Abstract
Silencing of androgen receptor (AR) signaling is a specific and effective mechanism to cure cancer of the prostate (CaP). In this study, the isolation and characterization of a compound from the aromatic berries of Pimenta dioica (allspice) that silences AR is presented. Potential antitumor activities of an aqueous allspice extract (AAE) and a compound purified from the extract were tested on CaP cells. AAE inhibited tumor cell proliferation and colony formation (50% growth inhibition ∼40–85 µg/ml) but not the viability of quiescent normal fibroblasts or non-tumorigenic prostate cells. In tumor cells, AAE inhibited cell cycle progression at G1/S, induced apoptosis or autophagy. Apoptosis was by caspase-dependent poly (ADP ribose) polymerase cleavage. A caspase-independent, apoptosis-inducing factor-mediated mechanism of apoptosis caused cell death in castration-resistant AR-positive or AR-negative CaP cells, such as CWR22RV1, PC-3 or DU145 cells. Treatment with AAE decreased the levels of AR messenger RNA (mRNA), protein and silenced AR activity in AR-positive cells. AR depletion was due to inhibition of AR promoter activity and mRNA stability. Delayed tumor growth (~55%) without measurable systemic toxicity was observed in LNCaP tumor-bearing mice treated with AAE by oral or intraperitoneal routes. LNCaP tumor tissues from AAE-treated mice revealed increased apoptosis as a potential mechanism of antitumor activity of AAE. The chemical identity of bioactive compound in AAE was established through multistep high-performance liquid chromatography fractionation, mass and Nuclear Magnetic Resonance spectroscopies. The compound, eugenol 5-O-β-(6′-galloylglucopyranoside) or ericifolin (EF), showed antiproliferative, pro-apoptosis and anti-AR transcription activities. These results demonstrate a potential use of AAE and EF against prostate cancer.
Introduction
Many aromatic tropical plants contain a rich assortment of secondary metabolites that are evolved to protect and preserve the nutrients from bacterial, fungal and insect infestations. These include alkaloids, glycosides, polyphenols, terpenes and terpenoids (1–3). Several compounds with pharmacological activities have been isolated from fresh leaves of Pimenta dioica (Family: Myrtaceae; alternate name: Jamaican pepper) and the dried, unripe berries, known as allspice, are marketed as an edible spice. Allspice, which tastes like a blend of cloves, nutmeg, cinnamon and pepper, is a common flavoring compound in Asian, Middle Eastern and Jamaican cuisines.
Most of the literature on the health benefits of Pimenta leaves is on the analgesic, antibacterial and antihypertensive properties present in organic or ethanolic extracts (4–7). Few studies have used allspice or the water extract from it as the starting material, although most health benefit of P. dioica is likely derived from consuming allspice. Two compounds, galloyl pedunculagin and casuarinin (3,8) have been isolated from P. dioica leaves, which have some cytotoxic and antibacterial properties. We reasoned that because allspice has universal culinary appeal and has high antioxidants with demonstrable analgesic, antibacterial and other beneficial pharmacological activities, identification of antitumor compounds should make allspice a potential source of a dietary, cancer-chemopreventive agent that is more palatable to patients at risk for prostate cancer or those with potential for disease recurrence.
Cancer of the prostate (CaP) is the most common non-skin cancer in American men (9). As the disease recurs over several years in a significant fraction of patients, it is a good target for chemoprevention. If began early, preventive agents may enhance the survival and quality of the patients’ life profoundly such that the disease, even if not completely eliminated, may pose little threat to life. Recurrent CaP following radiation therapy, surgery or both is incurable at present and total androgen ablation is the first line of therapy for this stage (10). All studies reported to date state that total androgen ablation leads to the CaP progression of castration-resistant stage at which time conventional chemotherapy is used with limited effect, prolonging life between 2 and 4 months. The transition from chemical castration-responsive to castration-resistant stage is the critical step in CaP progression and the prevention of castration-resistant CaP (CRPC) may bring significant improvement in morbidity and mortality associated with CaP. It is noteworthy that CRPC cells harbor androgen receptors (ARs; wild-type or mutated forms) and AR signaling, independent of androgen(s), which very likely contributes to the progression to a more aggressive disease (11). Therefore, the major strategy in containing CaP progression is plausibly by chemoprevention, or by disabling the activities of AR (12). Several mechanisms of growth and survival signaling influence the development of CRPC and the activation of AR, in the absence of high levels of androgens (13,14). It has been argued that total silencing of AR, preferably transcriptional, is an effective therapeutic avenue to most stages of CaP (15).
In this study, we demonstrate strong and potentially clinically applicable antiproliferative and antitumor activities of an aqueous allspice extract (AAE) and further establish that most of the antitumor activities of AAE were found in a single, purified bioactive compound from AAE, ericifolin (EF). EF reproduced antiproliferative and antiprostate cancer properties exhibited by AAE, suggesting that EF is one of the active anticancer compounds, if not the only one, present in allspice berries.
Materials and methods
Preparation of AAE
Certified-organic P.dioica berries (Oregon Spice Company, Portland, OR) were pulverized, boiled in distilled water at 100g/l for 10 min and clarified by filtration through a Whatman #1 paper. The filtered extract was lyophilized and designated as AAE. A solution of defined concentration was prepared in distilled water to test its biological activities.
Cell lines
The CaP cell lines (LNCaP, DU145 and PC-3, CW22RV1), an immortalized prostate epithelial cell line (RWPE-1) and a human lung normal fibroblast line (ATCC CCL-153) were originally obtained from ATCC (Manassas, VA) and maintained routinely as reported previously (16). All basal media were from Mediatech (Corning), Manassas VA. The primary prostate cancer cell line, LAPC-4 was a gift from Dr Sawyers (Memorial Cancer Center, New York, NY) and was cultured in Iscove’s Dulbecco’s modified Eagle’s medium with 15% fetal bovine serum (FBS) and 1nM 5α-dihydrotestosterone (DHT; Sigma Chemicals, St Louis, MO) as described previously (16). A primary prostate tumor cell line (Gleason 6) E006AA was a gift from Dr Koochekpour (Roswell Park Memorial Cancer Institute, Buffalo, NY) and the Androgen Repressed Cancer of Prostate (ARCaP) cell line was a gift from Dr Leland Chung (Cedars-Sinai Medical Center, Los Angeles, CA). The cell lines used in this study were authenticated for their origin by Genetica DNA Laboratories (Cincinnati, OH).
Cytotoxicity/cell proliferation assay
The anticlonogenic and antiproliferation activities of AAE were assayed on CaP and RWPE-1 cells by 2D colony-forming assays, cell counting, trypan blue exclusion and by a colorimetric 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay as described previously (17). AAE and EF had no absorption at 575/650nm, thus did not interfere with the absorption of dissolved formazan.
Cell cycle phase fractionation
CaP cells (1 × 105 cells/35mm dish) cultured for 24 h were incubated with AAE (0–150 µg/ml) for 24 or 48 h and were analyzed for cell cycle phase fractions based on DNA content by flow cytometry, as described previously (16).
Immunoblotting
Cell lysates from AAE-treated and AAE-untreated (control) cultures were analyzed by immunoblotting using antibodies and enhanced chemiluminescence detection reagents bought from commercial vendors (18). The protein band densities were quantified using imaging software (Carestream Technology, New Haven, CT). Blots were re-probed with horseradish peroxidase-labeled anti-β-actin antibody (Santa Cruz Technology, Santa Cruz, CA), by running the same samples in parallel and probing for β-actin, or using the predetermined amount of total proteins loaded from the cell fractions, as loading control.
Prostate-specific antigen and AR promoter activity measurements by luciferase reporter assays
LNCaP cells were transfected with AR-promoter-reporter or prostate-specific antigen (PSA)-promoter luciferase reporter plasmids (pLARS and pGL-3 PSA-luc) and TK-Renilla plasmid (Promega, Madison, WI) using Lipofectamine as described previously (19,20). Two days after transfections, cultures were exposed to AAE or EF for 4 h before measuring luciferase activity using the Duo Glo kit (Promega). Relative light units (activity) were normalized to that of TK-Renilla (21).
Tumor induction and growth measurement
Subcutaneous tumors were generated in athymic male mice (Harlan Labs, Indianapolis, IN) by injecting LNCaP cells (2 × 106 cells/0.2 ml/site) mixed with a basement membrane extract (pathogen-clear BME; Trevigen, Gaithersburg, MD) at 1:1 ratio. Cells were injected bilaterally into the dorsal flank of mice. A power analysis program (22) was used to determine the group size (>80% power) in hypothesis testing. AAE (100mg/kg) was given as gavage daily, or injected into peritoneum (100mg/kg, 3× per week), for 42 days, starting from the day of tumor cell injection. Tumor growth was monitored over time by measuring tumor volume twice a week (16). Potential systemic toxicity of AAE was monitored by changes in body weight during the course of treatment. On day 43, mice were euthanatized, blood and tumor tissues were collected for ex vivo analysis in the AAE intraperitoneal (i.p.)-treated groups.
