Abstract
Objective
Intraspinal microstimulation (ISMS) is a promising method for reanimating paralyzed limbs following neurological injury. ISMS within the cervical and lumbar spinal cord is capable of evoking a variety of highly-functional movements prior to injury, but the ability of ISMS to evoke forelimb movements after cervical spinal cord injury is unknown. Here we examine the forelimb movements and muscles activated by cervical ISMS both before and after contusion injury.
Approach
We documented the forelimb muscles activated and movements evoked via systematic stimulation of the rodent cervical spinal cord both before injury and three, six and nine weeks following a moderate C4/C5 lateralized contusion injury. Animals were anesthetized with isoflurane to permit construction of somatotopic maps of evoked movements, and quantify evoked muscle synergies between cervical segments C3 and T1.
Main results
When ISMS was delivered to the cervical spinal cord, a variety of responses were observed at 68% of locations tested, with a spatial distribution that generally corresponded to the location of motor neuron pools. Stimulus currents required to achieve movement and the number of sites where movements could be evoked were unchanged by spinal cord injury. A transient shift toward extension-dominated movements and restricted muscle synergies were observed at three and six weeks following injury, respectively. By nine weeks after injury, however, ISMS-evoked patterns were similar to spinally-intact animals.
Significance
The results demonstrate the potential for cervical ISMS to reanimate hand and arm function following spinal cord injury. Robust forelimb movements can be evoked both before and during the chronic stages of recovery from a clinically-relevant and sustained cervical contusion injury.
Keywords: neuroprosthesis, spinal stimulation, spinal cord injury, reanimation
Introduction
Intraspinal microstimulation (ISMS) is promising method for reanimating the limbs following injuries to the brain or spinal cord. In contrast with direct nerve or muscle stimulation, ISMS activates motor neurons trans-synaptically (Gaunt et al., 2006), producing smooth grading for force and fatigue resistant contractions (Mushahwar and Horch, 1998). ISMS delivered to the lumbar spinal cord is capable of evoking a range of specific and synergistic hind limb movements in the frog (Giszter et al., 2000; Giszter et al., 1993), rat (Bamford et al., 2005, 2010), and cat (Lemay and Grill, 2004; Mushahwar et al., 2002).
Stimulation delivered within the cervical spinal cord elicits a variety of hand and arm movements in spinally-intact primates (Moritz et al., 2007). Cervical ISMS readily produced movements of the thumb and fingers, often in highly-functional grasping synergies. In addition, long trains of ISMS in the intact primate cervical cord have been shown to produce functional movements using 1–2 stimulation sites (Zimmermann et al., 2011). In the primate, however, the somatotopic organization of evoked movements relative to cervical motor neuron anatomy (Jenny and Inukai, 1983) was much less apparent than in studies of the feline lumbar spinal cord (Guevremont and Mushahwar, 2008; Vanderhorst and Holstege, 1997).
Little is known about the effect of spinal cord injury on the ability to evoke movements via cervical ISMS – a critical prerequisite to clinical application. Several studies have examined the effect of acute or sub-acute complete spinal transections on the output effects of lumbar spinal stimulation (Mushahwar et al., 2004; Tresch and Bizzi, 1999). Tresch and colleagues (1999) observed that lumbar ISMS evoked predominantly flexor-withdrawal movements in the rat for up to three weeks after spinal cord injury, but did not examine later post-injury time points.
Here we examined the effect of ISMS within the cervical cord of the rat prior to injury and in the acute to sub-chronic phases following mid-cervical contusion injury, the most common clinically observed trauma to the spinal cord (National Spinal Cord Injury Statistics, 2012). Cervical ISMS evoked a variety of movements and muscle responses in intact and injured animals, following a transient shift to extensor synergies at three weeks after injury. Additionally, we observed a somatotopic organization of evoked movements similar to documented cervical motor neuron anatomy (McKenna et al., 2000; Tosolini and Morris, 2012). These results provide evidence for cervical ISMS as a promising means for reanimating the upper extremities following injury to the nervous system.
Methods
Overview
Intraspinal microstimulation (ISMS) was delivered at regularly spaced locations within the cervical spinal cord while evoked muscle activity and forelimb movements were recorded from 14 adult female Long-Evans rats (298 ± 17g; mean ± SD). These somatotopic maps of stimulation-evoked responses were obtained from five uninjured animals and three animals each at three, six, and nine weeks following a moderate, lateralized contusion injury between vertebral segments C4–C5. ISMS was delivered and responses were recorded during a terminal procedure for all animals. All procedures were approved by the IACUC at the University of Washington.
Spinal contusion injury
Nine animals received a moderate lateralized contusion injury to determine the effect of spinal cord injury on movements evoked by ISMS caudal to the lesion. Injuries were performed using a modified Ohio State injury device with 0.8mm displacement, 14ms dwell time at maximum displacement (McTigue et al., 1998; Stokes et al., 1992). All injured animals were assessed three weeks after injury and exhibited similar deficits including a pronounced flexion and lack of weight-bearing on the forelimb ipsilateral to injury, and excess tone/spasticity of the injured forepaw. Animals were then randomly assigned to undergo ISMS mapping at one of three post-injury time points (three, six or nine weeks after injury). Each animal was re-assessed immediately prior to ISMS mapping, and all animals continued to exhibit similar deficits in forelimb function.
