Abstract
Toxoplama gondii (Apicomplexa: Coccidia), an obligatory intracellular parasite with a unique capacity to invade virtually all nucleated cell type from warm-blooded vertebrate hosts. Despite the efficiency with which Toxoplasma enters its host cell, it remains unresolved if invasion occurs by direct penetration of the parasite or through phagocytosis. In the present work, electron microscopic study was designed to examine the entry process of Toxoplasma (RH strain) into macrophages and non phagocytic-host cells (Hela cells) and to observe the ultrastructure changes associated with intracellular parasitism. The results showed that both active invasion and phagocytosis were occurred and revealed that invasion is an ordered process that initiates with binding of the parasite at its apical end followed by tight-fitting invagination of the host cell membrane and a prominent constriction in the parasite at the site of penetration. The process ended by the professional parasitophorous vacuole that is distinct at the outset from those formed by phagocytosis in which once Toxoplasma triggered, phagocytic uptake can proceed by capture of the parasite within a loose fitting vacuole formed by localized membrane ruffling. The cytopathic effects of the parasite on macrophages and Hela cells were demonstrated within 5–15 h post-inoculation in the form of degenerative mitochondria, swelling Golgi apparatus and widening of endoplasmic reticulum indicating intracellular oedema. These changes were exaggerated and several cells were found dead after 48–72 h.
Keywords: Ultrastructure, Toxoplasma gondii, Tachyzoites, Mammalian cells
1. Introduction
Toxoplasmosis is one of the most common parasitic zoonoses caused by intracellular pathogenic protozoon Toxoplasma gondii which infects nearly one-third of world population. In most immunocompetent adults, toxoplasmosis is usually symptomless but in immunocompromised individuals (AIDS patients, transplant recipients, etc. the parasite can cause severe, life-threatening disease (Hokelek, 2009). The serious problem is also posed by congenital toxoplasmosis as a result of transplacental fetus infection in pregnant women leading to the classic triad of chorioretinitis, intracranial calcifications, and hydrocephalus (Montoya and Liesenfeld, 2004).
Toxoplasma has an extremely broad host range (Dubey and Beattie, 1988) and unique capacity to invade virtually all nucleated cell types from warm-blooded vertebrate hosts (Werk, 1985). Despite the efficiency with which Toxoplasma enters its host cell, it remains unresolved if invasion occurs by direct penetration of the parasite or by induction of a host-mediated endocytic event. This has been a matter of debate for many decades. Jones et al. (1972) and Werk (1985) considered that phagocytosis is the main path for the merozoites to enter into the cells. On the other hand several authors suggested that Toxoplasma merozoites penetrated the cytoplasmic membrane (Morisaki et al., 1995; Lee et al., 2001 and Butcher and Denkers, 2002).
Since there is still no available effective vaccine against toxoplasmosis in people, further studies on the mechanisms of T. gondii pathogenicity beginning with the events of its entry to the host cell are required for the identification and implementation of the new strategies of toxoplasmosis treatment.
Therefore, in the present work, an ultrastructural study was designed to demonstrate the mechanisms of entry of Toxoplsma tachyzoites into phagocytic and non-phagocytic cells and the accompanied cytopathic changes.
2. Material and methods
2.1. Parasite
T. gondii RH strain tachyzoites were laboratory maintained by two weekly intraperitoneal passages in Balb/c mice. In order to recover Toxoplasma parasites, the parasite-rich peritoneal fluid was diluted with 2 volume of minimal essential medium (MEM) and centrifuged at 70 g for 3 min to remove cells and debris. The supernate was then centrifuged at 900g for 10 min to deposit the free Toxoplasma (Lee et al., 2001).
The pellets of harvested parasite were:
-
a)
Resuspended in culture medium {MEM, containing 20% heat inactivated fetal calf serum (HIFCS)} at a concentration of 2 × 106 and were used within 1 h after the removal from the mouse peritoneal cavity for studying the active penetration experiment through macrophages and Hela cells.
-
b)
Resuspended and incubated in Phosphate Buffer Saline (PBS) for >2 h at 37 °C to enhance parasite phagocytosis by macrophage cells according to Morisaki et al. (1995).
2.2. The cells
Macrophages: mouse peritoneal macrophages were obtained from peritoneal exudates of Balb/C mice previously injected peritoneally with LPS (Lipopolysaccharide 10 ug/0.1 ml, Sigma) for 3 days prior to the peritoneal cavities lavage with heparinized phosphate- buffered saline (Lee et al., 2001). The isolated macrophages (1 × 10) were cultured in DMEM which was supplemented with 10% FCS and 20% conditioned L 929 cell medium (Vacsera, Egypt) were allowed to adhere to round glass cover slips and maintained for reaching confluent monolayer (Morisaki et al., 1995).
