Abstract
In the vast majority of studies utilizing adeno-associated virus (AAV) in central nervous system applications, including those published with spinal cord injury (SCI) models, AAV has been administered at the level of the cell body of neurons targeted for genetic modification, resulting in transduction of neurons in the vicinity of the injection site. However, as SCI interrupts many axon tracts, it may be more beneficial to transduce a diverse pool of supraspinal neurons. We determined if descending axons severed by SCI are capable of retrogradely transporting AAV to remotely transduce a variety of brain regions. Different AAV serotypes encoding the reporter green fluorescent protein (GFP) were injected into gray and white matter immediately rostral to a spinal transection site. This resulted in the transduction of thousands of neurons within the spinal cord and in multiple regions within the brainstem that project to spinal cord. In addition, we established that different serotypes had disparate regional specificity and that AAV5 transduced the most brain and spinal cord neurons. This is the first demonstration that retrograde transport of AAV by axons severed by SCI is an effective means to transduce a collection of supraspinal neurons. Thus, we identify a novel, minimally invasive means to transduce a variety of neuronal populations within both the spinal cord and the brain following SCI. This paradigm to broadly distribute viral vectors has the potential to be an important component of a combinatorial strategy to promote functional axonal regeneration.
Keywords: adeno-associated virus, propriospinal, retrograde transduction, spinal cord injury, supraspinal
Introduction
The limited capacity of adult mammalian central nervous system axons to regenerate following spinal cord injury (SCI) leads to substantial functional defects. One likely cause of this diminished growth ability is that the expression levels of proteins necessary for robust growth is dramatically lower in mature neurons than in younger, developing neurons.1,2 One tool that can modify gene expression in the mature central nervous system, potentially allowing for the overcoming of this significant hurdle, is recombinant viral vectors.
Adeno-associated virus (AAV) is one type of viral vector that is often used for gene therapy in the central nervous system because of its non-pathogenic nature, replication deficiency and high neuronal transduction efficiency. This helper-dependent, single-stranded DNA parvovirus has the ability to effectively transduce post-mitotic cells producing prolonged, stable gene expression without triggering an inflammatory response and toxicity.3,4 Several serotypes of AAV exist, each with distinct cellular tropisms determined by the surface features of the capsid.5 The ability of AAV to transduce fully differentiated neurons makes it an ideal candidate to manipulate gene expression in adult, supraspinal neurons to promote axon regeneration, synaptic plasticity and neuronal survival following the injury of their axons that project to spinal cord.
In various SCI models, application of AAV in the vicinity of targeted cell bodies results in their successful transduction.6,7,8 Because SCI interrupts many different axon tracts projecting from the brain to the spinal cord, promoting regeneration of several injured, descending tracts would likely restore more interrupted circuitry than regeneration of a specific tract, thereby possibly improving functional recovery. Therefore, it seems reasonable to imagine that it would be advantageous to simultaneously modify a variety of supraspinal neuronal pools affected by the injury. Recently, widespread transduction was achieved by injecting AAV-green fluorescent protein (GFP) into multiple sites throughout the brainstem.9 While this injection paradigm effectively transduces many neurons within the brainstem, it is also very invasive.
Interestingly, another means to transduce neurons using AAV is via retrograde transport. Injecting AAV into a target muscle10,11,12,13,14 or peripheral nerve15,16,17 results in the transduction of motoneurons and dorsal root ganglion neurons that innervate the targeted muscle or that send projections via the injected nerve. Whether AAV is taken up by central axons within the spinal cord after injury and is then retrogradely transported to transduce neurons within the brain has not been determined.
In these experiments, we sought to assess the effectiveness of a novel, less invasive approach to transduce multiple descending populations following SCI. We hypothesized that AAV injected into spinal cord tissue immediately rostral to an injury site would not only transduce local spinal neurons but would also be taken up by severed, descending tract axons and retrogradely transported to transduce diverse brain neurons that project to spinal cord. Successful retrograde transport would allow manipulation of gene expression in several remote neuronal populations upon administration into one location. In addition, because different AAV serotypes have dissimilar tropisms18,19 and potential for retrograde transport,20 we sought to identify which AAV serotype most efficiently retrogradely transduces neurons in brain after SCI.
Results
Immediately following a complete transection of the spinal cord at thoracic level 3 (T3), equivalent titers of AAV1-, AAV2-, AAV5-, AAV8-, or AAV9-CBA-GFP (GFP transgene under a chicken β-actin promoter; n = 3 per serotype) were injected into four different locations rostral to the transection site (Figure 1a) at two different depths per location (Figure 1b). This was done to “flood” with viral vectors tissue encompassing the intermediate gray matter and the lateral and ventral funiculi just rostral to the injury. One month later, the animals were sacrificed.
Figure 1.
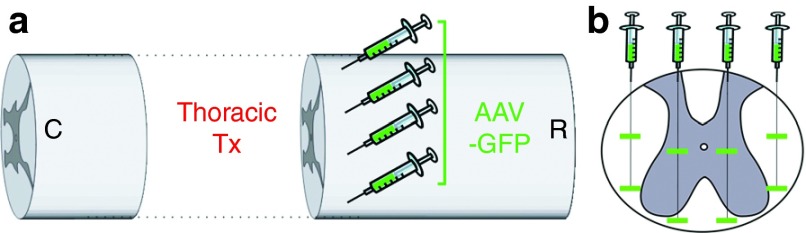
Schematic of intraspinal injection methodology. Equal titers and volumes of each of the tested AAV serotypes were injected into spinal cord tissue ~1 mm rostral to a fresh spinal transection site at T3. Injections of AAV1-, AAV2-, AAV5-, AAV8-, or AAV9-GFP were made at (a) four different locations, i.e., two lateral locations and two medial locations, and at (b) two different depths per location. Thus, there were a total of eight injections. AAV, adeno-associated virus; GFP, green fluorescent protein.
