Abstract
Excessive softening is the main factor limiting fruit shelf life and storage. It is generally acceptable now that softening of fruit which occurs during the ripening is due to synergistic actions of several enzymes on cell wall polysaccharides. As a subject for this study, we have assayed some glycosidase activities using three tomato species (Lycopersicon esculentum) contrasted for their texture phenotypes; the cherry tomato line Cervil (Solanum lycopersicum var. cerasiforme), a common taste tomato line Levovil (S. lycopersicum Mill.) and VilB a modern line, large, firmer and with good storage capability. Four glycosidase activities namely α-galactosidase, β-galactosidase, β-mannosidase and β-glucosidase were extracted from tomato’s cell wall of the three species. Cell wall protein from fruits pericarp was extracted and compared among the three cultivars at the following stages; 14 days post anthesis (14DPA) fruit; 21 days post anthesis (21DPA), turning (breaker), red and over ripe. When glycolytic activities were also compared among these cultivars at the precited development stages, gross variations were noticed from stage to stage and also from species to species in accordance with the fruit firmness status. Interestingly, VilB cultivar, the firmer among the other two, though possessed the highest total protein content, exhibited the lowest enzymatic activities. Taken together, these results may therefore allow us to conclude that studies of glycolytic activities in a single tomato cultivar cannot be generalized to all species. On the other hand, relating fruit development to glycosidase activities should logically be coupled to these enzymes from cell wall compartment.
Abbreviations: DPA, days post anthesis; VilB, a modern tomato line, large, firmer and with good storage capability; PME, pectin methylestrase; MES, 2-(N-morpholino) ethanesulfonic acid; BSA, bovine serum albumin; G6P-D, glucose-6 phosphate dehydrogenase; NADP, nicotinamide adenine dinucleotide phosphate
Keywords: Tomato cultivars, Texture, Glycosidases, Cell wall protein, Development stage
1. Introduction
Fruit ripening is genetically programed and involves physiological, biochemical, and structural changes, such as cell wall hydrolysis, pigment degradation and synthesis, carbohydrate metabolism, and generation of secondary metabolism compounds which influence fruit appearance, texture, flavor, and aroma (Mworia et al., 2011) and (Prasanna et al., 2007). The softening of fruits that occurs after harvest is a major factor that contributes to the losses of good percentage of all fresh produce grown world-wide. Many efforts to suppress expression of cell wall-degrading enzymes have not provided the insight needed to genetically engineer fruits whose softening can be adequately controlled (Meli et al., 2010) and (Giovannoni et al., 1989). Studying the biochemical mechanisms involved in this loss is a global effort to modify plants genetically so that they produce fruit that resists the softening process. It was believed that polygalacturonase (PG) and pectin methylestrase (PME) are principally responsible for fruit ripening (Giovannoni et al., 1989) and (Brummell and Harpster, 2001). However, several subsequent studies demonstrated that inhibition of neither PG nor PME could interfere with fruit ripening (Tieman and Handa, 1994; Tieman et al., 1992). Since wall rigidity and intercellular cohesion in fruit tissue have also been partially attributed to cross-linkages that contain arabinose and galactose (Wong, 2008). The modification of cross-linking polymers could contribute to the loss of wall structure and fruit firmness that occur during ripening. If this were so, softening could be at least partly attributable to the action of enzymes that cleave bonds between sugars other than galacturonic acid (Ahmed and Labavitch, 1980; Giovannoni et al., 1989). Reports are accumulating now emphasis on that fact that glycosidases play fundamental role in loosening of cell wall structure and finally fruit development and ripening in tomato (Brummell and Harpster, 2001; Wallner and Walker, 1975), berries (Hilz et al., 2006), coffee beans (Marraccini et al., 2005), mango (Ali et al., 1995), papaya (Manenoi and Paull, 2007) and several other fruits, for a review see Prasanna et al. (2007).