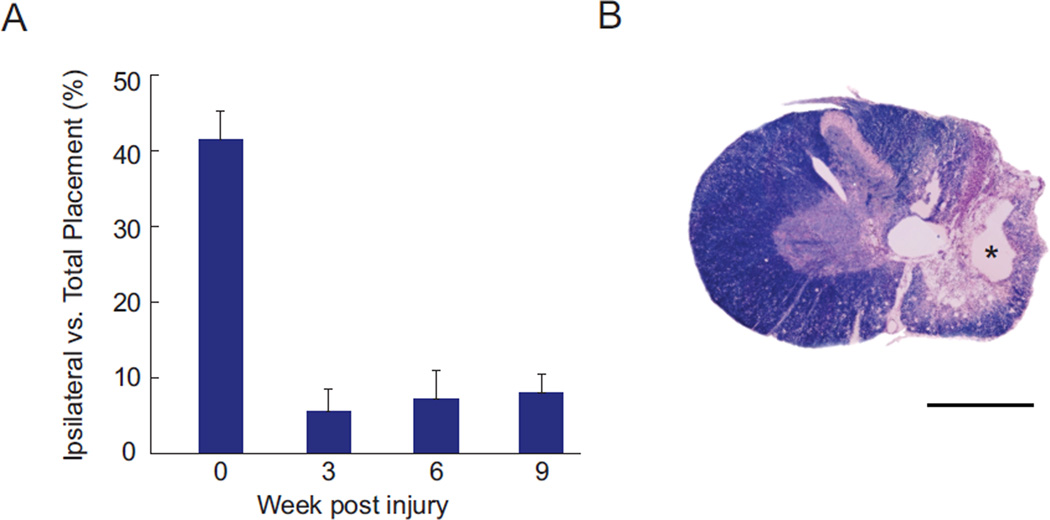
In order to further rule out the possibility that changes in spinal stimulation evoked movements were due to spontaneous recovery from injury, an additional group of six animals underwent identical spinal contusion injuries, and were assessed at each time point on the standard forelimb asymmetry test (Gensel et al., 2006; Liu et al., 1999; Schallert et al., 2000). When animals reared to explore an acrylic cylinder, the number of touches using their forepaw ipsilateral to injury was compared to the total number of forepaw touches. Figure 1A illustrates the substantial and sustained deficit caused by this injury. Prior to injury these animals use each forepaw approximately 40% of the time for support on the cylinder wall. At all time points after injury, however, animals use the ipsilateral to injury forepaw less than 10% of the time for support. Figure 1B illustrates the extent of the injury showing histology from a representative animal in this group. Tissue was stained for the presence of myelin (myelin stain: Eriochrome Cyanine R; Sigma) and cell bodies (cresyl violet with acetate; Sigma). Even 20 weeks after injury, a substantial cavity is present at the lesion epicenter, and demyelination is present in the majority of the fiber tracts surrounding the injury.
Figure 1.
Sustained forelimb deficits result from cervical contusion injuries. A: Forelimb asymmetry (cylinder) test demonstrates that animals use their ipsilateral to contusion forepaw for weight support less than 10% of the time after injury, compared to more than 40% of the time prior to injury (Mean ± SEM; N = 6 animals). B: Histology of a representative injury stained with cresyl violet (purple) and myelin stain (blue). Substantial cavitation is present at the injury epicenter (*), with wide-spread demyelination throughout the ipsilateral hemicord that persists for 20 weeks after injury. Scale bar in B is 1 mm.
Surgical procedure
Animals were anesthetized using a constant dose of 2% isoflurane in 100% O2 to maintain a stable level of anesthesia and eliminate spontaneous movements and muscle activity throughout the experiment. Ketoprofen (5 mg/kg) was given preoperatively, and body temperature was maintained using a water recirculating heating pad. The skin and muscles covering the cervical spinal cord were retracted to expose the dorsal lamina extending from vertebral segments C2 through T2. Animals were stabilized with a custom rodent spinal fixation frame attached to the dorsal processes of vertebral segments C2 and T2. Hemi-laminectomies were performed from C3 to T1 to expose the spinal cord, and the dura incised to permit electrode insertion without dimpling of the cord surface. Skin covering the forelimb was removed to aid with EMG electrode placement in the following muscles: deltoid (DEL), teres major (TMJ), triceps (TRI), biceps (BIC), extensor carpi radialis (ECR), extensor carpi ulnaris (ECU), flexor carpi radialis (FCR), flexor carpi ulnaris (FCU). Electrode placement was verified by stimulating the muscles and observing contractions.
Intraspinal microstimulation mapping
Stimuli were delivered using single tungsten microelectrodes (impedance 800–1000 kΩ at 1 kHz; FHC) positioned by a stereotaxic manipulator (Kopf Instruments) mounted on the spinal fixation frame. Constant current stimuli consisted of three biphasic square-wave pulses with 0.2-ms durations per phase, with pulses delivered at 300 Hz. Stimulation returned through a distance reference electrode placed under the skin above the hindquarters. Stimulation was delivered with an analog stimulus isolator (A-M systems) controlled by custom Lab VIEW software (National Instruments).