Chemical characterization and fractionation of AAE
We analyzed AAE for relative composition of polyphenols (23), tannins (24), alkaloids (25), nucleotides (26), carbohydrates and amino acids. Types of carbohydrates and amino acids were analyzed at a so-named contract core facility, UT Medical Branch, Galveston, TX. The initial fractionation of AAE was on Sephadex LH-20 to separate tannins from non-tannins and polyphenols. The fraction of tannins bound to the matrix was eluted with 70% aqueous acetone, whereas the unbound non-tannins were collected as flow through (27).
Identification of the major ingredient of AAE
The lower polyphenol batch fraction was subjected to further fractionation by high-performance liquid chromatography (HPLC) (28) and characterized by mass and Nuclear Magnetic Resonance spectroscopies (Supplementary Data, available at Carcinogenesis Online). Antiproliferation and anti-AR activity (using LNCaP cells)-driven purification and characterization were used throughout.
Statistical analysis
All experiments in which quantitative data are presented were performed at least three times, each with 3–6 replicate samples. Immunoblots and Immuno-histochemistry were repeated once. All data are presented as mean ± standard error of the mean (SEM), with n ≥ 3, with significance of each result determined with Student’s t-test or analysis of variance.
Results
AAE is cytotoxic to CaP cells and inhibits their clonogenic survival
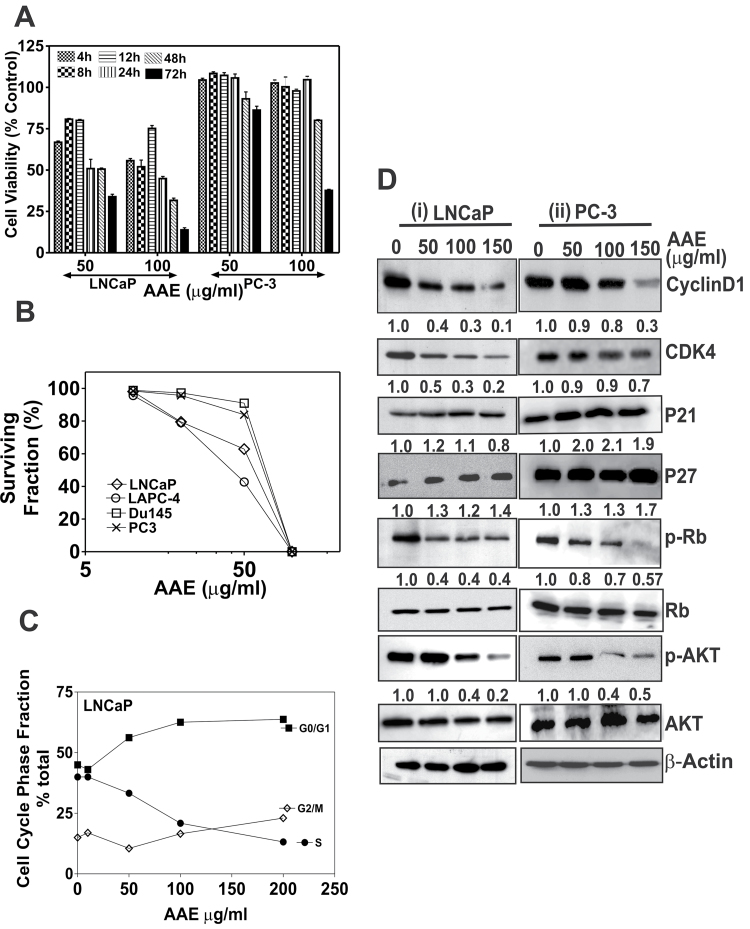
AAE was tested on human CaP cell lines (listed in Table I) for cytotoxicity, antiproliferative activities or both. The 50% growth inhibition (IC50) values of various CaP cells treated with AAE following 48 h of incubation are listed in Table I. The time-dependent cytotoxicity of AAE on two CaP cell lines is shown in Figure 1A. Furthermore, incubation with AAE continuously for 7–10 days caused inhibition of adherent CaP cell colony formation (proliferation). As shown in Figure 1B, continuous incubation with AAE strongly inhibited clonogenic growth of LNCaP, LAPC-4, PC-3 and DU145 cells (50% colony growth inhibition: ~40 µg/ml [LNCaP and LAPC-4 cells] or 85 µg/ml [PC-3 and DU145]).
Table I.
Cytotoxicity of AAE on CaP cells: cells cultured in 48-well clusters were incubated with AAE at 0–200 µg/ml for 48h. Cell viability was determined by measuring the amount of MTT converted to insoluble formazan and reading the solubilized formazan in a plate reader as described previously (16). IC50 dose was calculated from least-square analysis of survival curves using a program (16) (Prism 4; GraphPad, San Diego, CA). CM: RPMI1640 basal medium + FBS (10%) + gentamycin (10 µg/ml)
| Cell line | Culture medium (CM: RPMI basal medium + 10% FBS + gentamycin) | Concentration to achieve 50% survival (mean values, [IC50], µg/ml) |
|---|---|---|
| LNCaP | CM | 49.7 |
| LAPC-4 | CM | 77 |
| DU145 | CM | 84 |
| PC-3 | CM | 75 |
| PC-3AR | CM | 75 |
| E006AA | CM | 75 |
| CW22RV1 | CM | 125 |
| ARCaP | CM | 55 |
| RWPE-1 | Keratinocytes Serum-Free Medium + growth factors | 150 |
| HLF | CM | 40 |
| HLF | No FBS (RPMI + 0.1% bovine serum albumin) | No inhibition (IC50 > 200 µg/ml) |
Fig. 1.
AAE inhibits CaP cell proliferation, clonogenic survival and cell cycle progression. (A) Cytotoxicity of AAE on CaP cells in time-dependent manner. LNCaP and PC-3 cells were exposed to AAE for different time points. The time points used were 4, 8, 12, 24, 48 and 72 h. Cell viability was determined by MTT reduction assay, after incubation time points. Mean ± SEM (n = 5), data normalized to control. (B) Colony-forming assay of CaP cells treated with AAE. Cells from each of the four CaP cell lines were plated in replicate (3–5/concentration) in 35mm culture dishes at 500–5000 cells per dish and were incubated with AAE for 7–10 days with media changes every 3 days. Emergent colonies of surviving cells were stained with 0.1% crystal violet. Colonies containing ≥16 cells were manually counted with a hand-held counter (16). Data presented as surviving fraction (% of control) was calculated as a ratio of mean number of colonies at each AAE concentration to that of untreated cultures. Error bars are not shown for clarity. Standard deviations (SD) for all cell lines were always ≤10%. (C) AAE inhibits cell cycle progression from G1 to S phase in LNCaP cells. Cells cultured in 35mm dishes in complete medium were treated with various concentrations of AAE for 24 (Supplementary Table S1, available at Carcinogenesis Online) or 48 h, lysed in a hypotonic buffer containing propidium iodide dye (50 µg/ml) to make suspensions of dye-labeled nuclei. The DNA content of individual nuclei was analyzed on a Epics XL flow cytometer (Beckman-Coulter, Miami, FL) followed by analyzing the cell cycle fractions of the DNA histograms using a software as described previously (16). Data are shown for one experiment. Similar results were obtained in replicate experiments. (D) AAE affects cell cycle regulatory molecules; cell lysates from LNCaP and PC-3 cultures treated with AAE for 24 h were analyzed by immunoblotting. Level of each of the proteins (bands in each row) was normalized to that of untreated control (not against β-actin) as cytotoxicity was not observed in the first 24h. In addition to cell cycle regulatory molecules, levels of p-AKT were analyzed in treated culture samples. The band intensity was normalized to that of β-actin (loading control) and relative expression of each protein was compared with that of control (untreated) samples that are indicated in numbers below the bands.
We determined whether AAE is cytotoxic to both proliferating and quiescent cells using cultures of non-transformed lung fibroblasts (HLF, passage 74–77) (29). Results showed AAE is minimally cytotoxic to normal, non-proliferating (quiescent) cells (Table I). Although AAE was cytotoxic to proliferating HLF cells, it was significantly less toxic to quiescent HLF cells, indicating AAE affects cycling cells but not as much on resting (G0) cells. Similarly, AAE was less toxic to RWPE-1 cells (IC50 150 µg/ml; Table I).
