Abstract
Cyclic guanosine monophosphate (cGMP) is a key secondary messenger used in signal transduction in various types of sensory neurons. The importance of cGMP in the ASE gustatory receptor neurons of the nematode Caenorhabditis elegans was deduced by the observation that multiple receptor-type guanylyl cyclases (rGCs), encoded by the gcy genes, and two presently known cyclic nucleotide-gated ion channel subunits, encoded by the tax-2 and tax-4 genes, are essential for ASE-mediated gustatory behavior. We describe here specific mechanistic features of cGMP-mediated signal transduction in the ASE neurons. First, we assess the specificity of the sensory functions of individual rGC proteins. We have previously shown that multiple rGC proteins are expressed in a left/right asymmetric manner in the functionally lateralized ASE neurons and are required to sense distinct salt cues. Through domain swap experiments among three different rGC proteins, we show here that the specificity of individual rGC proteins lies in their extracellular domains and not in their intracellular, signal-transducing domains. Furthermore, we find that rGC proteins are also sufficient to confer salt sensory responses to other neurons. Both findings support the hypothesis that rGC proteins are salt receptor proteins. Second, we identify a novel, likely downstream effector of the rGC proteins in gustatory signal transduction, a previously uncharacterized cyclic nucleotide-gated (CNG) ion channel, encoded by the che-6 locus. che-6 mutants show defects in gustatory sensory transduction that are similar to defects observed in animals lacking the tax-2 and tax-4 CNG channels. In contrast, thermosensory signal transduction, which also requires tax-2 and tax-4, does not require che-6, but requires another CNG, cng-3. We propose that CHE-6 may form together with two other CNG subunits, TAX-2 and TAX-4, a gustatory neuron-specific heteromeric CNG channel complex.
Keywords: C. elegans, gcy genes, gustation
THE identification and subsequent molecular characterization of mutant Caenorhabditis elegans strains defective in sensing specific environmental parameters has revealed many components of signal transduction pathways in sensory neurons (Bargmann 2006; Sengupta 2007). Among the genes identified by mutant analysis are those coding for several distinct cyclic guanosine monophosphate (cGMP)-generating guanylyl cyclases (GCs), cGMP-dependent protein kinase, as well as cyclic nucleotide-gated (CNG) channels (Coburn and Bargmann 1996; Komatsu et al. 1996; Birnby et al. 2000; Daniels et al. 2000; L’Etoile and Bargmann 2000; Cheung et al. 2004; Gray et al. 2004; Inada et al. 2006; Pradel et al. 2007; Ortiz et al. 2009). These genes are expressed in different types of sensory neurons and are required for sensation of odorants, gustatory cues, temperature, bacterial pathogens, and ambient oxygen levels. cGMP has therefore emerged as a key signal transducer for various sensory modalities.
While the cGMP dependence of many sensory systems is now well established, sensory receptors that trigger the cGMP-dependent signaling cascades are only characterized only for some, but not all sensory modalities. In C. elegans, seven transmembrane olfactory receptors as well as photoreceptors couple to GCs and CNGs via heterotrimeric G proteins (Bargmann 2006; Liu et al. 2010). How other cGMP-dependent sensory modalities, such as taste or temperature, are coupled to GCs and CNGs is not understood simply because the respective receptor systems have not been well defined.
Taste is often categorized into five modalities: sweet, bitter, salty, sour, and umami (the taste of glutamate or amino acids). In vertebrates and invertebrates, sweet, bitter, and umami tastes are thought to be sensed by specific types of G-protein-coupled receptors (GPCRs) (Scott 2005). Attractive responses to low salt concentrations are mediated by ion channels of the epithelial sodium channel (EnaC) type (Chandrashekar et al., 2010). However, vertebrate ENaC channels are sodium selective, yet worms sense distinct types of salt cations and anions (Ward 1973; Ortiz et al. 2009); moreover, amiloride does not block the behavioral response of C. elegans to NaCl (Hukema 2006). Therefore, salt receptor molecules remain to be identified in C. elegans.
The ASE neurons, consisting of a pair of morphologically symmetric cells (ASEL and ASER) are the main taste receptor neurons in C. elegans. Laser ablation analysis demonstated that they are required to process a variety of distinct taste cues, including amino acids, salts, and other small molecules (Bargmann and Horvitz 1991). Many and perhaps all of the cues that are processed by the ASE neurons are sensed in a left/right asymmetric manner and also trigger distinct outputs (Pierce-Shimomura et al. 2001; Suzuki et al. 2008; Ortiz et al. 2009). For example, sodium ions are sensed by the ASEL neuron and trigger run behavior upon increases in sodium concentration, while chloride ions are sensed by the ASER neuron and trigger turning behavior upon decreases in chloride concentration (Suzuki et al. 2008). Previous genetic analyses have identified two CNG channel subunits, encoded by the ASEL/R-expressed tax-2 and tax-4 genes, as being required for all ASE-mediated sensory processes (Coburn and Bargmann 1996; Komatsu et al. 1996). Moreover, we have previously shown that cGMP-generating, receptor-type guanylyl cyclase (rGC) proteins, encoded by the gcy genes, are required for sensing a number of distinct salt ions (Ortiz et al. 2009). Curiously, with almost 30 representatives, rGC-encoding gcy genes have significantly expanded in Caenorhabditis genomes and almost one-third of them are expressed in a left/right asymmetric manner in the ASEL and ASER neurons (Ortiz et al. 2006).
Genetic loss-of-function analysis has shown that specific gcy genes endow ASEL and ASER with the ability to sense specific distinct salt ions (Figure 1A) (Ortiz et al. 2009). For example, ASER-expressed gcy-1 is required to efficiently respond to potassium, but not other salt ions, while gcy-4 is required for animals to respond efficiently to bromide and iodide ions (Ortiz et al. 2009). The sensory modality-specific function of gcy genes was unanticipated since work in other systems (e.g., the vertebrate retina; Koch et al. 2002) or worm olfactory and photosensory neurons (Bargmann 2006; Liu et al. 2010) suggests that rGC proteins may only serve as intermediary signal transducers that are activated by sensory modality-specific GPCRs through G proteins. The observation that individual C. elegans rGC proteins act to transduce specific salt sensory information within the ASE neurons suggests the intriguing possibility that transmembrane rGC proteins themselves may serve as salt receptor proteins. Receptor functions for rGC proteins are indeed well characterized in the vertebrate system, though not in sensory function. Rather, vertebrate rGC proteins act as receptors for small peptides in several distinct tissue types; these peptides stimulate the intracellular rGC activity of the receptor protein (Wedel and Garbers 2001; Potter 2011).
Figure 1.
GCY protein function. (A) Expression and function of gcy genes in the ASE gustatory neurons, as previously reported (Ortiz et al. 2006, 2009). (B) Schematic depiction of rGC domains. (C) Schematic of rGC chimeras generated and tested in this study.
In this article, we further investigate the hypothesis that C. elegans rGC proteins may themselves be salt receptors. To address this hypothesis, we asked whether the specificity in rGC function lies in their extracellular domain, as one would expect if they were receptor proteins, or whether specificity lies in their intracellular domain. The latter would be consistent with a possibility in which different rGC proteins couple to distinct upstream signaling inputs. We also test whether their ectopic expression in other sensory neurons endows these neurons with the capacity to respond to specific salt ions.
In an attempt to shed more light on rGC-mediated signal transduction in the ASE neurons, we also determined the molecular identity of a novel regulator of gustatory signal transduction, encoded by the che-6 gene. che-6 was identified as a chemotaxis mutant >30 years ago (Lewis and Hodgkin 1977). We find that che-6 encodes a CNG channel that likely acts directly downstream of rGC proteins. While this CNG is required for ASE salt transduction, it is not required for the transduction of several other sensory cues in other sensory neurons, in which we implicate instead a previously uncharacterized cng gene, cng-3.
Materials and Methods
Mutant alleles
che-5(e1073)IV, che-6(e1126)IV, che-7(e1128)V (Lewis and Hodgkin 1977), gcy-22(tm2364)V, gcy-4(tm1653)II, gcy-1(tm2669)II (Ortiz et al. 2009), che-1(ot66)I, cng-3(jh113)IV (Cho et al. 2004), tax-2(ot25)I (Sarin et al. 2007), tax-4(p678)III (Dusenbery et al. 1975), and che-6(tm5036)IV were kindly provided by Shohei Mitani, National Bioresource Project, Japan, and che-7(ok2373)V was kindly provided by the Oklahoma and Vancouver C. elegans knock out consortium.
DNA constructs for transgenic line construction
A list of transgenic lines can be found in Supporting Information, File S1.
Expression wild-type and chimeric GCY receptors:
The full-length rescue constructs carried by strains (gcy-1(tm2669)II; otEx5076, gcy-4(tm1653)II; otEx5101, gcy-22(tm2364)V; otEx5120) for gcy-1, gcy-4, and gcy-22, respectively, were made by using a PCR fragment covering the entire gene locus and injected into the gcy-1, gcy-4, and gcy-22, mutant background, respectively, as a simple array at 15 ng/µl with 5 ng/µl elt-2::gfp as a co-injection marker.
Chimera constructs were made by fusing PCR fragments from N2 genomic DNA that encode the extracellular domain and transmembrane domain of one GCY to the intracellular domain of another. Domains were predicted with the SMART server (Letunic et al. 2012). The junction sites for the individual GCY proteins are:
GCY-1: .....[.... LIMIIGCLCVI]trans[GKRAERARI.....]intra
GCY-4: .....[....AIAVTILILLAIII]trans [CMSSKIRNRR...]intra
GCY-22: .....[....AAALVLIIAVISTI]trans [VFLVRSKRQE...]intra.