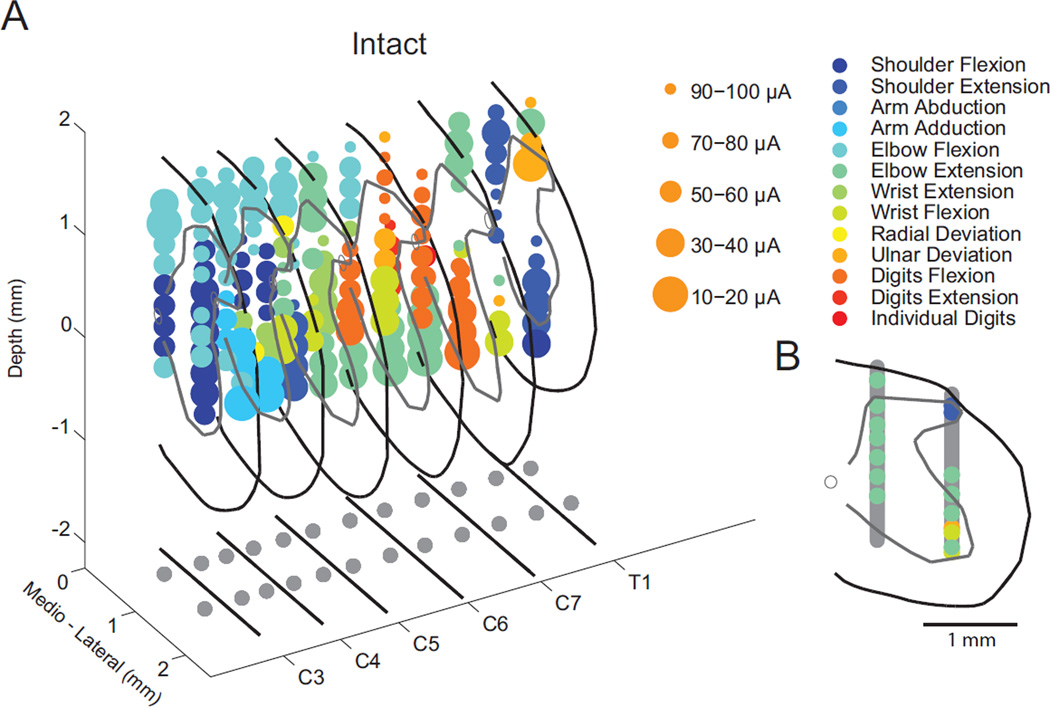
To explore the majority of the cervical spinal cord responsible for forelimb movement, electrodes were advanced ventrally in 24 tracks beginning at evenly distributed locations along the dorsal surface of the spinal cord. Electrode penetrations began at the intersection of a grid comprised of twelve rostrocaudal and two mediolateral coordinates (Figure 2A – bottom projection). Rostrocaudal coordinates for each segment were aligned with the rostral and caudal edges of each bony lamina from C3–T1. Mediolateral coordinates were set to 0.5 and 1.3 mm lateral to the midline as measured at the dorsal T1 and C2 vertebral processes (see Figure 2A bottom). Electrodes were advanced ventrally in 200 µm increments up to 1800 µm below the dorsal surface of the pia, with the goal of characterizing movements throughout the spinal grey matter (Figure 2B). At each site, stimulation intensity began at 10 µA and was increased in increments of 10 µA until a movement was observed or 100 µA stimulus current was reached.
Figure 2.
Location of electrode penetrations and evoked movements. A: 3D rendering of sites where spinal stimulation evoked forelimb movement in a spinally-intact animal. Marker size and color represent the stimulus current and the observed movement, respectively. The projection below illustrates the center of the bony lamina and the grid of 12 rostrocaudal by 2 mediolateral electrode penetrations. B: Example transverse plane reconstruction at segment C7. The black line denotes the pia matter, gray line outlines the gray matter, and the open circle is the central canal. Vertical gray bars illustrate the stimulation locations tested, with colored symbols appearing at locations where movements were evoked (as in part A).
EMG recording
ISMS-evoked electromyography (EMG) was recorded using a 16 channel low impedance headstage (Tucker-Davis Technologies) and custom acquisition software. When a movement was observed following stimulation, ten stimulus trains each separated by one second were delivered to determine associated muscle activity. EMG data were recorded at 24414 Hz, low-pass filtered at 12207 Hz (anti-aliasing filter), and saved to disk for offline analysis.
Tissue processing
At the conclusion of stimulation, animals were euthanized with an intraperitoneal injection of beuthanasia (200 mg/kg) and transcardially perfused with 10% formalin. Spinal cord tissue from select animals was sliced on a Leica VT1000S vibrating blade microtome in the transverse plane to provide a reference for animal size at the time of the experiments in order to scale images obtained from an atlas of the rat spinal cord (Watson et al., 2009) for data visualization.
Data analysis
Evoked muscle activity was rectified and compiled into stimulus triggered averages (StTAs) using custom MATLAB software (The MathWorks). StTAs were aligned to the initial stimulus pulse and included data from 100ms before to 500ms after the first stimulation occurred. A muscle was considered active when the average rectified EMG reached a peak greater than or equal to five standard deviations above that of the pre-stimulus baseline period (calculated in the interval −100 to 0ms) and had a total duration of at least 3ms above two standard deviations of baseline activity. When a muscle was activated by spinal stimulation, we calculated the onset, offset and duration of the response, as well as the area, peak amplitude and mean-percent increase (MPI) relative to baseline activity prior to stimulation.
Statistics
All statistical analyses were performed in MATLAB. Two-tailed unpaired T-tests were used to determine significance between experimental groups as all data satisfied the Lilliefors test of normality.