HeLa cells (Vacsera, Egypt) were maintained in Falcon plastic flasks in MEM with 10% HIFCS and passed by trypsinization when the cell density approached a confluent monolayer. 2 days before use for the experiment, approximately 2 × 105 HeLa cells were placed on round cover slips 5/8 inches in diameter in Falcon dishes and maintained at 37 °C in 5% CO2 and balanced air.
Techniques for the study of parasite entry were done through:
-
A)
Inoculation of macrophage cell monolayers covered with thin film of medium with either cooled fresh or effete, those incubated for >2 h in PBS), Toxoplasma inoculums containing 1 × 106 parasite/1ml of culture medium.
-
B)
Inoculation of HeLa cells monolayer with fresh cooled Toxoplasma inoculum containing 5 × 10/6 parasites/1ml of culture medium. Each cells’ monolayer (macrophages and HeLa cells) and Toxoplasma inoculums were placed in flat-bottomed sealed vials, centrifuged at 200g for 5 min at 4 °C, then transferred to a 37 °C water bath then processed for transmission electron microscope study (TEM) at different time intervals (10 min, 30 min, 2, 5, 15, 20, 48 and 72 h) according to Mercer and Birbeck (1966) and Jones et al. (1972). A Joel 100s electron microscope was used for the examination at an accelerating voltage of 60 KV.
3. Results
3.1. Active penetration of phagocytic and non phagocytic cells
-
I)
Entrance of Toxoplasma into macrophages: Intracellular Toxoplasma organisms were observed for 10 min after the inoculation of the macrophage cell culture. Following the initial contact at the parasite apical end and macrophage cell, the parasite penetrated the cell by narrowing at the point of passage through the plasma membrane. This active penetration was characterized by a noticeable constriction of the parasite cell at the junction of the host cell membrane and the Parasite (Fig. 1), there was no membrane or space between the intracellular part of the organism and the cytoplasm of the cell (Fig. 2). As tachyzoites penetrate the macrophage, they were surrounded by a membrane derived from the host cell plasmalemma which was destined to form the parasitophorous vacuoles (PV) and parasitophorous vacuole membrane (PVM). This membrane was found to be incomplete, and these vacuoles remained segregated. Two hours after inoculation, multiple infections of the cell culture with Toxoplasma parasites were detected (Fig. 3).
-
II)
Entrance of Toxoplasma into HeLa cells: The parasite was present in the cytoplasm of HeLa 10 min post inoculation. No distinct continuous space or membrane was present between the parasites and the cytoplasm of HeLa cells (Fig. 4). Mitochondria and rough endoplasmic reticulum were situated close to the PV (Fig. 5). Multiplications of the parasites within the vacuoles were seen 2–5 h post-inoculation (Fig. 6).
Figure 1.
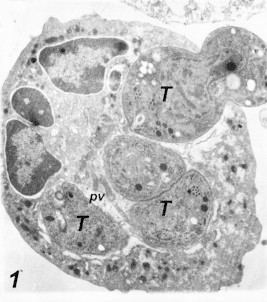
Entrance of Toxoplasma into macrophage 2 h post-infection. Notice the parasitic constriction at its entrance, multiple parasitic infections and the surrounding parasitophorous vacuoles (pv). 17,000×.
Figure 2.
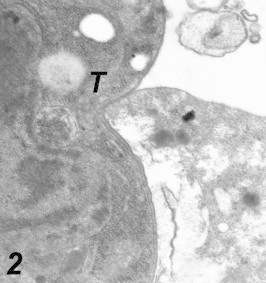
Higher magnification of the previous figure. Notice the absence of any space between the parasite and the cytoplasm of the macrophage. 55,000×.
Figure 3.
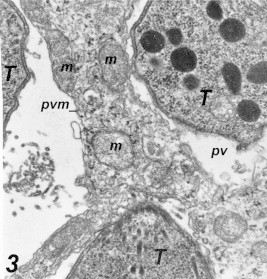
Multiple Toxoplasma parasites invading macrophage cell. The left upper parasite has well formed parasitophorous vacuole membrane (pvm) while both upper and the third one surrounded with incomplete pvm. Adjacent containing vacuoles remained segregated and did not undergo detectable fusion with each other 55,200×.
Figure 4.