AAE blocks cell cycle progression
As shown in Figure 1C, AAE significantly blocked LNCaP cells at G1/S interface. This block was dose-dependent and produced ∼45% increase in G0/G1 and ∼55% decrease in S-phase fractions exposed for 24 h at 100 µg/ml AAE (Figure 1C). Incubation with lower AAE concentration also caused cell cycle arrest over a longer period (48 h). Further, we estimated the levels of key cell cycle–regulated proteins in AAE-treated LNCaP and PC-3 cells. Levels of cell cycle–regulated proteins were more inhibited in LNCaP cells as compared with PC-3. We found a dose-dependent decrease in the levels of cyclinD1, CDK4 and phosphorylated-Rb in LNCaP cells; all strongly associated with cell cycle arrest at G1/S interface (Figure 1D). Moreover, we found an increase in the cell cycle inhibitor protein p21 in PC-3 (200% at 50 µg/ml), but not significantly in LNCaP cells. Similarly, we found ~30% increase in p27kip1 in both LNCaP and PC-3 cells, but the increase was not dose-dependent (Figure 1D). AAE also reduced phosphorylated-AKT (p-AKTSer473) levels in both LNCaP (60%) and PC-3 (57%) cells (Figure 1D).
AAE induces apoptosis in LNCaP cells
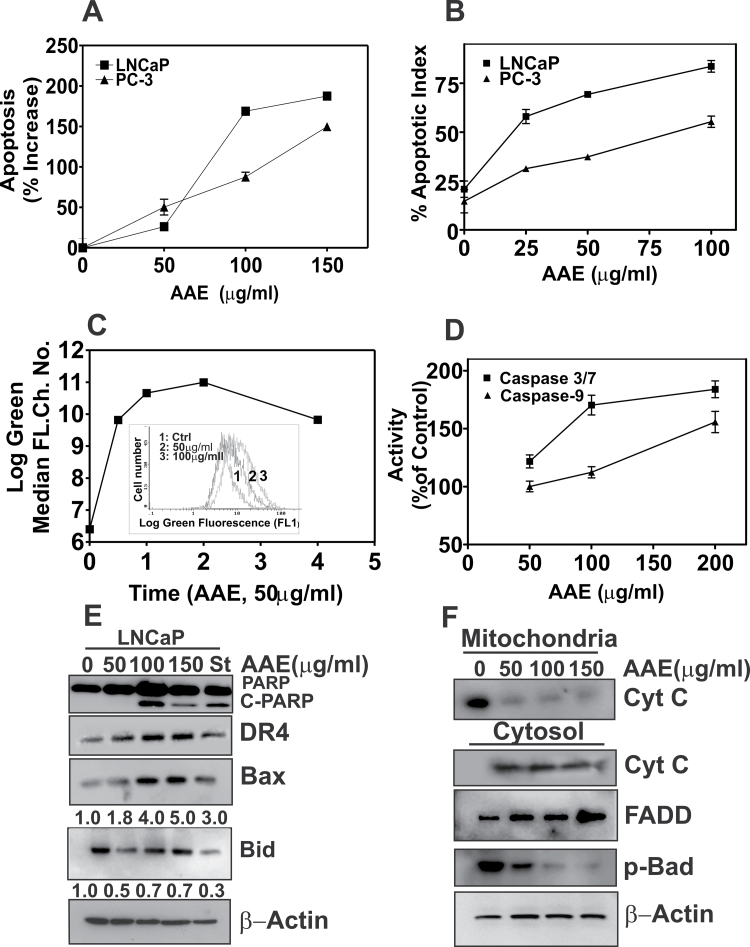
We found a significant increase in apoptosis in AAE-treated cultures, determined by an enzyme-linked immunosorbent assay (ELISA). There was ≥2-fold increase in H1 histone released in the culture medium due to chromatin fragmentation (Figure 2A). As H1 histone levels in culture supernatant is derived from both necrotic and apoptotic activities, we determined the free-nucleosome fractions in cell lysates, which represent apoptosis resulting from activation of caspase-dependent and -independent pathways (30). Further, to resolve whether apoptosis precedes, concurrent or succeeds cell cycle arrest, we followed cell death activity in a time- and dose-dependent manner. Cell cycle arrest was the predominant activity when LNCaP cultures were incubated at lower concentration (≤IC50) of AAE for 24 h. Apoptosis was significantly increased (~2× that of control) at doses higher than 50 µg/ml, as determined by cell cycle phase fraction and cell death ELISA, respectively. Apoptotic activity and percent of dead cells (unpermeabilized cells stained with propidium iodide) were comparable following incubation with AAE for 48 h, at concentration ranging from 50–200 µg/ml (Supplementary Table S1, available at Carcinogenesis Online). We further confirmed AAE induced apoptosis in CaP cells by Terminal Deoxynucleotide Transferase dUTP Nick End Labeling, (TUNEL) assay. As shown in Figure 2B, the percent of TUNEL-positive nuclei in AAE-treated LNCaP cells were significantly higher than that of PC-3 cells at any concentration of AAE; however, AAE increased TUNEL-positive population in both cultures.
Fig. 2.
The cytotoxicity of AAE is due to apoptosis. (A) Apoptosis in LNCaP and PC-3 cells treated with AAE for 48 h. Levels of H1 histone in cell lysates prepared from AAE-treated samples, indicative of apoptosis, were determined using a kit followed by the procedure described in the kit manual (Cell death ELISA Plus; Roche Applied Sciences, Indianapolis, IN). Results are expressed as % increase from untreated cultures (three replicates). Also see Supplementary Table S1, available at Carcinogenesis Online; (B) Apoptosis index determined by TUNEL assay of cells treated with AAE (48 h). LNCaP and PC-3 cell cultures were treated with AAE for 48 h and assayed for DNA fragmentation due to apoptosis using a TUNEL assay kit that fluorescently labels the cells undergoing apoptosis. Confocal imaging was used to enumerate fraction of cell population in a given field (total of 4 fields/treatment) that are fluorescent. Data are presented for a single experiment; similar data were obtained in two replicate experiments. Representative micrographs of AAE-treated and TUNEL-positive cells are presented in Supplementary Figure S1A, available at Carcinogenesis Online. (C) Decrease in mitochondrial membrane potential in AAE-treated LNCaP cells. Cell suspensions prepared from cultures treated with AAE over 48h were incubated with potential-sensitive mitochondrial dye JC-1 for 30min followed by determination of green fluorescence intensity by flow cytometry, as described (16). Time course of relative accumulation of monomeric JC-1 dye with and without AAE treatment is shown. Time was represented as hours in the graph. Inset: Levels of ROS in LNCaP cells after exposure to AAE. Increased accumulation of carboxy-6-carboxy-2′7′-dicholorohydroxy fluorescein is shown as right shift in median log fluorescence channel. (D) Caspases activation by AAE in LNCaP cells (24h). Caspases 3/7 and 9 activities were measured using a kit (Promega). Results are presented as mean ± SD, for three independent replicate experiments. (E) Levels of C-PARP, DR4, Bax, Bid and cleaved-BID (C-Bid) in LNCaP cells treated with AAE for 24h. Cell lysates prepared form AAE-treated cultures for 24 h were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting as described previously (16). Blots were re-probed for β-actin to normalize total protein loading. Doses of AAE are shown at top of the panel (0–150 µg/ml) and staurosporin (St) used as positive control for apoptosis induction. (F) AAE causes intrinsic apoptosis by cytochrome-C release from mitochondria. The mitochondria and cytosol fractions of AAE-treated LNCaP cells for 24 h were isolated and analyzed by immunoblotting. Note the increased levels of cytoplasmic-cytochrome-C in AAE-treated cell cultures. Increased levels of FADD and decreased level of phospho-Bad were also seen in cells treated with AAE for 24 h.
We next examined whether AAE-induced apoptosis is by extrinsic or intrinsic mechanism, or both (30). We measured alteration in mitochondrial permeability and depolarization using a potential-sensitive dye, JC-1 as described previously (16). As shown in Figure 2C, AAE (50 µg/ml) elevated the percentage of LNCaP cells with green fluorescence indicating depolarized mitochondria. LNCaP cells exposed to AAE showed a dose- and time-dependent increase in reactive oxygen species (ROS) determined by levels of intracellular accumulation of oxidized, membrane impermeable, 6-carboxy-2′7′-dicholorohydroxy fluorescein in cells labeled with carboxy-H2DCF-diacetate, as described previously (31). Increased levels of ROS were evident in LNCaP cells treated with AAE as indicated by increased green fluorescence (Figure 2C, inset).