The tomato (Solanum lycopersicum) is a major vegetable crop that has achieved tremendous popularity over the last century, with thousands of cultivars having been selected with varying fruit types, and for optimum growth in differing growing conditions. The domestication of the tomato S. lycopersicum and associated selective pressures eventually led to the large-fruited varieties cultivated today. Cultivated tomatoes vary in color intensity, shape, quality and size from tomberries, about 5 mm in diameter, through cherry tomatoes, about the same 1–2 cm (0.4–0.8 in.) size as the wild tomato to up to beefsteak tomatoes 10 cm (4 in.) or more in diameter (Bai and Lindhout, 2007; Barrett et al., 1998).
Glycosidases have been related to tomato development and ripening in several reports. However since, fruit ripening and development have been extensively, in part, related to the dynamic activities of the glycol-hydrolases localized to cell wall, it becomes more logical to study these enzymes from cell wall and not whole cell extract where they could also be present. Since tomato cultivars, currently commercialized, are of vast texture variations, no information on relating the texture of these cultivars with endogenous glycolytic hydrolases isolated from cell wall at different stages of development and ripening, are available. In this investigation, to our knowledge for first time glycosidases were isolated from cell walls from three texture contrasted tomato cultivars; the cherry tomato line Cervil (S. lycopersicum var. cerasiforme), a common taste tomato line Levovil (S. lycopersicum Mill.) and VilB a modern line, large, firmer and with good storage capacity, with the objective to check for any possible correlation between fruit texture and glycosidase activities not from whole cell extract, as previous studies reported, but from cell wall, in order to pave way for further understanding the mechanism underlying the softening process which restrict the longer shelf life of fresh fruits.
2. Materials and methods
2.1. Tomato growth conditions
Six plants per cultivar of Cervil, Levovil and VilB were grown in pots in a greenhouse under standard conditions. Tomatoes were collected at varying six stages of development and ripening; 14 days post-anthesis (14DPA), 21 days post-anthesis (21DPA), turning (breaker), red and over ripe. To harvest the first two stages, flowers were tagged at anthesis. For the latter three stages, fruits were harvested based on fruit color.
2.2. Protein extraction methods
2.2.1. Total protein extraction
Five hundred mg of pericarp powder material was directly extracted in 1.2 mL of Laemmli sample buffer (Laemmli, 1970) during 15 min at room temperature. After 15 min centrifugation at 5500g the protein content of the supernatant was assayed using the Biorad protein assay kit with bovine serum albumin (BSA) as a standard according to the manufacturer prescriptions.
2.2.2. Soluble cell wall proteins extraction
The method was adapted from Chivasa et al. (2002). Unless otherwise stated all steps were carried out at 4 °C. Red riped tomato pericarps were cut with a cleaned razor into small pieces and immediately immersed in ice-cold 20 mM K2HPO4 pH 6.0 buffer, excess buffer was blotted away from pericarp segments by filter paper before weighing. They were then rinsed twice with degassed ice-cold 3 mL/g 10 mM MES buffer, pH 5.5 and immersed in flasks containing the same solution. Rinsing buffer was discarded and tissue pieces were vacuum infiltrated at 60 kPa for 30 s for removal of gas trapped in veins. Ice-cold degassed 100 mM KCl, 10 mM MES, pH 5.5 buffer plus 5 μL of protease inhibitor cocktail (Sigma) was added on the basis of 5 mL per gram of plant material then soaked pericarp segments were subjected to 60 kPa vacuum for 3 min with gentle intermittent shaking. Vacuum was released; pericarp pieces were allowed to stand in the infiltration buffer for 3 min. Infiltration buffer was then removed and excess buffer was blotted away from tomato segments. Pericarp pieces were then transferred into 15 mL Falcon tube internally lined with mesh into a U-shape and previously holed at their bottom part. The whole unit was inserted into a 50 mL clean Falcon tube and centrifuged for 10 min at 1000 rpm. Infiltration juice collected at the bottom of the 50 mL Falcon tube from various vacuum infiltration experiments was collected, concentrated with Marosep centrifugal device (15 mL capacity/10 kDa membrane cutoff) followed by desalting by washing using three volume of 50 mM Na-Acetate buffer, pH 6 with 1 μL/mL protease inhibitor cocktail. Protein was quantified using Bio-Rad protein estimation kit and preserved at −80 °C till further use.