The resulting chimeric PCR products was then subcloned into pPD95.75 with the ASER-specific gcy-5 promoter (containing the sequence 305 bp upstream of the gcy-5 translational start site) using the restriction sites, added onto the PCR primers (noted below). The sites were designed to allow removal of GFP from the vector. Chimeras containing the extracellular domain of a specific GCY protein were injected into animals mutant for the respective gcy gene (at 25 ng/µl of DNA as a simple array together with 50 ng/µl of the elt-2::gfp marker). Three lines were scored for chemotaxis behavioral rescue and subsequently crossed into the gcy mutant background that matches the intracellular domain of the respective chimera and then tested for rescue of behavioral phenotypes. The gcy mutant genotypes were followed by PCR. Plasmids are as follows:
pHKS015: gcy-5prom::gcy-4Extra(aa1-aa518)::gcy-22Intra(aa462-aa1012)], cloned into pPD95.75, using KpnI/EcoRI, injected into gcy-4(tm1653) and crossed into gcy-22(tm2364). Array names: otEx5102-otEx5104.
pHKS016: [gcy-5prom::gcy-22Extra(aa1-aa461)::gcy-4Intra(aa519-aa1143)], cloned into pPD95.75, using AgeI/EcoRI, injected into gcy-22(tm2364) and crossed into gcy-4(tm1653). Array names: otEx5105-otEx5107.
pHKS017: [gcy-5prom::gcy-4Extra(aa1-aa518)::gcy-1Intra(aa520-aa1137)], cloned into pPD95.75, using KpnI/EcoRI, injected into gcy-4(tm1653) and crossed into gcy-1(tm2669). Array names: otEx5086-otEx5088.
pHKS018: [gcy-5prom::gcy-1Extra(aa1-aa519)::gcy-4Intra(aa519-aa1143)], cloned into pPD95.75, using AgeI/EcoRI, injected into gcy-1(tm2669) and crossed into gcy-4(tm1653). Array names: otEx5083-otEx5085.
pHKS019: [gcy-5prom::gcy-1Extra(aa1-aa519)::gcy-22Intra(aa462-aa1012)], cloned into pPD95.75, using AgeI/EcoRI, injected into gcy-1(tm2669) and crossed into gcy-22(tm2364). Array names: otEx5077-otEx5079.
pHKS020: [gcy-5prom::gcy-22Extra(aa1-aa461)::gcy-1Intra(aa520-aa1137)], cloned into pPD95.75, using AgeI/EcoRI, injected into gcy-22(tm2364) and crossed into gcy-1(tm2669). Array names: otEx5080-otEx5082.
Pansensory heterologous expression:
The gcy-4 and gcy-22 genomic loci (from start to stop codon) were PCR amplified from genomic DNA and subsequently subcloned into pPD95.75 containing the osm-6 promoter (containing sequences 2083 bp upstream of the osm-6 locus, relative to its translational start site) using the specific insertion sites noted below that removed the GFP from the vector. In both cases the DNA was injected at 25 ng/µl as a simple array and either elt-2::DsRed or elt-2::gfp was used as a co-injection marker at 50 ng/µl.
ASI-specific heterologous expression:
The gcy-4 and gcy-22 genomic loci (from start to stop codon) were PCR-amplified from genomic DNA and subsequently subcloned into the pPD95.77 vector containing the srg-47 promoter, kindly provided by Piali Sengupta. In the case of the gcy-4 and gcy-22 alone the DNA was injected at 25 ng/µL as a simple array and myo-2::gfp was used as a co-injection marker at 2 ng/µL. In the case of the double injection, the gcy-4 and gcy-22 were each injected as simple arrays at 2.5 ng/µL each with myo-2::gfp as a co-injection marker at 3 ng/µL.
pHKS031: srg-47prom::gcy-4, cloned into pPD95.77 using EagI/KpnI. Array names: otEx5349, otEx5351, otEx5352.
pHKS032: srg-47prom::gcy-22, cloned into pPD95.77 using EagI/KpnI. Array names: otEx5353-otEx5355.
Array names for simultaneous injection of pHKS031 and pHKS032 are otEx5358-otEx5361.
Transformation rescue of che mutants:
For che-7 rescue, fosmid WRM0620bH04 was injected into che-7(e1128) animals at 5 ng/µL, 3 ng/µL elt-2::gfp as a co-injection marker as well as 130 ng/µL genomic bacterial array (array name: otEx5063). The che-6 mutant phenotype was rescued with a genomic DNA clone and by expression with a cell-type specific promoter. For the genomic rescue, the che-6 locus was amplified from coordinates 2103929-2111708 (103 bp before the start site to 127 bp after the end of the last exon) by PCR and the PCR product was injected at 15 ng/µL with 5 ng/µL elt-2::gfp as a co-injection marker. Array names: otEx5064-otEx5066
pHKS021 (ceh-36prom::che-6) was generated using the pPD95.75 vector containing sequences 1852 bp upstream of the ceh-36 locus, relative to its translational start site. The full length che-6 that was generated by PCR from genomic N2 DNA was inserted using the AgeI/XhoI cut sites and the DNA was injected at 25 ng/µL as a simple array and 50 ng/µL of the elt-2::gfp marker. Array names: otEx5154-otEx5156.
che-6 reporter gene:
The che-6prom::gfp transcriptional reporter was made by PCR fusion as described previously (Hobert 2002). 772 bp of sequences upstream of che-6 were fused by PCR to a gfp::unc-543′UTR fragment using the two primers “che-6prom::gfp fusion forward” (GGGCAAATTCTGTGAACCATATTCCT) and “che-6prom::gfp fusion reverse” (GGAAACAGTTATGTTTGGTATATTGGG). The gfp::unc-543′UTR fragment was PCR amplified from the plasmid pPD95.75. The resulting PCR fusion was PCR purified and injected into wildtype (N2) animals at 80 ng/μl alongside the co-injection marker elt-2::gfp at 50 ng/μl. The resulting transgenic array name is hanEx24.
Chemotaxis assays
Two types of chemotaxis assays were used: radial gradient assays and population assays. A radial gradient assay was used if transgenic extrachromosomal DNA-containing animals were scored so that individual transgenic worms could be picked and assayed. In this assay, four animals were placed around the circumference of a salt gradient 1 cm away from the peak formed by application of 10 μl of 1M salt attractant spotted 14–16 hr before assay and a second 4-μl drop of 1 M salt attractant spotted 3–4 hr before assay. This assay is modified from previous single worm tracking assays (Pierce-Shimomura et al. 2001).The plates used in this assay were identical to those used in the population assays except for the concentration of the gradient. After the worms were placed on the plate around the circumference of the gradient the recording started within 1 min. Behavior was recorded continuously for 15 min using a USB microscope (GSI High-Definition Scientific Digital LED Microscope) over the plate while the cover of the Petri dish remained on to avoid drying. A circle of red LED lights around the plate illuminated the worms while the assays were carried out in the dark to increase the contrast for scoring. The videos were converted to Quicktime movies and subsequently scored for time spent within the peak of the gradient. In this case the chemotaxis index (CI) was calculated as gradient tracking assay CI = (time in seconds spent in the peak for 4 worms)/(total time). The total time was 3,600 sec to account for four worms multiplied by the length of the assay (900 s). For these assays n represented the average of two assays done in duplicate on the same day. Therefore n = 1 represents the average of two plates.
The population assay is based on a protocol described previously (Chang et al. 2004; Ortiz et al. 2009), with some minor modifications. Buffered agar (20 g/L agar, 1 mM CaCl2, 1 mM MgSO4, 5 mM KPO4) similar to the plates worms are maintained on for routine usage were used as assay plates with the exception of a saturating concentration of 100mM NH4Ac added to counteract the NH4+ and Ac− responses of individual ions. 10-cm diameter Petri dishes were filled with 10 mL and allowed to cool for 1–3 hr. A salt gradient was established opposite a control gradient (water) by adding 10 µl of 2.5 M salt solutions (adjusted to pH = 6 with either NH4OH or acetic acid) to the attractant spot, and 10 µl of ddH2O to the control spot. After 12–16 hr an additional 4 µL of salt solution and ddH2O was added. After 4 hr the assays were carried out by adding between 50–250 synchronized adult worms that had been washed 3 times with M9 buffer. Using a glass Pasteur pipette the worms were transferred to the center of the plate with minimal liquid. The remaining liquid was removed with a tissue so the worms were using normal taxis across the plate and not swimming motion in remaining liquid. Two to five minutes before the worms are placed on the plate, 2 µl of 1M sodium azide was added to the salt spot as well as the control spot. The worms naturally disperse from the center point and explore the plate. When wild-type worms encounter the salt gradient they move up the gradient and this anesthetizes the worms and locks them into their position. The worms that moved across the control spot by chance are accounted for in the equation for the chemotaxis index. The worms were left at between 20–23° for exactly 1 hr before being placed at 4° to be counted the next day. The chemotaxis index (CI) was calculated as population assay CI = (# worms in attractant − # worms in control)/(total # of worms). Worms that failed to move from the center spot were not counted in the assay. Each n represented the average of two assays done in duplicate on the same day. In this manner, a hypothetical n = 1 represents 100–500 worms.
For odortaxis assay, we also used a population assay, essentially as previously described (Colburn and Bargmann 1996). Specifically, we used buffered agar plates (20 g/L agar, 1 mM CaCl2, 1 mM MgSO4, 5 mM KPO4) for the assays and placed between 50-100 synchronized worms at the center point. This assay is quite similar to the radial gradient population assay in that the odortaxis test spot is placed diametrically opposite to the control spot. The sodium azide (2 µl of 1 M) was added at the control and odor points. The worms are then allowed to explore the plate for 1 hr at room temperature and the sodium azide acts to anesthetize the worms at each spot. Because the assays tests for odor the droplets were placed on the cover of the plate and once the worms were placed in the center the plate was intverted onto the cover. The benzaldehyde was diluted to 1:200 in ethanol. The control spot placed opposite is of the odorant in this case is ethanol alone. The odortaxis index is calculated in the same manner as the population assays for salt chemotaxis.
Statistical analyses
All statistical tests were completed GraphPad Prism 6. The data for all behavioral assays of chemotaxis indices were represented as the mean ± SEM. Comparisons were made using Student’s two-tailed t-test assuming equal variance when comparing less than 5 groups. When more than 2 comparisons were made a correction factor was utilized in the Student’s t-test to adjust the P-value for multiple comparisons. When testing more than 5 comparisons a one-way ANOVA was used comparing the mean of each group with the mutant mean and the Holm–Sidak correction was applied. The Holm–Sidak correction or Bonferroni adjustment for multiple comparisons was used based on the number of comparisons being made and the n value to minimize the elimination of false positive without creating false negatives in the process.