The mitochondrial membrane permeability and cytochrome-C release into the cytoplasm are regulated by the Bcl-2 family proteins (32). Mitochondrial permeability changes and formation of Bax/Bak oligomer cause release of cytochrome-C to cytoplasm leading to the formation of an apoptosome (apaf-1-oligomer). Cytochrome-C release leads to activation of caspase 9 and 3/7. Further, this leads to cleaved-poly (ADP ribose) polymerase (C-PARP) and DNA fragmentation (32,33). Caspase 3/7 and 9 activities increased by 81 and 50%, respectively, in AAE- (100 µg/ml) treated LNCaP cells, compared with the untreated (Figure 2D). Indeed, as shown in Figure 2E, there was a significant increase in C-PARP in AAE-treated LNCaP cells.
We next examined the expressions of pro-apoptotic and antiapoptotic Bcl-2 family proteins on LNCaP cells with or without AAE treatments. AAE reduced the antiapoptotic p-Bad protein level found in cytosol (70%; Figure 2F, bottom lane) and elevated the pro-apoptotic Bax (96%) protein (Figure 2E and F). Reduction of BH3-interacting domain protein (BID) in AAE-treated LNCaP cells implies that it is due to activation of caspase-8 into its truncated form. The death receptor protein DR4 and FADD are elevated in AAE-treated LNCaP cells. Further, cytochrome-C levels were decreased significantly in mitochondrial fraction with a concomitant increase in the cytosol, in LNCaP cells exposed to AAE for 24 h, indicating the release of cytochrome-C from depolarized mitochondria (Figure 2F, lower panel).
Cytotoxicity of AAE on castrate-resistant and AR-negative CaP cells is by caspase-independent mechanism
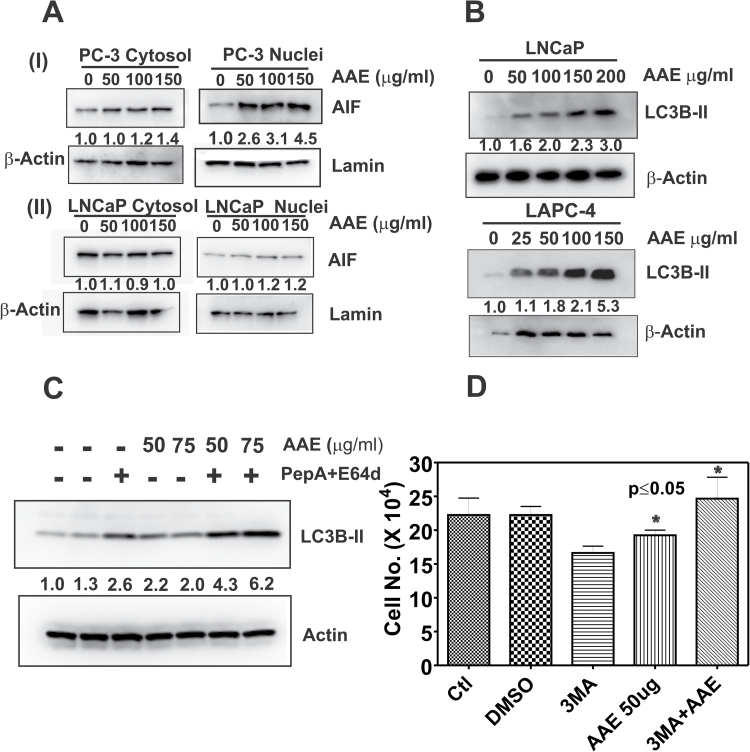
PC-3, DU145 and CWR22RV1 cells died by apoptosis upon exposure to AAE for 48h; see Figure 2A and B and Supplementary Figure S1A–C and S2A, available at Carcinogenesis Online. For example, as shown in Figure 2A, the fractions of cells in apoptosis were similar between LNCaP and PC-3 cells at a given dose of AAE. Similarly, AAE elevated the fraction of cells with depolarized mitochondria; 50% increase (AAE; 100 µg/ml) when compared with untreated (Supplementary Figure S1B, available at Carcinogenesis Online). However, we could not detect any C-PARP (see Supplementary Figure S1C) and activities of caspases 3/7 and 9 in AAE-treated PC-3 cells (Supplementary Figure S1D, available at Carcinogenesis Online). Similarly, no C-PARP was found in AAE-treated DU145 cells, and further, no caspase activation in DU145 or CWR22RV1 cells, the latter being AR-positive CRPC line (Supplementary Figure S2A–C, available at Carcinogenesis Online). Therefore, we examined the activation of the apoptosis-inducing factor (AIF), a mitochondrial protein that induces cell death by a caspase-independent mechanism (34,35). To identify AIF activity and nuclear translocation, we prepared the nuclear and cytosol fractions of AAE-treated PC-3 cells by density gradient fractionation. As shown in Figure 3A (I), we observed a dose-dependent increase in the levels of AIF in nuclear fractions in AAE-treated PC-3 cells. The levels of AIF in nuclear fractions increased by 260% at 50 µg/ml AAE (that is at IC50 level) to 450% (4.5-fold) at 150 µg/ml AAE in 24h. We also observed an increase of cytosolic AIF in AAE-treated PC-3 cells (by ~40% at 150 µg/ml of AAE, possibly due to the migration of AIF from mitochondria (Figure 3A (I)). Importantly, similar fractionation and probing the distribution of AIF in nuclear and cytosolic fractions of AAE-treated LNCaP cells showed little nuclear translocation of AIF, suggesting this is an unlikely mechanism in LNCaP cells (Figure 3A (ii)).
Fig. 3.
AAE induces apoptosis and autophagy in CaP cells. (A) Effect of AAE on AIF protein levels in (I) PC-3 and (II) LNCaP cells. Cells were treated with several concentrations of AAE for 24 h. Cytosol and nuclear fractions were prepared using a kit (Thermo Fisher Scientific, Pittsburgh, PA). Total proteins were estimated in each nuclear and cytoplasmic fraction using the BCA reagent (Bio-Rad) and equal amount of proteins were mixed with 2× sodium dodecyl sulfate–gel sample buffer and analyzed by western blotting. Blots were re-probed with β-actin and nuclear lamin to normalize for loading controls for cytoplasmic and nuclear fractions, respectively. Numbers shown below the blots are density of the protein blots normalized to that of untreated controls. (B) AAE induced autophagy in LNCaP and LAPC-4 cells. Cell lysates from LNCaP and LAPC-4 cells treated with AAE for 24 h were analyzed by immunoblotting. Blots were probed for membrane-bound form of LC3B (LC3B-II) using a specific antibody (Cell Signaling, Danvers, MA). A significant dose-dependent increase in LC3B-II (shown as the ratio of LC3B-II to β-actin below the blots) occurred following AAE treatment. (C) Specific increase in membrane-bound LC3B-II following AAE treatment in LAPC-4 cells. Cells were exposed to lysosomal protease inhibitors pepstatin A and E64D (PepA/E64D) for 4 h and LC3B-II levels were analyzed by immunoblotting. As PepA/E64D combination inhibited lysosomal degradation of LC3B-II, increased accumulation of LC3B-II resulting from AAE-induced autophagy flux was observed. (D) Inhibition of autophagy by 3-MA decreases AAE cytotoxicity in LAPC-4 cells. LAPC-4 cells were pretreated with 3-MA, an inhibitor of PI3K that drives autophagy. Cytotoxicity of AAE on LAPC-4 cells, treated with AAE (50 µg/ml) alone or with 3-MA (an inhibitor of autophagy) for 48 h, were estimated by cell counting. Data shown are mean ± SEM. Significant decrease in the efficacy of AAE was observed in the presence of 3-MA (P ≤ 0.05).
AAE increases autophagy-related protein LC3B
We examined whether autophagy, an independent mechanism of cell death, is associated with cytotoxicity of AAE. LNCaP and LAPC-4 cells treated with AAE were analyzed for the level of lysosomal protein LC3B-II, a universal indicator of autophagy in tumor cells (36). LC3B-II levels were low in untreated LNCaP and LAPC-4 cells, but rose rapidly with increasing concentration of AAE upon 24 h (Figure 3B). AAE-induced autophagy was further investigated in LAPC-4 cells to determine whether increased autophagy contributes to cytotoxicity of AAE in these cells. As shown in Figure 3C, further increase in levels of LC3B-II by the addition of lysosomal inhibitors pepstatin A and E64D, which inhibit the turnover of the marker (LC3B-II), indicated frank autophagy induction after AAE treatment. This was further corroborated when AAE induced cytotoxicity was abolished in the presence of an autophagy inhibitor 3-methyl adenine (3-MA). As shown in Figure 3D, AAE induced cytotoxicity in LAPC-4 cells was significantly diminished when cells were pre-incubated with 1 mM 3-MA for 4 h and then treated with AAE (50 µg/ml) for 48 h. In addition, we independently confirmed increased autophagy in AAE-treated cells by measuring the red/green fluorescence ratio of acridine orange-labeled-LAPC-4 cells (data not shown).
