Abstract
The physiological and developmental effects of harmine, a β-carboline alkaloid, on the insect pest Plodia interpunctella have been analyzed. When added at the larval diet, harmine induced a strong reduction of larvae weight, cannibalism between larvae, in addition to significant mortality. On the other hand, it caused a remarkable development disruption, manifested by both delay and reduction of pupation and adult emergence. Using spectrophotometric assays, we have shown that harmine ingestion provoked a severe reduction in protein, glycogen and lipid contents. Beside, when larvae fed harmine, the activity of the digestive enzyme α-amylase was strongly reduced. In conclusion, our experiments clearly show the susceptibility of P. interpunctella to harmine ingestion revealing the potent bioinsecticidal effect of harmine.
Keywords: Plodia interpunctella, Harmine, Toxicity, Energy reserves, α-Amylase
1. Introduction
Alkaloids have been reported as the most important group of natural substances (Bruneton, 1999), playing an important role in the ecology of organisms which synthesize them (Aniszewski, 2007). For instance, it has been suggested that they constitute part of the plant defences against phytophagous animals (Aniszewski, 2007) together with terpenoids, phenols, flavonoids, steroids, etc. (Hartmann, 1996; Bruneton, 1999; Cox, 2004; Kubo, 2006). The application of plant-derived insect growth regulators is known to be safe for man and environment in the integrated pest management program.
The applications of alkaloids are not limited to biological control of herbivores; they also possess pharmacological, veterinary and medical interest (Aniszewski, 2007). For example, some alkaloids belonging to β-carboline group have been shown to possess a wide range of pharmacological properties including antimicrobial (Aassila et al., 2003), anti-HIV (Ishida et al., 2001) and antiparasitic properties (Rivas et al., 1999; Mishra et al., 2009).
In fact, among medicinal plants, Peganum harmala (L.) (Siddiqui et al., 1987), Grewia bicolor (Roth) (Jaspers et al., 1986) and Passiflora incarnata (L.) (Bennati, 1971) are known for their exceptional wealth in alkaloids belonging to β-carboline type as harmine, harmane and harmaline.
Alkaloids are natural products widely distributed in plants and also found in alcoholic beverages, well-cooked foods and tobacco smoke. In addition, β-carboline have been reported as normal constituents of human tissues and body fluids (Yu et al., 2003). They exhibit a variety of biochemical, psychopharmacological, and behavioural effects in animals and humans (Airaksinen and Kari, 1981). While neurotoxicity of harmaline has been discussed in the literature (O’Hearn and Molliver, 1993; Cobuzzi et al., 1994), neuroprotective effects of harmaline and harmane have also been reported (Bonnet et al., 2000; Splettstoesser et al., 2005). Various authors have reported genotoxic activities of several carboline in prokaryotic and eukaryotic cells that have been attributed to their abilities to intercalate into DNA but studies on the genotoxic and on the cytotoxic potencies in human cells in vitro are not found in the literature (Jiménez et al., 2008).
The effects of methanolic extract of P. harmala ingestion were studied in Schistocerca gregaria (Forskal) (Abbassi et al., 2003) and Tribolium castaneum (Herbst) (Jbilou et al., 2008) and displayed a severe disruption of insect development.
The effects of pure alkaloid compounds application on insect pests are poorly documented compared to other plant compound families. Therefore, we have chosen to test the insecticidal activity of harmine, a β-carboline alkaloid, on the Indian meal moth, Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). In previous work, we have tested the effects of another β-carboline alkaloid, harmaline, on P. interpunctella (Rharrabe et al., 2007). With this study, we want to see if the minimal structural differences existing between these two β-carboline alkaloid molecules (harmine and hamaline) significantly affected their toxicity toward P. interpunctella. For that, we determine the effects of harmine on P. interpunctella development, on its energy reserves (protein, glycogen and lipid content) as well as on the activity of a key α-amylase digestive enzyme.
P. interpunctella is a major economic pest of stored products (dates, wheat, sorghum, barley, almonds, pistachios, walnuts, etc.) and has a worldwide distribution (Mohandass et al., 2007), and its larvae have been recorded feeding upon and contaminating many commodities (Simmons and Nelson, 1975).
2. Materials and methods
2.1. Insect culture
The Indian meal moth P. interpunctella used in this research were collected as larvae from infested dates from Errachidia province in the south-east region of Morocco. The larvae were reared in the laboratory on wheat flour under standard conditions at 28 ± 2 °C, 60 ± 5% relative humidity (r.h.) and a (16L:8D) photoperiod. Emerging adults were removed and allowed to mate in new 0.25-L glass containers half full of wheat flour, as a medium. Eggs were allowed to develop in their oviposition sites. In the present study, fifth-instar larvae were used and the duration of P. interpunctella development was 46 days.
