Abstract
The genioglossus (GG) muscle is considered the principal protruder muscle of the tongue that dilates and stiffens the pharyngeal airway. We recorded whole muscle and single motor unit (MU) activities in healthy adults performing progressive intensity exercise on a cycle ergometer. Tungsten microelectrodes were inserted percutaneously into the GG of 11 subjects (20–40 years) to record electromyographic (EMG) activities and pulmonary ventilation (VI) at rest and at workload increments up to 300 W. Increases in respiratory drive were associated with increases in VI, mean inspiratory flow (Vt/Ti) and tonic and phasic components of the GG EMG activity. In contrast, individual MUs typically showed expiration-related decreases in firing as exercise intensity increased. We suggest the decrease in MU activity may occur secondary to afferent feedback from lungs/chest wall and that compensation for more negative inspiratory airway pressures generated during heavy exercise occurs primarily via recruitment of previously silent MUs.
Key points
Motor units (MUs) are the fundamental element of force generation. Here we document the MU activities of the airway dilator muscle genioglossus (GG) during breathing at rest and in heavy exercise.
Based on our observations we suggest that tonic MU activity, albeit with varying degrees of respiratory modulation, is the preponderant activity in the GG motoneuron pool of healthy young adults at rest in the upright position.
Second, we show that exercise-driven increases in respiratory drive are associated with increased GG electromyographic activity principally due to recruitment of previously silent MUs and to a shift in the firing behaviour of already active MUs to inspiratory phase-dependent bursting.
Such expiration-related inhibition may arise secondary to an exercise-related central enhancement of motoneuron excitability that amplifies the response to afferent input.
Introduction
Upper airway muscles play a critical role in stabilizing the pharyngeal airway in advance of negative intraluminal pressures associated with inspiration (Bailey, 2011). The same muscles also participate in numerous non-respiratory functions, including swallowing, speaking and chewing (Sawczuk & Mosier, 2001). The genioglossus (GG) muscle of the human tongue has long served as a proxy for upper airway musculature more generally, in large part due to its accessibility. Despite an extensive literature that documents the role of the GG as an airway dilator muscle, basic questions remain concerning the transmission of respiratory drive to the hypoglossal motoneuron (HMN) pool in general. For example, animal studies indicate that increased blood CO2 substantially increases HMN firing rates (Hwang et al. 1983; Mitra & Cherniack, 1983; John et al. 2005) whereas in human subjects, GG motor units (MUs) maintain stable firing rates under comparable conditions (Richardson & Bailey, 2010). Thus, there is conflicting information regarding the mechanisms of force production evoked by increased respiratory drive. The absence of firing rate modulation observed in human subjects may reflect a lower overall level of drive onto the HMN pool (Hwang et al. 1983; Mitra & Cherniack, 1983; John et al. 2005).
In light of the aforementioned findings, we postulated that exercise provides a suitable framework within which to observe firing rate modulation. Previous studies in the human upper airway show enhanced excitability of upper airway muscle motoneurons (MNs) during exercise compared with rebreathing or hypercapnia (Sullivan et al. 1996; Shi et al. 1998; Williams et al. 2000). Thus, feedback arising in the periphery from exercising muscles, lungs and heart or centrally from motor cortex (Krogh & Lindhard, 1913; Fink et al. 1995) may have a larger impact on the activities of the HMN pool than chemoreceptor stimulation or airway resistance alone (Williams et al. 2000; Richardson & Bailey, 2010). Accordingly, we examined human GG whole muscle and single MU activities during cycling and tested the hypothesis that GG MU activities would on average increase during exercise. Furthermore, given that the neural drive and mechanical activities associated with respiration are inherently phasic, we predicted that single MU activity should show changes in phasic firing rate modulation in addition to any sustained or tonic effects. Preliminary reports have been published (Walls et al. 2011).
Methods
All procedures were approved by the Human Subjects Protection Program at the University of Arizona. Subjects gave their written informed consent prior to their participation in the study. Subjects were healthy, without history of neurological or respiratory pathology, major surgery or medications that could affect nervous or respiratory system function. All subjects were physically active and reported performing ∼5.0 h of exercise per week.
General procedures
Measures were obtained while subjects sat upright on a variable-resistance cycle ergometer (Model 828E, Monark, Vansbro, Sweden; Fig. 1A). Subjects were fitted with an oronasal facemask that allowed for oral and nasal breathing (Series 7450, Hans Rudolph, Shawnee, KS, USA). The mask was held in place with a head cap (Series 7450, Hans Rudolph), and self-sealed to the face. The seal was verified by a vacuum leak test. Inspiratory and expiratory airflow was measured using a heated pneumotachograph (Model 4813 PNT, Hans Rudolph) attached in series to the mask and connected to a differential pressure transducer (Model PT5, Grass Instruments, West Warwick, RI, USA). The resistance of this circuit was 2.8 cmH2O l−1 s. Mask pressure was measured with a second differential pressure transducer (Model PT5, Grass Instruments) connected to the mask via Tygon tubing. Total dead space of the system from mask opening to tubing opening ranged from 99 to 143 ml depending upon mask size. Fractional concentrations of inspired and expired CO2 and O2 were measured at the mask via rapidly responding analysers (Models 17515A and 17518A, Vacumed, Ventura, CA, USA). Gas analysers were calibrated prior to each experiment against a medically calibrated gas mix. All signals were monitored and recorded on a Spike2 data-acquisition and analysis system (Cambridge Electronic Design (CED), Cambridge, UK).
Figure 1. Experimental set-up.
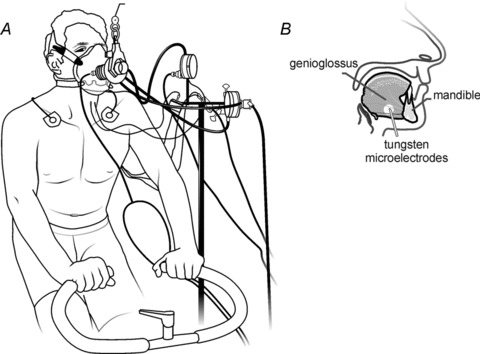
A, schematic representation of experimental set-up. B, schematic lateral view of the mandible and genioglossus (GG) muscle showing approximate insertion sites and angle of electrode insertion.