Thermotaxis
Our linear thermal gradients apparatus is a larger and improved version of the one we previously designed (Ryu and Samuel 2002). Each end of an anodized aluminum slab (24 inches × 12 inches × ¼ inch) was fixed at a specific temperature under thermal electric control (Oven Industries). A 22 cm × 22 cm agar plate was placed in the middle of aluminum slab to establish a linear thermal gradient of 18–22° across the agar surface. In each assay, 15–20 young adult worms raised at 20° were washed in NGM buffer (ref) before being released in the middle of the agar surface (20°). Videos were captured using a CCD camera (Mightex Systems, BCE-B050-U) at 2 Hz for 20 min.
Whole genome sequencing
We sequenced che-5, che-6 and che-7 mutant animals, obtained from the CGC, with an Illumina GA2 genome analyzer. We used MAQGene for WGS data analysis (Bigelow et al. 2009). After subtraction of background variants found in two other sequenced che strains (che-5, che-6 and che-7), we found six missense mutations on LGIV in the che-6(e1126), where che-6 had been previously mapped to (located in inx-18, C23H5.7, gbb-2, Y52D5A.2, inx-7, C46G7.3). No splice site or nonsense mutations were found. In the case of che-7(e1128) animals, we identified one nonsense and 22 missense mutations. In che-5 mutants, we found 7 missense mutations on LGIV and tested available alleles of several candidates that failed to mimic the defective chemotaxis behavioral phenotype of che-5. We did not pursue this mutant further.
Heterologous expression and GC assays of GC proteins in tissue culture
GCY-4 and GCY-22 DNA fragments were synthesized in a human codon-optimized manner, and were subcloned, together with an FLAG epitope, into pcDNA3.1 expression vector. Constructs were transfected into CHO cells and the GC activity assay was performed as previously described (Guo et al. 2007, 2009). Cells were cultured in growth medium to ∼95% confluency and were washed in a buffer containing 50 mM NH4Ac and 200 mM sucrose pH 7 (plus 1 mM IBMX). Membrane preparations were made. 100 mM of NaCl, NaBr, NaPO4, or NaI were used for treatment. After 20 min, the membrane preparations were lysed in 0.1 M HCl and assayed for cGMP concentration using Direct cGMP EIA Kits (NewEast Biosciences).
Results
Chimeric GCY receptor experiments demonstrate that specificity of rGC function lies in the extracellular domain
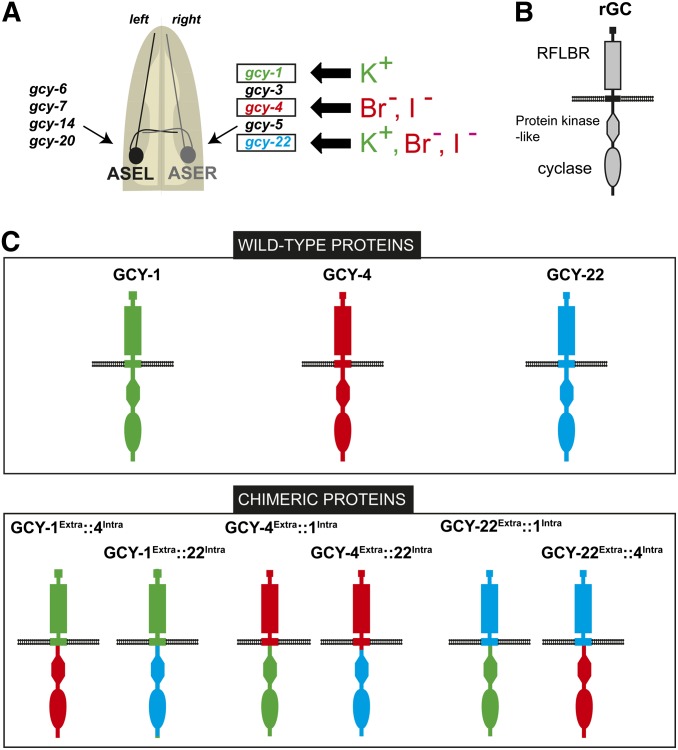
Figure 1B shows the general structure of receptor-type rGC proteins. They contain a large extracellular domain and many rGC proteins show similarity in this domain to small ligand binding bacterial proteins (“RFLBR domain” = receptor family ligand-binding region). On the intracellular side, rGC proteins contain a protein kinase-like domain and a cyclase domain. We chose to analyze the requirement of the extracellular and intracellular domain for the specific function of three different rGC proteins, GCY-1, GCY-4 and GCY-22. GCY-1 is expressed in ASER, not ASEL and is required for an efficient response to potassium ions, but not other ASER-sensed ions (Ortiz et al. 2006, 2009). GCY-4 is also expressed in ASER and is required for an efficient response to bromide and iodide ions, but not other ASER-sensed ions (Ortiz et al. 2006, 2009). GCY-22 is required for the processing of all ASER-sensed ions. Based on the previous suggestion that rGCs may form heterodimers (Morton 2004), we have proposed that GCY-22 may be a common subunit of the normally dimeric rGC proteins (Ortiz et al. 2009). As indicated in Figure 1C, we generated chimeric expression proteins in which the intra- and extracellular domains of all three proteins are swapped in all possible combinations. We generated stable transgenic lines that express each of these constructs, as well as wild-type controls, using the ASER-specific gcy-5 promoter. We then crossed transgenic lines into gcy-1, gcy-4, and gcy-22 mutant backgrounds to ask which of these constructs rescue the respective mutant phenotype.
We find that the potassium response defect in gcy-1 mutant animals is rescued by gcy-1Extra::22Intra (extracellular domain of GCY-1, intracellular domain of GCY-22) and by gcy-1Extra::4Intra chimeras (three extrachromosomal lines each; Figure 2). In contrast, the gcy-1 defect is not rescued by gcy-4Extra::1Intra or by gcy-22Extra::1Intra chimeras (three extrachromosomal lines each; Figure 2). These results demonstrate that the specificity determinant of gcy-1 function resides in the extracellular domain of the GCY-1 protein.
Figure 2.
Chimera rescue experiments demonstrate the importance of the extracellular domain of GCY-1. Results of population salt chemotaxis assays are shown for wild-type, mutant, and transgenic strains. Three independent lines of receptor chimeras were tested for whether they can rescue gcy-1 mutant defects. Analysis was completed using a one-way ANOVA with repeated measures comparing the mean of each group to the mean of the mutant gcy-1. Error bars indicate SEM. The Holm–Sidak correction was used to adjust for multiple comparisons and α = 0.1. All P-values reported are the adjusted value after the correction was applied. P-values: ****P < 0.0001, **P < 0.01, *P < 0.05; NS, not significant (P > 0.05). n = 6 with each sample being a duplicate of two plates with four worms per plate. Assays were done blind to the genotype under test.
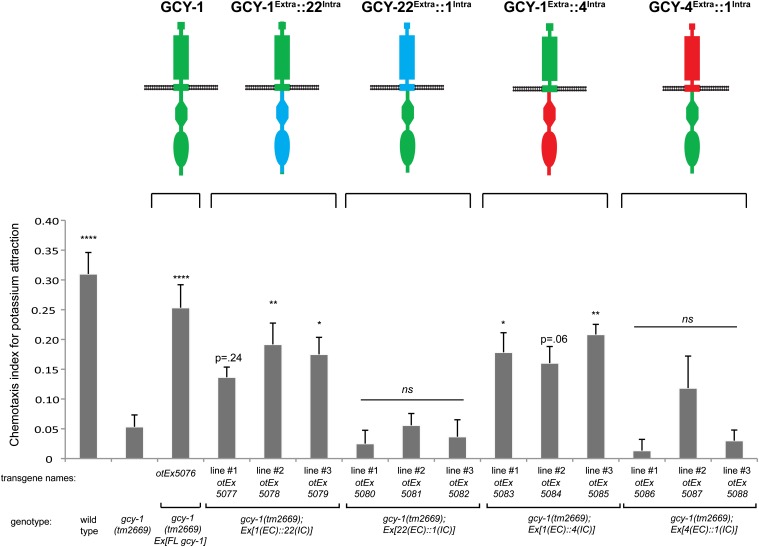
The same theme is readily apparent upon the analysis of iodide response defect in gcy-4 mutant animals expressing various gcy-4 chimeric constructs. The gcy-4 defects are rescued by gcy-4Extra::22Intra and by gcy-4Extra::1Intra chimeras (three extrachromosomal lines each; Figure 3). In contrast, no rescue is observed in gcy-4 mutants expressing the gcy-22Extra::1Intra or by gcy-22Extra::4Intra chimeras (each three extrachromosomal lines; Figure 3).
Figure 3.
Chimera rescue experiments demonstrate the importance of the extracellular domain of GCY-4. Results of population salt chemotaxis assays are shown for wild-type, mutant, and transgenic strains. Three independent lines of receptor chimeras were tested for whether they can rescue gcy-4 mutant defects. Analysis was completed using a one-way ANOVA with repeated measures comparing the mean of each group to the mean of the mutant gcy-4. Error bars indicate SEM. The Holm–Sidak correction was used to adjust for multiple comparisons and α = 0.05. All P-values reported are the adjusted value after the correction was applied. P-values: **P < 0.0001, *P < 0.001; NS, not significant (P > 0.01). Error bars indicate SEM. n = 6 with each sample being a duplicate of two plates with four worms per plate. Assays were done blind to the genotype under test.
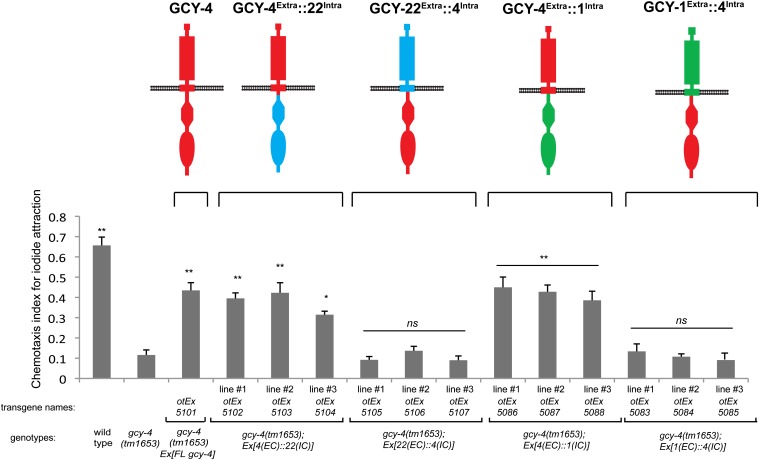
Lastly, the chloride response defect of gcy-22 mutant animals (only observed in gcy-22, but not gcy-1 or gcy-4 mutant animals) is rescued by gcy-22Extra::1Intra and by gcy-22Extra::4Intra chimeras, but not by gcy-1Extra::22Intra or by gcy-4Extra::22Intra chimeras (three extrachromosomal lines each; Figure 4A). This result is in accordance with all other chimera experiments, again showing that the specificity of rGC protein function resides in their extracellular domains.