2.2. Treatment
Harmine (Fluka) was dissolved in 5% methanol in distilled water. A volume of 5 mL was incorporated into 5 g of wheat flour to a final concentration of 250, 500, 750 and 1000 ppm. For control (0 ppm) larvae 5 mL of 5% of methanol alone in distilled water alone was added to wheat flour. The solvent was evaporated from the diet at 35 °C in an oven for 48 h. Starved group (also used as a control group) was performed by transferring larvae into empty Petri-dishes to differentiate between toxic rather than simple deterrency effect of harmine.
2.3. Post-embryonic development
Fifth-instar larvae were starved for 24 h prior to use, to induce a high feeding rate. Then 10 larvae were placed in the Petri-dish in the presence of 5 g of treated or control diet. Four replicates were done for treated, starved and control larvae. Observations including larval weight, cannibalism between larvae, larval mortality, pupation and adult emergence, were made every second day during 26 days. Mortality was determined by brown colour with no observable movements. Cannibalism was noted by the disappearance of larval bodies from the Petri dishes. Larvae were maintained under standard conditions (28 ± 2 °C, 60 ± 5% r.h. and a 16L:8D) during the whole experiment.
2.4. Biochemical analyses
For biochemical analyses, fifth-instar larvae were reared individually and taken 7 days after the beginning of the treatment. Each sample was composed of three cold-anaesthetized larvae. Soluble protein, glycogen and lipids contents were quantified spectrophotometrically. The activity of α-amylase was analysed by spectrophotometry and directly on polyacrylamide gel electrophoresis. Ten replicates were done for the whole analyses and for each dosis.
2.4.1. Protein determination
For protein measurements, larvae were homogenized in 1 mL of Tris–HCl buffer (50 mM, pH 7). After centrifugation at 9000g for 20 min, a volume of 200 μL was used for protein according to the method of Bradford (1976) using bovine serum albumin as standard.
2.4.2. Glycogen determination
Glycogen content was quantified by the method of Roe (1955) using anthrone reagent. Larvae were homogenized with 1 mL of ethanol saturated with sodium sulphate, then centrifuged at 1000g for 10 min and the supernatants were discarded. The pellets were resuspended in 0.5 mL of ethanol (70%), then centrifuged as before, and the supernatants were discarded. The final pellets were heated to drive off residual ethanol, then dissolved in 0.5 mL 30% potassium hydroxide and heated at 100 °C for 15 min. The digests were allowed to cool. One millilitre of absolute ethanol was added, then tubes were centrifuged at 1000g for 10 min. The supernatants were carefully removed and the pellets were dissolved in 0.5 mL of distilled water. Two millilitres of anthrone reagent (0.05% in sulphuric acid) were then added. After mixing, samples were heated at 90 °C for 15 min and then rapidly cooled. The absorbance was read at 620 nm and glycogen levels were calculated by reference to a standard curve.
2.4.3. Lipid determination
Lipid extraction was realized according to the method of Van Handel (1965). Larvae were homogenized in 1 mL of chloroform/methanol (1:1, v/v). The homogenates were centrifuged at 1000g for 10 min at 4 °C. The supernatants were mixed with 1 mL of chloroform and 0.5 mL of distilled water and centrifuged at 1500g for 1 h at 4 °C. The aqueous phase was eliminated. The organic phase was extracted again with distilled water (0.5 mL). Organic phase was evaporated until dryness. Lipid content was quantified by the method of Zöllner and Krich (1996). The samples were digested with 1 mL of sulphuric acid at 100 °C for 10 min. The tubes were cooled and 5 mL of sulphosphovanillin reagent (orthophosphoric acid/0.6% aqueous vanillin solution, 4:1) was added to the mixture. After 40 min, the absorbance was measured at 530 nm and lipid level was calculated by reference to a standard curve using cholesteryl palmitate.
2.4.4. α-Amylase assay
Assay of α-amylase activity (α-1,4-glucan-4-glucanohydrolases, EC 3.2.1.1) was performed according to Valencia et al. (2000) with some modifications. Larvae were homogenized in 100 μL of sodium citrate NaCl–CaCl2 (10 mM), buffer (pH 8). The mixture was added to 100 μL starch (0.5%). Samples were incubated at 37 °C for 15 min. Iodine reagent (2.5 μL of 0.02% I2 and 0.2% KI) was then added and the sample was centrifuged at 4000g for 10 min. The absorbance was read at 580 nm. The enzymatic activity was expressed by the quantity of starch consumed per unit of time by larvae (ng/min/larva).
2.4.5. In gel α-amylase assay
Polyacrylamide gel electrophoresis was carried out in a vertical slab gel apparatus at 4 °C as described by Laemmli (1970). Samples were homogenized in 50 μL of buffer (0.5 M Tris, 10% glycerol and 0.1% bromophenol blue) and run on a 12% polyacrylamide gel in Tris–glycine buffer (pH 8.8), with constant current set at 100 mA. A zymogram of amylase activity was carried out as described by Campos et al. (1989). After separation, the gel was transferred into a solution of 2.5% Triton X-100 and left overnight, and then transferred to a substrate/buffer solution (1% (w/v) gelatinized potato starch, acetate (100 mM), NaCl (20 mM), CaCl2 (0.2 mM) at pH 8.8) and incubated at 30 °C for 30 min. After rinsing the gel in distilled water, amylolytic activity was stopped by transferring the gel to the staining solution (1.3% (w/v) I2 and 3% (w/v) KI). After coloration, light bands against the dark background indicated the presence of α-amylase activity.