2.2.3. Calculation of fruit water content
Equal amount of fresh pericarp of Cervil, Levovil and VilB was washed with distilled water and placed in open previously weighed container, the container was then placed in an oven at 70 °C and the pericarp was dried to a constant weight. The container, plus dry tissue were weighed and recorded. The pericarp dry weight was determined by subtraction of the empty container from container with the dried tissues.
2.2.4. Protein estimation
Protein content was quantified with Bio-Rad Bradford assay protein using BSA as a standard.
2.2.5. Wall protein purity assessment
This was carried out by following the intracellular marker glucose-6 phosphate as reported (Li et al., 1989) in brief: in 1 mL reaction volumes. Glucose-6 phosphate dehydrogenase (G6P-D, EC 1.1.1.49) in a reaction mixture containing 25 μM Tricine, pH 8, 25 μM glucose-6-phosphate, 0.1 M MgCl2, 25 μM NADP, and enzyme, following the reduction of NADP+ at 340 nm.
2.2.6. Protein electrophoresis
Cell wall proteins were separated by SDS–PAGE according to Laemmli (1970). 40 μg protein samples were loaded on 13% acrylamide SDS–PAGE gels. Gels were stained with coomassie colloidal as per Scheler et al. procedures (Christian Scheler et al., 1998). Briefly, proteins were first fixed ca. 1 h in 50% ethanol, 2% phosphoric acid. Gels were then washed 1 h in 2% phosphoric acid. Gels were submitted to a sensitization step for 20 min in 17% ethanol, 15% ammonium sulfate, and 2% phosphoric acid, and 0.1% Coomassie colloidal was then added to this buffer. After 3 days of staining, gels were washed 10 min in deionized water, 10 min in 20% ethanol, and finally, 10 min in deionized water.
2.2.7. Estimation of glycosidase activities
β-Mannosidase, β- and α-galactosidase and β-glucosidase were determined according to Li and Li (1970). One unit of enzyme was expressed as μmole of ρ-nitrophenol liberated per mL per min under the assay conditions using a molar extinction coefficient of 1.77 × 104 for ρ-nitrophenol (Li, 1967).
3. Results and discussion
Plant cell wall has been long known to be a common residence for many glycosidases and acid hydrolases (Asamizu et al., 1981; Murray and Bandurski, 1975; Pierrot and Wielink, 1977). However, since glycosidases are also documented to be localized in other cellular compartments like mitochondria, protein bodies, plastid, etc. (Nikus et al., 2001; Sekhar and DeMason, 1990; Thornton, 2005), it was urging to start our work with isolation of glycosidases from cell wall. A mild isolation strategy that meant for purifying wall proteins with maintaining cytoplasmic compartment intact was employed. To validate cell wall protein purity, we assayed wall extract for activity of the intracellular marker glucose-6 phosphate dehydrogenase (G6P-D). The obtained result for purity confirmation is shown in Table 1, as it is clear from these results that the obtained wall protein purity was of acceptable limit as only 0.1% of total cellular G6P-D activity was detected in the extract. Almost the same intracellular contamination limit was obtained in the wall protein extracted from other cultivars. The wall protein extract obtained from Cervil, Levovil and VilB varied in volume and protein quantity in which Cervil was the richest in protein content followed by VilB and finally Levovil. The highest juice volume was obtained for Levovil which is justifiable on the high softness characterizes this cultivar as compared to Cervil and VilB (Table 2). To gain an idea about total water content in the three varieties, we calculated the water accumulation percentage at the varying development stages (Fig. 1); maximum water accumulation was detected in breaker stage for both Levovil and VilB. Cervil had the least water accumulation as compared to the formers.
Table 1.
Wall protein purity confirmation with detection of intracellular glucose-6 phosphate dehydrogenase.
| Extract | Activity (nM/min/g) | % Activity |
|---|---|---|
| Whole cell | 770 | 100 |
| Vacuum infiltrate (soluble wall protein) | 0.76 | 0.1 |
Table 2.