AAE depletes AR levels
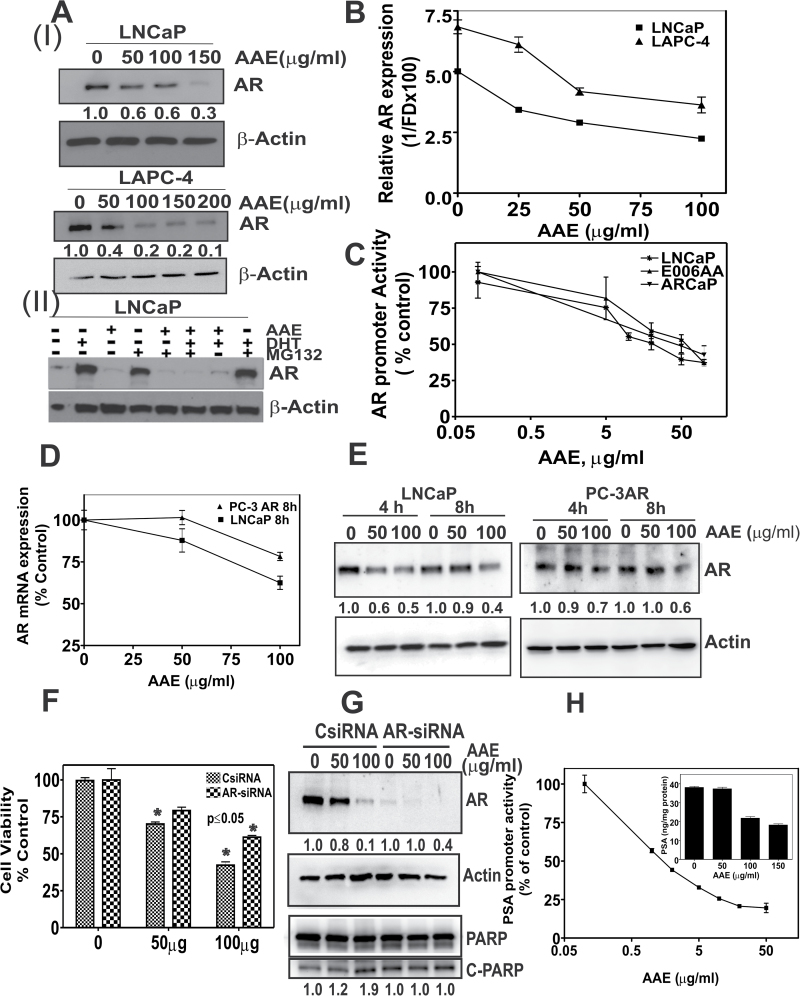
Effects of AAE on AR expression in LNCaP cells were investigated as AR activity drives G1 to S-phase cell cycle transition in androgen-responsive AR-positive cells. We reasoned that AAE might inhibit AR signaling. We found an AAE dose-dependent decrease (IC50 50 µg/ml) in AR protein levels in LNCaP and LAPC-4 cells (Figure 4A (I)). We investigated further, whether this is due to AR protein stability or inhibition of its transcription. To determine the former, we examined AR levels in LNCaP cells that were treated with AAE (50 µg/ml) in the presence or absence of MG132, a 26S proteasome inhibitor (37), and with or without DHT. As shown previously (38), AR levels are higher in DHT- (1 nM) treated cells, due to the stabilization of AR, see Figure 4A (II). However, in AAE-treated cells, AR levels are lower in the presence or absence of DHT. The decrease in AR levels in AAE-treated cells was not rescued by treatment with 10 µM MG132, suggesting, AAE is unlikely to destabilize AR protein.
Fig. 4.
AAE down modulates AR in CaP cells. (A) (I) Immunoblots of AR from AAE-treated LNCaP and LAPC-4 cells. Cells were incubated with indicated levels of AR for 24 h, lysed, total proteins were estimated with BCA reagent and lysates with estimated equal amount of total proteins were loaded onto SDS–polyacrylamide gel and immunoblotted to detect AR levels followed by re-probe for β-actin as loading control. Band densities normalized to that of control (untreated) sample are indicated below the bands. Band densities corresponding to AR decreased up to 70% in LNCaP cells and 90% in LAPC-4 cells due to incubation with AAE. (II) AAE does not interfere with proteasomal degradation of AR. LNCaP cells were exposed to AAE for 24 h in androgen-free culture medium, followed by 4h incubation with or without DHT and the 26S-proteasomal inhibitor MG132. Levels of AR in cell lysates were estimated by immunoblotting. Numbers at the bottom of the blot represent normalized AR protein blot density normalized to that of untreated control (corresponding to with or without androgen containing medium). All blots were re-probed with β-actin to ensure equal amount of total protein loading on the gel. (B) AAE inhibits AR mRNA levels: real-time quantitative PCR of AR mRNA from LNCaP and LAPC-4 cells treated with AAE for 24 h. Significant decrease in AR mRNA was noted in a AAE dose-dependent manner. (C) AAE affects AR promoter activity in AR+ CaP cells. All three cell lines (LNCaP, ARCaP and E006AA) were transfected with AR-promoter-luciferase plasmid and cultured for 44h. Cells were then incubated with various levels of AAE at 4 h before measuring luciferase activity, as described in Materials and methods. Data shown are mean ± SDs from three separate experiments. A dose-dependent decrease in AR promoter activity was observed in all three tested cell lines. (D) Effect of AAE on AR mRNA levels in a cell line expressing constitutively active promoter-driven AR transcription (PC-3AR cells). Quantitative PCR of AR mRNA isolated from LNCaP and PC-3AR cells exposed to AAE for 8 h. (E) AR protein expression on PC-3AR and LNCaP cells after 4 and 8 h exposure of AAE. Protein levels were analyzed by western blotting as in Figure 4A. (F) Increase in cell viability ofAAE-treated LNCaP cells with AR gene silencing. AR gene was silenced in LNCaP cells by RNA interference using SMARTpool AR siRNA (25nM) purchased from Dharmacom/Thermo Scientific (Chicago, IL) and viability was estimated by MTT assay following exposure to AAE for 48h. *P ≤ 0.05 compared with cells transfected with control siRNA alone. (G) AR-dependent apoptosis induction by AAE. As shown in the top panel, AR protein was reduced by >90% by AR siRNA (25 nM) transfection and it was perhaps further reduced by incubation with AAE up to 60% of that observed for AR siRNA transfectants alone. Bottom panel shows levels of total and C-PARP in the transfected cells, indicating increased apoptosis activity by AR RNA interference alone and no further increase in C-PARP. Relative band densities are indicated at the bottom of the respective blots. (H) PSA transcriptional activity in AAE-treated LNCaP cells. Inset: PSA levels in the conditioned medium of LNCaP cells treated with different doses of AAE for 24 h. PSA was measured using an ELISA kit (Calbiotech, Spring Valley, CA).
AAE inhibits AR messenger RNA transcription
Levels of AR messenger RNA (mRNA) in LNCaP and LAPC-4 cells following AAE treatment were determined using real-time quantitative PCR (38). As shown in Figure 4B, we found a dose-dependent decrease in AR mRNA in both cell lines. This decrease in AR mRNA in AAE-treated cells may be due to inhibition of AR transcription, AR mRNA stability or both. The relative levels of AR promoter activity were measured by an AR-promoter-reporter (AR-LUC) assay with or without AAE treatment. As shown in Figure 4C, exposure to AAE reduced the AR promoter activity in a dose-dependent manner. AAE did not exhibit luminescence or quench luminescence on its own, as determined in a cell-free system (data not shown). AR promoter activity was inhibited at AAE ≤ 10 µg/ml, much lower concentration than that required for growth inhibition. AR transcriptional activity in other AR-expressing CaP cell lines, ARCaP and E006AA (39), was also inhibited by AAE (Figure 4C).
To further validate the specificity of AAE to inhibit AR promoter activity to AR-expressing, androgen-responsive cell lines that contain native AR promoter, we measured the AR mRNA levels in a stable PC-3 AR cell line expressing AR under the control of an exogenous promoter (e.g. cytomegalovirus promoter) (40). As shown in Figure 4D, levels of mRNA in PC-3AR cells were reduced slightly (~20%) at 100 µg/ml AAE as compared with 40% in LNCaP cells that express native AR promoter. The relative levels of AR protein in AAE-treated LNCaP and PC-3AR cells were dissimilar (Figure 4E). There was a ~40% reduction in AR protein in LNCaP cells versus <10% in PC-3AR cells exposed to AAE (50 µg/ml, 4h). However, at higher concentration of AAE, AR protein levels in LNCaP and PC-3AR cells were reduced by 50 and 30%, respectively, after 4 h exposure.