Electromyographic (EMG) recordings
EMG recordings were obtained from the GG muscle that arises from the medial aspect of the mandible anteriorly and fans posteriorly to insert into the central mass of the tongue (Takemoto, 2001). For each subject, the depth to the inferior border of the GG muscle was determined via ultrasound (ProSound 3500, Aloka, Tokyo, Japan; Eastwood et al. 2003). Whole muscle and single MU EMG activities were obtained using tungsten microelectrodes (100 kΩ and 10 MΩ at 1 kHz, 1–5 μm tip diameter, 250 μm shaft diameter, Frederick Haer, Bowdoinham, ME, USA) inserted percutaneously into the GG (Bailey et al. 2007a; Pittman & Bailey, 2009; Richardson & Bailey, 2010; Laine & Bailey, 2011; Laine et al. 2012; Fig. 1B). Surface electrodes (4 mm diameter Ag–AgCl) attached to the skin overlying each mastoid process served as reference electrodes for both whole muscle and MU recordings. Electrodes were positioned ∼0.5–1.0 cm from the midline and ∼2.0 cm from the other electrode. A standard EKG electrode (Model 2259–3, 3M) attached to the skin overlying the clavicle served as ground. Whole muscle EMG signals were sampled at 10 kHz, amplified (1000×) and band-pass filtered from 0.3 to 3.0 kHz (Model P511, Grass Instruments). Single MUs were sampled at 20 kHz, amplified (1000×) and band-pass filtered from 0.3 to 3.0 kHz (Model P511, Grass Instruments). The signals were acquired and stored using a Cambridge Electronic Design 1401 interface and Spike2 software (CED).
EMG and respiratory-related signals were obtained with the subject seated quietly on the bicycle ergometer and subsequently during loadless pedalling (0 W). The workload was then increased in steps of 15–50 W. The initial workload and the magnitude of the step increase were determined on the basis of individual subject size and fitness. Each workload was maintained for a minimum of 10 breaths and subjects were asked to maintain a pedalling rate between 70 and 80 r.p.m. throughout. Exercise stopped when the required power output increased to the point where subjects could no longer maintain the required pedalling rate or the fidelity of the MU recording deteriorated or the subject fatigued, whichever occurred first.
Data analysis
Data were analysed offline in Spike2 and MATLAB (MathWorks, Natick, MA, USA) using customized scripts. Measures of inspiratory flow (i.e. combined oral and nasal flow) were integrated to obtain inspiratory tidal volume (VT) and ventilation (VI). Mean inspiratory flow was calculated per breath as VT/TI (ml s−1) to derive the mean inspiratory flow, an accepted index of respiratory drive (Milic-Emili & Grunstein, 1976; Boggs & Tenney, 1984), at each workload.
Amplified and filtered GG whole muscle EMG signals were rectified, integrated and smoothed offline with a time constant of 400 ms using customized software (Spike2, CED). Single MU action potentials were discriminated using a commercial template-matching algorithm based upon waveform shape and amplitude (Spike2, CED). Results of the automated spike sorting procedure were checked by visual inspection and, where necessary, erroneous spike sorting was corrected manually. Single MU spike trains were converted to instantaneous frequency traces and the firing rate determined in a 400 ms moving window.
For both the whole muscle and the MU recordings, the mean, minimum and maximum level of activity (root mean square amplitude or firing rate) were determined over the duration of each whole breath (inspiration and expiration). The values calculated within each workload were normalized (expressed as percentage change from baseline) and averaged across breaths. This method allowed us to track the magnitude and direction of any changes in tonic or phasic EMG/MU activity, without assuming consistent timing of minimum and maximum activity with respect to the respiratory cycle. In other words, this method will accurately track alteration in GG activity even if those changes are not uniformly timed across the pool of MUs.
To characterize the consistency of within-breath firing rate variations with respect to the respiratory cycle, we used the eta (η2) index (Netick & Orem, 1981). This measure quantifies the ‘respiratory modulation’ of MU firing rates on a scale from 0 to 1, where 1 indicates that all firing rate variance is breath-phase related, and 0 indicates no consistent relationship between firing rate and breath phase (Orem & Dick, 1983; Bailey et al. 2007a). The standard method requires that breaths be divided into a number of time segments, under the assumption that each time segment corresponds to a particular phase of the breath. In our case, breath durations and breath-phase durations were likely to be variable across breaths and workloads. To account for this, we divided breaths into five phases according to inspired (I) and expired (E) volume (0–40% (I); 40–80% (I); 80–20% (I–E); 20–60% (E); 60–100% (E)). This modification does not alter the calculation or meaning of the eta statistic, but does allow a more flexible division of breaths into phases. Division of breaths into equal-volume phases also allowed us to track, across workloads, the average firing rate of MUs at each phase of the respiratory cycle.
For statistical evaluation, changes in respiratory effort (Vt/Ti) from baseline were binned into four groups corresponding to <30, 30–60, 60–90 and >90% change. A non-parametric ANOVA (Kruskal–Wallis test) was used to determine if MU or whole muscle EMG activity changed significantly across workload bins. We assessed the relationship between respiratory effort and MU or whole muscle EMG measures using a Pearson's correlation. The absolute-value change in MU or whole muscle EMG measures (i.e. minimum, maximum and mean) were compared with each other across workloads using paired t-tests. A significance level of P < 0.05 was adopted for all comparisons.
Results
We recorded a total of 46 single MUs in 11 healthy volunteers (six men and five women: average age 23.7 ± 5.8 years; height: 1.74 ± 0.10 m; weight: 64.7 ± 11.0 kg; body mass index: 21.2 ± 1.8). We did not control for stage of menstrual cycle in female participants as there is good evidence that phase of the menstrual cycle does not affect exercise performance or exercise-related cardiorespiratory variables (Jurkowski et al. 1981; Horvath & Drinkwater, 1982; Smekal et al. 2007). All subjects were within normal limits for measures of pulmonary function, i.e. forced vital capacity (FVC, 4.7 ± 0.9), forced expiratory volume in 1 s (FEV1.0, 4.0 ± 0.7) and FEV1.0/FVC ratio (85.3 ± 6.0%) indicating that subjects had normal lung mechanics (West, 1982; Gulsvik et al. 2001a,b; Anthonisen et al. 2002).
Figure 2 shows representative recordings of GG EMG whole muscle and single MU activities, mask pressure, flow, and fractional CO2 and O2 obtained at baseline (left) and during cycling at a 160 W workload (right) in a single subject. Note that whole muscle and single MU GG EMG activity is tonic in baseline with little evidence of respiratory modulation. At higher workloads, single GG MUs discharged a volley of action potentials per breath cycle. In this individual, there was a near doubling of ventilation from baseline to 160 W achieved via parallel increases in tidal volume and breathing frequency.
Figure 2. Representative recordings.