Summary for wall protein from the three tomato cultivars Cervil, Levovil and VilB.
| Gentype | FM⁎ (g) | Juice (mL) | Protein (μg/mL) | Total protein (mg) | Protein (μg/g FM) |
|---|---|---|---|---|---|
| Cervil | 360 | 16 | 84 | 1350 | 4 |
| Levovil | 360 | 26 | 23 | 612 | 2 |
| VilB | 360 | 20 | 52 | 1040 | 3 |
FM: Fresh material.
Figure 1.
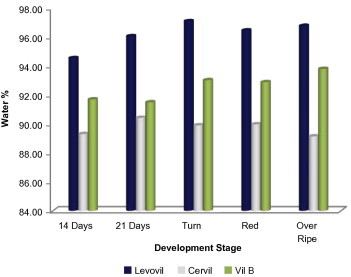
Percentage of total cellular water content in the three cultivars. Calculation for each genotype was done in three independent experiments. Details on experimental conditions are given in Section 2.
Electrophoretic pattern analysis of the wall protein extracts from the three cultivars exhibited, unexpectedly, bands patterns variation. Cervil and VilB electrophoretic pattern shared some degree of resemblances. However, Levovil pattern was very different as compared to Cervil and VilB patterns. Since Cervil and Levovil are relatively soft as compared to VilB, which is very firm with highest storage capacity, we expected VilB to exhibit a different electrophoretic protein profile as compared to Cervil and Levovil, but that was not the case as shown in Fig. 2.
Figure 2.
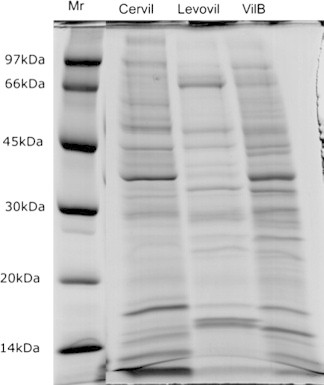
Polyacrylamide gel electrophoresis of cell wall protein from Cervil, Levovil and VilB tomato cultivars: 40 μg protein samples were loaded on 13% acrylamide SDS–PAGE gels. Gels were stained with coomassie colloidal (see experimental body). Protein markers used were: phosphorylase (97 kDa), BSA (66 kDa), egg albumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20 kDa) and α-lactalbumin (14 kDa).
To correlate between texture and glycosidase activities we intensionally chosen to study the activities of α-galactosidase, β-galactosidase, β-mannosidase and β-glucosidase, for three reasons firstly their known localization in cell wall, secondly direct involvement in ripening mechanism and finally their easy detection. Plants were grown and fruits were collected at five varying development stages; 14 days post-anthesis (14DPA), 21 days post-anthesis (21DPA), at turning (when fruit just starts getting red), red (fully red fruit) and over ripe (when fruit becomes very soft). All of these enzymes were detected in both total fruit protein and cell extract, however, with some variations. Strong activities were exhibited by β- and α-galactosidase whereas β-mannosidase and β-glucosidase were, comparatively, of weak activities.
3.1. Total protein content
In the current study, we freshly collected fruits and immediately processed for total whole cell protein content as shown in the experimental body. Evaluation of protein content at each development stage is exhibited in Fig. 3. Protein content (dry matter) at each developmental stage exhibited interesting results in which the three cultivars accumulated maximum protein content at 14DPA (stage of cell expansion) (Mapelli et al., 1978). The highest protein content was noticed for Cervil followed by VilB and finally Levovil. Protein content, in all cultivars, sharply declined following cell expansion stage to reach minimum level at the onset of fruit ripening (turning). Another elevation was observed as the fruit progressed toward over ripe stage.
Figure 3.
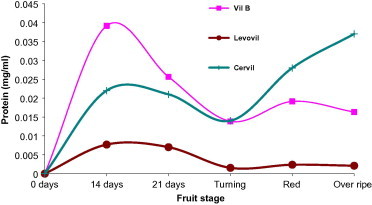
Protein concentration (dry matter) at varying development stages. Each point is a mean of three independent experiments.