Intraspinal injection of AAV rostral to a SCI site transduces many spinal cord neurons
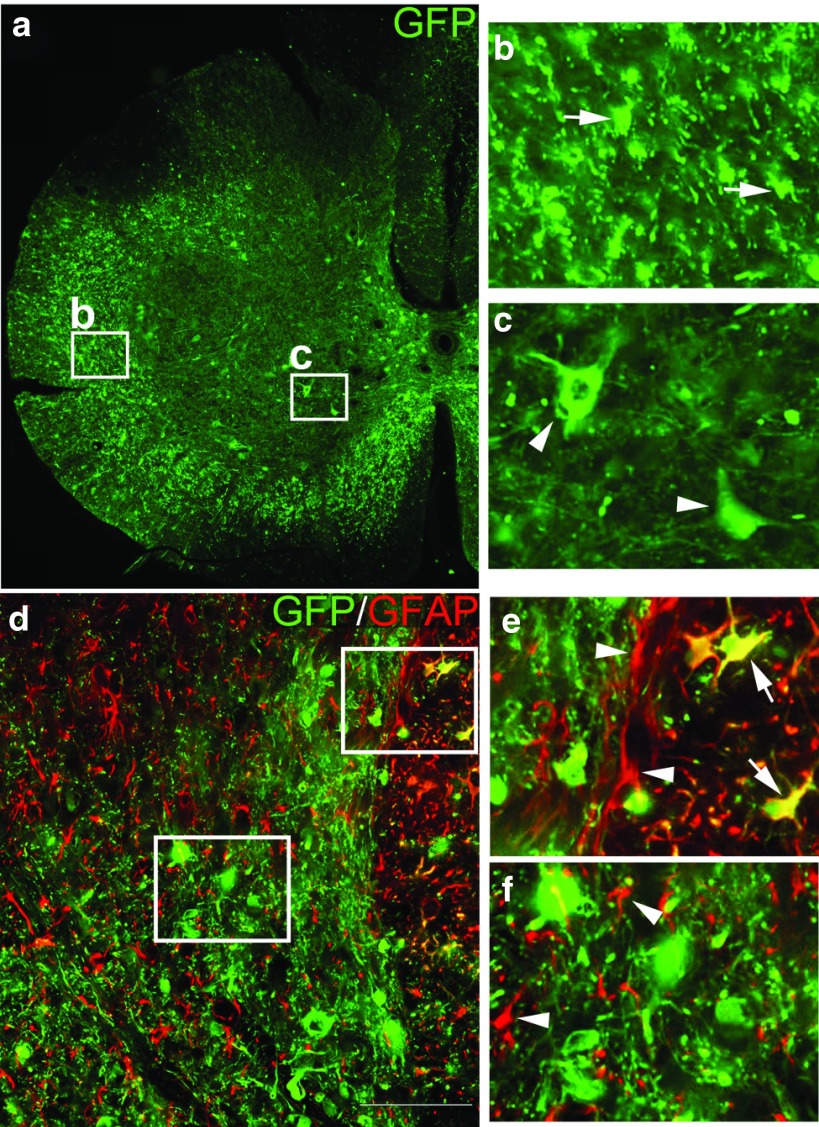
Intraspinal injection of all tested AAV serotypes resulted in transduction of spinal cord neurons rostral to a SCI site. In animals from all groups, numerous GFP+ neurons were observed in gray matter (Figure 2a,c) above the injury. Moreover, many GFP+ axons (visualized as more punctate than linear staining in Figure 2a,b because the axons are seen in cross-section) were observed in the lateral and ventral white matter rostral to the transection site. Most GFP+ cells within the spinal cord had a neuronal phenotype (Figure 2a,c,d) but a few GFP+ cells colocalized with the astrocytic marker glial fibrillary acidic protein (Figure 2e, arrows). Nonetheless, the vast majority of glial fibrillary acidic protein-positive cells did not coexpress GFP (Figure 2e, arrowheads), even in regions containing many GFP+ neurons (Figure 2f, arrowheads). These data indicate that each serotype transduced more neurons than astrocytes, agreeing with literature that AAV with a non-cell–specific promoter preferentially transduces neurons and not glia.21,22
Figure 2.
Intraspinal AAV injection rostral to an SCI site transduces spinal cord neurons. (a,d) Transverse sections of spinal cord above the injury site were processed for immunohistochemistry for the reporter protein GFP (green) and the astrocytic marker GFAP (red). One month following intraspinal injection of AAV, the reporter gene GFP is expressed in neurons within gray matter (a, arrowheads in c) and in many axonal profiles (cut in cross-section) throughout white matter (a, arrows in b) located rostral to the transection site. AAV preferentially transduces neurons as only a few GFAP+ astrocytes also express GFP (d, arrows in e). The vast majority of GFAP+ astrocytes did not express GFP (d, arrowheads in e), even in areas where there was abundant neuronal expression of GFP (d, arrowheads in f). AAV, adeno-associated virus; GFP, green fluorescent protein; GFAP, glial fibrillary acidic protein; SCI, spinal cord injury.