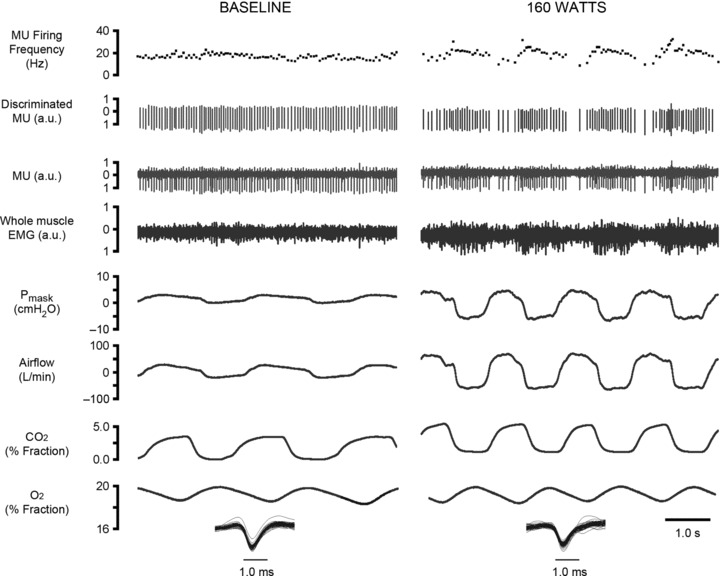
Recordings obtained from a subject in the baseline condition (left) and at 160 W (right). Left and right panels show, from the top, instantaneous motor unit firing rate, discriminated GG MU activity, original GG MU activity, whole muscle GG EMG, mask pressure (cmH2O), airflow (l min−1), and inspired and expired CO2 and O2 (% fraction).
To quantify the magnitude of each subject's ventilatory response as a function of power (watts) output we determined mean inspiratory flow (ml s−1) at baseline and at successive workloads. The results of these analyses for all subjects are presented in Fig. 3A. As anticipated, mean inspiratory flow (Vt/Ti) rose linearly as a function of increasing power output (Pearson's r= 0.8765, P < 0.01). The average firing rate of individual GG MUs did not show a similar trend (Fig. 3B). Rather, phasic (per breath) alterations in GG activity were elicited by increased respiratory effort.
Figure 3. Mean inspiratory flow and single MU firing frequency.
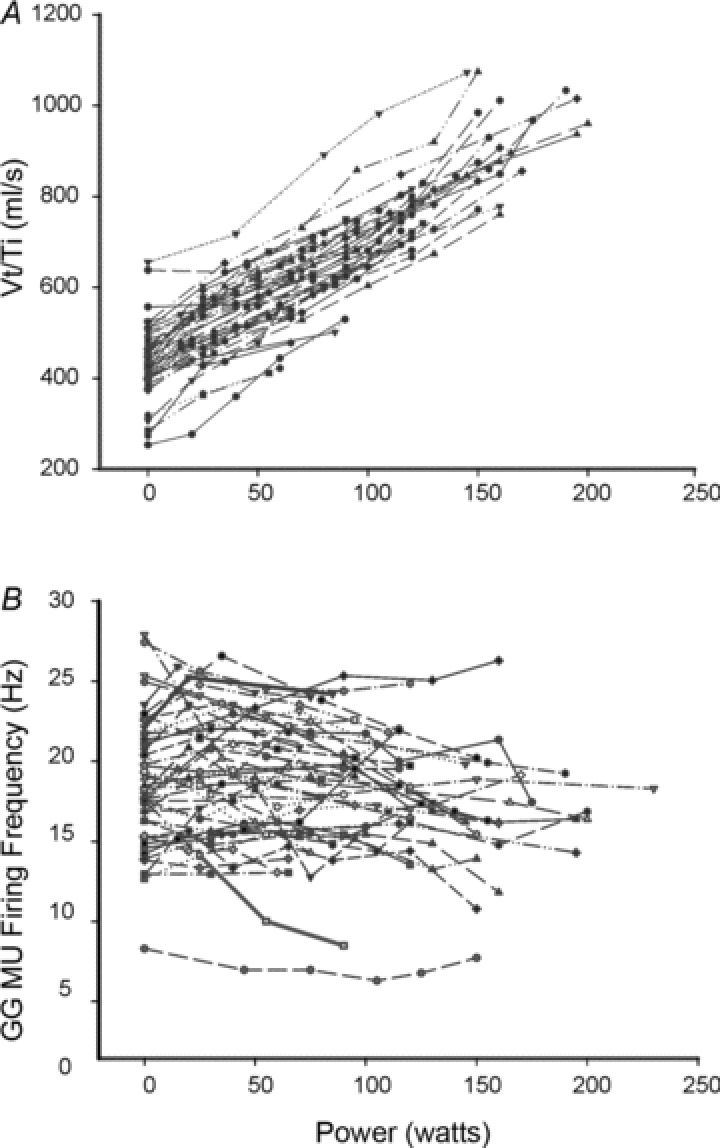
A, mean inspiratory flow (Vt/Ti) as a function of power (watts) output for individual subject cycling trials. B, individual subject cycling trials (each line/symbol indicating a different subject) showing single MU firing frequency (Hz) as a function of power (watts). Note that average firing frequency refers to the per-breath (i.e. across both phases of the respiratory cycle) average (10 breaths) for each of 46 MUs at each workload.
As shown in the grouped data presented in Fig. 4, the maximum MU firing rate per breath did not increase (upper right panel); indeed, average firing rates dropped slightly despite increasing Vt/Ti (P= 0.039). Minimum MU firing rates declined markedly (i.e. ∼ 8.0 Hz) under the same conditions (P≤ 0.01, Fig. 4), in many instances dropping to zero during expiration. The results of a paired t-test confirmed this observation and showed the absolute percentage change in minimum firing rate was greater than the percentage change in maximum firing rate at each workload (P < 0.01). Both the group minimum (expiratory) (P < 0.01) and the group maximum (inspiratory) (P < 0.01) components of the whole muscle GG EMG increased with increasing Vt/Ti, although the magnitude of the inspiratory component of whole muscle EMG (% baseline) typically exceeded that for the expiratory component across workloads (P < 0.01, paired t-test).
Figure 4. Minimum and peak MU firing rates as a function of respiratory effort.