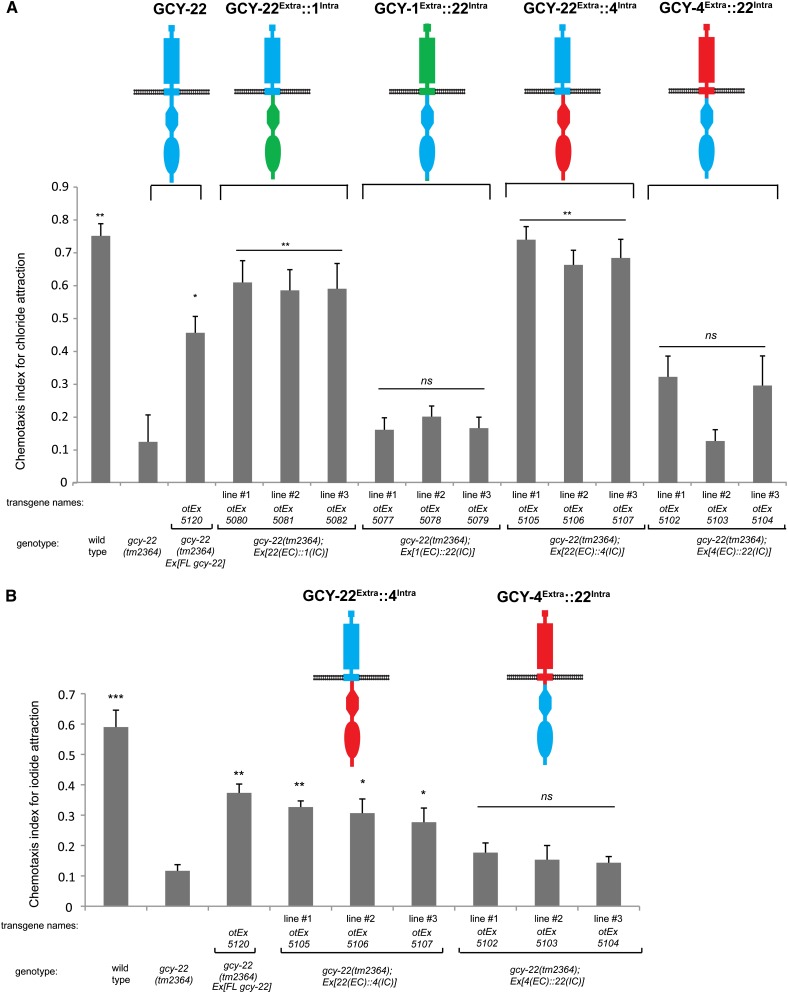
Figure 4.
Chimera rescue experiments demonstrate the importance of the extracellular domain of GCY-22. Results of population salt chemotaxis assays are shown for wild-type, mutant, and transgenic strains. Three independent lines of receptor chimeras were tested for whether they can rescue gcy-22 mutant defects. For both A and B, error bars indicate SEM. Analysis was completed using a one-way ANOVA with repeated measures comparing the mean of each group to the mean of the gcy-22 mutant. Error bars indicate SEM. The Holm–Sidak correction was used to adjust for multiple comparisons and α = 0.05. All P-values reported are the adjusted value after the correction was applied. (A) GCY-22 chimeras in chloride response. P-values: **P < 0.0001, *P < 0.001; NS, not significant (P > 0.01). n = 6 with each sample being the average of the duplicate of two plates with four worms per plate. (B) GCY-22 chimeras in iodide response. P-values: **P < 0.0001, **P < 0.01, *P < 0.05; NS, not significant (P > 0.05). n = 3 with each sample being the average of the duplicate of two plates with four worms per plate.
In contrast to GCY-4 (involved in iodide response, but not potassium or chloride response) and GCY-1 (involved in potassium response, but not iodide or chloride response), GCY-22 is involved in the response to chloride, potassium and iodide (Ortiz et al. 2009). We therefore asked whether the involvement of GCY-22 in the iodide response is, like the response to chloride, dependent on the extracellular domain of GCY-22 or whether in this case, the extracellular domain of iodide-sensing GCY-4 could substitute for the extracellular domain of GCY-22. We find that it is again the extracellular domain of GCY-22 that is required to rescue the gcy-22 mutant phenotype (Figure 4B). A chimera with the extracellular domain of GCY-4, even though required to rescue the iodide defects of gcy-4 mutants, is not able to substitute for GCY-22 function (Figure 4B).
GCY-4 and GCY-22 are sufficient to impose salt responsiveness onto other neuron types
If rGC proteins were indeed direct chemoreceptors, misexpression of rGC proteins in other neuron types should confer salt responsiveness to these neurons. To test this possibility, we pursued the following strategy. We used the che-1 genetic mutant background in which the development and function of the ASE neurons is abrogated (Uchida et al. 2003; Etchberger et al. 2007). che-1 mutant animals are unable to respond to salt cues (Figure 5). We then attempted to restore salt responsiveness by misexpressing rGC proteins in all other worm sensory neurons, using the osm-6 promoter. Because of the absence of ASE (che-1 mutant background), these animals will only be able to respond to salt cues if any other sensory neuron is now able to confer salt responsiveness. We chose specifically the osm-6 promoter because it is active in all sensory neurons, including ASE (Collet et al. 1998), therefore allowing us to test through mutant rescue assays whether the expression construct indeed produces functional protein, as detailed below. We also anticipated that a broadly expressed promoter may hedge our bets to hit a neuron that could provide a functional response.
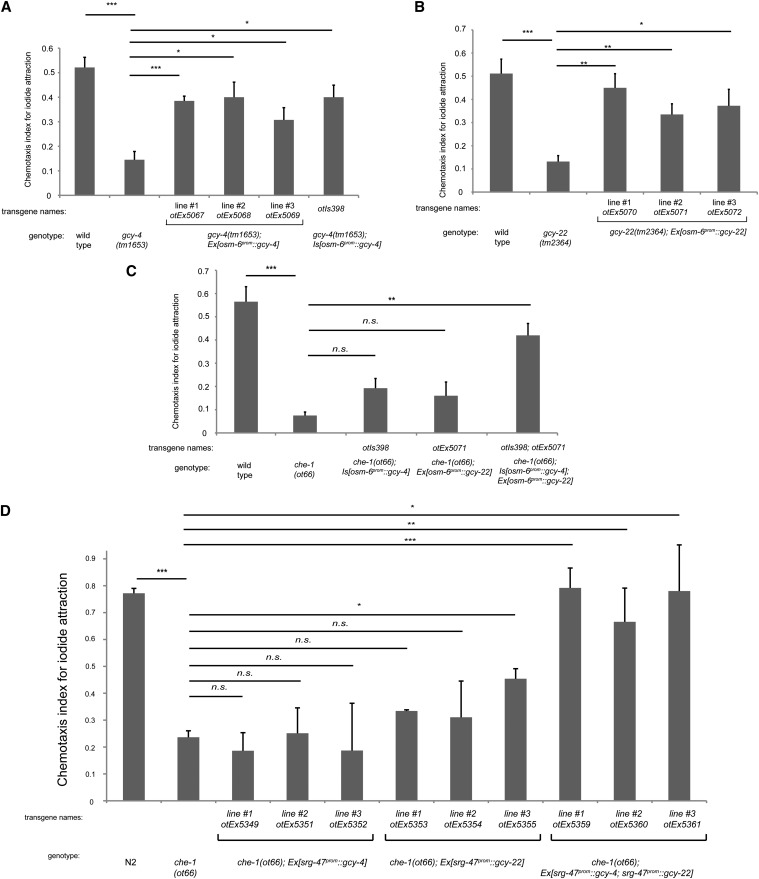
Figure 5.
Ectopic expression of gcy-4 and gcy-22 confers iodide responsiveness on ASE-deficient animals. (A) Pansensory expression of gcy-4 rescues the gcy-4 mutant phenotype, as observed with the independent extrachromosomal arrays expressing osm-6prom::gcy-4 and one chromosomal integrant generated from one of the extrachromosomal arrays. (B) Pansensory expression of gcy-22 rescues the gcy-22 mutant phenotype, as observed with the independent extrachromosomal arrays expressing osm-6prom::gcy-22. Panel A and B are control experiments that establish the functionality of the individual transgenes. (C) A transgenic strain that expresses the integrated osm-6prom::gcy-4 array from A or one extrachromosomal osm-6prom::gcy-22 array is not able to rescue the loss of ASE neuron functionally (che-1 mutant background in which ASE fail to differentiate and do not express gcy-4 or gcy-22). However, combining the osm-6prom::gcy-4 integrated array with the osm-6prom::gcy-22 extrachromosomal array results in rescue of the che-1 mutant phenotype (last bar). (D) ASI-specific expression of GCY-4 and GCY-22 compensates for loss of che-1. Three lines that coexpress srg-47prom::gcy-4 and srg-47prom::gcy-22 arrays are able to rescue the chemotaxis defect of che-1 mutants to iodide. In all panels, error bars indicate the SEM. Analysis was completed using a one-way ANOVA with repeated measures comparing the mean of each group to the mean of the mutant. Error bars indicate SEM. The Holm\x{2013}Sidak correction was used to adjust for multiple comparisons and a = 0.01. All P-values reported are the adjusted value after the correction was applied. P-values: ***P < 0.001, **P < 0.01, *P < 0.05; NS, not significant (P < 0.05). In A\x{2013}C, n = 4 and in D, n = 3 with each sample being the average of the duplicate of two plates with four worms per plate. Assays were done blind to the genotype under test.