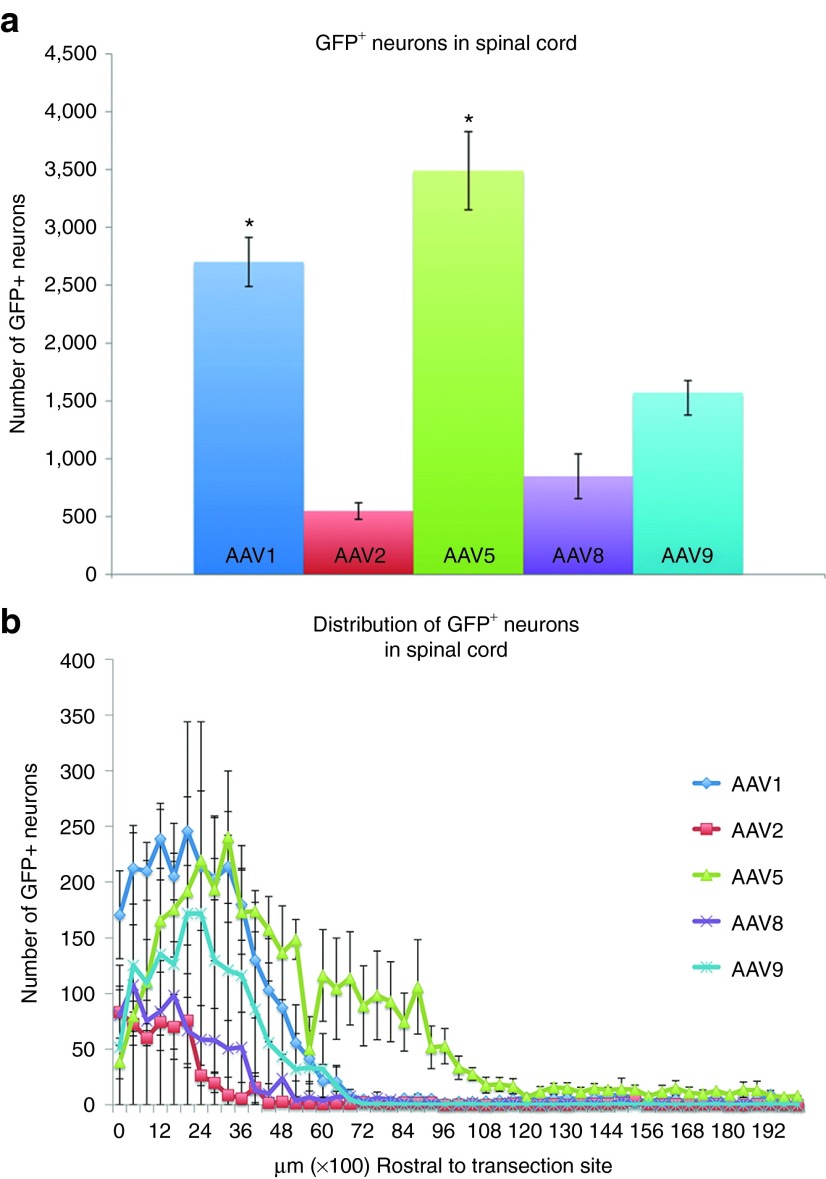
To determine if different AAV serotypes transduce spinal cord neurons to disparate degrees, all GFP+ neurons in a subset of spinal cord sections rostral to the spinal transection site (every 400 μm; Figure 3a) were counted. There were 2,700 ± 212.3 GFP+ spinal cord neurons in animals injected with AAV1 and 3,439 ± 338.3 neurons in animals injected with AAV5. Both AAV1 and AAV5 were significantly better at transducing spinal cord neurons than the other tested serotypes (P < 0.05). There were 548 ± 71.3 GFP+ spinal neurons in animals injected with AAV2, 848 ± 193.3 in animals injected with AAV8 and 1,572 ± 104.5 in animals injected with AAV9. All serotypes were able to transduce spinal cord neurons as far as 5–6 mm rostral to the injury site (Figure 3b). Moreover, neurons as far as 10 mm rostral to the transection site were transduced following AAV5 injection.
Figure 3.
Quantification of transduced, GFP+ neurons within spinal cord following intraspinal injection. GFP+ neurons in serially collected spinal cord tissue sections (one section every 400 µm rostral to the injury site) from animals injected with each of the tested AAV serotypes were manually counted. (a) Total numbers of GFP+ neurons between groups were compared for statistical significance using a one-way analysis of variance test followed by post-hoc Tukey's tests. The number of GFP+ neurons following AAV1 or AAV5 was significantly higher than with AAV2, AAV8, or AAV9 (*P < 0.05; mean ± SEM, n = 3). There was no significant difference between AAV1 and AAV5. (b) With all tested serotypes, most of the GFP+ neurons were found fairly close to the injection site (within ~5 mm). In AAV5-injected animals, GFP+ neurons were found as far as 10 mm away. AAV, adeno-associated virus; GFP, green fluorescent protein.