2.5. Statistical analysis
Statistical analyses were performed using Statistica Software (Statistica, 1997). All data were checked for normality using Shapiro–Wilk W-static and for homogeneity of variance by Levene’s test. Difference in larval weight were analysed from day 2 to 10 by a repeated-measures Friedman test and day six by the Kruskal–Wallis test to compare all the several independent groups. Cannibalism, larval mortality, pupation and adult emergence data (percentages) were analyzed by repeated-measures ANOVA test, after logit transformation, followed by Dunnett’s test for multiple comparisons with respect to the control. Biochemical parameters data were analyzed using one-way ANOVA followed by Dunnett’s test. The significance was tested at the P = 0.05 level.
3. Results
3.1. Effects of harmine on development parameters
3.1.1. Effect on larval weight
The results of weight change in treated, starved and control larvae are shown in Fig. 1. A progressive decrease in treated larvae weight was observed during the experiment. Overall 10 days after the beginning of the treatment, compared on initial weight, the weight loss was 26%, 30%, 32% and 39% for 250, 500, 750 and 1000 ppm, respectively. For starved larvae, the weight loss was more pronounced. In contrast, control larvae gained 54% in weight compared to their initial weight. Difference between groups from day 2 to 10 is highly significant (Friedman test) being the treatment of 250, 500, 750 and 1000 ppm as well as starved significantly different from control group at day six.
Figure 1.
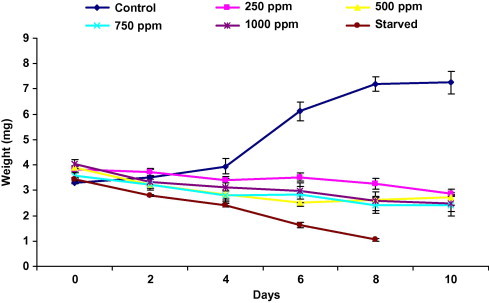
Effect of ingestion diet treated with harmine and starvation on larval weight evolution of fifth instar Plodia interpunctella larvae (n = 4 of 10 larvae). ((N = 52, df = 4) = 33.23, P < 0.00000, Coeff. of concordance = 0.16, r = 0.14 Friedman test.)
3.1.2. Effect on cannibalism
The presence of harmine in the larval diet evoked cannibalistic behaviour between larvae (Fig. 2). We noted the disappearance of larval bodies in the treated batches from the second day. After 8 days, it was 30% for 250 and 500 ppm, 32.5% and 7.5% for 750 and 1000 ppm, respectively. Cannibalism appeared in the fourth day for larva treated with 1000 ppm with 2.5% and remained stable during the experiment. For starved larva it reached its maximum at day eight (30%).
Figure 2.
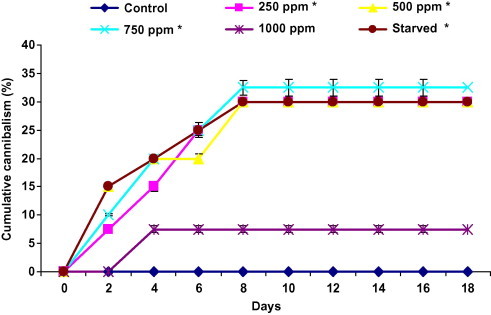
Effect of ingestion diet treated with harmine and starvation on cumulative cannibalism of fifth instar Plodia interpunctella larvae (n = 4 of 10 larvae). Asterisks significantly different with respect to control (Dunnett’s, P ⩽ 0.05).
3.1.3. Effect on larval mortality
Harmine provoked a high larval mortality (Fig. 3). Death of larvae started 2 days after the beginning of the experiment for larvae treated with 500, 750 and 1000 ppm, and at day four for control and 250 ppm. The mortality was 50%, 65%, 60% and 90% for the concentration of 250, 500, 750 and 1000 ppm, respectively. In starved larvae, the mortality appeared just 2 days after treatment and it reached 70% just after 6 days. Mortality of control larvae did not exceed 10% during the observation period.
Figure 3.
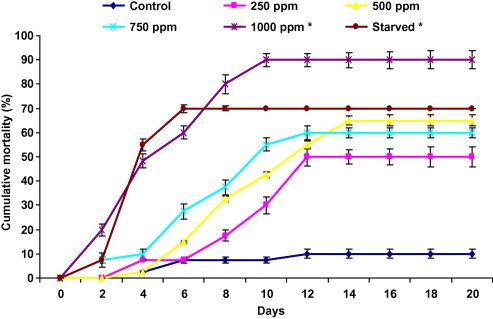
Effect of ingestion diet treated with harmine and starvation on cumulative larval mortality of fifth instar Plodia interpunctella larvae (n = 4 of 10 larvae). Asterisks significantly different with respect to control (Dunnett’s, P ⩽ 0.05).