We used the GCY-4 protein, which is normally required for bromide and iodide response and the presumed common subunit GCY-22. We generated transgenic animals that express a chromosomally integrated osm-6prom::gcy-4 expression construct. We confirmed that this transgene produces functional protein by its ability to rescue the iodide response defect of gcy-4 mutant animals (Figure 5A; note that this controls illustrates the usefulness of the osm-6 promoter). We then transferred the array from the gcy-4 mutant background into a che-1 mutant background. che-1; Is[osm-6prom::gcy-4] animals are unable to respond to bromide/iodide, suggesting that gcy-4 alone is not sufficient to confer bromide/iodide responsiveness (Figure 5C). Similarly, we generated animals with an extrachromosomal array that contains a osm-6prom::gcy-22 expression construct. We confirmed that this transgene is able to rescue the iodide response defect of gcy-22 mutant animals (Figure 5B) and then transferred the array from the gcy-22 mutant background into the che-1 background. Like che-1; Is[osm-6prom::gcy-4] animals, che-1; Ex[osm-6prom::gcy-22] animals are also not able to respond to iodide (Figure 5C). However, when we crossed the Is[osm-6prom::gcy-4] and Ex[osm-6prom::gcy-22] transgenes together, again in the context of a che-1 mutant background, we find that the resulting double transgenic che-1; Is[osm-6prom::gcy-4]; Ex[osm-6prom::gcy-22] animals are able to respond to iodide (Figure 5C). These results demonstrate that gcy-4, in combination with gcy-22, is capable of imposing iodide responsiveness to other sensory neuron types and are consistent with the possibility that GCY-4/GCY-22 constitute a heteromeric iodide receptor complex. We can at present not exclude the possiblity that GCY-4 and GCY-22 cooperate in distinct neurons to impose a chemosensory response.
It is surprising that pansensory expression of GCY-4/GCY-22 with the osm-6 driver permits salt attraction since the activation of a number of sensory neurons (e.g., AWB, ASH or ADL) are thought to mediate repulsive behavior (Bargmann 2006). Perhaps any potential repulsive response of these neurons is overwhelmed by the expression of GCY-4/22 in attractive neurons. Also, GCY-4/22 may only be appropriately transported in some but not other neurons.
To further pursue reconstitutation experiments, but now in a more cell-type specific manner, we expressed GCY-4 and GCY-22 alone and in combination in the ASI sensory neurons, using the srg-47prom driver. The ASI neurons have sensory ending that, like, for example the gustatory ASE and ASG neurons, are exposed to the environment and sense a variety of distinct stimuli (Bargmann 2006). The gcy-4 and gcy-22 transgenes were expressed in a che-1 mutant background to ask whether the loss of ASE-mediated iodide attraction can rescue the ASI-expression of GCY-4/GCY-22. We find that srg-47prom::gcy-4 or srg-47prom::gcy-22 alone does not provide substantial rescue of the iodide attraction phenotype of che-1 mutants, but coexpression of srg-47prom::gcy-4 and srg-47prom::gcy-22 does provide significant rescue (Figure 5D). A conceptually similar reconstitution experiment in the AWC olfactory neurons resulted in no rescue, consistent with the fact that the AWC dendritic endings are not exposed to the environment (data not shown). Taken together, the ASI-specific reconstitution experiment provides further support for the hypothesis that GCY-4 and GCY-22 may collaborate, possibly as a heterodimer, to confer a chemosensory function.
Lastly, we attempted to measure salt-inducible GCY protein activity in heterologous cell culture aassys (Guo et al. 2007, 2009). We co-expressed GCY-4 and GCY-22 proteins in Chinese hamster ovary (CHO) cells and tested whether guanylyl cyclase activity could be directly stimulated by bromide, chloride, or sodium. No significant stimulations were observed (Figure S1), but given the negative nature of these results, they cannot rule out that GCY-4/GCY-22 constitute a bromide/iodide receptor complex.
In conclusion, pansensory GCY-4/GCY-22 expression is able to restore iodide attraction of animals that contain no functional ASE neuron, which supports, but does not ultimately prove the notion that GCY-4/GCY-22 form a functional iodide receptor.
che-6 is a cyclic nucleotide gated ion channel likely acting as an effector of rGC proteins in the ASE neurons
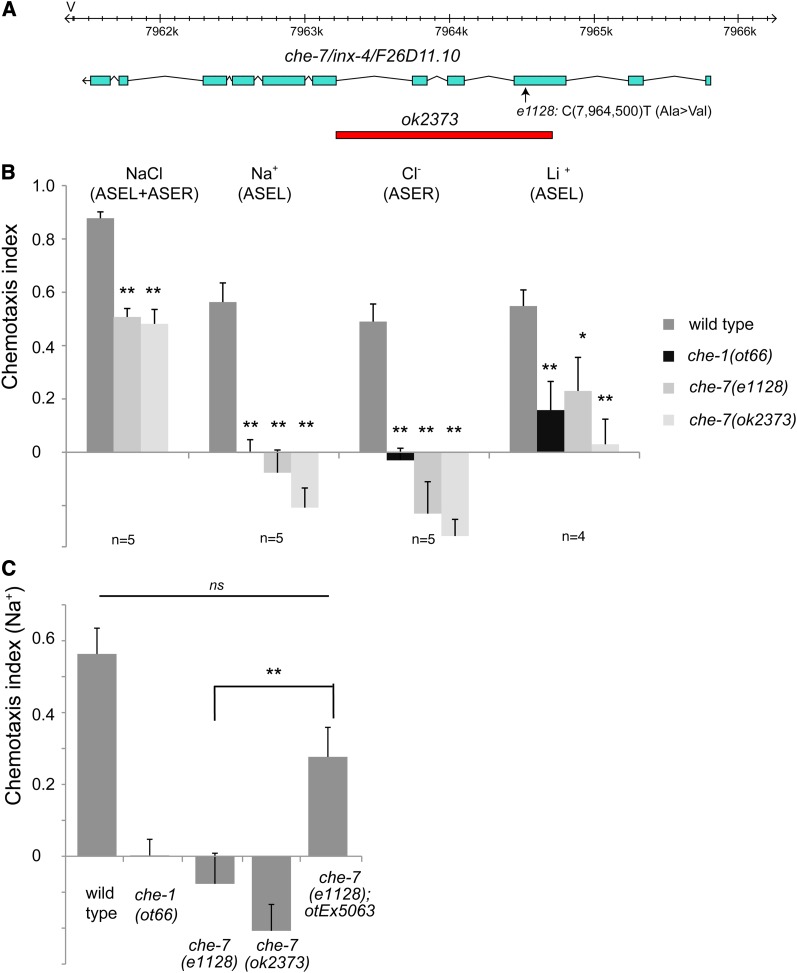
To identify additional molecules involved in rGC-mediated gustatory signal transduction in the ASE neurons, we determined the molecular identity of two as yet uncloned chemotaxis (che) mutants, che-6 and che-7, using whole genome sequencing (WGS). che-6 and che-7 were isolated in screens for mutants unable to respond to sodium and chloride ions (Lewis and Hodgkin 1977) but have not since been further analyzed. We find that che-7 mutant animals carry a mutation in inx-4, which codes for a gap junction component broadly expressed in the nervous system (Figure 6, A–C). Based on its molecular identity and expression pattern (several head neurons, but not ASE; Altun et al. 2009), this gene likely acts downstream of primary signal transduction events and we did not pursue its characterization any further. In contrast, we find che-6 to indeed code for another gustatory signaling component and we therefore chose to focus on the characterization of che-6.
Figure 6.
che-7 corresponds to the inx-4 gene. Three lines of evidence for che-7 being inx-4 are shown here, one for each panel. (A) che-7(e1128) animals carry a mutation in the inx-4 gene (Ala→Val at 7964500) within the third exon. The missense mutation lies within the third transmembrane domain. (B) Chemotaxis defects of che-7(e1128) animals are similar to those of inx-4(ok2373) animals. The assay used here is a population assay. Each n shown in the figure is the average of two assays done in duplicate on the same day and each assay has between 50 and 250 worms. Statistics were measured using unpaired Student’s t-test assuming equal variance and the Bonferroni correction was used to adjust the P-values. Error bars indicate the SEM. P-values: **P < 0.01, *P < 0.05; NS, not significant (P > 0.05). (C) The che-7 chemotaxis defect can be rescued with a fosmid (WRM0620bH04) covering the inx-4 locus, contained on the otEx5063 array. n = 5 with each sample being a duplicate of two plates with four worms per plate. Statistics were measured using unpaired Student’s t-test assuming equal variance and the Bonferroni correction was used to adjust the P-values. Error bars indicate the SEM. P-values: **P < 0.01, *P < 0.05; NS, not significant (P > 0.05).