AAV is retrogradely transported by axons injured by SCI and robustly transduces various supraspinal brain neurons
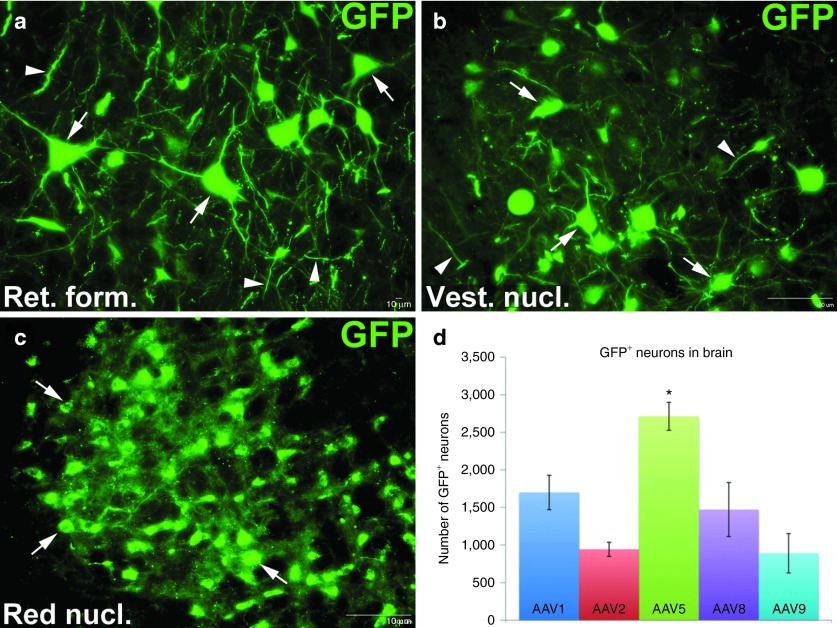
To determine if AAV injected immediately rostral to a SCI site is taken up by injured axons and retrogradely transported to transduce neurons within the brain, GFP+ neurons in brain sections every 400 μm apart were counted. Virtually all GFP+ neurons were located within the brainstem. We found many GFP+ neurons in the reticular formation (Figure 4a, arrows), vestibular nucleus (Figure 4b, arrows) and red nucleus (Figure 4c, arrows). Moreover, we found many GFP+ processes in these same areas (Figure 4, arrowheads), some appearing to emerge from GFP+ somas. GFP+ neurons were observed in all brains, indicating that each of the tested AAV serotypes is capable of being retrogradely transported by injured axons to transduce brain neurons (Figure 4d). However, there was a significantly greater number (P < 0.05) of GFP+ neurons in the brains of animals intraspinally injected with AAV5 (2,713 ± 186.0) than AAV1 (1,700 ± 229.3), AAV2 (943.3 ± 93.8), AAV8 (1,471.7 ± 359.3) or AAV9 (890.7 ± 262.1). We did not observe any transduced neurons within cortex (data not shown).
Figure 4.
Retrograde transduction of brainstem neurons following intraspinal AAV injection after spinal cord injury. One month after AAV delivery, serially collected transverse sections of brain was processed for immunohistochemistry for GFP (green). Many GFP+ neurons were found in different brainstem regions, including the (a) reticular formation (Ret. form.), (b) the vestibular nucleus (Vest. nucl.), and (c) the red nucleus (Red nucl.). (d) All GFP+ neurons in a series of tissue sections (one section every 400 µm) encompassing all of the brain were counted. Differences in total numbers of GFP+ between AAV serotype groups was analyzed for statistical significance using a one-way analysis of variance and post-hoc Tukey's tests. The most GFP+ neurons were found in the brains of animals injected with AAV5 (P < 0.05; mean ± SEM, n = 3). No additional significant differences were found amongst the other serotypes. AAV, adeno-associated virus; GFP, green fluorescent protein.
To determine if the tested serotypes had different tropisms for various supraspinal neuronal pools, we assessed where each GFP+ neuron in the brain was located. Not unexpectedly, we found that different serotypes had regional specificity. Significantly more neurons within the reticular formation (Figure 5a) that project via the reticulospinal tract were transduced with AAV5 (1,873 ± 168.7) than AAV1 (743 ± 130.4), AAV2 (441.7 ± 55.9), AAV8 (844.7 ± 207.8) or AAV9 (378.7 ± 174.5; P < 0.05). All AAV serotypes resulted in statistically equal numbers of GFP+ neurons in the vestibular nuclei (Figure 5b) that form the vestibulospinal tract. AAV1 transduced 436.7 ± 132 neurons, AAV2 transduced 228.3 ± 51.1, AAV5 transduced 523.7 ± 70.1, AAV8 transduced 276.7 ± 129.1, and AAV9 transduced 239 ± 127.5. Injecting AAV1 into the spinal cord transduced significantly more neurons in the raphe nuclei (18.4 ± 2.2) that form the raphespinal tract than AAV5 (9 ± 5.5; P < 0.05; Figure 5c). We did not observe any GFP+ neurons in the raphe after injecting AAV2, AAV8 or AAV9 rostral to the spinal transection. All AAV serotypes were transported by injured rubrospinal tract axons to result in GFP expression in neurons within the red nucleus (Figure 5d). In the subset of sections analyzed, there were 211 ± 14 GFP+ neurons in the red nucleus of animals injected with AAV1, 273.3 ± 16.5 injected with AAV2, 307.3 ± 53.5 injected with AAV5, 350.5 ± 61.2 injected with AAV8 and 273 ± 48.1 injected with AAV9. There were no significant differences between groups in this region.
Figure 5.
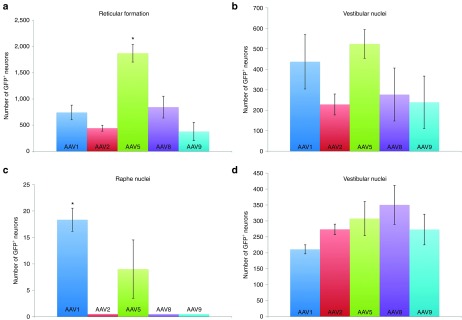
Brain regional differences in retrograde transduction efficiencies. Using the same brain tissue sections used for the analysis depicted in Figure 4, the location of each GFP+neuron was assessed and totaled. (a) AAV5 resulted in the most GFP+ neurons in the reticular formation (*P < 0.05; mean ± SEM, n = 3). All tested serotypes transduced statistically equivalent numbers of neurons in (b) the vestibular nuclei and (d) the red nucleus. (c) Animals intraspinally injected with AAV1 had the most GFP+ neurons within the raphe nuclei (P < 0.05). AAV, adeno-associated virus; GFP, green fluorescent protein.