As the mechanism by which AAE exerts cytotoxicity is different in AR-expressing LNCaP cells when compared with AR-negative PC-3 cells, we sought to establish whether altering the levels of AR in LNCaP and introducing AR in PC-3 cells also alters the mechanism by which cells die following AAE exposure. We silenced the AR expression in LNCaP by small interfering RNA (siRNA) AR knock-out. We observed 80 and 90% decreases in AR mRNA at 48h of AR siRNA transfection, respectively (see Supplementary Figure S3A, available at Carcinogenesis Online). Cytotoxicity of AAE was significantly decreased (>50%) in LNCaP cells with reduced AR levels (Figure 4F). C-PARP was higher in AR-downregulated LNCaP cells. However, C-PARP level did not increase in AR-knockdown cells treated with AAE (Figure 4G).
AAE affects AR target gene and AR transcription
We used a luciferase promoter reporter assay for PSA (38) to measure the levels of the AR-regulated mRNA transcription in LNCaP cells following AAE treatment. We found a significant decrease in the PSA promoter activity by AAE (Figure 4H). In addition, we also observed 43% (at AAE at 100 µg/ml) and 53% (AAE at 150 µg/ml) decreases in secreted PSA levels in AAE-treated cultures, respectively (Figure 4H, insert).
Antitumor activity of AAE
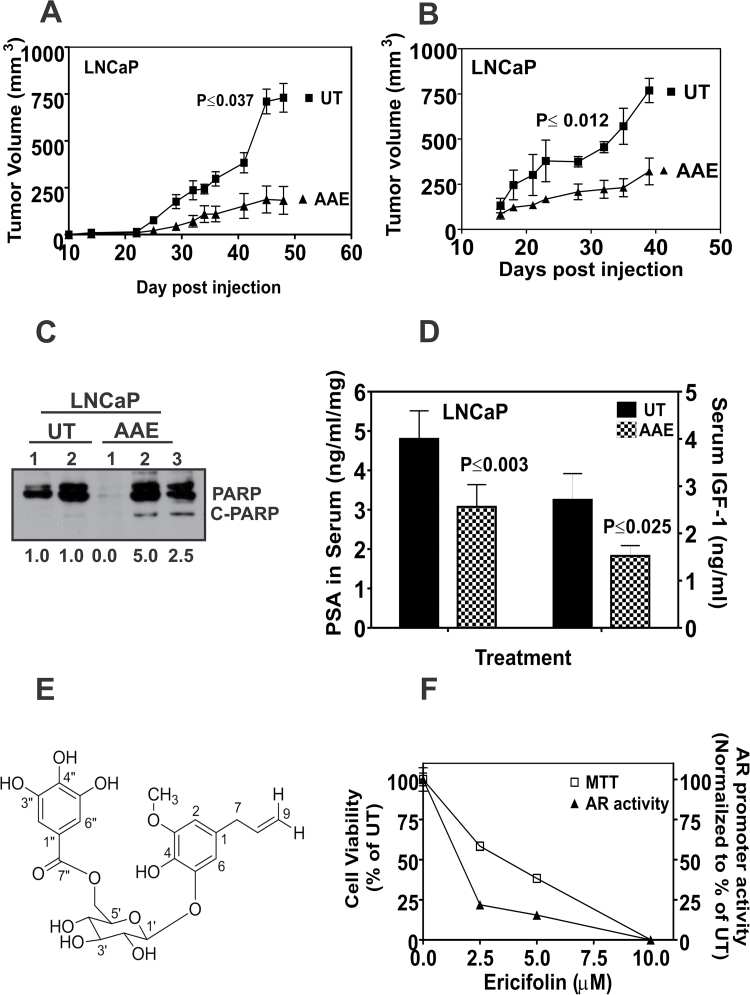
We tested the antitumor activity of AAE in vivo using LNCaP subcutaneous tumors generated in athymic mice. Mice were randomly divided into two groups after LNCaP cells injection and gavaged daily with water or AAE (100mg/kg). Incidences of tumors in LNCaP xenograft were 90 and 100% in both control and treated groups of mice. There was no significant change in the weights of mice during the course of treatment, indicating non-toxicity of AAE. Analyses of the changes in tumor volumes between control and AAE-treatment groups showed a lower tumor growth rate and a significant (62.3%, P < 0.037, pairwise t-test) decrease in tumor volume at the time of termination in AAE-treated group (Figure 5A).
Fig. 5.
AAE inhibits in vivo growth of LNCaP in xenografts. (A) Growth of LNCaP tumors in male athymic nude mice with or without AAE gavage. Tumor volume was measured using hand-held microcalipers twice weekly as described in Materials and methods. Mice were gavaged daily, starting on the day of tumor cell injections for 50 days, with freshly prepared solution of AAE (100mg/kg). All tumor-bearing mice were euthanized 50 days after tumor cell injections. Symbols shown represent mean tumor volumes (vertical bars: SD, n = 8). (B) Growth of LNCaP tumors in athymic mice treated with AAE (50mg/kg by i.p. injection, 3×/week) or with saline injection (control). Experiment details are similar to that in Figure 5A. Mice were injected i.p. with AAE, three times a week at 50mg/kg starting from the day of tumor cell injection for 6 weeks. Significance of decreased tumor growth was tested by analysis of variance. The probability of observed difference in tumor volumes between AAE treated and control is due to chance is indicated (P ≤ 0.037 and P ≤ 0.012). (C) Expression of apoptotic biomarker, C-PARP in tumor tissues. Tumor tissues collected at necropsy were analyzed for C-PARP by extracting total proteins using a tissue homogenizer and lysing the tissue in NP-40 lysis buffer. Data shown for (D) serum IGF-1 and PSA levels in control and AAE-treated mice with LNCaP xenografts, IGF-1 and PSA levels in the sera of tumor-bearing mice were assayed using ELISA kits and expressed as ng/ml of serum. Data shown are mean ± SD (n = 3) for all treatments. (E) Identification of EF in AAE. EF was purified from AAE as described in Supplementary Data, available at Carcinogenesis Online. Approximately, 3.5mg of EF was purified from 1g of AAE; purity was confirmed using HPLC fractionation spectra followed by mass spectroscopy. Deduced chemical structure of eugenol-O-β-(6-galloylglucopyranoside) is shown. (F) EF purified from AAE inhibits cell proliferation and AR levels in LNCaP cells. LNCaP cells incubated with indicated concentrations of EF were assayed for cytotoxicity using MTT after 48 h. AR promoter reporter activity was assayed following 4 h treatment with EF, as described in Materials and methods. EF decreased viability of LNCaP cells (left ordinate) and AR transcriptional activity (right ordinate) (mean ± SEM, n = 5).
Another study, where AAE was dosed by i.p. injection into LNCaP tumor-bearing mice showed significantly delayed (~58%) tumor growth, again without systemic toxicity due to AAE (Figure 5B). Ex vivo analyses of LNCaP tumor tissue showed increased apoptosis as determined by C-PARP (Figure 5C) in AAE-treated group compared with that of vehicle control groups. As an independent measure of antitumor activity of AAE on LNCaP tumors, we analyzed PSA and IGF-1 in sera from untreated and AAE-treated-LNCaP tumor-bearing mice (41). The levels of PSA (58%) and IGF-1 (44%) were significantly lower in AAE-treated mice at termination (Figure 5D).
Structural elucidation of antiproliferative compound on AAE
The structural elucidation of the bioactive compound present in AAE is described in detail in Supplementary Table S3 and Figures S4 and S5, available at Carcinogenesis Online). The structure was identified as eugenol 5-O-β-(6′-galloylglucopyranoside) (Figure 5E). This compound was previously identified as EF from Melaleuca ericifolia (42). EF was shown to have antibacterial activity against both Gram-positive and Gram-negative pathogenic bacteria. Marzouk et al. (3) reported identification of 18 polyphenols and tannins from P. dioica leaves and found two tannins (galloyl pedunculagin and casuarinin) with antiproliferative properties against breast (MCF-7) and colon tumor (HCC-116) cells, they did not identify EF in the leaves of P.dioica and did not report analysis of allspice (the berries of P.dioica).
EF induces apoptosis and affects growth on LNCaP cells
EF from AAE was investigated to determine whether it retained the activities found in AAE. We tested the effect of EF on cell cycle, cell cycle–regulated proteins, AR mRNA and apoptosis at concentration similar to that found in AAE. Cells treated with EF showed reduced levels of cyclin D1, pRb, CDK4 and p-AKT (Supplementary Figure S3C, available at Carcinogenesis Online). EF-treated LNCaP cells underwent apoptotic cell death (2-fold increase in free nucleosomes) and a 2-fold increase in C-PARP (Supplementary Figure S3C, available at Carcinogenesis Online). Further, EF inhibited the AR expression level in LNCaP cells, repressed AR mRNA level and AR promoter activities (Figure 5F).