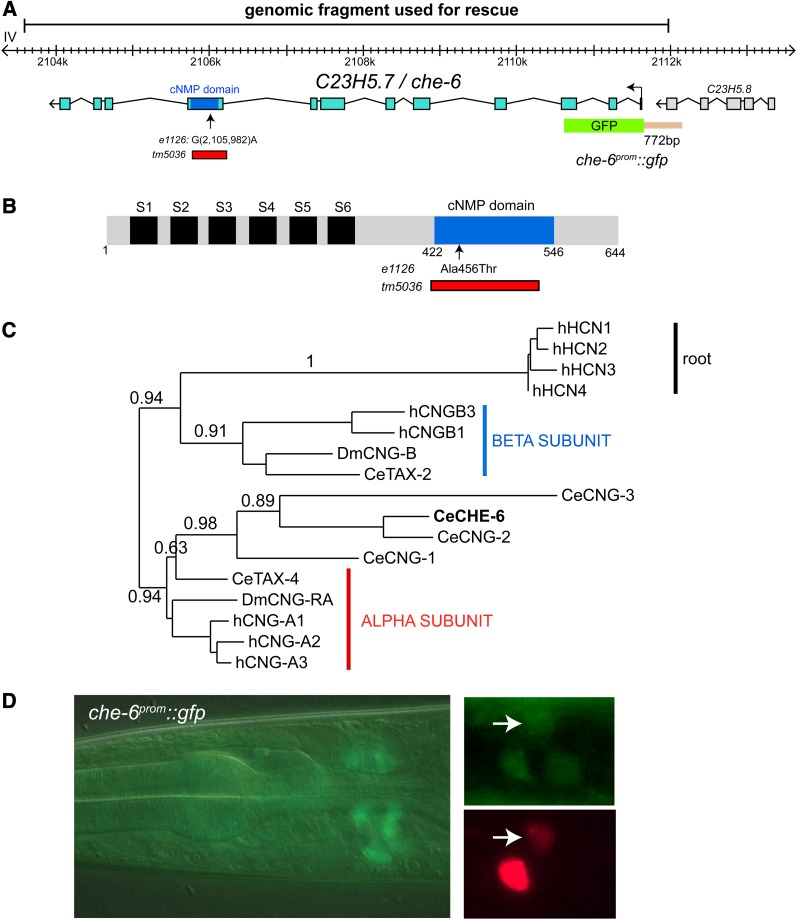
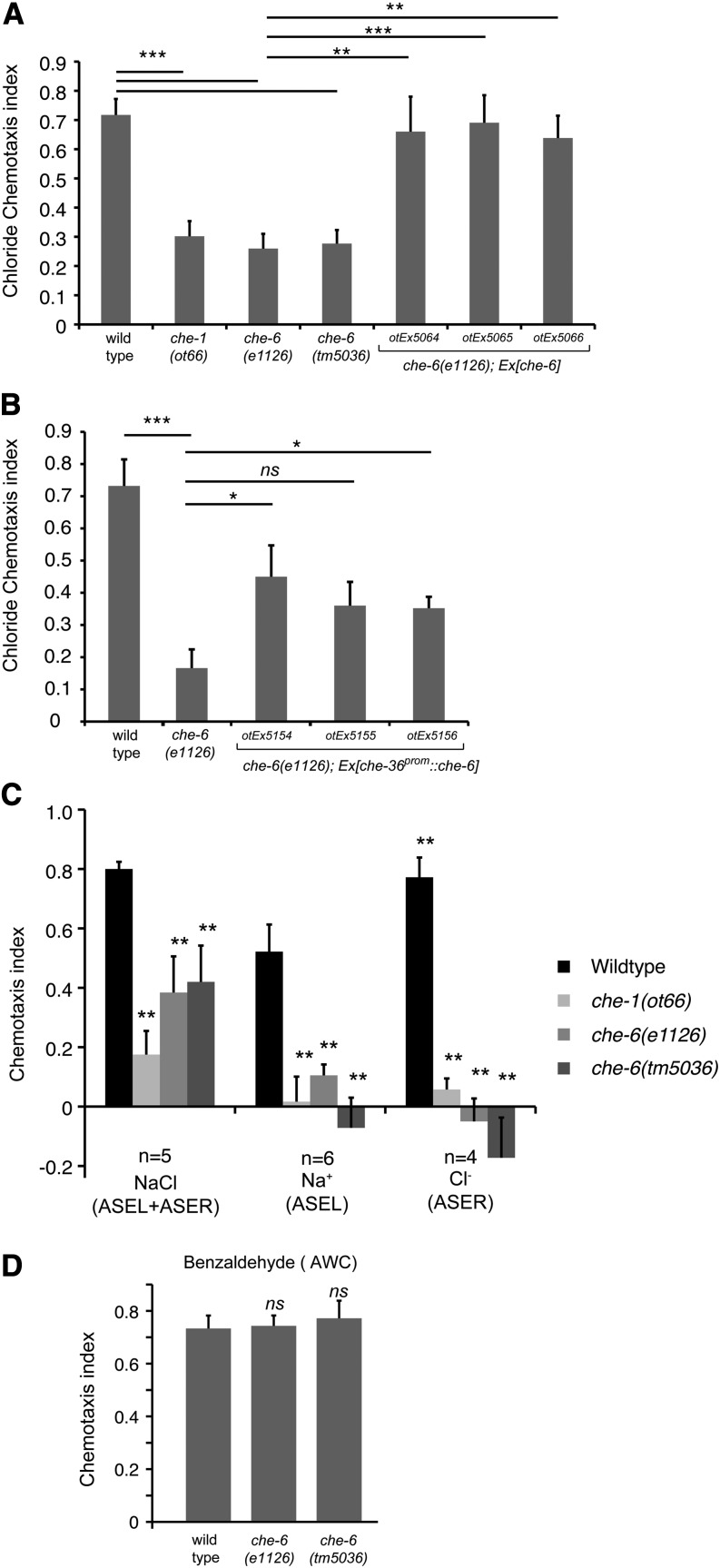
Specifically, we found through WGS that the previously identified che-6(e1126)IV strain bears mutations in six different coding loci on chromosome IV, one of them a mutation in the previously uncharacterized cng-4/C23H5.7 gene (Materiala and Methods; Figure 7, A and B), which encodes a predicted cyclic nucleotide-gated ion channel. The e1126 mutation is a missense mutation in the nucleotide-binding domain (Figure 7, A and B). The chemotaxis defect of che-6(e1126)IV can be rescued by supplying a piece of genomic DNA that contains the wild-type copy of the cng-4 locus (Figure 8A). Animals carrying a deletion allele, tm5036, that removes the critical cyclic nucleotide-binding domain of the cng-4 gene, kindly provided by Shohei Mitani and the National Bioresource Project of Japan, show the same phenotype as the che-6(e1126) mutant animals (Figure 8A). From here on we refer to cng-4 as che-6.
Figure 7.
che-6 codes for a cyclic nucleotide-gated ion channel. (A) che-6 gene structure and alleles. The alanine-encoding codon mutated in e1126 to a threonine-encoding codon resides in the nucleotide-binding domain. Generally, within nucleotide-binding domains, this position is either an alanine or a glycine (Kaupp and Seifert 2002). (B) Schematic protein structure of CHE-6s. (C) Phylogenetic tree built at the www.phylogeny.fr suite (Dereeper et al. 2008). Full-length protein sequence was used an Hyperpolarization-activated cyclic nucleotide-gated channel (HCN; no worm homologs) were used to root the tree. A similar clustering is observed if only the cNMP domain or the ion channel domain is used (Figure S2). (D) che-6prom::gfp expression pattern. The left panels show the overview of expression in several adult head neurons. The two smaller panels on the right show an image of the head region of a transgenic animal coexpressing che-6prom::gfp (hanEx24) and ASE + AWC-expressed ceh-36::mCherry (otIs264), revealing overlap of expression in ASE and AWC.
Figure 8.
Chemotaxis defects of che-6 mutant animals. (A) Two different che-6 alleles show similar chemotaxis defects as observed upon loss of the ASE neurons (che-1 mutants (Uchida et al. 2003) and transformation rescue of the che-6 mutant phenotype with a piece of genomic DNA illustrated in Figure 7A. Error bars indicate SEM. Analysis was completed using a one-way ANOVA comparing the mean of each group to the mean of the mutant che-6 (e1126). The Holm–Sidak correction was used to adjust for multiple comparisons and α = 0.01. All P-values reported are the adjusted value after the correction was applied. P-values: ***P < 0.001, **P < 0.01, *P < 0.05 ; NS, not significant (P > 0.05). n = 4–13 replicates with each n being the average of the duplicate of two plates with four worms per plate. (B) Rescue of the che-6 mutant phenotype with a transgene that expresses che-6 under control of the ceh-36 promoter in ASE and AWC. n = 5 with each sample being the average of the a duplicate of two plates with four worms per plate. (C) che-6 mutants fail to respond to ASEL and ASER-sensed cues. The assay used here is a population assay. n = 3–5 and each n shown in the figure is an average of two assay plates done in duplicate on the same day with between 50 and 250 worms per plate. (D) che-6 mutants show a normal response to the AWC-sensed olfactory cue benzaldehyde. The assay used here is a population assay. n = 3 with each n being the average of two assay plates done in duplicate on the same day with between 50 and 250 worms per plate. For B and D, statistics were measured using unpaired Student’s t-test assuming equal variance comparing the mean of each group to the mean of the mutant che-6 (e1126) and the Bonferroni correction was used to adjust the P-values. Error bars indicate the SEM. P-values: **P < 0.01, *P < 0.05; NS, not significant (P > 0.05).
The C. elegans genome codes for a total of six predicted CNG channels (Kaupp and Seifert 2002). Sequence analysis indicates that one of them, tax-4, is a homolog of the α-type subunit of CNGs, while another one, tax-2, is a β-type subunit (Figure 7C) (Kaupp and Seifert 2002). Both tax-2 and tax-4 have been extensively characterized in terms of function and expression (Coburn and Bargmann 1996; Komatsu et al. 1996). The four remaining CNGs are more divergent but have a somewhat greater overall sequence similarity to the α-type, based on the sequence comparison of the entire proteins or individual domains (Figure 7C; Figure S2). However, only two of the four proteins, CNG-1 and CNG-3, contain a negatively charged amino acid in the ion-conducting pore, which is a characteristic feature of α-subunits. Three of them, CNG-1, CNG-2, and CNG-3, contain leucines in their extreme C termini that are predicted to form coiled coils, another defining feature of α-subunits. Whether any of these four proteins fulfill the classic definition of an α-subunit of being able to assemble ion-conducting channels on their own, remains to be shown.
Deletion alleles of two of the four divergent CNGs, cng-1 and cng-3, have been functionally characterized previously, showing no defects in salt chemotaxis and olfaction, respectively (Cho et al. 2004, 2005). The remaining two CNGs, cng-2 and che-6 (previously called cng-4), have not been functionally characterized to date.
che-6 is expressed and functions in ASE
Sensory neuron-specific expression profiles in subsets of amphid neuron pairs have previously been described for tax-2, tax-4, cng-1, and cng-3 (Coburn and Bargmann 1996; Komatsu et al. 1996; Cho et al. 2004, 2005), but not for cng-4/che-6. We generated a che-6 reporter gene fusion that contains 722 nucleotides upstream of the start codon and that encompasses all intergenic sequences to the next upstream gene (schematically shown in Figure 7A). This intergenic region contains a putative cis-regulatory motif, the “ASE motif” (GAAGCC), which is found in many genes expressed in the ASE neurons and is a binding site for the terminal selector transcription factor che-1 (Etchberger et al. 2007). We found that this reporter is expressed weakly in approximately five neuron pairs, one of them the ASE neuron pair (Figure 7D).