These data demonstrate that multiple supraspinal neuronal pools that project to spinal cord are effectively retrogradely transduced when AAV is intraspinally injected after SCI. Moreover, these data indicate all AAV serotypes tested are capable of being retrogradely transported by injured axons.
Discussion
While various treatments have successfully promoted some axonal regeneration after SCI, gene therapy may help surmount the decreased expression of regeneration-associated genes in adult neurons to significantly enhance their intrinsic growth potential.23,24,25 We chose to focus on AAV because it is non-pathogenic, able to transduce post-mitotic cells, preferentially transduces neurons rather than glia and does not trigger an immune or gliotic response.3,4,26
Because multiple descending populations are affected by SCI, it is plausible that increasing the expression of growth-promoting genes in a wide population of supraspinal neurons that project to spinal cord could further improve functionally relevant axonal regeneration. One means of accomplishing an extensive transduction of brain neurons in a SCI model is to inject AAV into multiple locations throughout the brainstem,9 which is home to many neuronal pools that project to spinal cord. While this method is effective, it is very invasive and involves 19 separate injections.
While AAV effectively transduces neurons when applied to the cell body, it also can be retrogradely transported following injection into peripheral nerve10,11,12,13 or muscle (after uptake at synaptic terminals) 15,16,17 to transduce neurons remote from the injection site. Retrograde transport of AAV appears to be dependent upon interaction with the motor protein dynein that, in turn, binds to and travels along microtubules.27 Indeed, colchicine, which inhibits microtubule polymerization and induces microtubule disassembly,28 prevents the transduction of spinal motoneurons following AAV injection into peripheral nerve.17
In this study, we sought to determine if we could take advantage of the ability of AAV to be retrogradely transported to transduce a large and diverse population of neurons whose axons are injured by SCI while concurrently minimizing additional trauma due to delivery of viral vectors. To this end, AAV was intraspinally injected immediately rostral to a fresh SCI site. As one would expect, thousands of GFP+ neurons were found within the spinal cord itself. While this is likely primarily due to the transduction of spinal neurons by virus that diffused within the parenchyma away from the injection sites, the fact that some GFP+ neurons were located 10 mm away (Figure 3b) suggests that some propriospinal neurons were also retrogradely transduced.
In addition, we found thousands of GFP+ neurons in brain regions far distant from the injury and injection sites in upper thoracic spinal cord. These data indicate that acutely injured, descending axons are capable of retrogradely transporting intraspinally-delivered AAV to effectively transduce neurons within the brainstem that project to spinal cord. This process is likely due (at least partly) to active endocytosis because axonal membranes typically seal up within 30 minutes after injury29 and the injections took about an hour to complete. If uptake of virus into injured axons was merely passive, it seems likely that we would have noticed more GFP+ neurons in tissue on the side that was injected into first, before membrane sealing, which we did not observe (data not shown). It is important to note that only a fraction of all brain sections were analyzed (one section every 400 μm). Thus, it is possible and likely that our GFP+ neuron counts underestimate the absolute number of neurons that were transduced in this fashion. In addition, using higher titers of virus may also increase the number of transduced neurons.
We are confident that the expression of the reporter gene GFP in the brain resulted from transport of AAV and not from uptake by cell bodies of excess viral vector circulating in cerebral spinal fluid because we found no evidence of GFP+ neurons surrounding the ventricles (data not shown). We only observed GFP+ neurons within the spinal cord or in brain regions that project down to spinal cord. In the latter case, GFP+ neurons were found in the reticular formation, the vestibular nuclei, the red nucleus and, to a lesser extent, the raphe nuclei. This indicates that AAV was taken up and transported by axons forming the reticulospinal, vestibulospinal, rubrospinal and raphespinal tracts present in the lateral and ventral funiculi.
While other groups have found that transport of several different AAV serotypes within the brain is possible,30,31,32 to our knowledge, these data are the first to demonstrate that retrograde transport of AAV after SCI can remotely transduce neurons. While the direction of transport (retrograde versus anterograde) is not always clear,31 we are confident that in this study, transduction in the brainstem is due to retrograde transport. Neurons residing within the spinal cord do not project to the red nucleus or the raphe,33 so anterograde transport of AAV to these regions is not likely. Furthermore, while some spinal neurons project via the spinoreticular tract to the reticular formation and via the spinovestibular tract to the vestibular nuclei, the vast majority of these neurons reside in cervical (for spinoreticular and spinovestibular tracts) and lumbar spinal cord (for spinoreticular tract only),33 far from the injury and injection site within thoracic spinal cord. Furthermore, we did not observe GFP+, transduced neurons within the dorsal column nuclei (data not shown), which is where ascending dorsal column axons terminate, providing additional support that transduction within the brain is due to retrograde and not anterograde transport.