3.1.4. Effect on pupation
Pupation of control larvae started at the eighth day of the experiment (Fig. 4). The percentage of cumulated pupation increased during the observation period and reached 90% over 16 days. Meanwhile, in treated larvae a delay of 2 days was recorded compared to the control. After 14 days the pupation reached the maximum and it was 20%, 5%, 7.5% and 2.5% for 250, 500, 750 and 1000 ppm, respectively. Pupation was completely abolished in starved larvae.
Figure 4.
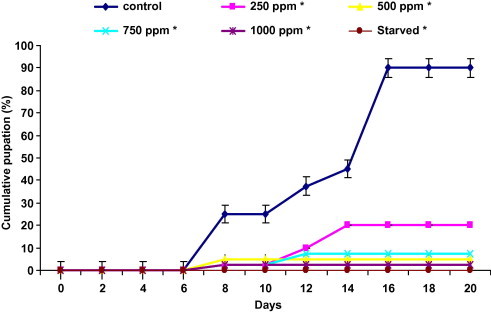
Effect of ingestion diet treated with harmine and starvation on cumulative pupation of fifth instar Plodia interpunctella larvae (n = 4 of 10 larvae). Asterisks significantly different with respect to control (Dunnett’s, P ⩽ 0.05).
3.1.5. Effect on adult emergence
As for pupation, this developmental parameter was significantly affected by the presence of harmine in the larval diet (Fig. 5). From these pupae, in insects treated at 500, 750 and 1000 ppm, adult emergence was completely inhibited. For control adult emergence appeared 16 days after the beginning of the experiment (80%), it reached its maximum at day 22 (100%). For 250 ppm of harmine, we noted a delay of 2 days when compared with controls, at the end of the experiment, the percentage of adult emergence was 10% for 250 ppm.
Figure 5.
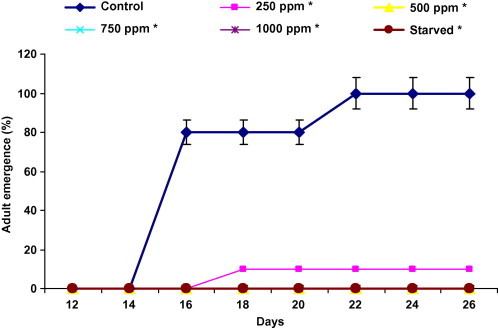
Effect of ingestion diet treated with harmine and starvation on cumulative adult emergence of fifth instar Plodia interpunctella larvae (n = 4 of 10 larvae). Asterisks significantly different with respect to control (Dunnett’s, P ⩽ 0.05).
3.2. Effects of harmine on biochemical parameters
Biochemical results showed clearly that harmine induced a decrease in protein, glycogen and lipid contents compared to control larvae (Table 1). It also provoked a decrease of α-amylase activity (Fig. 6 and Table 1).
Table 1.
Effect of ingestion diet treated with harmine and starvation on protein, glycogen and lipid content as well as the activity of α-amylase enzyme of fifth instar Plodia interpunctella larvae 7 days after exposure (n = 10).
| Protein (μg/larvae) | Glycogen (μg/larvae) | Lipid (μg/larvae) | α-Amylase activitya | |
|---|---|---|---|---|
| Control | 544 ± 22 | 36 ± 4.3 | 846 ± 27 | 10 ± 0.8 |
| 250 ppm | 337 ± 24⁎ | 14 ± 3.2⁎ | 865 ± 24 | 7.3 ± 0.7⁎ |
| 500 ppm | 177 ± 13⁎ | 12 ± 2.3⁎ | 730 ± 15⁎ | 7.3 ± 0.7⁎ |
| 750 ppm | 155 ± 17⁎ | 8.2 ± 1.5⁎ | 827 ± 17 | 6.6 ± 0.6⁎ |
| 1000 ppm | 119 ± 12⁎ | 5.9 ± 1.2⁎ | 761 ± 12⁎ | 4.3 ± 0.6⁎ |
| Starved | 187 ± 17⁎ | 2.9 ± 0.6⁎ | 570 ± 25⁎ | 7.0 ± 0.7⁎ |
| Control harmine in vitro | – | – | – | 10 ± 0.9 |
Specific activity as ng of substrate hydrolyzed/min/larvae.
Significantly different with respect to control (Dunnett’s, P ⩽ 0.05).
Figure 6.
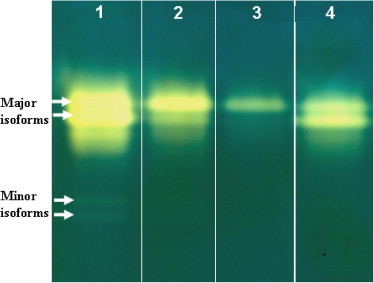
Effect of ingestion diet treated with harmine and starvation on the activity of α-amylase enzyme of fifth instar Plodia interpunctella larvae. In gel expression. Lane 1: control; lane 2: 250 ppm; lane 3: 1000 ppm; lane 4: starved larvae.
