus. J Bacteriol. 1986:166. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma C, Kokai-Kun J. Comparison of four methods for determining lysostaphin susceptibility of various strains of Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:3256–3263. doi: 10.1128/AAC.49.8.3256-3263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden DJ, Jedrzejas MJ, Galperin MY. Amidase domains from bacterial and phage autolysins define a family of gamma-D, L-glutamate- specific amidohydrolases. Trends Biochem Sci. 2003;28:230–234. doi: 10.1016/s0968-0004(03)00062-8. [DOI] [PubMed] [Google Scholar]
- Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage {phi}11 and {phi}12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol. 2007;73:347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer J, Stomp A, Vouk M, Bitzer D. Predicting Shine– Dalgarno sequence locations exposes genome annotation errors. PLoS Comput Biol. 2006;2:e57. doi: 10.1371/journal.pcbi.0020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strommenger B, Cuny C, Werner G, Witte W. Obvious lack of association between dynamics of epidemic methicillin-resistant Staphylococcus aureus in central Europe and agr specificity groups. Eur J Clin Microbiol Infect Dis. 2004;23:15–19. doi: 10.1007/s10096-003-1046-8. [DOI] [PubMed] [Google Scholar]