While we observed many GFP+ neurons within brainstem, we did not find any evidence that neurons within primary motor cortex that project to spinal cord via the corticospinal tract were transduced. This is a bit surprising given that these neurons are well-transduced following intracortical injections of AAV122 and AAV8,34 two of the serotypes tested in this study. While it is not entirely clear if the lack of transduction in this study is due to a poor ability of these axons to take up and retrogradely transport the vectors or virus not diffusing to the dorsal funiculus, Figure 2a suggests that there was sufficient vector present in the dorsal funiculus, where the primary component of the corticospinal tract is present in the rat. Furthermore, when a mixture of horseradish peroxidase and adenovirus encoding for lacZ was injected into lumbar spinal cord of naive mice, there were many more horseradish peroxidase-positive than β-galactosidase+ neurons in motor cortex, indicating that corticospinal tract axons either endocytosed or transported horseradish peroxidase and the adenovirus at different rates.35 These data along with the lack of transduction of primary motor cortical neurons in our study suggest that corticospinal tract axons may inherently be difficult to transduce via retrograde transport of viral vectors, be it AAV or adenovirus. It also indicates that transduction efficiencies following delivering virus to the cell body can be quite different from after delivering virus to the axon.
Because our protocol entails injecting AAV into the spinal cord, we are able to more specifically target descending tracts than if we were to inject virus throughout brainstem, which will transduce neurons that project to spinal cord as well as other areas within the brain. Interestingly, retrograde transport of AAV has been reported in nonhuman primate,31,36 suggesting that retrograde transport of AAV is not specific to small animal models and is likely possible in the human. Another potential advantage of this paradigm for future clinical application is that as surgical intervention in the lesion vicinity is already likely in people who sustained SCI,37 it is foreseeable that AAV could be injected into tissue immediately rostral to the injury site during this surgical procedure, negating the need for an additional surgery.
Although it is beyond the scope of the present study, which was focused on ascertaining whether AAV was transported by injured axons, at all, it would be interesting to determine if injury itself affects transport or transduction efficiency. Furthermore, while we found that our injection paradigm results in robust neuronal transduction of brainstem neurons in an acute SCI model, it would be very important to assess if AAV is also taken up and retrogradely transported by chronically injured axons, as studies have demonstrated retrograde transport in long-injured axons is impaired.38,39,40 This information would allow us to better understand if this AAV injection paradigm could possibly be used to treat the millions of people within the United States alone already living with some form of SCI.41
As alluded to above, one potential application of this AAV injection paradigm is to enhance axonal regeneration after SCI. Our labuses a well-established grafting model in which segments of peripheral nerve are transplanted to fill the lesion cavity with a growth-supportive environment.42 Some axonal tracts, including the populations that we found to be well-transduced in this study (e.g., reticulospinal, vestibulospinal, rubrospinal, and propriospinal pathways) regenerate fairly well into these peripheral nerve grafts.43 A significant challenge has proven to be getting axons to emerge from the graft to reinnervate spinal cord tissue. This step is necessary if these axons are to reform functionally relevant synapses upon target neurons.43,44,45,46 Injecting AAV rostral to a SCI site could be one means to overexpress regeneration-associated genes1,47 or knockdown genes that negatively regulate growth48,49,50 specifically in neurons that regenerate into the graft (e.g., those originating from the reticular formation, vestibular nuclei, and red nucleus) to enhance their axons' ability to traverse the distal graft–host interface.
We also found that there were transduction efficiency differences between the tested serotypes, with AAV1 and AAV5 transducing the most neurons within spinal cord and AAV5 transducing the most neurons within brain. It is not yet known if this is due to distinct, capsid-dependent5,51 neurotropisms of the various AAV serotypes18 or differential ability of AAV serotypes to be retrogradely transported.20 Thus, for SCI, AAV5 seems like the best viral vector to use since it results inefficient transduction of a diverse population of both descending neurons and propriospinal neurons, whose regeneration and/or plasticity can also potentially mediate functional recovery.52,53 However, with continued improvements in our understanding of AAV capsids, it is possible that another serotype will be discovered or developed that will surpass AAV5 in usefulness for our very specific application (i.e., retrograde transduction after SCI). In addition, the further refinement of inducible promoter systems to better control transgene expression in specific neuronal populations will greatly affect the potential of using AAV to promote axonal regeneration in the clinic.
Overall, we have identified a novel, minimally invasive method to effectively transduce a variety of neuronal populations within both the spinal cord and the brain following SCI. While the field is still in the nascent stages of using gene therapy as a means to help repair the injured spinal cord, our findings demonstrate the ability to simultaneously and specifically affect multiple neurons after injecting AAV5 into one location, i.e., intraspinally into tissue just rostral to a SCI site. This paradigm to broadly distribute viral vectors has the potential to be an important component of an eventual “bench-to-bedside” combinatorial strategy to promote functional axonal regeneration.
Materials and methods
AAV vectors. All single-stranded AAV vectors were obtained from the University of North Carolina's Gene Therapy Center (Chapel Hill, NC) and encoded for the reporter gene GFP under the control of a chicken β-actin promoter. AAV serotypes used were AAV1, AAV2, AAV5, AAV8, and AAV9. All viral vectors used were hybrids in that the replication gene was from AAV2 and the capsid gene was specific to the particular serotype.