3.2. β-Glucosidase activity
Beta-d-glucoside glucohydrolase, EC 3.2.1.21, is the enzyme which acts upon β1–4 bonds linking two glucose or glucose-substituted molecules (i.e., the disaccharide cellobiose). In this investigation weak activity was detected in the three cultivars with a relative higher activity in Levovil. The week activity of β-glucosidase in tomato may justify the scarcity on literature related to tomato β-glucosidase. Among the three tomato species, β-glucosidase showed clear activity disparity at the five ripening stages. Interestingly, throughout Cervil development stages, the enzyme retained a plateau activity (Fig. 4). In the previous report, this enzyme had been shown to localize in both periplasm as well as cytoplasm, with no clear assignment in cell wall during ripening (Odoux et al., 2003). This may clarify the feeble activity of this enzyme in our cell wall preparation.
Figure 4.
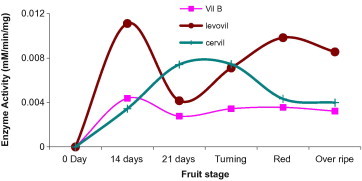
Beta-glucosidase activity in varying development and ripening stages of Cervil, Levovil and VilB cultivars. Each point is a mean of three independent experiments.
3.3. β-Mannosidase activity
When activity of β-mannosidase at the five ripening stages was assayed in the three cultivars, activity disparity was noticed. These variations made any interpretation difficult. Glycosidases are usually found in isoforms that are expressed at different development stages (Giannakouros et al., 1991; Jagadeesh et al., 2004b; Morant et al., 2008). β-Mannosidase from tomato seed was purified and studied to genetic level, in which a single gene was reported to code for this enzyme in tomato (Mo and Bewley, 2002) (Fig. 5). Studies on tomato seeds β-mannosidase and endo-β-mannanase by Mo and Bewley indicated that these enzymes are involved in the mobilization of the mannan-containing cell walls of the tomato seed endosperm (Mo and Bewley, 2002). However, another report published by Bewley and his colleagues who worked with tomato (Lycopersicon esculentum Mill.) fruit of the cultivar Trust, had shown the enzyme to tightly bound to the cell wall and removal of which would require a high salt buffer and is only detectable in the early stages of fruit development (Bewley et al., 2000). Since we used a buffer of a mild salt strength and non-destructive experimental procedures, our results jointly taken with the work of Mo and Bewley (2002) may reasonably allow us to conclude either the enzyme is sparingly extracted under our buffer system or the enzyme may not be playing a role in the development and ripening of tomato.
Figure 5.
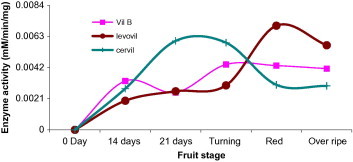
Beta-mannosidase activity in varying development and ripening stages of Cervil, Levovil and VilB cultivars. Each point is a mean of three independent experiments.
3.4. α-Galactosidase activity
Alpha-galactosidase is known to reside in both cell wall and cytoplasm (Bassel et al., 2001; Marraccini et al., 2005). In cell wall the enzyme involves in the modification or degradation of plant galactomannans and thereby assists in fruit softening. In the present study, we have found the enzyme to elevate, for Levovil and Cervil, during both development (14DAP) and ripening (turning) stages. On the other hand, unlike the formers, VilB enzyme showed little activity rising in the ripening stage. Since this enzyme is believed to take part in tomato ripening (Jagadeesh et al., 2004a) the weak enzyme activity exhibited in the firmest cultivar VilB becomes justifiable. Levovil, which is fleshy and very soft with minimal shelf life, possesses the highest α-galactosidase activity as compared to others (Fig. 6).
Figure 6.
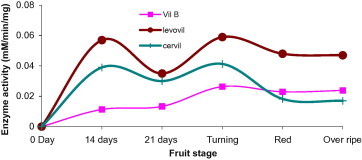
Alpha-galactosidase activity in varying development and ripening stages of Cervil, Levovil and VilB cultivars. Each point is a mean of three independent experiments.