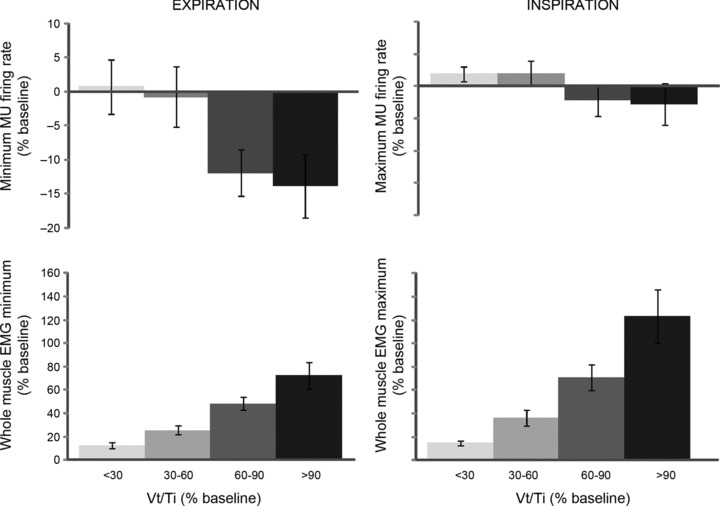
Group data depicting minimum (i.e. expiration) and peak (i.e. inspiration) MU firing rates as a function of respiratory effort level (Vt/Ti, % baseline) (top most plots). Values represent average interspike intervals derived from 46 single MUs calculated over 10 breaths at each workload. Lower plots depict the change (% baseline) in minimum (expiratory) and peak (inspiratory) components of whole muscle GG EMG as a function of mean inspiratory flow (Vt/Ti, % baseline). Values represent group averages calculated over 10 breaths at each workload.
Figure 5 depicts frequency histograms of all GG MUs as a function of eta (η2) value at baseline and maximum workload. The majority of units exhibited moderate–weak respiratory modulation at baseline (η2= 0.54) that shifted to moderate–strong modulation (η2= 0.64) at the highest workloads (P < 0.01). The eta statistic reported here measures the consistency of within-breath firing rate fluctuations across many breaths, but does not provide information about the within-breath firing rate profile for the units analysed. The individual cycle-triggered firing rate histograms shown in Fig. 6 indicate that for these units, a decrease in firing rate occurred specifically during expiration, while inspiratory firing rates remained stable across workloads.
Figure 5. The population of GG motor units as a function of the consistency and strength of their respiratory activity.
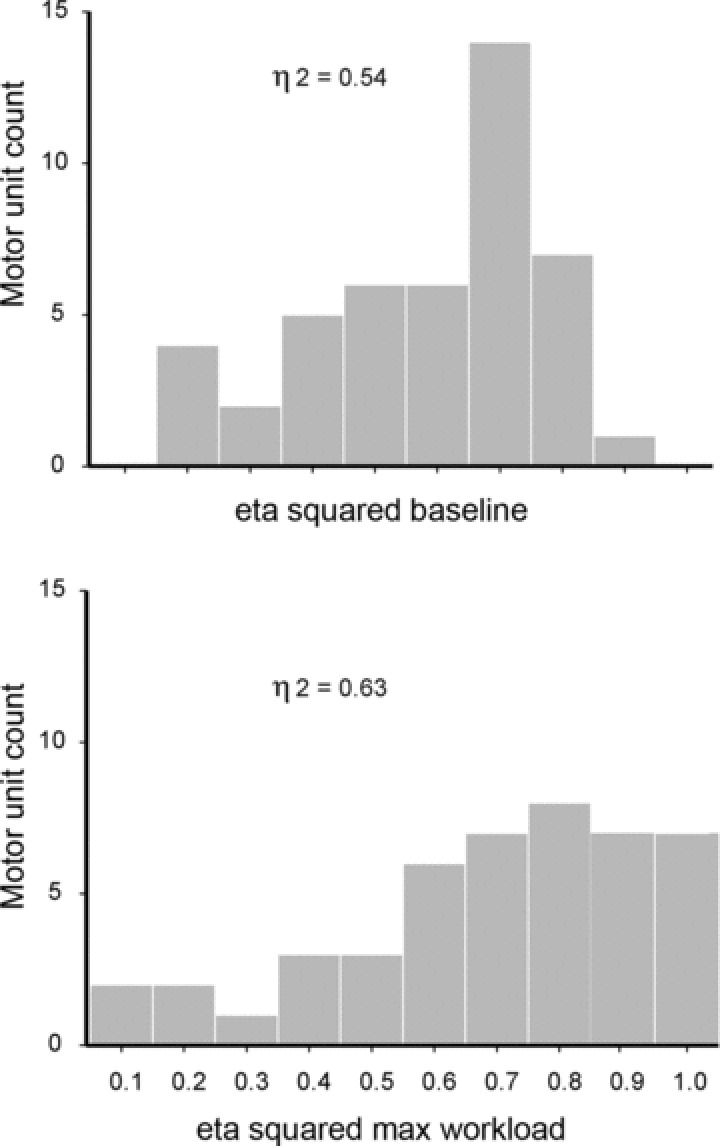
Frequency histograms of the population of GG motor units (MUs) as a function of the consistency and strength of their respiratory activity as reflected by eta (η2) values at baseline and maximum workloads.
Figure 6. Cycle triggered histograms obtained from three subjects.
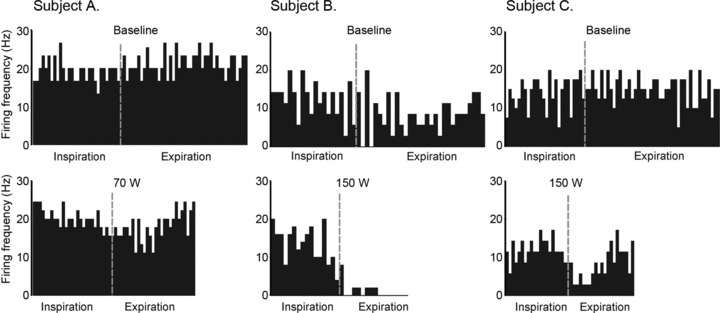
Histograms depict single MU firing frequencies (Hz) for inspiratory and expiratory phases averaged over 10 breaths at baseline (0 W) and at mid (70 W) and peak workloads (150 W).
To assess the dependence of firing rate on breath phase over the population of units, we calculated the average firing rate in each of the five breath phases used to calculate eta. Figure 7 shows the results of this analysis for each of the four workload bins. Consistent with the individual examples, the population results show a progressive decline in expiratory firing rate, peaking at mid-expiration. In contrast, mid-inspiratory firing rates remained stable across workloads. Note that the occurrence of minimal firing rates during mid to late expiration (phases 4 and 5) changed from ∼22% of the population at baseline to ∼44% at highest workload. Combined with magnitude information contained in Figs 4 and 7, it is clear that expiratory inhibition was the predominant exercise-induced effect on GG MU firing.
Figure 7. Change in MU firing frequency as a function of breath phase at each respiratory effort level.