Surgical procedures. All procedures complied with Drexel University's Institutional Animal Care and Use Committee and National Institutes of Health guidelines for experimentation with laboratory animals. Adult female Sprague–Dawley rats (225–250 g; Charles River, Wilmington, MA) were anesthetized with isoflurane inhalation. The dorsal surface of thoracic level 3 (T3) spinal cord was exposed by laminectomy. The dura was incised and one vertebral body length (2–3 mm) of spinal cord was removed via aspiration. Hemostasis was achieved with Gelfoam pledgets placed into the cavity. The dura was closed using a 10-0 suture. To target lateral and ventral funiculi and gray matter 1 μl of AAV1-, AAV2-, AAV 5-, AAV8-, or AAV9-GFP (n = 3 per group, 109 transducing unit) was slowly injected into four different site locations at two different depths ~1 mm rostral to the transection using a glass micropipette attached to a Hamilton syringe44 (Figure 1a). Specifically, AAV was bilaterally injected just medial to the lateral edge of the spinal cord at a depth of 1 and 2 mm and 1 mm lateral to midline, and 2.5 and 1.5 mm deep (Figure 1b). Thus, there were a total of eight sites. The laminectomy site was covered with a silastic membrane (BioBrane; UDL Laboratories, Rockford, IL). The overlying musculature was closed using 4-0 sutures, and the skin was closed using wound clips. One month later, animals were perfused using 4% paraformaldehyde. All animals were administered lactated Ringer's, buprenorphine (0.05 mg/kg) for postoperative pain management, and cephazolin (160 mg/kg) for 7 days to prevent infection. In addition, bladders were expressed manually two- to three-times a day until the voiding reflex returned (~14 days).
Histology. The brain and spinal cord rostral to the transection were dissected and post-fixed overnight in 4% paraformaldehyde at 4 °C. The tissue then was cryoprotected in 30% sucrose before sectioning on a cryostat. Transverse sections (50 µm) were serially cut. Every eighth section (every 400 µm) was mounted onto glass slides and coverslipped using FluorSave (EMD Millipore, Billerica, MA).
In addition, some sections were blocked in 5% normal goat serum, 1% bovine serum albumin, 0.1% Triton X-100 in phosphate-buffered saline for 1 hour at room temperature before incubation in the appropriate primary antibody overnight at room temperate. The primary antibodies used were against GFP (1:1,000; Millipore, Billerica, MA) and glial fibrillary acidic protein (1:1,000; Millipore). Sections were rinsed in phosphate-buffered saline and incubated in the appropriate secondary antibody for 2 hours at room temperature. Secondary antibodies used were conjugated to Alexa 488 or 594 (1:500; Life Technologies, Grand Island, NY). Sections were rinsed in phosphate-buffered saline, mounted onto glass sides, and coverslipped. Slides were imaged using an Olympus BX51 and a Leica DM5500B microscope (Leica Microsystems, Wetzlar, Germany).
Quantification of GFP+ transduced neurons. To quantify transduced spinal cord neurons, all GFP+ neurons in sections 400 µm apart were manually counted. Differences in the number of GFP+ neurons amongst AAV serotype groups was assessed for significance using a one-way analysis of variance and post-hoc Tukey's tests.
To quantify transduced neurons within the brain, all GFP+ neurons in sections 200 µm apart were counted and their location noted. Statistical significance was determined using one-way analysis of variance and post-hoc Tukey's tests (GraphPad, La Jolla, CA). All quantification is presented as averages ± SEM.
Acknowledgments
Funding for this study was provided by the Craig H. Neilsen Foundation (V.J.T.) and the Spinal Cord Research Center at Drexel University College of Medicine. The authors declared no conflict of interest.
References
- Blackmore MG. Molecular control of axon growth: insights from comparative gene profiling and high-throughput screening. Int Rev Neurobiol. 2012;105:39–70. doi: 10.1016/B978-0-12-398309-1.00004-4. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for CNS gene therapy. Ther Deliv. 2010;1:517–534. doi: 10.4155/tde.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiol Dis. 2012;48:179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Blits B, Dijkhuizen PA, te Beek ET, Bakker A, van Heerikhuize JJ, et al. Adeno-associated viral vector-mediated gene transfer of brain-derived neurotrophic factor reverses atrophy of rubrospinal neurons following both acute and chronic spinal cord injury. Neurobiol Dis. 2004;15:394–406. doi: 10.1016/j.nbd.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu J, Apostolova I, Skup M, Irintchev A, Kügler S, et al. Adeno-associated virus-mediated L1 expression promotes functional recovery after spinal cord injury. Brain. 2007;130 Pt 4:954–969. doi: 10.1093/brain/awm049. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Lam C, Plunet W, Oschipok LW, Hauswirth W, et al. Brain-derived neurotrophic factor gene transfer with adeno-associated viral and lentiviral vectors prevents rubrospinal neuronal atrophy and stimulates regeneration-associated gene expression after acute cervical spinal cord injury. Spine. 2007;32:1164–1173. doi: 10.1097/BRS.0b013e318053ec35. [DOI] [PubMed] [Google Scholar]
- Williams RR, Pearse DD, Tresco PA, Bunge MB. The assessment of adeno-associated vectors as potential intrinsic treatments for brainstem axon regeneration. J Gene Med. 2012;14:20–34. doi: 10.1002/jgm.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun J, Puzis R, Pearse DD, Gage FH, Bunge MB. Muscle injection of AAV-NT3 promotes anatomical reorganization of CST axons and improves behavioral outcome following SCI. J Neurotrauma. 2009;26:941–953. doi: 10.1089/neu.2008.0807. [DOI] [PubMed] [Google Scholar]