3.2.1. Effect on protein, glycogen and lipid
Protein content was severely reduced in all groups as compared with the control group at all doses (P ⩽ 0.05); this content is less reduced for starved larvae than for 250 ppm of harmine (Table 1).
Glycogen level was remarkably reduced in all groups and starved larvae compared with the control group in a dose-dependent manner (Table 1) (P ⩽ 0.05).
Harmine ingestion had a negative effect on total lipid (Table 1). It was reduced for the doses 500 and 100 ppm compared to control larvae. In larvae fed 250 ppm, the lipid content was not affected. In contrast, for starved larvae, the lipid content was strongly reduced. The Dunnett’s test showed that the difference between control and larvae fed 500 and 1000 ppm was significant, as well as between control and starved larvae (P ⩽ 0.05).
3.2.2. Effect on α-amylase activity
Larvae fed harmine showed a dose dependent lowering of α-amylase as compared to those fed control diet (Table 1). In addition, the α-amylase activity is more reduced for the 1000 ppm group than for the starved group. Zymographic analysis of α-amylase activity (Fig. 6) revealed that control larvae have four distinct α-amylase bands: two closely associated with high intensity and two minor bands with much lower intensity. In starved and treated larvae, the minor isoforms were not observed and the major ones had a lower intensity. The dosis of 1000 ppm provoked the most potent effect.
4. Discussion
4.1. Effects of harmine on development parameters
The data presented in this report clearly show that P. interpunctella is susceptible to harmine ingestion. Indeed, harmine toxicity was manifested in larval weight loss (Fig. 1), cannibalism (Fig. 2) and survival (Fig. 3) in addition to noticeable insect development disruption. In fact, the ingestion of harmine by P. interpunctella larvae provoked two categories of metamorphic disorders: delay in pupation (Fig. 4) of survivors and almost lack complete adult emergence (Fig. 5). Similar perturbations were reported in insects feeding alkaloids. For example, the effect of ingestion of alkaloids extract from P. harmala was studied in S. gregaria and it caused a weight loss, a significant mortality and a severe sexual maturity disruption in both males and females adults (Abbassi et al., 2003). The effects of P. harmala methanolic extract provoked larval growth inhibition in T. castaneum (Jbilou et al., 2008). Heinz et al. (1996) showed that harmane and harmaline reduced growth and feeding in the fifth-instar larvae of Trichoplusia ni (Hübner). Similar disturbances have been observed in many phytophagous insects after ingestion of various allelochemicals belonging to other chemical families, as for example, azadirachtin (family of limonoids – Mordue and Blackwell, 1993) and different phytoecdysteroids (family of steroids – Marion-Poll et al., 2005). Overall, many plant secondary metabolites affect insect behaviour, development and reproduction (Cox, 2004; Kubo, 2006).
P. interpunctella presents different susceptibility to other allelochemicals. Indeed, after 20-hydroxyecdysone ingestion, significant mortality, large weight loss and high cannibalism between larvae were recorded. On the other hand, the 20-hydroxyecdysone induced precocious pupation and adult emergence (Rharrabe et al., 2009). With azadirachtin treatment, an important larval and pupal mortality was noted in P. interpunctella (Rharrabe et al., 2008).
In previous work, we have tested the effects of harmaline (another β-carboline alkaloid) on P. interpunctella (Rharrabe et al., 2007). Indeed, although there is small structural difference between harmine (C13H12ON2) and harmaline (C13H15ON2), their toxicity towards P. interpunctella was remarkably affected. In general, harmaline was more toxic than harmine. The main differences with the present study were: the weight loss is more important for the treatment with harmaline than for harmine. Moreover, harmaline causes an important larval mortality at 250 ppm, prevented pupation and inhibits adult emergence.
Thus, the effects of allelochemicals on insect pests not only depend on the type of family of compound but also depend on their structure.
4.2. Effect of harmine on nutriment level (protein, glycogen, lipids and α-amylase)
Following harmine ingestion, a significant drop of energy reserves was observed, especially in protein and glycogen contents (Table 1). In contrast, the effect on lipid content (Table 1) was much weaker; possibly because the lipids metabolism is less important in larvae instar if we compared with adult instar. In general, the lipid content of the fat body increases continuously during the larval period of holometabolous insects and it is mobilised in adult life stages to support flight, to travel, to support embryogenesis and in general roles such as energy provision, membrane formation and repair (Beenakkers et al., 1985; Arrese et al., 2001; Canavoso et al., 2001; Ziegler and Van Antwerpen, 2006). In addition, there is no remarkable effect on lipids, possibly because we have analyzed the total lipids. Perhaps if we analyse each class of lipids (as triacylglycerols, diacylglycerols and phospholipids) separately we could observe a significant decrease in one or more class following harmine treatment.