Results
Evoked movements
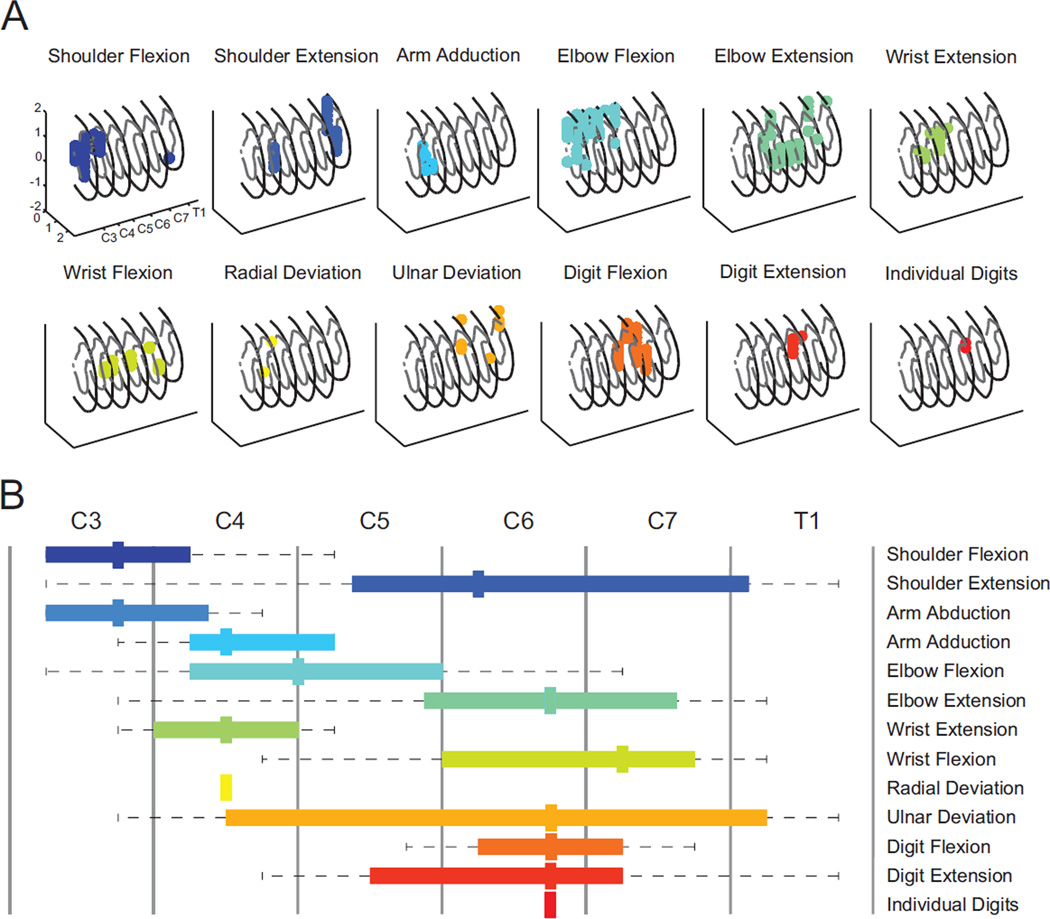
Cervical intraspinal stimulation typically evoked at least 12 distinct forelimb movements from sites in localized clusters within largely non-overlapping regions of the spinal cord (Figure 3A). Figure 3B summarizes the rostrocaudal organization of movement for all spinally-intact animals. A somatotopic, but slightly expanded pattern of evoked movements is evident when compared to the location of motor neuron pools within the rodent cervical spinal cord (McKenna et al., 2000; Tosolini and Morris, 2012).
Figure 3.
Location of stimulus evoked forelimb movements within the cervical spinal cord. A: 3D rendering of sites that evoked specific forelimb movement in a spinally-intact animal; each panel represents a single movement. Arm abduction was not observed for this animal. Numerical units indicate millimeters. B: Average anterior-posterior organization of movements evoked via spinal stimulation in all five spinally-intact animals. Somatotopic organization is suggested by the median location (thick mark), and upper and lower quartiles (colored bar). Dashed lines indicate the entire range over which a movement was evoked, which extends beyond published reports of motor neuron locations (McKenna et al., 2000; Tosolini and Morris, 2012), suggesting stimulation may also activate spinal interneurons and nearby axons.
Intraspinal stimulation evoked movements at 67.9 ± 15.2% (mean ± SD) of sites tested in the spinally-intact animals. Prior to injury, the potential for ISMS to evoke movements at each of the four forelimb joints observed was similar (shoulder, 18.1± 3.2% of sites tested; elbow, 22.6± 6.9%; wrist, 9.2± 1.1%; digits,13.2± 3.3%; Figure 4).
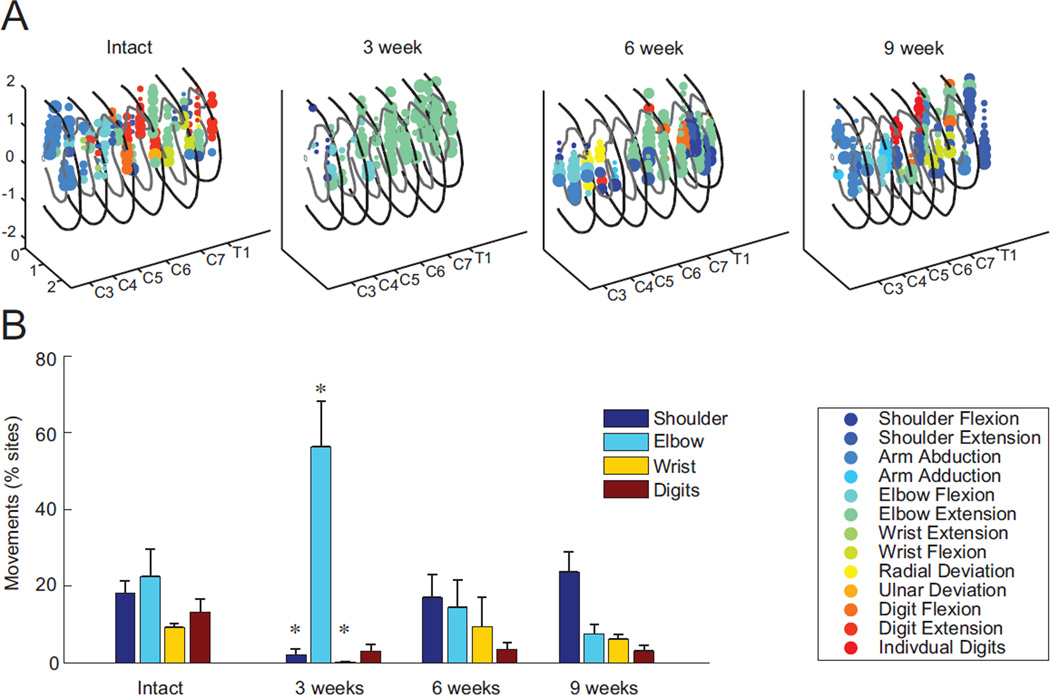
Figure 4.
Proportion of observed movements before and after cervical spinal cord injury. A: Reconstruction of forelimb movements evoked by cervical intraspinal microstimulation (ISMS) in example animals prior to spinal injury and at three, six and nine weeks after mid-cervical contusion injury (format identical to Figure 2A). While a variety of movements could be evoked prior to injury, as indicated by the range of colored symbols, extension of the elbow was dominant in animals three weeks after spinal injury. The variety of movements begins to return by six and nine weeks. B: For all animals in the study, the number of movements evoked via spinal stimulation about each forelimb joint as a percentage of total sites tested across each condition (Mean ± SEM; N = 5 spinally-intact animals, N = 3 animals per group at each post-injury time point, * denotes p < 0.05 compared to the spinally-intact animals).