3.5. β-Galactosidase activity
Beta-galactosidase (EC 3.2.1.23) activity is characterized by the ability to hydrolyze terminal nonreducing β-d-galactosyl residues from β-d-galactoside polymers. At least seven isoforms of this enzyme have been reported to get expressed at different fruit development stages. β-Galactosidase activity had shown to increase in parallel with tissue ripening (Smith and Gross, 2000) and genetically inhibition of TBG4 gene of several other genes for the enzyme delayed fruit softening (Carey et al., 2001). In the current investigation, the enzyme activity was increased sharply just before the breaker stage when the fruit starts to get red. This increase was clear in case of both Cervil and Levovil, which are known for their shorter shelf life, whereas VilB which is characterized by high degree of firmness and preservation period showed minimal activity for the enzyme at development and ripening stages (Fig. 7). These results will again emphasize on the implication of β-galactosidase in the fast fruit softening.
Figure 7.
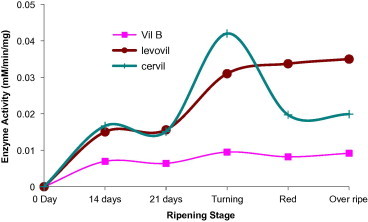
Beta-galactosidase activity in varying development and ripening stages of Cervil, Levovil and VilB cultivars. Each point is a mean of three independent experiments.
To further investigate on the wall localization of β-galactosidase. We assayed the enzyme activity in both cell wall and whole cell extract for three cultivars at ripening stage. High increase in the enzyme activity was obtained for wall enzyme as compared to whole cell enzyme activity. As expected, Levovil possessed highest activity followed by Cervil and finally by the firmest cultivar VilB (Fig. 8).
Figure 8.
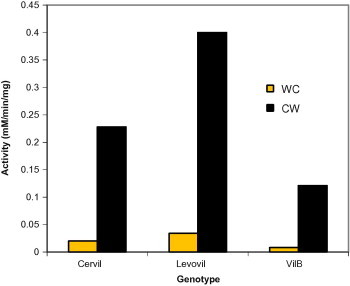
Comparison between activities of beta-galactosidase in the cell wall and whole cell extract. WC – whole cell extract; CW – cell wall extract.
4. Conclusion
Taken together, these results may allow us to conclude that studies of glycolytic activities in a single tomato cultivar cannot be generalized to whole species. On the other hand, it is more logical to correlate fruit ripening and development to wall glycosidases rather than to whole cell glycosidase activities.
Acknowledgements
The authors acknowledge support from (ACTION FA0603), EU-SOL (FOOD-CT-2006-016214) ANR QUALITOM-FIL (ANR-06-PNRA009). Konozy EH wishes to acknowledge the Postdoc fellowship awarded to him by INRA. We would also like to acknowledge the excellent technical assistance rendered by Ms. Esther Pelpoir and Mrs. Karine Leyre. We are finally very grateful to Mrs. Yolande Carretero for taking care of the plants.
References
- Ahmed A.E., Labavitch J.M. Cell wall metabolism in ripening fruit: II. Changes in carbohydrate-degrading enzymes in ripening ‘Bartlett’ pears. Plant Physiol. 1980;65:1014–1016. doi: 10.1104/pp.65.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Z.M., Armugam S., Lazan H. β-Galactosidase and its significance in ripening mango fruit. Phytochemistry. 1995;38:1109–1114. doi: 10.1016/0031-9422(94)00804-3. [DOI] [PubMed] [Google Scholar]
- Asamizu T., Inoue Y., Nishi A. Glycosidases in carrot cells in suspension culture: localization and activity change during growth. Plant Cell Physiol. 1981;22:469–478. [Google Scholar]
- Bai Y., Lindhout P. Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Ann. Bot. 2007;100:1085–1094. doi: 10.1093/aob/mcm150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D.M., Garcia E., Wayne J.E. Textural modification of processing tomatoes. Crit. Rev. Food Sci. Nutr. 1998;38:173–258. doi: 10.1080/10408699891274192. [DOI] [PubMed] [Google Scholar]
- Bassel G.W., Mullen R.T., Bewley J.D. Alpha-Galactosidase is synthesized in tomato seeds during development and is localized in the protein storage vacuoles. Can. J. Bot. 2001;79:1417–1424. [Google Scholar]