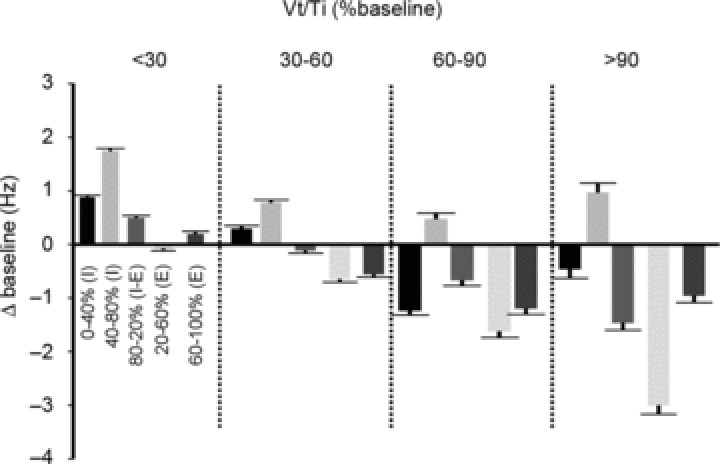
Group data depicting the average (SE) change in MU firing frequency (Hz) as a function of breath phase at each respiratory effort level (Vt/Ti). Values represent MU firing rates for each of five phases of the breath corresponding to inspired (I) and expired (E) volumes: 0–40% (I); 40–80% (I); 80–20% (I–E); 20–60% (E); 60–100% (E), and at each respiratory effort level (i.e. <30, 30–60, 60–90 and >90%).
Last, as expected based on the exercise-induced increases in whole muscle GG EMG activity we documented numerous instances of the recruitment of previously silent MUs into activity. Of this number, we were successful in discriminating for analysis 16 additional MUs. Although such recruited MUs exhibited tonic (4/16) or phasic activity (12/16) the activities appeared inherently flexible, precluding categorization (see for example Fig. 8B).
Figure 8. Representative recordings obtained in two subjects cycling at different workloads.
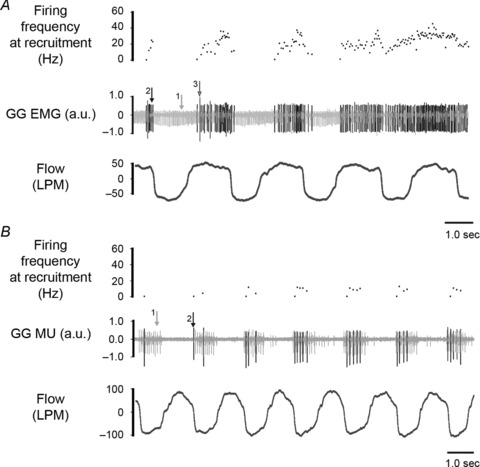
A, representative recordings of airflow (l min−1), electromyographic (EMG) activities and instantaneous firing frequency (Hz) of recruited MUs during cycling at 50 W workload. At this workload MU 1 (light grey) was tracked from baseline and is tonically active throughout the breath cycle. Two newly recruited MUs appear as larger amplitude potentials (arrows 2 and 3) and are active on inspiration. Note that MU 2 shifts from phasic activity during expiration to tonic activity and MU 3 ceases activity shortly after activation. B, representative recording from a subject cycling at 150 W workload. As for A, MU 1 (light grey) was tracked from the baseline condition and at 150 W is active during the inspiratory phase of the breath cycle. Note that a second MU (arrow 2) recruited into activity also discharges phasically on inspiration.
Discussion
Refinement of a technique first described by Bellemare et al. (1983) permitted us to obtain single MU recordings from healthy men and women performing exercise on a cycle ergometer. To the best of our knowledge, these are the first data depicting firing patterns of upper airway MNs during dynamic muscular exercise in human subjects. Although the magnitude of background tonic (i.e. active throughout the respiratory cycle) and inspiratory phasic components of GG EMG whole muscle activity increased with increasing workload, average GG MU firing rates did not change. Rather, GG MU bursting shifted from a tonic activity pattern toward an inspiratory phasic activity pattern. The abrupt termination of GG MU discharge during expiration associated with higher respiratory drive suggests an exercise-related inhibition of central drive or lung inflation-mediated inhibition of respiratory motor activity.
Critique of the methods
Single MU recording techniques have inherent advantages and disadvantages. Relative to traditional hook-wire electrodes, we have found tungsten microelectrodes to offer superior stability and, in the case of high-impedance electrodes, the ability to continue to track a single MU despite increases in whole muscle EMG that previously obscured the MU signal (Richardson & Bailey, 2010). By the same token, high-impedance electrodes necessarily restrict detection of newly recruited MUs, except in instances where a previously silent MU happens to lie immediately adjacent to the electrode tip. Thus, although we are able to record MUs during exercise we have a limited ability to systematically evaluate recruitment from individual MU recordings. Nevertheless, the contrast between whole muscle EMG and the activities of those MUs that were active at baseline yields some information concerning recruitment (see below).
We urged subjects to successively higher workloads, although the final workload attained during the protocol typically did not represent that subject's maximum (see Fig. 3). Based on each subject's ventilation and power output we estimate that the output ranged between 40 and 80% of their maximum power output. Nevertheless, given the between-subject variability in the workload attained during the protocol we expressed respiratory drive (Vt/Ti) as a proportion of the baseline condition (% baseline) rather than as a proportion of maximum workload (% max). This method permits a more intuitive comparison between changes in ventilation and changes in EMG and single MU activities.
In the current study, subjects remained upright for all recordings. With few exceptions (Tsuiki et al. 2000), previous studies of respiratory-related GG MU activities have been conducted with subjects supine (Saboisky et al. 2006, 2007, 2010; Bailey et al. 2007b; Lo et al. 2007; Martic & Podnar, 2008; Wilkinson et al. 2008; Eckert et al. 2009; Jordan et al. 2009; Nicholas et al. 2010; Richardson & Bailey, 2010; Laine et al. 2012). Although we observed tonic and phasic GG MU activities, the range of firing patterns evident in supine is not evident in upright. Importantly, our results are consistent with those of Tsuiki et al. (2000) who documented only inspiratory/expiratory (i.e. tonic) and phasic inspiratory GG MUs in upright and with the activity patterns reported in geniohyoid (Brown et al. 2011) muscle MUs also in upright. Our results indicate that tonic firing with a moderate degree of respiratory modulation would appear to be the predominant activity in the GG MN pool of healthy young adults at rest in the upright position.