ISMS-evoked responses after cervical contusion injury
Spinal stimulation evoked movements at a similar percentage of sites from spinally-intact animals and from animals tested at each of the three time points after spinal cord injury (p > 0.68). The pattern of evoked movements, however, differed transiently three weeks after injury before returning towards a pre-injury distribution by nine weeks after injury (Figure 4). Three weeks after injury, the distribution of ISMS-evoked movements changed to predominately movements of the elbow (56.3±11.8%), with decreased shoulder (2.0±1.7%) and wrist movements (0.2± 0.2 %; p < 0.05; Figure 4B). Extension of the elbow dominated the movements evoked three weeks after injury (87% of elbow movements; Figure 4A), contrasting sharply with the typically even distribution of elbow flexion (49%) and extension (51%) evoked from spinally-intact animals. While movements about the elbow exhibited the largest change after injury, there was also a transient shift towards extension movements at all joints three weeks after injury (81% of all in-plane movements). By six and nine weeks after injury, the relative balance of flexion and extension returned (52% and 67% extension, respectively), which were no different than the spinally intact animals (55% extension, p > 0.42).
By six and nine weeks after injury there was no longer any statistical difference in the distribution of forelimb movements compared to the spinally-intact animals (Figure 4B). There remained, however, a slight trend toward decreased wrist and digit movements at all times after injury. Representative maps of stimulation sites and associated movements at each time point illustrate a similar variety of movements comparing spinally intact animals with animals six and nine weeks after injury (Figure 4A). After injury, these figures also illustrate a subtle absence of movements near the injury site on the dorsal surface of the spinal cord spanning segments C3–C5.
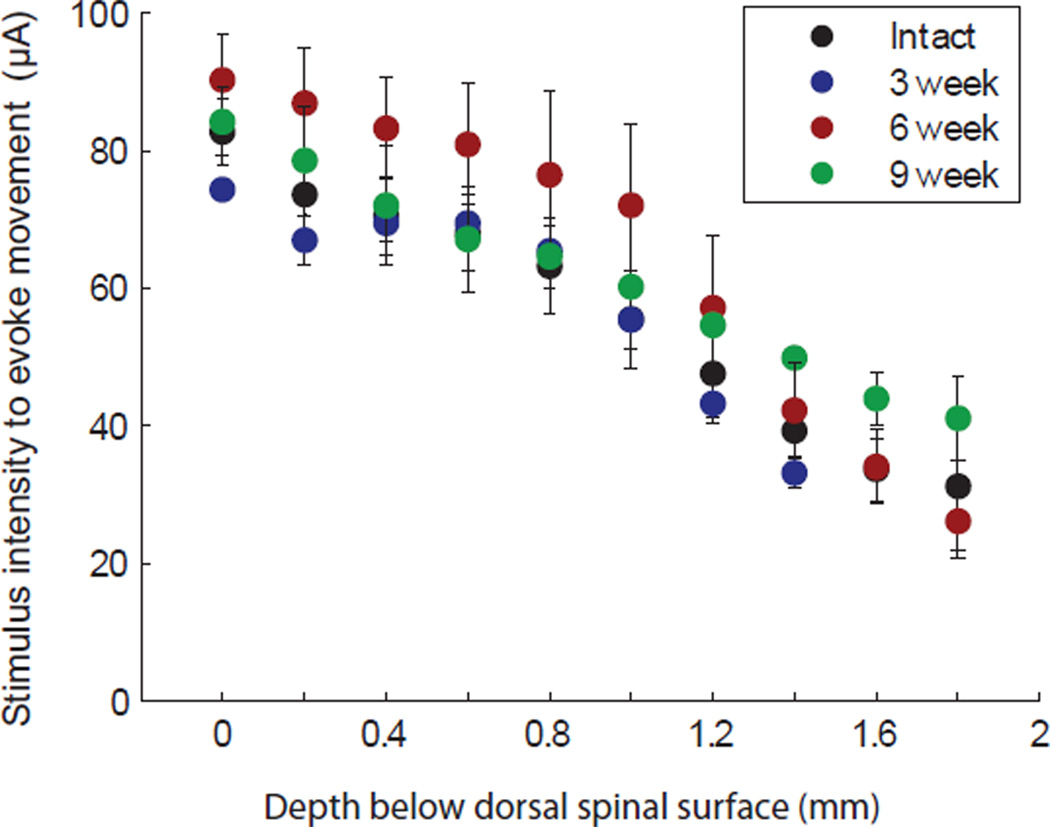
The average stimulus current necessary for evoking a movement in intact animals was 50.2 ± 7.8µA (mean ±SD). There was no difference in stimulus current required to evoke movement at any time point after injury (p > 0.51). As electrodes approached the motor neuron pools in the ventral horn of the spinal cord, threshold to evoke a movement similarly decreased in all animal groups (Figure 5). There was a negative and approximately linear relationship between dorsoventral location within the spinal cord and stimulus current required to evoke a movement (R2 > 0.82, p < 0.002).
Figure 5.
Stimulus current required to evoke a movement relative to dorsoventral position within the spinal cord. Average movement threshold decreased in an approximately linear fashion for all animal groups as electrodes approached the ventral horn motor neuron pools (Mean ± SEM).