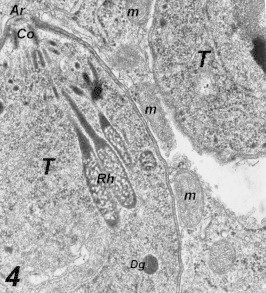
Multiple infections of a HeLa cell by Toxoplasma 2 h post-infection. No vacuole around the left one and incomplete membrane around the two parasites. Notice the fine structure f the parasite, Apical ring (Ar), Conoid (Co), Rhoptry (Rh), and Dense granule (Dg). 55,200×.
Figure 5.
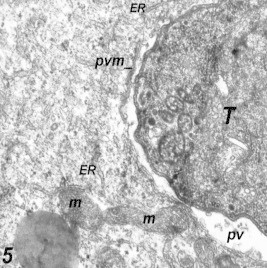
HeLa cells 2 h post-infection. Notice partial surrounding membrane (pvm). Mitochondria (m) and endoplasmic reticulum (ER) were seen surrounding the parasitic vacuole (PV). 55,200×.
Figure 6.
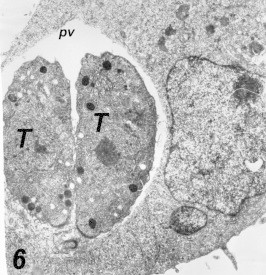
Intracytoplasmic parasitic vacuole in a HeLa cell 15 h post-infection, bounded by partial membrane. Notice multiplication of the parasite inside the vacuole. 19,200×.
3.2. Entrance of T. gondii treated with PBS into macrophage by phagocytosis
The ability of Toxoplasma parasite to invade host cells was greatly decreased as a result of its incubation with PBS for >2 h. Phagocytosis was characterized by extensive, localized ruffling of the phagocytic cell membrane that captured the parasite (Fig. 7) within a loose-fitting phagocytic vacuole surrounding with a complete membrane. Adjacent parasite-containing phagosomes were tended coalesce (Fig. 8).
Figure 7.
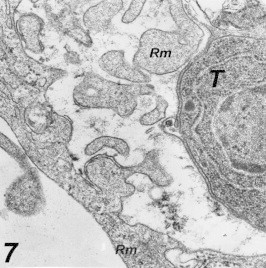
Extensive membrane ruffling (Rm) as seen by cross-sections within the membrane-lining the phagocytic vacuole in a macrophage 48 h post-infection 55,200×.
Figure 8.
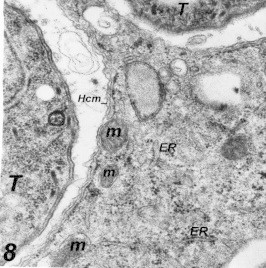
Complete host cell membrane (Hcm) surrounding the phagocytic vacuole in a macrophage 2 h post-infection. Notice multiple infections, mitochondria (m) and endoplasmic reticulum (ER) surrounding the parasites (T).
3.3. Cytopathic effect of Toxoplasma on the infected cells
Many changes were observed after Toxoplasma parasites inoculation to macrophages and HeLa cells, the arrangement of rough endoplasmic reticulum (ER) around the parasitic vacuole (PV) which appeared as incomplete membrane (Fig. 8). Mitochondria were seen surrounding some of the (PV), also widening of Golgi apparatus of the infected cells was a constant finding. Vacuolization of the cytoplasm with early degenerative changes of both nucleus and mitochondria of the infected cells and several cells were seen dead (Figs. 9–11).
Figure 9.
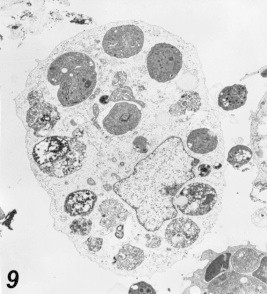
Multiple infections of macrophage 48 h post-infection, showing several tachyzoites, one in endodygony and others are free in the cytoplasm. PVM was no longer evident. Notice the intracellular oedema and the dark degenerating mitochondria. 7800×.
Figure 10.
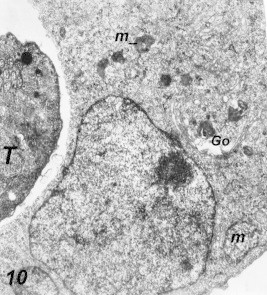
Pathogenic features in infected HeLa cells 15 h post-infection. Notice dying mitochondria (m) and widening of Golgi apparatus (Go). 35,000×.
Figure 11.
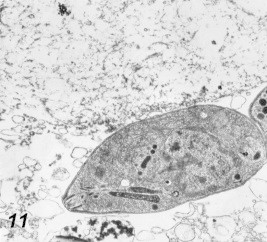
Dead macrophage 48 h post-infection. 28,000×.