Overall, the depletion of energy reserves could be due to high mobilization of these metabolites to compensate the lack of nutriments caused by the drug stress owing to a cytotoxic effect on midgut epithelial cells as well as to a reduction of their synthesis. In fact, we have demonstrated that harmaline ingestion caused a very marked cytotoxicity and severe disorganization of proteosynthetic organelles of the midgut epithelial cells of P. interpunctella larvae (Rharrabe et al., 2007).
Investigations on various insect species have shown a depression or an inhibition of energy reserves including lipids content. For example, after ingestion of jatropherol-I by Bombyx mori (L.) (Jing et al., 2005), after Neem Oil treatment in Choristoneura rosaceana (Harris) (Smirle et al., 1996) and after azadirachtin treatment in some insects as Locusta migratoria (L.) (Rembold et al., 1987), Labidura riparia (Pallas) (Sayah et al., 1996) and Spodoptera litura (F.) (Huang et al., 2004). As well as in P. interpunctella after ingestion of harmaline (Rharrabe et al., 2007), of azadirachtin (Rharrabe et al., 2008) and of 20-hydroxyecdysone (Rharrabe et al., 2009).
Our results show that α-amylase activity (Table 1) is reduced after harmine ingestion. This reduction was not due to an interaction between harmine and enzyme (there is no difference between in vivo and in vitro results). Thus, the reduction of the activity of this enzyme could be due to a cytotoxic effect of harmine on midgut epithelial cells as is the case of harmaline.
Many plant defence compounds include enzyme inhibitors that act on insect gut digestive enzymes as hydrolases, proteinases and α-amylases (Ryan, 1990; Franco et al., 2002). Indeed, several authors reported that, for example, the ingestion of azadirachtin by S. litura (and Cnaphalocrocis medinalis Guenée) (Senthil Nathan et al., 2005a,b) caused a decrease in gut enzyme activities.
Starved P. interpunctella larvae also displayed a marked disruption of development and a severe decrease in energy reserves and α-amylase activity. However, the intensity of these perturbations was more pronounced than those caused by harmine ingestion. For example, the weight loss was more pronounced in starved larvae than in larvae fed harmine. On the other hand, the insect development to pupal stage was completely prevented in starved larvae.
5. Conclusion
In summary, this work establishes clearly the susceptibility of P. interpunctella to harmine ingestion. It would be of interest to check the activity of alkaloids mixture on P. interpunctella in order to determine if there is a synergic effect. Indeed, experiments are in progress to determine the effects of harmine/harmaline mixture on P. interpunctella.
The use of alkaloids as bioinsectidal is not an alternative for the methods used currently for pest control but they represent interesting molecules to improve the integrated pest management program, to decrease the parasitic pressure exerted by insects on plants by using other efficient and environmental-friendly practices.
Acknowledgements
This work was supported by the program “CEEM-Pôle d’Excellence Régional-AUF” project (FST, Tangiers, Morocco). We are grateful to Professor Félix ORTEGO ALONSO (Cento de Investigaciones Biologicas, Madrid) for critical reading of the manuscript and for fruitful discussions.
References
- Aassila H., Bourguet-Kondracki M.L., Rifai S., Fassouane A., Guyot M. Identification of harman as the antibiotic compound produced by a tunicate-associated bacterium. Mar. Biotechnol. 2003;5:163–166. doi: 10.1007/s10126-002-0060-7. [DOI] [PubMed] [Google Scholar]
- Abbassi K., Atay-Kadiri Z., Ghaout S. Biological effects of alkaloids extracted from three plants of Moroccan arid areas on the desert locust. Physiol. Entomol. 2003;28:232–236. [Google Scholar]
- Airaksinen M.M., Kari I. β-Carbolines, psychoactive compounds in the mammalian body. Part II: effects. Med. Biol. 1981;59:190–211. [PubMed] [Google Scholar]
- Aniszewski, T., 2007. Alkaloids – Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role. Elsevier Science, Amsterdam–Oxford.