To corroborate the cellular focus of che-6 action, we expressed the che-6 locus in che-6 mutant animals under control of the ceh-36 promoter, which is active in the ASE gustatory neurons and the AWC olfactory neurons (Lanjuin et al. 2003). We find that two out of three lines show rescue of the che-6 mutant chemotaxis phenotype (Figure 8B), indicating that che-6 acts in the ASE neurons to control chemosensory behavior.
che-6 affects gustatory, but not olfactory or thermosensory behavior, and cng-3 affects thermosensory behavior
We tested the effect of loss of che-6 on additional sensory modalities. We examined salt chemotaxis, which is primarily mediated by the ASE neurons [as done previously by Lewis and Hodgkin (1977), but now done with different assays], thermotaxis primarily mediated by the AFD thermosensory neurons (Mori and Ohshima 1995) and olfactory attraction mediated by the AWC sensory neurons (Bargmann et al. 1993). We confirmed that che-6 mutant animals are defective in salt chemotaxis as determined by our chemotaxis assay system (Figure 8C). Salt chemotaxis defects extend to ions sensed by either ASEL (sodium) or ASER (chloride) (Figure 8C). However, we found that che-6 mutants show no defects in AWC-mediated olfactory behavior (Figure 8D) and no defects in thermotaxis behavior (Figure 9; Figure S3).
Figure 9.
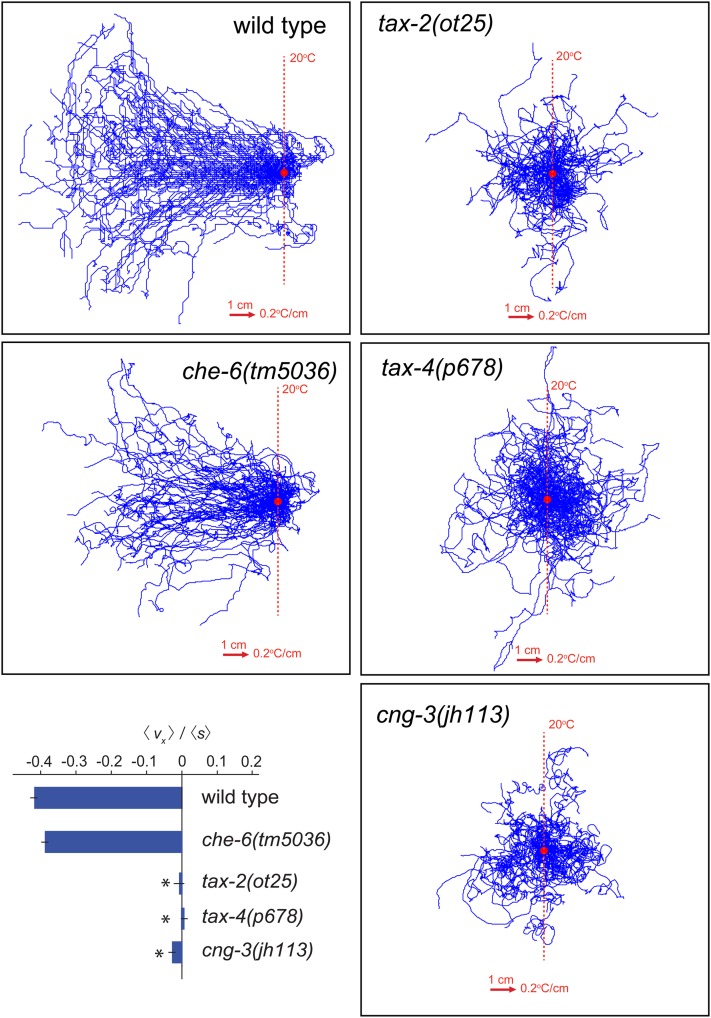
Thermotaxis behavior of the CNG channel mutants che-6 and cng-3. Representative trajectories and navigational indexes (boxed inset) of wild-type N2 (n = 100), che-6(tm5036) (n = 60), tax-2(ot25) (n = 60), tax-4(p678) (n = 60), and cng-3(jh113) (n = 40) animals navigating linear spatial thermal gradients (0.2°/cm) on the surface of 22 cm × 22 cm plates. Worms grown at 15° were started at 20°. Trajectories were aligned to have the same starting point for presentation purposes. Navigational indexes are defined as <vg >/<s>. <vg > indicates the mean velocity in the direction up the gradient. <s> indicates crawling speed along trajectories. An alternative data representation is shown in Figure S3.
While we observed no thermotaxis defects in che-6 mutants, we observed thermotaxis defects in animals lacking the cng-3 gene (Figure 9; Figure S3), which was previously shown to be expressed in the AFD thermosensory neurons (Cho et al. 2004). Since tax-2 and tax-4 channels also show defects in both thermotaxis and salt chemotaxis (Komatsu et al. 1996) (Figure 9; Figure S3), these findings suggest that che-6 and cng-3 form sensory modality-specific subunits with the more broadly acting tax-2 and tax-4 (see Discussion).
Discussion
Mechanisms of salt sensation: the rGC proteins
In combination with our previous work (Ortiz et al. 2009), this work has provided genetic evidence in support of the notion that rGC proteins may work as direct receptors for salt ions. We have shown previously through genetic loss-of-function analysis that individual gcy genes are required for the efficient response of animals to specific salt ions, both on the level of behavior as well as neuronal activity (Ortiz et al. 2009). We have extended these observations here by showing (a) that the ion-selectivity in rGC protein function lies in their extracellular domain and (b) that rGC proteins can confer salt responsiveness to other sensory neurons.
GCY-1 and GCY-4 may operate as direct sensors of potassium and iodide, respectively. GCY-22 may operate as a direct sensor of chloride. The involvement of GCY-22 in iodide and potassium sensation, for which the extracellular domain is again essential, may lie in GCY-22 being a common subunit for the usually hetero- or homodimeric rGC proteins, as previously speculated (Ortiz et al. 2009). That is, GCY-22 may form a heterodimeric iodide receptor with GCY-4, a heterodimeric potassium receptor with GCY-1 and it may form a heterodimeric chloride receptor with an as yet uncharacterized GCY subunit. Since none of the other presently known ASE-expressed GCY proteins are involved in chloride chemotaxis, it is also conceivable that GCY-22 may constitute a homodimeric chloride receptor on its own. It is important to point out that a heterodimeric constitution of rGCs protein still awaits biochemical proof and that our genetic data are nothing more than consistent with such a hypothetical model.
A receptor function of rGCs, rather than a more intermediary, signal-transducing role, is also consistent with the notion that gustatory sensory transduction in the ASE neurons appears independent of GPCR signaling in which rGCs are normally embedded in as signaling intermediates (Bargmann 2006). A GPCR-coupling of rGCs is unlikely in ASE neurons since ASE-mediated chemosensation is independent of all characterized heterotrimeric G proteins (Jansen et al. 1999, 2002), is independent of the GPCR-regulatory kinase GRK-2 (Fukuto et al. 2004), and is independent of the ODR-4/ODR-8 GPCR trafficking system (Dwyer et al. 1998).
There are other rGCs that may operate as direct sensory receptors. Based on genetic loss-of-function analysis, the three rGC proteins GCY-8, GCY-18, and GCY -23 are candidate thermosensors in the AFD thermosensory neurons (Inada et al. 2006; Ramot et al. 2008; Wasserman et al. 2011) and GCY-9 is a candidate carbon dioxide receptor (Hallem et al. 2011; Brandt et al. 2012). In none of these cases, however, has it been tested whether the extracellular domains are dispensable for function, as it is the case for an rGC, ODR-1, in olfactory signal transduction (L’Etoile and Bargmann 2000) or whether the extracellular domains are required for function, as we show here for the gustatory rGCs. A direct receptor function of rGC proteins would also be reminiscent of the function of soluble GCs (sGCs) as direct sensory receptors for another ambient cue, oxygen. In this case, the ligand sensor domain is a heme binding domain (Gray et al. 2004; Cheung et al. 2005). Ligand binding activates the cyclase resulting in cGMP production that in turn activates the TAX-2/TAX-4 CNG complex in oxygen-sensing neurons.
Biochemical studies showing that changes in salt concentration can activate cyclase activity of the rGC proteins in a heterologous in vitro system would be the ultimate proof for receptor function, but our attempts to detect sensory stimulus-induced activation have so far not been successful. There are also no reports of in vitro activation of other candidate rGC sensory receptors through defined sensory stimuli (such as the GCY-8, -9, -18, -23 proteins mentioned above). Our failure to detect salt-stimulated activity in vitro could be the result of several different complications associated with correct expression, localization, and folding of C. elegans proteins in vertebrate cell culture or the absence of accessory subunits. In the absence of such biochemical data, alternative scenarios for rGC function cannot be excluded. For example, the extracellular domain of individual ASE-expressed GCY proteins may not itself be involved in salt sensation, but may be required to couple to the extracellular domain of other, specific salt-sensing proteins. Our GCY-4/GCY-22 reconstitution experiments could be explained by such specific salt sensor being expressed, but not normally functioning, in other sensory neurons. However, such a model seems less parsimonious than a direct role of rGC proteins in salt sensation.
A role for rGCs as direct sensory receptors should also be viewed from the perspective of the expansion of rGCs in nematode genomes (27 genes in C. elegans vs. 5 in humans), their apparent sequence diversity in different nematodes, and their predominant expression in sensory neurons (at least 25 out of 27 rGC-encoding genes are expressed in sensory neurons) (Ortiz et al. 2006). Species-specific expansions and diversification are general features of sensory receptor gene families (consider, for example, GPCR-type odorant receptors) and provide a reflection of the highly diverse and species-specific sensory environments that different organisms find themselves in. It will be interesting to determine the spectrum of sensory cues for other rGC proteins.
Mechanisms of salt-triggered signal transduction: the CNG channels
A nodal point in signal transduction in the ASE neurons is the cGMP-triggered gating of ion channels of the CNG family. Two CNGs acting in salt transduction in ASE were previously identified, TAX-2 (a β-subunit) and TAX-4 (an α-subunit) (Coburn and Bargmann 1996; Komatsu et al. 1996) and we have identified here a third CNG acting in ASE-mediated salt transduction, CHE-6. CNGs are known to be tetrameric channels composed of multiple distinct types of subunits (Kaupp and Seifert 2002). In rat olfactory neurons, tetrameric CNG channels are composed of three distinct subunits (Bonigk et al. 1999). Based on this precedent, we propose that the CNG channel in the ASE neurons is composed of TAX-2, TAX-4, and CHE-6 subunits.