Muscle responses
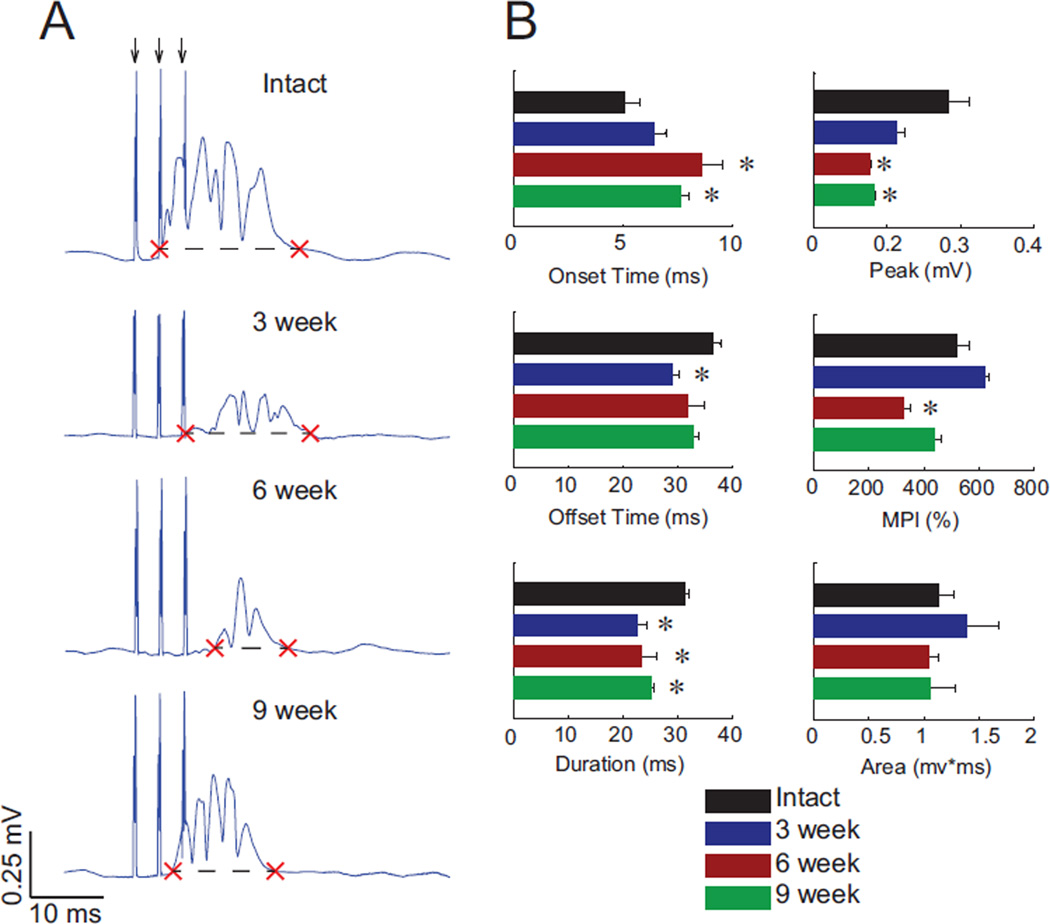
Stimulus triggered averages (StTAs) of EMG activity at the lowest stimulus intensity necessary for evoking a visible movement (i.e., movement threshold) were used to determine which muscles were active at each stimulation site within the cervical spinal cord. Figure 6A shows example stimulus-evoked activity from one animal in each group. When a muscle was activated by spinal stimulation, we determined the onset, offset, and duration of the response (Figure 6A, red Xs), as well as the area, peak amplitude, and mean-percent increase (MPI) during the active response period. Figure 6B compares these stimulus evoked parameters among the four groups of animals. ISMS evoked marginally shorter EMG bursts following injury due to small delays in the onset of activity. The magnitude of evoked EMG activity was also generally smaller after injury, although all measures trend toward values measured in spinally-intact animals by nine weeks after injury.
Figure 6.
Stimulus-triggered averages (StTA) of ISMS-induced muscle activity reveal progressive changes after spinal cord injury. A: Example StTAs from each experimental group: uninjured, and three, six, and nine weeks post-injury. Timing of the three stimulus pulses are shown by the arrows at top, and stimulus artifacts corresponding to each pulse are visible unless partly obscured by the evoked activity. Red X marks indicate onset and offset of response, and the dashed line denotes two standard deviations above baseline. B: Evoked EMG parameters from each experimental group for all muscles activated by ISMS (Mean ± SEM; * denotes p < 0.05 compared to spinally-intact animals).
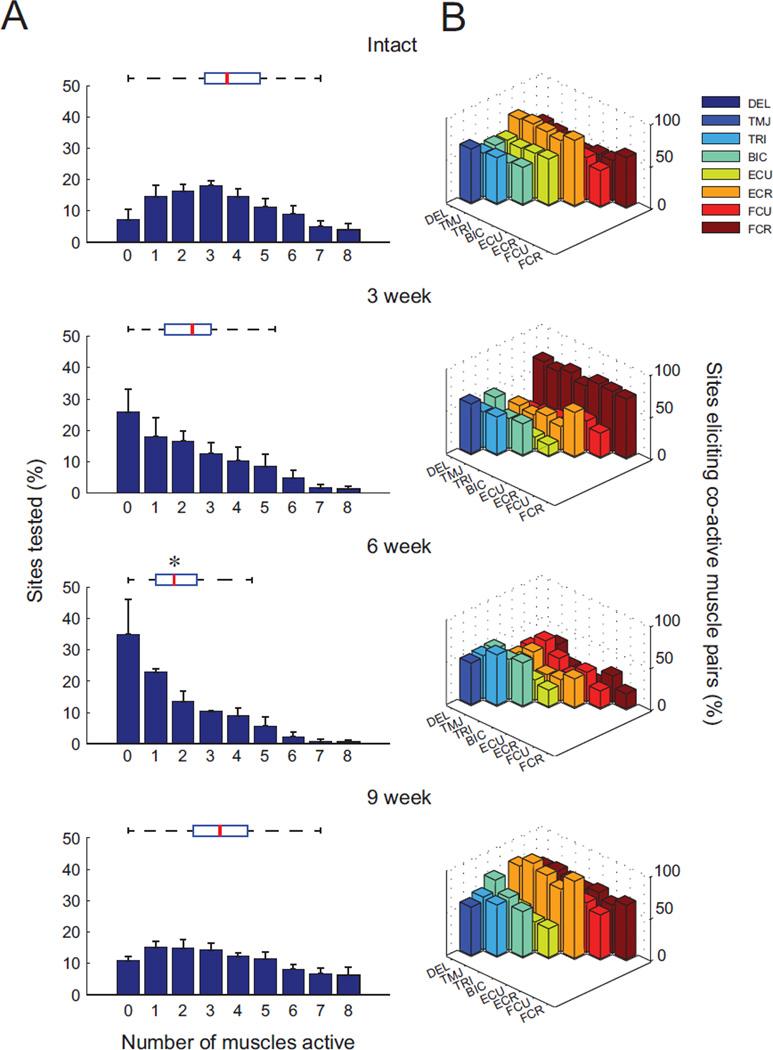
Multiple muscles were often coactivated by cervical ISMS at threshold intensities sufficient for producing a single forelimb movement. For the spinally-intact animals, an average of 3.9 ± 1.7 (mean ± SD) muscles were activated at each stimulation location when using the minimum current necessary for producing an observable movement (Figure 7A). At six weeks post-injury, the incidence of coactivation by ISMS was significantly reduced at each stimulation site (1.7 ± 0.9; p < 0.05). By nine weeks after injury, however, the number of coactive muscles returned to 3.4 ± 1.6, nearly identical to the uninjured animals (p = 0.96). The shape of the overall distribution of coactive muscles also returned toward that observed for the spinally-intact animals by nine weeks after injury (Figure 7A).