- Bewley J.D., Banik M., Bourgault R., Feurtado J.A., Toorop P., Hilhorst H.W.M. Endo-β-mannanase activity increases in the skin and outer pericarp of tomato fruits during ripening. J. Exp. Bot. 2000;51:529–538. doi: 10.1093/jexbot/51.344.529. [DOI] [PubMed] [Google Scholar]
- Brummell D.A., Harpster M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001;47:311–339. [PubMed] [Google Scholar]
- Carey A.T., Smith D.L., Harrison E., Bird C.R., Gross K.C., Seymour G.B., Tucker G.A. Down-regulation of a ripening-related β-galactosidase gene (TBG1) in transgenic tomato fruits. J. Exp. Bot. 2001;52:663–668. doi: 10.1093/jexbot/52.357.663. [DOI] [PubMed] [Google Scholar]
- Chivasa S., Ndimba B.K., Simon W.J., Robertson D., Yu X.-L., Knox J.P., Bolwell P., Slabas A.R. Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis. 2002;23:1754–1765. doi: 10.1002/1522-2683(200206)23:11<1754::AID-ELPS1754>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Christian Scheler S.L., Pan Zaoming., Li Xin.-Ping., Salnikow Johannes., Jungblut Peter. Peptide mass fingerprint sequence coverage from differently stained proteins on two-dimensional electrophoresis patterns by matrix assisted laser desorption/ionization-mass spectrometry (MALDI-MS) Electrophoresis. 1998;19:918–927. doi: 10.1002/elps.1150190607. [DOI] [PubMed] [Google Scholar]
- Giannakouros T., Karagiorgos A., Simos G. Expression of β-galactosidase multiple forms during barley (Hordeum vulgare) seed germination. Separation and characterization of enzyme isoforms. Physiol. Plant. 1991;82:413–418. [Google Scholar]
- Giovannoni J.J., DellaPenna D., Bennett A.B., Fischer R.L. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell Online. 1989;1:53–63. doi: 10.1105/tpc.1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz H., Lille M., Poutanen K., Schols H.A., Voragen A.G.J. Combined enzymatic and high-pressure processing affect cell wall polysaccharides in berries. J. Agric. Food Chem. 2006;54:1322–1328. doi: 10.1021/jf052401+. [DOI] [PubMed] [Google Scholar]
- Jagadeesh B.H., Prabha T.N., Srinivasan K. Activities of glycosidases during fruit development and ripening of tomato (Lycopersicum esculantum L.): implication in fruit ripening. Plant Sci. 2004;166:1451–1459. [Google Scholar]
- Jagadeesh B.H., Prabha T.N., Srinivasan K. Activities of β-hexosaminidase and α-mannosidase during development and ripening of bell capsicum (Capsicum annuum var. variata) Plant Sci. 2004;167:1263–1271. [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y.-T. Studies on the glycosidases in jack bean meal. J. Biol. Chem. 1967;242:5474–5480. [PubMed] [Google Scholar]
- Li S.-C., Li Y.-T. Studies on the glycosidases of jack bean meal. J. Biol. Chem. 1970;245:5153–5160. [PubMed] [Google Scholar]
- Li Z.-C., McClure J.W., Hagerman A.E. Soluble and bound apoplastic activity for peroxidase, β-d-glucosidase, malate dehydrogenase, and nonspecific arylesterase, in barley (Hordeum vulgare L.) and oat (Avena sativa L.) primary leaves. Plant Physiol. 1989;90:185–190. doi: 10.1104/pp.90.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenoi A., Paull R.E. Papaya fruit softening, endoxylanase gene expression, protein and activity. Physiol. Plant. 2007;131:470–480. doi: 10.1111/j.1399-3054.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Mapelli S., Frova C., Torti G., Soressi G.P. Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol. 1978;19:1281–1288. [Google Scholar]