Our genetic analysis suggests that CNG channels assemble and transduce signals in a cell-type specific manner. ASE-mediated salt sensation requires tax-2, tax-4, and che-6, but not cng-3. AFD-mediated thermosensory transduction requires tax-2 and tax-4 (Coburn and Bargmann 1996; Komatsu et al. 1996), but not che-6 (this article). Instead, cng-3 is required for efficient thermotaxis (this article). CNG channels may therefore have sensory- and cell-type specific compositions, with a CHE-6/TAX-4/TAX-2 channel in ASE and a CNG-3/TAX-4/TAX-2 channel in AFD. The olfactory AWC neurons also require TAX-2 and TAX-4 (Coburn and Bargmann 1996; Komatsu et al. 1996), but neither che-6 (this article) nor cng-3 (Cho et al. 2004); these neurons may employ a yet different CNG subunit, perhaps the as yet uncharacterized CNG-2 protein. Sensory neuron-type specific subunit compositions have also been described in vertebrates (Kaupp and Seifert 2002).
In conclusion, our studies have deepened our understanding of salt-induced sensory transduction, providing support for the hypothesis of rGC proteins functioning as direct salt receptors and identifying a key effector component of rGC-triggered signal transduction.
Supplementary Material
Acknowledgments
We thank Alex Boyanov for running our whole genome sequencing (WGS) operations, Richard J. Poole for the WGS data analysis, Qi Chen for generating transgenic strains, Kelly Howell for help with transgenic lines, Piali Sengupta and Yuichi Iino for helpful comments on the manuscript, Piali Sengupta for the srg-47 promoter, the Caenorhabditis Genetics Center for providing the che strains, Shohei Mitani at Tokyo Women’s Medical University School of Medicine for kindly providing the che-6(tm5036) allele, and the Vancouver/Oklahoma Caenorhabditis elegans knockout consortium directed by Don Moerman for the che-7(ok2373) allele. This work was funded by the National Institutes of Health (NIH) (R01NS050266 to O.H. and R01GM084191 to X.-Y.H.), an NIH pioneer award (A.D.T.S.), the National Science Foundation (A.D.T.S.), and the Howard Hughes Medical Institute (O.H.).
Footnotes
Communicating editor: P. Sengupta
Literature Cited
- Altun Z. F., Chen B., Wang Z. W., Hall D. H., 2009. High resolution map of Caenorhabditis elegans gap junction proteins. Dev. Dyn. 238: 1936–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., 2006. Chemosensation in C. elegans (Oct. 25, 2006), WormBook, ed. The C. elegans Research Community, WormBook, PMID: 18050433/wormbook. http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R., 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hartwieg E., Horvitz H. R., 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527 [DOI] [PubMed] [Google Scholar]
- Bigelow H., Doitsidou M., Sarin S., Hobert O., 2009. MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat. Methods 6: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnby D. A., Link E. M., Vowels J. J., Tian H., Colacurcio P. L., et al. , 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155: 85–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonigk W., Bradley J., Muller F., Sesti F., Boekhoff I., et al. , 1999. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 19: 5332–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. P., Aziz-Zaman S., Juozaityte V., Martinez-Velazquez L. A., Petersen J. G., et al. , 2012. A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PLoS ONE 7: e34014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Johnston R. J., Frokjaer-Jensen C., Lockery S., Hobert O., 2004. MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature 430: 785–789 [DOI] [PubMed] [Google Scholar]
- Chandrashekar N., Roper S. D., 2010. The cell biology of taste. J. Cell Biol. (PMID 20107438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung B. H., Arellano-Carbajal F., Rybicki I., de Bono M., 2004. Soluble guanylate cyclases act in neurons exposed to the body fluid to promote C. elegans aggregation behavior. Curr. Biol. 14: 1105–1111 [DOI] [PubMed] [Google Scholar]
- Cheung B. H., Cohen M., Rogers C., Albayram O., de Bono M., 2005. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr. Biol. 15: 905–917 [DOI] [PubMed] [Google Scholar]
- Cho S. W., Choi K. Y., Park C. S., 2004. A new putative cyclic nucleotide-gated channel gene, cng-3, is critical for thermotolerance in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 325: 525–531 [DOI] [PubMed] [Google Scholar]
- Cho S. W., Cho J. H., Song H. O., Park C. S., 2005. Identification and characterization of a putative cyclic nucleotide-gated channel, CNG-1, in C. elegans. Mol. Cells 19: 149–154 [PubMed] [Google Scholar]
- Coburn C. M., Bargmann C. I., 1996. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706 [DOI] [PubMed] [Google Scholar]
- Collet J., Spike C. A., Lundquist E. A., Shaw J. E., Herman R. K., 1998. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148: 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels S. A., Ailion M., Thomas J. H., Sengupta P., 2000. egl-4 acts through a transforming growth factor-beta/SMAD pathway in caenorhabditis elegans to regulate multiple neuronal circuits in response to sensory cues. Genetics 156: 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., et al. , 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36: W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery D. B., Sheridan R. E., Russell R. L., 1975. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics 80: 297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer N. D., Troemel E. R., Sengupta P., Bargmann C. I., 1998. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell 93: 455–466 [DOI] [PubMed] [Google Scholar]
- Etchberger J. F., Lorch A., Sleumer M. C., Zapf R., Jones S. J., et al. , 2007. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 21: 1653–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto H. S., Ferkey D. M., Apicella A. J., Lans H., Sharmeen T., et al. , 2004. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron 42: 581–593 [DOI] [PubMed] [Google Scholar]
- Gray J. M., Karow D. S., Lu H., Chang A. J., Chang J. S., et al. , 2004. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430: 317–322 [DOI] [PubMed] [Google Scholar]
- Guo D., Tan Y. C., Wang D., Madhusoodanan K. S., Zheng Y., et al. , 2007. A Rac-cGMP signaling pathway. Cell 128: 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Zhang J. J., Huang X. Y., 2009. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry 48: 4417–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Spencer W. C., McWhirter R. D., Zeller G., Henz S. R., et al. , 2011. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730 [DOI] [PubMed] [Google Scholar]
- Hukema R. K., 2006. Gustatory behaviour in Caenorhabditis elegans, pp. 176 in MGC Department of Cell Biology and Genetics. Erasmus MC, Rotterdam, The Netherlands [Google Scholar]
- Inada H., Ito H., Satterlee J., Sengupta P., Matsumoto K., et al. , 2006. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 172: 2239–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G., Thijssen K. L., Werner P., van der Horst M., Hazendonk E., et al. , 1999. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat. Genet. 21: 414–419 [DOI] [PubMed] [Google Scholar]
- Jansen G., Weinkove D., Plasterk R. H., 2002. The G-protein gamma subunit gpc-1 of the nematode C.elegans is involved in taste adaptation. EMBO J. 21: 986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Seifert R., 2002. Cyclic nucleotide-gated ion channels. Physiol. Rev. 82: 769–824 [DOI] [PubMed] [Google Scholar]
- Koch K. W., Duda T., Sharma R. K., 2002. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol. Cell. Biochem. 230: 97–106 [PubMed] [Google Scholar]
- Komatsu H., Mori I., Rhee J. S., Akaike N., Ohshima Y., 1996. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718 [DOI] [PubMed] [Google Scholar]
- Lanjuin A., VanHoven M. K., Bargmann C. I., Thompson J. K., Sengupta P., 2003. Otx/otd homeobox genes specify distinct sensory neuron identities in C. elegans. Dev. Cell 5: 621–633 [DOI] [PubMed] [Google Scholar]
- L’Etoile N. D., Bargmann C. I., 2000. Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron 25: 575–586 [DOI] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P., 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40: D302–D305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Hodgkin J. A., 1977. Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans. J. Comp. Neurol. 172: 489–510 [DOI] [PubMed] [Google Scholar]
- Liu J., Ward A., Gao J., Dong Y., Nishio N., et al. , 2010. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nat. Neurosci. 13: 715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Ohshima Y., 1995. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348 [DOI] [PubMed] [Google Scholar]
- Morton D. B., 2004. Invertebrates yield a plethora of atypical guanylyl cyclases. Mol. Neurobiol. 29: 97–116 [DOI] [PubMed] [Google Scholar]
- Ortiz C. O., Etchberger J. F., Posy S. L., Frokjaer-Jensen C., Lockery S., et al. , 2006. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics 173: 131–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. O., Faumont S., Takayama J., Ahmed H. K., Goldsmith A. D., et al. , 2009. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr. Biol. 19: 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura J. T., Faumont S., Gaston M. R., Pearson B. J., Lockery S. R., 2001. The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410: 694–698 [DOI] [PubMed] [Google Scholar]
- Potter L. R., 2011. Guanylyl cyclase structure, function and regulation. Cell. Signal. 23: 1921–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E., Zhang Y., Pujol N., Matsuyama T., Bargmann C. I., et al. , 2007. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104: 2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D., MacInnis B. L., Goodman M. B., 2008. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 11: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C., Gutknecht S., Delay E., Kinnamon S., 2006. Detection of NaCl and KCl in TRPV1 knockout mice. Chem. Senses 31: 813–820 [DOI] [PubMed] [Google Scholar]
- Ryu W. S., Samuel A. D., 2002. Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined Thermal stimuli. J. Neurosci. 22: 5727–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., M. O’Meara M, E. B. Flowers, C. Antonio, R. J. Poole et al, 2007. Genetic screens for Caenorhabditis elegans mutants defective in left/right asymmetric neuronal fate specification. Genetics 176: 2109–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K., 2005. Taste recognition: food for thought. Neuron 48: 455–464 [DOI] [PubMed] [Google Scholar]
- Sengupta P., 2007. Generation and modulation of chemosensory behaviors in C. elegans. Pflugers Arch. 454: 721–734 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Thiele T. R., Faumont S., Ezcurra M., Lockery S. R., et al. , 2008. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454: 114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida O., Nakano H., Koga M., Ohshima Y., 2003. The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development 130: 1215–1224 [DOI] [PubMed] [Google Scholar]
- Ward S., 1973. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc. Natl. Acad. Sci. USA 70: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. M., Beverly M., Bell H. W., Sengupta P., 2011. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr. Biol. 21: 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel B., Garbers D., 2001. The guanylyl cyclase family at Y2K. Annu. Rev. Physiol. 63: 215–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.