- Benkhelifa-Ziyyat S, Besse A, Roda M, Duque S, Astord S, Carcenac R, et al. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol Ther. 2013;21:282–290. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Qiao C, Wang CH, Li J, Li J, Yuan Z, et al. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum Gene Ther. 2010;21:87–97. doi: 10.1089/hum.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YY, Wang LJ, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, et al. Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport. Neurosci Res. 2003;45:33–40. doi: 10.1016/s0168-0102(02)00195-5. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Kitay B, Boyce VS, Kaspar BK, Pearse DD, Gage FH, et al. Intramuscular AAV delivery of NT-3 alters synaptic transmission to motoneurons in adult rats. Eur J Neurosci. 2010;32:997–1005. doi: 10.1111/j.1460-9568.2010.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16:296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Noordmans AJ, Song DK, Imperiale MJ, Rubin A, Leone P, et al. Adeno-associated viral vector gene expression in the adult rat spinal cord following remote vector delivery. Neurobiol Dis. 2003;14:535–541. doi: 10.1016/j.nbd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Willmarth NE, Song DK, Feldman EL, Imperiale MJ. Intraneural colchicine inhibition of adenoviral and adeno-associated viral vector remote spinal cord gene delivery. Neurosurgery. 2003;52:381–7; discussion 387. doi: 10.1227/01.neu.0000044459.24519.3e. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20:348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCarty DM, Bruce AT, Suzuki K. Oligodendrocyte-specific gene expression in mouse brain: use of a myelin-forming cell type-specific promoter in an adeno-associated virus. J Neurosci Res. 1999;55:504–513. doi: 10.1002/(SICI)1097-4547(19990215)55:4<504::AID-JNR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hutson TH, Verhaagen J, Yáñez-Muñoz RJ, Moon LD. Corticospinal tract transduction: a comparison of seven adeno-associated viral vector serotypes and a non-integrating lentiviral vector. Gene Ther. 2012;19:49–60. doi: 10.1038/gt.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore MG, Moore DL, Smith RP, Goldberg JL, Bixby JL, Lemmon VP. High content screening of cortical neurons identifies novel regulators of axon growth. Mol Cell Neurosci. 2010;44:43–54. doi: 10.1016/j.mcn.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol. 2010;20:510–518. doi: 10.1016/j.conb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ. Adeno-Associated Virus (AAV) Vectors in the CNS. Curr Gene Ther. 2011;11:181–188. doi: 10.2174/156652311795684759. [DOI] [PubMed] [Google Scholar]
- Kelkar S, De BP, Gao G, Wilson JM, Crystal RG, Leopold PL. A common mechanism for cytoplasmic dynein-dependent microtubule binding shared among adeno-associated virus and adenovirus serotypes. J Virol. 2006;80:7781–7785. doi: 10.1128/JVI.00481-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie SB. Interactions of colchicine with tubulin. Pharmacol Ther. 1991;51:377–401. doi: 10.1016/0163-7258(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Shi R, Asano T, Vining NC, Blight AR. Control of membrane sealing in injured mammalian spinal cord axons. J Neurophysiol. 2000;84:1763–1769. doi: 10.1152/jn.2000.84.4.1763. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci USA. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamizu Y, Okada T, Kawasaki K, Ishibashi H, Yuasa S, Takeda S, et al. Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience. 2011;193:249–258. doi: 10.1016/j.neuroscience.2011.06.080. [DOI] [PubMed] [Google Scholar]
- Kayalioglu G. The Spinal Cord: Projections From the Spinal Cord to the Brain. Academic Press: London, UK; 2009. pp. pp. 148–167. [Google Scholar]
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, et al. Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci USA. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Yamamoto T, Okado H, Nibu K, Terashima T. Retrograde labeling of mouse spinal descending tracts by a recombinant adenovirus. Arch Histol Cytol. 2003;66:209–220. doi: 10.1679/aohc.66.209. [DOI] [PubMed] [Google Scholar]
- Towne C, Schneider BL, Kieran D, Redmond DE, Jr, Aebischer P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17:141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371–1399. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conta Steencken AC, Smirnov I, Stelzner DJ. Cell survival or cell death: differential vulnerability of long descending and thoracic propriospinal neurons to low thoracic axotomy in the adult rat. Neuroscience. 2011;194:359–371. doi: 10.1016/j.neuroscience.2011.05.052. [DOI] [PubMed] [Google Scholar]
- Peyronnard JM, Charron L, Lavoie J, Messier JP. Differences in horseradish peroxidase labeling of sensory, motor and sympathetic neurons following chronic axotomy of the rat sural nerve. Brain Res. 1986;364:137–150. doi: 10.1016/0006-8993(86)90994-7. [DOI] [PubMed] [Google Scholar]
- Petrosyan HA, Hunanyan AS, Alessi V, Schnell L, Levine J, Arvanian VL. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J Neurosci. 2013;33:4032–4043. doi: 10.1523/JNEUROSCI.4702-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDRF Prevalence of Paralysis, < http://www.christopherreeve.org/site/ c.mtKZKgMWKwG/b.5184255/k.6D74/Prevalence_of_Paralysis.htm > ( 2010
- Houle JD, Amin A, Cote MP, Lemay M, Miller K, Sandrow H, et al. Combining peripheral nerve grafting and matrix modulation to repair the injured rat spinal cord. J Vis Exp. 2009. p. pii: 1324. [DOI] [PMC free article] [PubMed]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Houlé JD. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol. 2008;211:315–319. doi: 10.1016/j.expneurol.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, et al. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol. 2013;239:91–100. doi: 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, et al. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulsara KR, Iskandar BJ, Villavicencio AT, Skene JH. A new millenium for spinal cord regeneration: growth-associated genes. Spine. 2002;27:1946–1949. doi: 10.1097/00007632-200209010-00030. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, Ferraro GB, Fournier AE. Rho signaling and axon regeneration. Int Rev Neurobiol. 2012;105:117–140. doi: 10.1016/B978-0-12-398309-1.00007-X. [DOI] [PubMed] [Google Scholar]
- Vandenberghe LH, Breous E, Nam HJ, Gao G, Xiao R, Sandhu A, et al. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther. 2009;16:1416–1428. doi: 10.1038/gt.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]