- Marraccini P., Rogers W.J., Caillet V., Deshayes A., Granato D., Lausanne F., Lechat S., Pridmore D., Pétiard V. Biochemical and molecular characterization of α-d-galactosidase from coffee beans. Plant Physiol. Biochem. 2005;43:909–920. doi: 10.1016/j.plaphy.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Meli V.S., Ghosh S., Prabha T.N., Chakraborty N., Chakraborty S., Datta A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc. Natl. Acad. Sci. 2010;107:2413–2418. doi: 10.1073/pnas.0909329107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B., Bewley D. ß-Mannosidase (EC 3.2.1.25) activity during and following germination of tomato (Lycopersicon esculentum Mill.) seeds. Purification, cloning and characterization. Planta. 2002;215:141–152. doi: 10.1007/s00425-001-0725-x. [DOI] [PubMed] [Google Scholar]
- Morant A.V., Bjarnholt N., Kragh M.E., Kjærgaard C.H., Jørgensen K., Paquette S.M., Piotrowski M., Imberty A., Olsen C.E., Møller B.L., Bak S. The β-glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus. Plant Physiol. 2008;147:1072–1091. doi: 10.1104/pp.107.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.K., Bandurski R.S. Correlative studies of cell wall enzymes and growth. Plant Physiol. 1975;56:143–147. doi: 10.1104/pp.56.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mworia, E.G., Yoshikawa, T., Salikon, N., Oda, C., Asiche, W.O., Yokotani, N., Abe, D., Ushijima, K., Nakano, R., Kubo, Y., 2011. Low-temperature-modulated fruit ripening is independent of ethylene in ‘Sanuki Gold’ kiwifruit. J. Exp. Bot. [DOI] [PMC free article] [PubMed]
- Nikus J., Daniel G., Jonsson L.M.V. Subcellular localization of β-glucosidase in rye, maize and wheat seedlings. Physiol. Plant. 2001;111:466–472. doi: 10.1034/j.1399-3054.2001.1110406.x. [DOI] [PubMed] [Google Scholar]
- Odoux E., Escoute J., Verdeil J.L., Brillouet J.M. Localization of β-d-glucosidase activity and glucovanillin in vanilla bean (Vanilla planifolia Andrews) Ann. Bot. 2003;92:437–444. doi: 10.1093/aob/mcg150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot H., Wielink J.E. Localization of glycosidases in the wall of living cells from cultured Convolvulus arvensis tissue. Planta. 1977;137:235–242. doi: 10.1007/BF00388156. [DOI] [PubMed] [Google Scholar]
- Prasanna V., Prabha T.N., Tharanathan R.N. Fruit ripening phenomena – an overview. Crit. Rev. Food Sci. Nutr. 2007;47:1–19. doi: 10.1080/10408390600976841. [DOI] [PubMed] [Google Scholar]
- Sekhar K.N.C., DeMason D.A. Identification and immunocytochemical localization of α-galactosidase in resting and germinated date palm (Phoenix dactylifera L.) seeds. Planta. 1990;181:53–61. doi: 10.1007/BF00202324. [DOI] [PubMed] [Google Scholar]
- Smith D.L., Gross K.C. A family of at least seven β-galactosidase genes is expressed during tomato fruit development. Plant Physiol. 2000;123:1173–1184. doi: 10.1104/pp.123.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton C.R. Use of monoclonal antibodies to quantify the dynamics of α-galactosidase and endo-1,4-β-glucanase production by Trichoderma hamatum during saprotrophic growth and sporulation in peat. Environ. Microbiol. 2005;7:737–749. doi: 10.1111/j.1462-2920.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- Tieman D.M., Handa A.K. Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol. 1994;106:429–436. doi: 10.1104/pp.106.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman D.M., Harriman R.W., Ramamohan G., Handa A.K. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell Online. 1992;4:667–679. doi: 10.1105/tpc.4.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S.J., Walker J.E. Glycosidases in cell wall-degrading extracts of ripening tomato fruits. Plant Physiol. 1975;55:94–98. doi: 10.1104/pp.55.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. Enzymatic deconstruction of backbone structures of the ramified regions in pectins. Protein. J. 2008;27:30–42. doi: 10.1007/s10930-007-9105-0. [DOI] [PubMed] [Google Scholar]