Determinants of rate coding and recruitment
Recruitment of new MUs and modulation of the firing rates of already active MUs are the two mechanisms available to the nervous system for regulation of muscle force. Previous studies of respiratory muscles in human subjects indicate the relative roles of rate coding and recruitment vary across muscles and that increments in muscle output are achieved by different means. For example, diaphragm muscle MUs exhibit clear increases in firing rate (i.e. from 11.0 to 17.7 Hz) during partial rebreathing whereas in scalene and parasternal intercostal muscle MU recruitment is prominent (Adrian & Bronk, 1928; Gandevia et al. 1999).
Previous studies of GG MUs in rebreathing or hypercapnia show modest (i.e. ∼2.0 Hz; Saboisky et al. 2010) or no evidence of firing rate modulation (Nicholas et al. 2010; Richardson & Bailey, 2010). There are several possibilities that may account for the absence of firing rate increase. Recruitment is considered the principal method of force modulation when neural drive onto a MN pool is modest and the number of active MNs is low. This is of relevance for the current study if we consider that in healthy young adults at rest (and upright), whole muscle GG EMG is characterized by low-level tonic activity that approaches ∼5.0% of maximum output. Note that even when the drive to breathe increases the magnitude of whole muscle GG EMG only approaches ∼20–40% of maximum (Williams et al. 2000; Richardson & Bailey, 2010). Thus, at moderate levels of neural drive recruitment may be the more energetically favourable means of contraction strength increase (Nicholas et al. 2010; Richardson & Bailey, 2010).
The firing rates and behaviour of GG MUs in voluntary movement also provide a valuable contrast and further support for this hypothesis. The magnitude of the GG whole muscle output in a voluntary tongue protrusion approaches ∼60% of maximum output in the generation of modest physiologicaL forces (i.e. ∼10 g force typical of speech production; Pittman & Bailey, 2009) and fall well short of the potential force that can be developed by the human tongue (i.e. 5–30 N; Scardella et al. 1993; Blumen et al. 2002). Under these circumstances, there is both recruitment and a substantial increase in firing rate (i.e. ∼11–20 Hz; Bailey et al. 2007b; Pittman & Bailey, 2009). Thus, frequency modulation is evident given sufficient levels of neural drive. Evidence that GG MNs may be more potently activated by voluntary (i.e. cortical) vs. involuntary (i.e. respiratory) input is also suggested by recent work showing a higher degree of correlated activity between groups of GG MUs in voluntary movement than in breathing (Laine & Bailey, 2011).
The size of an MN pool also may determine whether rate coding or recruitment is favoured. Current estimates, based on total number of myelinated nerve fibres (O’Kusky & Norman, 1995) and MN counts (Atsumi & Miyatake, 1987), indicate the XII motor nuclei comprises some ∼8–9000 MNs per side. This compares with estimates from studies in limb including thenar (∼340 ± 87; Stein & Yang, 1990), biceps brachii (911 ± 254; Stein & Yang, 1990) and vastus lateralis (229 ± 108; Galea et al. 2001) muscles. When compared with these spinal MN pools, the sheer number of XII MNs may contribute some bias to the system and favour recruitment at all but the very highest levels of neural drive.
Potential mechanisms
In addition to finding that exercise-induced increases in GG EMG activity occur via recruitment rather than rate modulating previously active MUs, we report a distinct phasic inhibition of MU firing during expiration. Based on the current observations and previously published findings, CO2 or rebreathing would not appear to elicit comparable levels of respiratory effort and/or lung expansion. Thus, the ventilation and tidal volumes reported by Richardson & Bailey (2010) in steady-state hypercapnia are lower than the values reported here at the highest workloads, i.e. ∼630 ml and 15.4 l min−1 (Richardson & Bailey, 2010) as compared with ∼850 ml and 26.6 l min−1 for workloads over 150 W (current findings). Moreover, a previous study of upper nasal dilator muscles yielded similar results, i.e. exercise is a more potent stimulus to airway activities than hypercapnia (Sullivan et al. 1996). On this basis we speculate that the emergence of inhibition during exercise may be attributable to central motor command (Krogh & Lindhard, 1913) that enhances the overall excitability of upper airway MNs and/or amplifies their response to afferent input (Sullivan et al. 1996). However, the precise mechanism underlying the inhibition of GG MUs is a subject for future research, and will probably require the tracking of recruited MUs as well as those active at baseline.
GG EMG activities are subject to modulation from pulmonary stretch receptors (Brouillette & Thach, 1980; Sica et al. 1984; van Lunteren et al. 1984) and baroreceptor stimulation (Salamone et al. 1983; Garpestad et al. 1992; Wasicko et al. 1993). In exercise, the threshold for termination of inspiration (i.e. the inspiratory off-switch) occurs at tidal volumes twice the resting value (Kay et al. 1975; Martin & Weil, 1979; Lind & Hesser, 1984; Ainsworth et al. 1992) or at volumes approaching 40% of the individual's inspiratory capacity (Iber et al. 1995). In the current study, tidal volumes approached and in some cases exceeded twice the resting value, but none exceeded 40% of peak inspiratory capacity. On this basis, vagal feedback from pulmonary stretch receptors appears as a credible source of respiratory muscle inhibition. The potential for afferent activity during inspiration to affect expiratory (motor)neuron activity (see Fig. 4) has been noted previously and may indicate conditioning of upper airway muscle activities by volume feedback (Leevers et al. 1993) or by the intensity of central respiratory drive (Fregosi & Bartlett, 1988).
An alternativee source of inhibition of respiratory motor output is feedback from baroreceptors. Baroreceptor stimulation elicits a sustained reduction in GG EMG (Garpestad et al. 1992). And, because exercise elicits a comparable increase in mean arterial pressure (i.e. ∼10–15 mmHg; Moran et al. 1995; Fowler, 1969; Wieling et al. 1996) a similar suppressive effect on GG EMG activity may be anticipated. However, because the increase in blood pressure is sustained (or increased) at each workload and because beat to beat variations in pressure operate on a much shorter timescale than breathing it is unlikely that the suppression of MU firing rates noted here can be ascribed to this mechanism.
Conclusion
To our knowledge, this is the first study to characterize the effects of exercise-induced modulation of respiratory drive on the recruitment and activity patterns of upper airway MUs in human subjects. Our results show that exercise-driven increases in respiratory drive are associated with increased GG EMG activity principally due to recruitment of previously silent MUs and to changes in the firing behaviour of already active MUs to breath phase-dependent bursting. We suggest that the significance of recruitment in this case is a function of the HMN pool size as well as the comparatively low levels of excitation possible from automatic, i.e. respiratory-related, inputs. The switch in firing pattern in active MUs may occur secondary to an increase in cortical drive, i.e. voluntary assistance of respiration that is unique to the exercise condition. Thus, in exercise unlike hypercapnia, central motor command could enhance MN excitability and/or amplify the response to afferent input. The collective inhibition of MN activity during early to mid expiration may ensure a coordinated inspiratory bursting in advance of large negative inspiratory pressures generated by the respiratory pump.