Discussion
The results presented in the preceding pages establish that an AAE has specific antiproliferative and cytotoxic activities against human prostate cancer cells in vitro and their xenografts in vivo. CaP cells that express AR and are responsive to androgen are more sensitive to AAE than CRPC cells. Further, regardless of the AR expression, cells that are likely to respond to androgen depletion (LNCaP and LAPC-4) were more sensitive than the cells that express non-signaling form of AR (CWR22RV1 or E006AA). Moreover, we also demonstrated that mechanism of cytotoxicity of AAE differs in AR-positive cells from that of CRPC cells; caspase-dependent apoptosis in AR-responsive cells but caspase-independent, AIF-dependent mechanism of cell death in CRPC cells (e.g. PC-3). Importantly, AAE was effective in decreasing the growth of LNCaP tumors by oral administration or i.p. injection. We succeeded in identifying at least one active compound, EF that had both antiproliferative and anti-AR activities.
We found that potent cytotoxicity of AAE and the purified single agent, EF, in LNCaP cells is mediated by cell cycle arrest and apoptosis. As shown in Figure 1C and D, cell cycle arrest was the predominant effect at AAE concentration <100 µg/ml, but apoptosis and autophagy were more dominant at higher concentrations, even before cells were blocked at G1. It has been reported before that downregulation of AR or androgen depletion leads to cell cycle arrest at G1 (43). However, unlike serum-starved normal fibroblast (HLF), in androgen-starved LNCaP cells, which are arrested at G1, AAE caused apoptotic cell death (see Supplementary Figure S2B, available at Carcinogenesis Online). Our efforts to determine whether sensitivity of AAE varies among cells that express high levels of AR but are mitogenically unresponsive to androgens (e.g. CW22RV1 and E006AA) (39,44) showed no consistent pattern. The AAE was significantly less potent in these two cells when compared with that of LNCaP cells. However, IC50 values were comparable between LNCaP and ARCaP cells, and further, IC50 of LAPC-4 was similar to that of CWR22RV1 cells (Table I). Furthermore, AAE was significantly less potent in cells that do not express AR, such as PC-3 and DU145. At this time, we do not know why CRPC cells are less responsive to AAE. It could be that the divergent cell death processes (caspase-dependent and independent apoptosis and autophagy) play a role in the potency of AAE against these cells.
The potent anti-AR activity of AAE in cells that respond to AR signaling may be one mechanism of AAE’s increased potency against androgen-responsive AR-positive cells. Further, it is possible that more abundant antiapoptotic machinery (e.g. increased bcl2-mediated resistance) present in CRPC may be responsible for increased resistance to AAE. However, we restrain from over-interpreting these results as the test agent AAE is a mixture of several potential bioactive compounds. One possibility is that AAE interferes with mitogenic signaling of AR in androgen-responsive CaP cells and, therefore, decreased sensitivity of AAE in CRPC cells may be due to the loss of this component of AR signaling.
The main mechanism of AAE cytotoxicity was by apoptosis in majority of cell lines. Among the three cell lines we examined, AAE induced cell death was accompanied by free-radical generated mitochondrial depolarization and PARP cleavage. This mechanism is similar to that of many plant-derived antitumor agents, such as green-tea polyphenols (e.g. epicatechin gallate and epigallocatechin 3-gallate); capsaicin, indole-3-carbinol and sulforaphane are shown to induce apoptosis in tumor cells via free-radical generation (45,46). As EF is a polyphenolic compound, it is likely to induce similar biochemical pathway leading to toxic level of ROS in tumor cells. In addition, the activation of caspases 7, 9 and 3 by AAE is caused by BID cleavage resulting from cytochrome-c release, which in turn may be due to excessive ROS production. However, AAE caused caspase-independent pathway of apoptosis involving AIF activation in PC-3 cells that is intriguing. There is little difference in caspase-induced apoptosis repertoire between LNCaP and PC-3 cells (16).
In recent years, autophagy, a cell-survival mechanism widely noted in tumor cells, has become a means to kill tumor cells as well by external stimulation. In this regard, our observation that AAE induced cell death by excessive autophagy in LAPC-4 cells provides another means of antitumor potential of AAE (Figure 3B–D). Contribution of autophagy as a significant inducer of cell death is derived from the observation of reduced cytotoxicity of AAE in LAPC-4 cells when cells were pre-incubated with a well-characterized autophagy inhibitor 3-MA (47). The actual mechanism of AAE-triggered autophagy is likely due to an upstream event, such as increased endoplasmic reticulum stress. We reserve a separate study in the future to delineate this novel cell death pathway induced by AAE. We found both LNCaP and PC-3 cells incubated with AAE showed reduced levels of p-AKT (Figure 1D) indicating, AAE is likely to affect multiple survival pathways in tumor cells, which frequently express redundant mechanisms of survival in response to various cytotoxic stimuli (48).
Several studies have focused to identify natural inhibitor of AR due to its critical role in CaP cell growth and progression (44). Most other plant-derived dietary anticancer compounds have been shown to affect AR by accelerated AR protein degradation (48). Perhaps, we are the first to report that AAE depletes AR expression by inhibiting AR promoter activity. Even though many novel natural compounds are emerging, EF is distinctive due to its inhibition of AR transcription and inhibition of AR-mediated gene expression, such as PSA mRNA transcription (Figure 4H).
The demonstration of antitumor activity of AAE by oral gavage or via i.p. injection in LNCaP xenografts suggests its potential bioavailability and clinical application. Strong antitumor activity by oral administration of AAE demonstrates the significant and potential use of AAE as a chemodietary agent. Further, we did not detect any toxicity following oral or i.p. administration at the indicated dose. The mechanism of antitumor activity was potentially due to increased apoptosis, primarily by caspase activation mechanism. Because AR amplification and hypersensitivity to androgen are associated with human CRPC, oral bioavailability of AAE makes it potentially a unique dietary chemopreventive agent against CRPC, the incurable stage of CaP.
Allspice is used as a food flavoring agent around the world in several cuisines; however, there is no reported epidemiological evidence that allspice consumption is associated with reduced cancer risk. This may be partly due to amount consumed is insignificant and such studies are not done in other geographic regions such as middle-east and Jamaica, where the consumption is significant. The lack of epidemiological data on the anticancer effects of allspice may also be due to other compounding causes, which may neutralize its benefits, e.g. concurrently consuming excess amount of charred meat. It is possible to use AAE as a dietary supplement with minimal adverse reaction. Both epidemiologic and case control studies have shown that higher intake of plant flavonoids reduces the risk of cancer (49). Together its water-extractable property, delectable flavor and minimal systemic toxicity, AAE and perhaps EF show potential for adjuvant therapy or for chemodietary prevention of recurrent prostate cancer.
Supplementary material
Supplementary Data, Tables 1–3 and Figures 1–5 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (R01AT003544 and 1R01CA156776-01 to B.L.L.); Veterans Administration MERIT (VA5312.01 to B.L.L.).
Conflict of Interest Statement: None declared.
Supplementary Material
Acknowledgements
The authors thank Daniel Munoz, Dr Vinata Lokeshwar and members of her lab for critical discussion and use of their instruments. Authors are grateful to Dr Francisco Raymo and Ms Stefania Impellizzeri (Department of Chemistry, University of Miami, FL) for initial help with the purification of AAE. Authors also thank Benny J.Fernandez and Dr Arumugam Jayakumar (VA Hospital, Miami, FL) for their help in fractionation of AAE using HPLC. B.L.L. is indebted to Prof. Lionel Sternberg (Department of Biology, University of Miami, FL) for sharing HPLC instrument.
Glossary
Abbreviations:
- AAE
aqueous allspice extract
- AIF
apoptosis-inducing factor
- AR
androgen receptor
- ARCaP
androgen repressed cancer of prostate
- BID
BH3-interacting domain protein
- CaP
cancer of the prostate
- CM
culture medium
- C-PARP
cleaved-PARP
- CRPC
castration-resistant CaP
- DHT
5α-dihydrotestosterone
- EF
ericifolin
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- HPLC
high-performance liquid chromatography
- IC50
50% growth inhibition
- i.p.
intraperitoneal
- 3-MA
3-methyl adenine
- mRNA
messenger RNA
- MTT
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide
- PARP
poly (ADP ribose) polymerase
- PSA
prostate-specific antigen
- ROS
reactive oxygen species
- SD
standard deviation
- SEM
standard error of the mean
- siRNA
small interfering RNA
- TUNEL
Terminal Deoxynucleotide Transferase dUTP Nick End Labeling.