- Arrese E.L., Canavoso L.E., Jouni Z.E., Pennington J.E., Tsuchida K., Wells M.A. Lipid storage and mobilization in insects: current status and future directions. Insect Biochem. Mol. Biol. 2001;31:7–17. doi: 10.1016/s0965-1748(00)00102-8. [DOI] [PubMed] [Google Scholar]
- Beenakkers A.M.Th., Van der Horst D.J., Van Marrewijk W.J.A. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lip. Res. 1985;24:19–67. doi: 10.1016/0163-7827(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Bennati E. Quantitative determination of harmane and harmine in the extract of Passiflora incarnata. Boll. Chim. Farm. 1971;110:664–669. [PubMed] [Google Scholar]
- Bonnet U., Leniger T., Wiemann M. Moclobemide reduces intracellular pH and neuronal activity CA3 neurones in guinea-pig hippocampal slices—implication for its neuroprotective properties. Neuropharmacology. 2000;39:2067–2074. doi: 10.1016/s0028-3908(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruneton J. Technique and Documentation; Paris: 1999. Pharmacognosie, Phytochimie, Plantes Médicinales. [Google Scholar]
- Campos F.A.P., Xavier-Filho J., Silva C.P., Ary M.B. Resolution and partial characterization of proteinases and α-amylases from midguts of larvae of the bruchid beetle Callosobruchus maculatus (F) Comp. Biochem. Physiol. B. 1989;92:51–57. [Google Scholar]
- Canavoso L.E., Jouni Z.E., Karnas K.J., Pennington J.E., Wells M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- Cobuzzi J.R., Neafsey E.J., Collins M.A. Differential cytotoxicities of N-methyl-beta-arbolinium analogues of MPP+ in PC12 cells: insights into potential neurotoxicants in Parkinson’s disease. J. Neurochem. 1994;62:1503–1510. doi: 10.1046/j.1471-4159.1994.62041503.x. [DOI] [PubMed] [Google Scholar]
- Cox P.D. Potential for using semiochemicals to protect stored products from insect infestation. J. Stor. Prod. Res. 2004;40:1–25. [Google Scholar]
- Franco O.L., Rigden D.J., Melo F.R., Grossi-de-Sá M.F. Plant α-amylase inhibitors and their interaction with insect α-amylase. Structure, function and potential for crop protection. Eur. J. Biochem. 2002;269:397–412. doi: 10.1046/j.0014-2956.2001.02656.x. [DOI] [PubMed] [Google Scholar]
- Hartmann T. Diversity and variability of plant secondary metabolism—a mechanistic view. Entomol. Exp. Appl. 1996;80:177–188. [Google Scholar]
- Heinz C.A., Zangerl A.R., Berenbaum M.R. Effects of natural and synthetic neuroactive substances on the growth and feeding of cabbage looper, Trichoplusia ni. Entomol. Exp. Appl. 1996;80:443–451. [Google Scholar]
- Huang Z., Shi P., Dai J., Du J. Protein metabolism in Spodoptera litura (F.) is influenced by the botanical insecticide azadirachtin. Pest. Biochem. Physiol. 2004;80:85–93. [Google Scholar]
- Ishida J., Wang H.K., Masayoshi O., Cosentino C.L., Hu C.Q., Lee K.H. Anti-aids agents 46. Anti-HIV activity of harman, an anti-HIV principle from symplocos setchuensis and its derivatives. J. Nat. Prod. 2001;64:958–960. doi: 10.1021/np0101189. [DOI] [PubMed] [Google Scholar]
- Jaspers M.W., Bashir A.K., Zwaving J.H., Malingre T.M. Investigation of Grewia bicolor Juss. J. Ethnopharmacol. 1986;17:205–211. doi: 10.1016/0378-8741(86)90109-1. [DOI] [PubMed] [Google Scholar]
- Jbilou R., Amri H., Bouayad N., Ghailani N., Ennabili A., Sayah F. Insecticidal effects of extracts of seven plant species on larval development, α-amylase activity and offspring production of Tribolium castaneum (Herbst) (Insecta: Coleoptera: Tenebrionidae) Bioresour. Technol. 2008;99:959–964. doi: 10.1016/j.biortech.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Jiménez J., Riverón-Negrete L., Abdullaev F., Espinosa-Aguirre J., Rodríguez-Arnaiz R. Cytotoxicity of the beta-carboline alkaloids harmine and harmaline in human cell assays in vitro. Exp. Toxicol. Pathol. 2008;4–5:381–389. doi: 10.1016/j.etp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Jing L., Fang Y., Ying X., Wenxing H., Meng X., Syed M., Syed N., Fang C. Toxic impact of ingested Jatropherol-I on selected enzymatic activities and the ultrastructure of midgut cells in silkworm, Bombyx mori L. J. Appl. Entomol. 2005;129:98–104. [Google Scholar]
- Kubo I. New concept to search for alternate insect control agents from plants. In: Rai M., Carpinella M., editors. vol. 3. Elsevier; Amsterdam: 2006. pp. 61–80. (Naturally Occurring Bioactive Compounds). [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marion-Poll, F., Dinan, L., Lafont, R., 2005. The role of phytoecdysteroids in the control of phytophagous insects. In: Regnault-Roger, C.J.R., Philogène, B., Vincent, C. (Eds.), Biopesticides of Plant Origin. Lavoisier, pp. 87–103.