Acknowledgments
This work was completed as part of the degree requirements for Masters in Physiological Sciences awarded to C.E.W. The authors acknowledge the input of committee members Drs Doug Keen and Ralph Fregosi.
Glossary
- EMG
electromyographic
- FVC
forced vital capacity
- FEV1.0
forced expiratory volume in 1 s
- GG
genioglossus
- HMN
hypoglossal motoneuron
- MN
motoneuron
- MU
motor unit
Additional information
Competing interests
None.
Author contributions
Conception and design of experiments EFB and CEW. Collection, analysis and interpretation of data; CEW, CML, IJK and EFB. Draft and revision of the article; EFB, CML, IJK.
Funding
The project described was supported by Grant Number 009587 from the NIDCD (to E.F.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
References
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibres: Part I. Impulses in single fibres of the phrenic nerve. J Physiol. 1928;66:81–101. doi: 10.1113/jphysiol.1928.sp002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth DM, Smith CA, Johnson BD, Eicker SW, Henderson KS, Dempsey JA. Vagal modulation of respiratory muscle activity in awake dogs during exercise and hypercapnia. J Appl Physiol. 1992;72:1362–1367. doi: 10.1152/jappl.1992.72.4.1362. [DOI] [PubMed] [Google Scholar]
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;73:25–31. doi: 10.1007/BF00695498. [DOI] [PubMed] [Google Scholar]
- Bailey EF. Activities of human genioglossus motor units. Respir Physiol Neurobiol. 2011;179:14–22. doi: 10.1016/j.resp.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007a;98:3284–3291. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007b;97:933–936. doi: 10.1152/jn.00737.2006. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J Neurophysiol. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Blumen MB, Perez de La Sota A, Quera-Salva MA, Frachet B, Chabolle F, Lofaso F. Genioglossal electromyogram during maintained contraction in normal humans. Eur J Appl Physiol. 2002;88:170–177. doi: 10.1007/s00421-002-0697-y. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Tenney SM. Scaling respiratory pattern and respiratory ‘drive’. Respir Physiol. 1984;58:245–251. doi: 10.1016/0034-5687(84)90001-x. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. J Appl Physiol. 1980;49:801–808. doi: 10.1152/jappl.1980.49.5.801. [DOI] [PubMed] [Google Scholar]
- Brown EC, Hudson AL, Butler JE, McKenzie DK, Bilston LE, Gandevia SC. Single motor unit recordings in human geniohyoid reveal minimal respiratory activity during quiet breathing. J Appl Physiol. 2011;110:1054–1059. doi: 10.1152/japplphysiol.00454.2010. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–1858. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Adams L, Watson JD, Innes JA, Wuyam B, Kobayashi I, Corfield DR, Murphy K, Jones T, Frackowiak RS, Guz A. Hyperpnoea during and immediately after exercise in man: evidence of motor cortical involvement. J Physiol. 1995;489:663–675. doi: 10.1113/jphysiol.1995.sp021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler NO. The normal pulmonary arterial pressure-flow relationships during exercise. Am J Med. 1969;47:1–6. doi: 10.1016/0002-9343(69)90235-6. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Bartlett D., Jr Central inspiratory influence on abdominal expiratory nerve activity. J Appl Physiol. 1988;65:1647–1654. doi: 10.1152/jappl.1988.65.4.1647. [DOI] [PubMed] [Google Scholar]
- Galea V, Fehlings D, Kirsch S, McComas A. Depletion and sizes of motor units in spinal muscular atrophy. Muscle Nerve. 2001;24:1168–1172. doi: 10.1002/mus.1128. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med. 1999;160:1598–1603. doi: 10.1164/ajrccm.160.5.9904023. [DOI] [PubMed] [Google Scholar]
- Garpestad E, Basner RC, Ringler J, Lilly J, Schwartzstein R, Weinberger SE, Weiss JW. Phenylephrine-induced hypertension acutely decreases genioglossus EMG activity in awake humans. J Appl Physiol. 1992;72:110–115. doi: 10.1152/jappl.1992.72.1.110. [DOI] [PubMed] [Google Scholar]
- Gulsvik A, Beckett LA, Bakke P, Humerfelt S, Omenaas E, Speizer FE. Standardized submaximal exercise testing in never smokers: a normative study. Clin Physiol. 2001a;21:629–636. doi: 10.1046/j.1365-2281.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- Gulsvik A, Tosteson T, Bakke P, Humerfelt S, Weiss ST, Speizer FE. Expiratory and inspiratory forced vital capacity and one-second forced volume in asymptomatic never-smokers in Norway. Clin Physiol. 2001b;21:648–660. doi: 10.1046/j.1365-2281.2001.00377.x. [DOI] [PubMed] [Google Scholar]
- Horvath SM, Drinkwater BL. Thermoregulation and the menstrual cycle. Aviat Space Environ Med. 1982;53:790–794. [PubMed] [Google Scholar]
- Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- Iber C, Simon P, Skatrud JB, Mahowald MW, Dempsey JA. The Breuer–Hering reflex in humans. Effects of pulmonary denervation and hypocapnia. Am J Respir Crit Care Med. 1995;152:217–224. doi: 10.1164/ajrccm.152.1.7599827. [DOI] [PubMed] [Google Scholar]
- John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med. 2005;172:1331–1337. doi: 10.1164/rccm.200505-790OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowski JE, Jones NL, Toews CJ, Sutton JR. Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol. 1981;51:1493–1499. doi: 10.1152/jappl.1981.51.6.1493. [DOI] [PubMed] [Google Scholar]
- Kay JD, Petersen ES, Vejby-Christensen H. Mean and breath-by-breath pattern of breathing in man during steady-state exercise. J Physiol. 1975;251:657–669. doi: 10.1113/jphysiol.1975.sp011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Bailey EF. Common synaptic input to the human hypoglossal motor nucleus. J Neurophysiol. 2011;105:380–387. doi: 10.1152/jn.00766.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Nickerson LA, Bailey EF. Cortical entrainment of human hypoglossal motor unit activities. J Neurophysiol. 2012;107:493–499. doi: 10.1152/jn.00769.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers AM, Simon PM, Xi L, Dempsey JA. Apnoea following normocapnic mechanical ventilation in awake mammals: a demonstration of control system inertia. J Physiol. 1993;472:749–768. doi: 10.1113/jphysiol.1993.sp019971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind F, Hesser CM. Breathing pattern and lung volumes during exercise. Acta Physiol Scand. 1984;120:123–129. doi: 10.1111/j.1748-1716.1984.tb07381.x. [DOI] [PubMed] [Google Scholar]
- Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62:799–805. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martic V, Podnar S. Reference data for quantitative motor unit potential analysis in the genioglossus muscle. Muscle Nerve. 2008;38:939–940. doi: 10.1002/mus.21011. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Weil JV. CO2 and exercise tidal volume. J Appl Physiol. 1979;46:322–325. doi: 10.1152/jappl.1979.46.2.322. [DOI] [PubMed] [Google Scholar]
- Milic-Emili J, Grunstein MM. Drive and timing components of ventilation. Chest. 1976;70:131–133. doi: 10.1378/chest.70.1_supplement.131. [DOI] [PubMed] [Google Scholar]
- Mitra J, Cherniack NS. The effects of hypercapnia and hypoxia on single hypoglossal nerve fibre activity. Respir Physiol. 1983;54:55–66. doi: 10.1016/0034-5687(83)90113-5. [DOI] [PubMed] [Google Scholar]
- Moran D, Epstein Y, Keren G, Laor A, Sherez J, Shapiro Y. Calculation of mean arterial pressure during exercise as a function of heart rate. Appl Human Sci. 1995;14:293–295. doi: 10.2114/ahs.14.293. [DOI] [PubMed] [Google Scholar]
- Netick A, Orem J. Erroneous classification of neuronal activity by the respiratory modulation index. Neurosci Lett. 1981;21:301–306. doi: 10.1016/0304-3940(81)90221-4. [DOI] [PubMed] [Google Scholar]
- Nicholas CL, Bei B, Worsnop C, Malhotra A, Jordan AS, Saboisky JP, Chan JK, Duckworth E, White DP, Trinder J. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. Sleep. 2010;33:1529–1538. [PMC free article] [PubMed] [Google Scholar]
- O’Kusky JR, Norman MG. Sudden infant death syndrome: increased number of synapses in the hypoglossal nucleus. J Neuropathol Exp Neurol. 1995;54:627–634. doi: 10.1097/00005072-199509000-00003. [DOI] [PubMed] [Google Scholar]
- Orem J, Dick T. Consistency and signal strength of respiratory neuronal activity. J Neurophysiol. 1983;50:1098–1107. doi: 10.1152/jn.1983.50.5.1098. [DOI] [PubMed] [Google Scholar]
- Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol. 2009;101:276–282. doi: 10.1152/jn.91065.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol. 2010;103:1315–1321. doi: 10.1152/jn.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol. 2007;585:135–146. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol. 2010;109:1939–1949. doi: 10.1152/japplphysiol.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JA, Strohl KP, Weiner DM, Mitra J, Cherniack NS. Cranial and phrenic nerve responses to changes in systemic blood pressure. J Appl Physiol. 1983;55:61–68. doi: 10.1152/jappl.1983.55.1.61. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- Scardella AT, Krawciw N, Petrozzino JJ, Co MA, Santiago TV, Edelman NH. Strength and endurance characteristics of the normal human genioglossus. Am Rev Respir Dis. 1993;148:179–184. doi: 10.1164/ajrccm/148.1.179. [DOI] [PubMed] [Google Scholar]
- Shi YX, Seto-Poon M, Wheatley JR. Breathing route dependence of upper airway muscle activity during hyperpnea. J Appl Physiol. 1998;84:1701–1706. doi: 10.1152/jappl.1998.84.5.1701. [DOI] [PubMed] [Google Scholar]
- Sica AL, Cohen MI, Donnelly DF, Zhang H. Hypoglossal motoneuron responses to pulmonary and superior laryngeal afferent inputs. Respir Physiol. 1984;56:339–357. doi: 10.1016/0034-5687(84)90069-0. [DOI] [PubMed] [Google Scholar]
- Smekal G, von Duvillard SP, Frigo P, Tegelhofer T, Pokan R, Hofmann P, Tschan H, Baron R, Wonisch M, Renezeder K, Bachl N. Menstrual cycle: no effect on exercise cardiorespiratory variables or blood lactate concentration. Med Sci Sports Exerc. 2007;39:1098–1106. doi: 10.1249/mss.0b013e31805371e7. [DOI] [PubMed] [Google Scholar]
- Stein RB, Yang JF. Methods for estimating the number of motor units in human muscles. Ann Neurol. 1990;28:487–495. doi: 10.1002/ana.410280404. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Fuller D, Fregosi RF. Control of nasal dilator muscle activities during exercise: role of nasopharyngeal afferents. J Appl Physiol. 1996;80:1520–1527. doi: 10.1152/jappl.1996.80.5.1520. [DOI] [PubMed] [Google Scholar]
- Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modelling. J Speech Lang Hear Res. 2001;44:95–107. doi: 10.1044/1092-4388(2001/009). [DOI] [PubMed] [Google Scholar]
- Tsuiki S, Ono T, Ishiwata Y, Kuroda T. Functional divergence of human genioglossus motor units with respiratory-related activity. Eur Respir J. 2000;15:906–910. doi: 10.1034/j.1399-3003.2000.15e16.x. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Strohl KP, Parker DM, Bruce EN, Van de Graaff WB, Cherniack NS. Phasic volume-related feedback on upper airway muscle activity. J Appl Physiol. 1984;56:730–736. doi: 10.1152/jappl.1984.56.3.730. [DOI] [PubMed] [Google Scholar]
- Walls CE, Laine CM, Kidder IJ, Berger GK, Bailey EF. Neuromuscular control of the tongue during exercise. FASEB J. 2011;25:653.1. [Google Scholar]
- Wasicko MJ, Knuth SL, Leiter JC. Response of genioglossus EMG activity to passive tilt in men. J Appl Physiol. 1993;74:73–81. doi: 10.1152/jappl.1993.74.1.73. [DOI] [PubMed] [Google Scholar]
- West JB. Pulmonary Pathophysiology: The Essentials. Baltimore, MD: Williams and Wilkins; 1982. [Google Scholar]
- Wieling W, Harms MP, ten Harkel AD, van Lieshout JJ, Sprangers RL. Circulatory response evoked by a 3 s bout of dynamic leg exercise in humans. J Physiol. 1996;494:601–611. doi: 10.1113/jphysiol.1996.sp021518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol. 2000;89:590–598. doi: 10.1152/jappl.2000.89.2.590. [DOI] [PubMed] [Google Scholar]