Figure 7.
Multiple muscles simultaneously activated by cervical ISMS. A: Histograms illustrating the percentage of sites where a given number of muscles were coactivated by ISMS. Box & whisker plots above each histogram show the median (red lines), upper and lower quartiles (box), and distribution (dashed lines) for each group of animals. Note that the distributions are similar for the spinally-intact and nine week injured animals despite a transient shift toward fewer coactive muscles at three and six weeks after injury (* denotes p < 0.05 compared to intact animals). B: Specific patterns of stimulus-evoked muscle coactivity, showing the percentage of times where a second muscle (indicated by a colored bar) was active normalized to the number of times the first muscle (indicated by axis label) was active. Extensor carpi radialis (ECR), shown in orange, was the most coactive muscle in both the spinally-intact animals and in animals 9 weeks after injury. For both panels, N = 5 spinally-intact animals, N = 3 animals per group at each post-injury time point.
To examine trends in muscle coactivity, we plotted the number of times two muscles were simultaneously active at the XY intersection points in Figure 7B. In the spinally-intact animals, the extensor carpi radialis (ECR; orange) was the most common muscle to participate in a coactive pair, and was active 73 ± 3% of the time another muscle was active. Three and six weeks after injury, ECR participation in coactive pairings significantly decreased (p < 0.04). Three weeks after injury, flexor carpi radialis (FCR; brown) was the most coactive muscle, participating in 68 ± 4% of paired muscle activations. Six weeks after injury, no muscle was coactive more than 55 ± 6.2% of the time, whereas by nine weeks following injury ECR was again the most commonly coactive muscle (83 ± 7 %), similar to the intact animals (p = 0.41).
Discussion
Our results emphasize the wide range of forelimb movements achieved with intraspinal microstimulation of the cervical spinal cord both before and after cervical contusion injury. While evoked movements show a transient shift toward extensor dominance for several weeks after injury, the pre-injury distribution of movements and their underlying muscle activity is largely restored by nine weeks post-injury. Movements were evoked by ISMS at locations within the cervical spinal cord that generally correspond to the locations of motor neuron pools, albeit with an expanded distribution which is likely due to the activation of local axons (see below). Multiple muscles were commonly coactivated by ISMS, demonstrating a variety of synergies that could be useful for reanimation of the limbs following cervical spinal cord injury in human patients.
Comparison with somatotopic organization of cervical motor neurons
Stimulation within the rodent cervical spinal cord evoked movements at broadly similar locations compared to the rostrocaudal distribution of motor neurons. Figure 3B was designed to facilitate comparison with previously documented cervical motor neuron locations in the rodent spinal cord (McKenna et al., 2000; Tosolini and Morris, 2012). While we were not able to record EMG from all muscles reported in these earlier studies, the movements reported here can be attributed to the major muscle groups identified in previous motor neurons labeling studies. For example, flexion of the elbow is likely driven by the biceps muscle, with representations in the C3–C5 spinal segments in both the present study and via neuroanatomical labeling of the corresponding motor neuron pools. Similarly, extension of the elbow is observed in the C5–T1 region along with triceps motor neurons described by Tosolini et al (2012), although the triceps representation was more limited to the middle of this range in the report by McKenna and colleagues (2000).
Although the locations of ISMS-evoked movements are in general agreement with the location of motor neuron pools, stimulation evoked movements from a slightly more distributed range within the spinal cord. This is likely due to the fact that electrical stimulation activates axons at a lower stimulus current than is required for direct activation of neuron cell bodies (Gustafsson and Jankowska, 1976). For example, stimulation within the feline lumbar spinal cord is known to activate sensory afferents as evidenced by antidromic activation of the dorsal root ganglion. Subsequent orthodromic activity has been observed several spinal segments away, likely leveraging the divergent but muscle-specific projections of the Ia-reflex circuits to activate specific motor neuron populations that lie across multiple segments of the spinal cord (Gaunt et al., 2006).