4. Discussion
The present study revealed fundamental differences between Toxoplasma invasion and phagocytosis that were evident in the pattern of entry, the kinetics of vacuole formation and the ultimate fate of the vacuole within the host cell. These have been carried out by bringing both parasites and phagocytic (Macrophages) and non phagocytic cells (HeLa cells) into close contact. Concerning the mechanism of active cell penetration by Toxoplasma, Kasper and Mineo (1994) declared that the initial step of the parasite invasion process is recognition and attachment of the target cell and upon encountering the host cell, Toxoplasma will survey the membrane until an appropriate point of attachment is recognized by its apical pole. Sibley et al. (1994) showed that the attachment and penetration of host cells by T. gondii zoites (tachyzoites, bradyzoites, sporozoites and merozoites) appear similar to those described for other coccidian parasites. The same authors added that the mechanical events involved in zoites attachment and penetration include (i) gliding of the zoite, (ii) probing of the host cell with the concoidal tip of the zoite, (iii) indenting the host cell plasmalemma, (iv) forming a moving junction that moves posteriorly along the zoites as it penetrates into the host cell, and (v) partially exocytosing micronemes, rhoptries, and dense granules. Kasper and Mineo (1994) and Karsten et al. (2004) reported that zoites of T. gondii can penetrate a variety of cell types from a wide range of hosts, indicating that the biochemical receptors involved in attachment and penetration are probably common to those host animal cells. Host cell receptors consisting of laminin, lectin, and SAGI are involved in T. gondii tachyzoite attachment of penetration.
Several distinctive features of Toxoplasma entry of macrophages or HeLa cells were recorded in the present study, narrowing of the anterior end, tight-fitting between the parasite and the invaded cell and prominent constriction that occurred in the parasite at the site of penetration (Figs. 1 and 2), the same observations were recorded by Speer et al. (1997). Morisaki et al. (1995) stated that the use of time-lapse video microscopy clearly reveals that this hourglass constriction remains fixed as the parasite is propelled forward and enters the host cell. The moving junction is likely formed by an interaction between the anterior end of the parasite and some component on the host cell membrane. By capping this attachment zone from anterior to posterior, the parasite moves forward into a vacuole formed by invagination of the host cell plasma membrane (Fig. 3). The same authors added that the significance of the moving junction may lie in modulation of the contents of the parasitophorous vacuoles that is formed by invasion. More direct analysis indicates that several abundant host cell proteins are internalized during Toxoplasma invasion, but these components are rapidly removed and remain absent from mature PVs. It is possible that the exclusion of key host cell molecules during the formation of their removal shortly thereafter is responsible for the resistance of these compartments to endocytic fusion (Sibley et al., 1994).
A great deal of research on the formation of the PV has been conducted. As tachyzoites penetrate host cells, a number of the parasite proteins associate with it, including rhoptry proteins ROP2, ROP4, and ROP7. ROP2 is located on the host cell cytoplasmic side of the PVM, suggesting that it plays a role in host-parasite biochemical communication (Beckers et al., 1994). Schwab et al. (1994) and Sibley (1995) added that Dense-granule proteins (GRA) are secreted into the PV after tachyzoite penetration; these proteins establish a parasite-friendly environment within the host cell cytoplasm that is conductive to parasite replication.
The parasite–host cell interaction is more complicated in phagocytic cells since a competition occurs between the active invasion and passive phagocytosis. Regarding the process of phagocytosis, Morisaki et al. (1995) reported that when extra cellular Toxoplasma parasites were incubated in PBS for >2 h, their ability to invade cells was greatly decreased; that is, leading to an increase in uptake is by phagocytosis. In the present study, the same observation was undertaken to follow the course of internalization (phagocytosis) of the effete parasite by macrophages. The present study revealed that when the effete parasites were added to macrophage cells, a localized extensive folding of the phagocytic cell membrane that captured the parasite in a loosing-fitting compartment (phagocytic vacuole) with distinctive complete membrane surrounding the organism (Figs. 7 and 8). This result is consistent with studies on phaocytosis by Greenberg et al. (1990) and Joiner et al. (1990). Phagocytosis of Toxoplasma as described by Morisaki et al. (1995) was chaotic rather a highly ordered event and this event was morphologically identical for both effete and live organism. The authors added that on closer examination of the time-lapse recordings, it was evident that live parasites often remained loosely adherent to host cells without invading and without inducing phagocytosis, sometimes for many minutes. The avoidance of phagocytosis by live parasites indicates that Toxoplasma may actively prevent phagocytosis possibly by altering host cell signaling.