- Mishra B.B., Kale R.R., Singh R.K., Tiwari V.K. Alkaloids: future prospective to combat leishmaniasis. Fitoterapia. 2009;80:81–90. doi: 10.1016/j.fitote.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Mohandass S., Arthur F.H., Zhu K.Y., Throne J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stor. Prod. Res. 2007;43:302–311. [Google Scholar]
- Mordue A.J.L., Blackwell A. Azadirachtin: an update. J. Insect Physiol. 1993;39:903–924. [Google Scholar]
- O’Hearn E., Molliver M.E. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55:303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- Rembold, H., Uhl, M., Müller, Th., 1987. Effect of azadirachtin A on hormone titres during the gonadotrophic cycle of Locusta migratoria. In: Schmutterer, H., Ascher, K.R.S. (Eds.), Proceedings of the Third International Neem Conference, Nairobi, Kenya. German Agency for Technical Cooperation, Eschborn, Germany, pp. 289–298.
- Rharrabe K., Amri H., Bouayad N., Sayah F. Effects of azadirachtin on post-embryonic development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae) J. Stor. Prod. Res. 2008;44:290–294. [Google Scholar]
- Rharrabe K., Bakrim A., Ghailani N., Sayah F. Bioinsecticidal effect of harmaline on Plodia interpunctella development (Lepidoptera: Pyralidae) Pest. Biochem. Physiol. 2007;89:137–145. [Google Scholar]
- Rharrabe K., Bouayad N., Sayah F. Effects of ingested 20-hydroxyecdysone on development and midgut epithelial cells of Plodia interpunctella (Lepidoptera, Pyralidae) Pest. Biochem. Physiol. 2009;93:112–119. [Google Scholar]
- Rivas P., Cassels B.K., Morillo A., Repetto Y. Effects of some beta-carboline alkaloids on intact Trypanosoma cruzi epimastigotes. Comp. Biochem. Physiol. C. 1999;122:27–31. doi: 10.1016/s0742-8413(98)10069-5. [DOI] [PubMed] [Google Scholar]
- Roe J.H. Determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955;212:335–338. [PubMed] [Google Scholar]
- Ryan C.A. Protease inhibitors in plants: genes for improving defences against insects and pathogens. Annu. Rev. Phytopathol. 1990;28:425–449. [Google Scholar]
- Sayah F., Fayet C., Idaomar M., Karlinsky A. Effect of azadirachtin on vitellogenesis of Labidura riparia (Insecta, Dermaptera) Tissue Cell. 1996;28:741–749. doi: 10.1016/s0040-8166(96)80077-2. [DOI] [PubMed] [Google Scholar]
- Senthil Nathan S., Kalaivani K., Chung P.G. The effects of azadirachtin and nucleopolyhedrovirus on midgut enzymatic profile of Spodoptera litura Fab (Lepidoptera: Noctuidae) Pest. Biochem. Physiol. 2005;83:46–57. [Google Scholar]
- Senthil Nathan S., Kalaivani K., Murugan K., Chung P.G. The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guenée) the rice leaffolder. Pest. Biochem. Physiol. 2005;81:113–122. [Google Scholar]
- Siddiqui S., Khan O.Y., Siddiqui B.S., Faizi S. Harmaline, a β-carboline alkaloid from Peganum harmala. Phytochemistry. 1987;26:1548–1550. [Google Scholar]
- Simmons, P., Nelson, H.D., 1975. Insects on Dried Fruits. Agriculture Handbook. USDA, Agricultural Research Service.
- Smirle M.J., Lowery D.T., Zurowski C.L. Influence of Neem Oil on detoxication enzyme activity in the obliquebanded leafroller, Choristoneura rosaceana. Pest. Biochem. Physiol. 1996;56:220–230. [Google Scholar]
- Splettstoesser F., Bonnet U., Wiemann M., Bingmann D., Büsselberg D. Modulation of voltage-gated channel currents by harmaline and harmane. Br. J. Pharmacol. 2005;144:52–58. doi: 10.1038/sj.bjp.0706024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistica, 1997. Statistica Release 5.1. Statistica Statsoft Inc., Tulsa, OK, USA.
- Valencia A., Bustillo A.E., Ossa G.E., Chrispeels M.J. Alpha-amylases of the coffee berry borer (Hypothenemus hampei) and their inhibition by two plant amylase inhibitors. Insect Biochem. Mol. Biol. 2000;30:207–213. doi: 10.1016/s0965-1748(99)00115-0. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Micro-separation of glycogen, sugars and lipids. Anal. Biochem. 1965;11:266–271. doi: 10.1016/0003-2697(65)90014-x. [DOI] [PubMed] [Google Scholar]
- Yu A.M., Idle J.R., Krausz K.W., Küpfer A., Gonzalez F.J. Contribution of individual cytochrome P450 isozymes to the Odemethylation of the psychotropic beta-carboline alkaloids harmaline and harmine. J. Pharmacol. Exp. Ther. 2003;305:315–322. doi: 10.1124/jpet.102.047050. [DOI] [PubMed] [Google Scholar]
- Ziegler R., Van Antwerpen R. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 2006;36:264–272. doi: 10.1016/j.ibmb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zöllner N., Krich K. The quantitative determination of lipids (micromethod) by means of sulphosphovanillin reaction common to many natural lipids (all known plasma lipids) Zeit. Ges. Exp. Med. 1996;135:545–561. [Google Scholar]