References
- 1. Ross J.A., et al. (2002). Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr., 22, 19–34 [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal B.B., et al. (2009). Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med., 234, 825–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzouk M.S., et al. (2007). Anticancer and antioxidant tannins from Pimenta dioica leaves. Z. Naturforsch. C., 62, 526–536 [DOI] [PubMed] [Google Scholar]
- 4. Suárez A., et al. (1997). Cardiovascular effects of ethanolic and aqueous extracts of Pimenta dioica in Sprague-Dawley rats. J. Ethnopharmacol., 55, 107–111 [DOI] [PubMed] [Google Scholar]
- 5. Suárez A., et al. (2000). Hypotensive action of an aqueous extract of Pimenta dioica (Myrtaceae) in rats. Rev. Biol. Trop., 48, 53–58 [PubMed] [Google Scholar]
- 6. Nakatani N. (2000). Phenolic antioxidants from herbs and spices. Biofactors., 13, 141–146 [DOI] [PubMed] [Google Scholar]
- 7. Kikuzaki H., et al. (2000). Galloylglucosides from berries of Pimenta dioica . J. Nat. Prod., 63, 749–752 [DOI] [PubMed] [Google Scholar]
- 8. Tanaka T., et al. (1993) Eugenin and galloyl pedunculagin were isolated from Platycarya strobilaceae. Chem. Pharm. Bull., 41, 1708–1716 [Google Scholar]
- 9. Siegel R., et al. (2012). Cancer statistics, 2012. CA. Cancer J. Clin., 62, 10–29 [DOI] [PubMed] [Google Scholar]
- 10. Debes J.D., et al. (2004). Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med., 351, 1488–1490 [DOI] [PubMed] [Google Scholar]
- 11. Nelson W.G., et al. (2003). Prostate cancer. N. Engl. J. Med., 349, 366–381 [DOI] [PubMed] [Google Scholar]
- 12. Chen C.D., et al. (2004). Molecular determinants of resistance to antiandrogen therapy. Nat. Med., 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y., et al. (2008). Targeting the androgen receptor pathway in prostate cancer. Curr. Opin. Pharmacol., 8, 440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clegg N.J., et al. (2012) ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res., 72, 1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scher H.I., et al. (2005). Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol., 23, 8253–8261 [DOI] [PubMed] [Google Scholar]
- 16. Lokeshwar B.L., et al. (2002). Inhibition of cell proliferation, invasion, tumor growth and metastasis by an oral non-antimicrobial tetracycline analog (COL-3) in a metastatic prostate cancer model. Int. J. Cancer, 98, 297–309 [DOI] [PubMed] [Google Scholar]
- 17. Dandekar D.S., et al. (2004). Inhibition of cyclooxygenase (COX)-2 expression by Tet-inducible COX-2 antisense cDNA in hormone-refractory prostate cancer significantly slows tumor growth and improves efficacy of chemotherapeutic drugs. Clin. Cancer Res., 10, 8037–8047 [DOI] [PubMed] [Google Scholar]
- 18. Shamaladevi N., et al. (2009). CXC receptor-1 silencing inhibits androgen-independent prostate cancer. Cancer Res., 69, 8265–8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L.G., et al. (2004). Androgen receptor level controlled by a suppressor complex lost in an androgen-independent prostate cancer cell line. Oncogene, 23, 5175–5184 [DOI] [PubMed] [Google Scholar]
- 20. Perez-Stable C.M., et al. (2000). A role for GATA transcription factors in the androgen regulation of the prostate-specific antigen gene enhancer. Mol. Cell. Endocrinol., 167, 43–53 [DOI] [PubMed] [Google Scholar]
- 21. Singh R.K., et al. (2009). Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol. Cancer, 8, 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dupont W.D., et al. (1990). Power and sample size calculations. A review and computer program. Control. Clin. Trials, 11, 116–128 [DOI] [PubMed] [Google Scholar]
- 23. Singleton D.L., et al. (1965). Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult., ., 16, 144–158 [Google Scholar]
- 24. König M., et al. (1994). Ellagitannins and complex tannins from Quercus petraea bark. J. Nat. Prod., 57, 1411–1415 [DOI] [PubMed] [Google Scholar]
- 25. Van Berkel G.J., et al. (2007). Thin-layer chromatography/desorption electrospray ionization mass spectrometry: investigation of goldenseal alkaloids. Anal. Chem., 79, 2778–2789 [DOI] [PubMed] [Google Scholar]
- 26. Nieman R.H., et al. (1963). Spectrophotometric estimation of nucleic acid of plant leaves. Plant Physiol., 38, 31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strumeyer D.H., et al. (1975). Condensed tannins in grain sorghum: isolation, fractionation, and characterization. J. Agric. Food Chem., 23, 909–914 [DOI] [PubMed] [Google Scholar]
- 28. He S., et al. (2007). Isolation and purification of antioxidative isomeric polyphenols from the roots for parthenocissus laetevirens by counter-current chromatography. J., Chromatogr A, 1151, 175–17–9 [DOI] [PubMed] [Google Scholar]
- 29. Lokeshwar B.L., et al. (1995). Enhancement of radiation response of prostatic carcinoma by taxol: therapeutic potential for late-stage malignancy. Anticancer Res., 15, 93–98 [PubMed] [Google Scholar]
- 30. Kim R. (2005). Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer, 103, 1551–1560 [DOI] [PubMed] [Google Scholar]
- 31. Lokeshwar B.L., et al. (2001). Cytotoxic activity and inhibition of tumor cell invasion by derivatives of a chemically modified tetracycline CMT-3 (COL-3). Curr. Med. Chem., 8, 271–279 [DOI] [PubMed] [Google Scholar]
- 32. Cory S., et al. (2002). The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer, 2, 647–656 [DOI] [PubMed] [Google Scholar]
- 33. Li J., et al. (2008). Caspases in apoptosis and beyond. Oncogene, 27, 6194–6206 [DOI] [PubMed] [Google Scholar]
- 34. Joza N., et al. (2009). AIF: not just an apoptosis-inducing factor. Ann. N. Y. Acad. Sci., 1171, 2–11 [DOI] [PubMed] [Google Scholar]
- 35. Lee J.H., et al. (2010). EGCG induces apoptosis in human laryngeal epidermoid carcinoma Hep2 cells via mitochondria with the release of apoptosis-inducing factor and endonuclease G. Cancer Lett., 290, 68–75 [DOI] [PubMed] [Google Scholar]
- 36. Tanida I., et al. (2008) LC3 and autophagy. In Deretic V. (ed.) Methods in Molecular Biology: Autophagosome and Phagosome, Humana Press, Totowa, New Jersey: pp.77–88 [Google Scholar]
- 37. Lee D.H., et al. (1996). Selective inhibitors of the proteasome-dependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J. Biol. Chem., 271, 27280–27284 [DOI] [PubMed] [Google Scholar]
- 38. Araki S., et al. (2007). Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res., 67, 6854–6862 [DOI] [PubMed] [Google Scholar]
- 39. Koochekpour S., et al. (2004). Establishment and characterization of a primary androgen-responsive African-American prostate cancer cell line, E006AA. Prostate, 60, 141–152 [DOI] [PubMed] [Google Scholar]
- 40. Dai J.L., et al. (1996). Androgenic up-regulation of androgen receptor cDNA expression in androgen-independent prostate cancer cells. Steroids, 61, 531–539 [DOI] [PubMed] [Google Scholar]
- 41. Adhami V.M., et al. (2007). Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo . Clin. Cancer Res., 13, 1611–1619 [DOI] [PubMed] [Google Scholar]
- 42. Hussein S.A., et al. (2007). Ericifolin: an eugenol 5-O-galloylglucoside and other phenolics from Melaleuca ericifolia . Phytochemistry, 68, 1464–1470 [DOI] [PubMed] [Google Scholar]
- 43. Burnstein K.L. (2005). Regulation of androgen receptor levels: implications for prostate cancer progression and therapy. J. Cell. Biochem., 95, 657–669 [DOI] [PubMed] [Google Scholar]
- 44. Zhau H.Y., et al. (1996). Androgen-repressed phenotype in human prostate cancer. Proc. Natl. Acad. Sci. U. S. A., 93, 15152–15157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singh M., et al. (2011). Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappaB and Akt activation in human cervical cancer cells. Oncol. Res., 19, 245–257 [DOI] [PubMed] [Google Scholar]
- 46. Rodríguez-Ramiro I., et al. (2011). Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr., 50, 313–322 [DOI] [PubMed] [Google Scholar]
- 47. Tanida I., et al. (2005). Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy, 1, 84–91 [DOI] [PubMed] [Google Scholar]
- 48. Chu C.P., et al. (2009). Suppression of androgen receptor signaling and prostate specific antigen expression by (-)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett., 275, 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bosetti C., et al. (2005). Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Biomarkers Prev., 14, 805–808 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.