Signs of degeneration of parasite-infected cells were recorded in the present study, these degenerative changes were demonstrated in the cytoplasm of the infected macrophages and HeLa cells 5 h after infection, exaggerated by 15–20 h (Figs. 9 and 10) and cell death after 48–72 h (Fig. 11). These changes were in the form of vacuolization of the cytoplasm and intracellular oedema due to the swelling of endoplasmic reticulum and degenerated mitochondria. These findings are in accordance with Hirch et al. (1974); they suggested that a certain substance produced by the parasite or metabolic products harm the host cell in the same way.
On conclusion, the present study revealed that (a) both active penetration and phagocytosis are used by T. gondii parasites in their entry to mammalian cells and each process is fundamentally different from the other one and (b) the study of the parasite-host cell interaction in vitro at the cellular level is critically important. Further studies on the mechanism of T. gondii pathogenicity are required for the identification of new strategies of toxoplasmosis control.
References
- Beckers C.J.M., Dubremetz J.F., Mercereau-Paijalon O., Joiner K.A. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher B.A., Denkers E.Y. Mechanism of entry determines the ability of T. gondii to inhibit macrophage proinflammatory cytokine production. Infect. Immun. 2002;70(9):5216–5224. doi: 10.1128/IAI.70.9.5216-5224.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, J. P., Beattie C. P. 1988. Toxoplasmosis of animals and man. CRC Press, Inc., Boca Raton, Fla.
- Greenberg S., Burridge K., Silverstein S.C. Colocalization ofF-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J. Exp. Med. 1990;172:1853–1856. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T.G., Jones T.C., Len L. Interaction in vitro between Toxoplasma gondii and mouse cells. Ciba foundation N. 25 Symposium on parasites in the immunized host. Mech. Survival. 1974:205–223. [Google Scholar]
- Hokelek, M., 2009. Toxoplasmosis. The Author: Dr Murat Hokelek, Ondokuz Mayis university Medical school, Turkey emedicine, infectious diseases (parasitic diseases) from webMD.
- Joiner K.A., Furhman S.A., Miettinen H.M., Kaspe L.H., Mellman I. Toxoplasma gondii: fusion competence of Parasitophorous vacuoles in Fc receptors transfected fobroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Jones T.C., Yeh S., Hirsch J.G. The interaction of Toxoplasma gondii and mammalian cells. I. mechanism of entry and intracellular fate of the parasite. J. Exp. Med. 1972;136:1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten V., Hegde R.S., Sinai A.P., Yang M., Joiner K.A. Transmembrane domain modulates sorting of membrane proteins in Toxoplasma gondii. J. Biol. Chem. 2004;279:26052–26057. doi: 10.1074/jbc.M400480200. [DOI] [PubMed] [Google Scholar]
- Kasper L.H., Mineo J.R. Attachment and invasion of host cells by Toxoplasma gondii. Parasitol. Today. 1994;10:184–188. doi: 10.1016/0169-4758(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Lee B.Y., Ahn M.H., Km H.C., Min D.Y. Toxoplasma gondii: ultrastructural localization of specific antigen and inhibition of intracellular multiplication by monoclonal antibodies. Korean J. Parasitol. 2001;39(1):67–75. doi: 10.3347/kjp.2001.39.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer E.N., Birbeck M.S. 2nd ed. Blackwell Scientific Publications; Oxford: 1966. Electron Microscopy. A Hand Book for Biologists. [Google Scholar]
- Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Morisaki J.H., Heuser J.E., Sibley L.D. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci. 1995;108:2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- Schwab J.C., Beckers M., Joiner K.A. The parasitophrous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L.D., Dobrowolski J., Morisaki J.H., Heuser J.E. Invasion and intracellular survival by Toxoplasma gondii. In: Russel D.G., editor. In Strategies For Intracellular Survival of Microbes. Bailliere; Tindall, London: 1994. pp. 245–264. [Google Scholar]
- Sibley L.D. Invasion of vertebrate cells by Toxoplasma gondii. Trends Cell Biol. 1995;5:129–132. doi: 10.1016/s0962-8924(00)88964-3. [DOI] [PubMed] [Google Scholar]
- Speer C.A., Dubey J.P., Blixt J.A., Prokop K. Time lapse video microscopy and ultrastructure of penetrating sporozoites, types 1 and 2 parasitophorous vacuoles, and the transformation of sporozites to tachyzoites of the VEG strain of Toxoplasma gondii. J. Parasitol. 1997;83:565–574. [PubMed] [Google Scholar]
- Werk R. How does Toxoplasma gondii enter host cells? Rev. Infect. Dis. 1985;7:449–457. doi: 10.1093/clinids/7.4.449. [DOI] [PubMed] [Google Scholar]