The present study of ISMS within the rat cervical spinal cord demonstrates much greater somatotopic organization of evoked movement than our previous study within the primate cervical spinal cord (Moritz et al., 2007). This difference is likely due to two factors. First, stimulation in the primates was restricted to a circumscribed range of the cervical spinal cord (C6–T1) owing to constraints on the size of the chronic electrode chamber that could be used. This limited snapshot represented approximately half of the range explored in the present study, and given the increased spatial distribution of evoked movement via ISMS, further experiments may be needed to reveal any somatotopic organization in the primate spinal cord. Second, the primate cervical spinal cord is known to contain direct corticomotoneuronal projections absent in rodents, especially to motor neurons controlling the digits (Nakajima et al., 2000; Yang and Lemon, 2003). Given that ISMS preferentially activates axons, the predominance of fibers of passage in the primate cervical spinal cord may further blur the organization of evoked movements (Moritz et al., 2007). Nonetheless, the generally somatotopic organization of evoked movements in the rodent cervical spinal cord parallels the well-organized somatotopy of ISMS-evoked activity observed in the lumbar spinal cord of rats, cats and frogs (Bamford et al., 2005; Giszter et al., 2000; Lemay and Grill, 2004; Mushahwar and Horch, 2000).
Effects of ISMS after injury
Perhaps the most encouraging finding of the present study is that stimulation within the cervical spinal cord continues to evoke a range of forelimb movements after chronic spinal contusion injury, despite a transient shift to extensor synergies shortly after injury. Changes in ISMS-evoked responses within the lumbar spinal cord have also been noted in the acute (Mushahwar et al., 2004) and sub-acute time period following mid-thoracic transection injury (Tresch and Bizzi, 1999). In both of these studies, a flexor-withdrawal pattern of the hind limbs was observed at nearly all sites examined. Tresch and Bizzi (1999) observed the persistence of this flexor pattern for one to three weeks following transection in rodents, but later time points were not explored. This hind limb flexor response may be analogous to the predominance of forelimb extension observed three weeks after injury in the present study. The opposite effects between forelimb and hind limb may be explained by common observations of extensor tone of the lower extremity and flexor tone of the upper extremity after damage to the upper motor neurons descending via the corticospinal tract (aka ‘decerebrate rigidity’; Purves et al., 2001). The return of an approximate balance of flexor and extensor movement by 9 weeks after injury in the present study suggests that the spinal cord circuitry may return to a state more amenable to treatment via ISMS.
Synergies evoked by ISMS
Complex muscle synergies elicited via stimulation of a single location in the spinal cord is a hallmark of intraspinal microstimulation. We observed a range of movements and synergies evoked via ISMS within the cervical spinal cord of uninjured rats (Figure 7B). Despite a transient shift in elicitation patterns at three weeks following injury, response profiles similar to those of uninjured animals were observed by nine weeks post-injury. Notably, despite a predominance of elbow extension movement evoked by stimulation three weeks after injury, we did not observe a concomitant increase in triceps muscle EMG. This is likely explained by the compartmentalized motor pool innervation of the three heads of the triceps muscle (Lucas-Osma and Collazos-Castro, 2009), as we recorded differential EMG from only one head of the triceps in each animal. It is nonetheless interesting to observe that such differential activation of sub-compartments of the same muscle may be achieved via spinal stimulation, and underscores the selectivity of ISMS.
Robust and complex forelimb synergies were also observed in our previous study stimulating within the cervical spinal cord of monkeys (Moritz et al., 2007), although the predominance of digit movements was much greater in primates as may be expected based on their enhanced corticomotoneuronal projections to the digits(Cheney and Fetz, 1985; Lawrence et al., 1985). In both species, the ability to activate multiple forelimb muscles in complex synergies from within the cervical spinal cord before and after injury is encouraging for future clinical applications of ISMS.
Conclusion
Stimulation within the cervical spinal cord evokes a wide variety of forelimb movements and muscle responses before and after contusion injury. Although evoked movements transiently regress to an extensor synergy shortly after injury, the variety of responses to cervical intraspinal microstimulation generally returns by six and nine weeks after injury. These findings provide promising evidence for the clinical utility of intraspinal microstimulation as a means for reanimation of the upper extremities following paralysis resulting from cervical spinal cord injury, or damage to the cortex or brainstem.
Acknowledgments
The authors thank Eric Secrist for critical input on experimental design, Joe Lyman for assistance with data collection and Sarah Mondello for histological analysis. This work was supported by an NIH/NINDS EUREKA award (1R01NS066357), an American Heart & Stroke Association Scientist Development Grant (NCRP 09SDG2230091), a DARPA Young Faculty Award (D12AP00251) and the Center for Sensorimotor Neural Engineering (CNSE), a National Science Foundation Engineering Research Center (EEC-1028725). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Science Foundation, or other funding agencies.
Footnotes
Conflict of interest
Authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bamford JA, Putman CT, Mushahwar VK. Intraspinal microstimulation preferentially recruits fatigue-resistant muscle fibres and generates gradual force in rat. J Physiol. 2005;569:873–884. doi: 10.1113/jphysiol.2005.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Putman CT, Mushahwar VK. Muscle plasticity in rat following spinal transection and chronic intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2010;19:79–83. doi: 10.1109/TNSRE.2010.2052832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol. 2006;96:2995–3005. doi: 10.1152/jn.00061.2006. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Loeb E, Mussa-Ivaldi FA, Bizzi E. Repeatable spatial maps of a few force and joint torque patterns elicited by microstimulation applied throughout the lumbar spinal cord of the spinal frog. Human Movement Science. 2000;19:597–626. [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog's spinal cord. J Neurosci. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevremont L, Mushahwar VK. D a Bronzino . Neural Engineering: Research, Industry and the Clinical Perspective. CRC Press; 2008. pp. 19-1–19-26. [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J Neurosci. 1983;3:567–575. doi: 10.1523/JNEUROSCI.03-03-00567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Porter R, Redman SJ. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J Comp Neurol. 1985;232:499–510. doi: 10.1002/cne.902320407. [DOI] [PubMed] [Google Scholar]
- Lemay MA, Grill WM. Modularity of motor output evoked by intraspinal microstimulation in cats. J Neurophysiol. 2004;91:502–514. doi: 10.1152/jn.00235.2003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Osma AM, Collazos-Castro JE. Compartmentalization in the triceps brachii motoneuron nucleus and its relation to muscle architecture. J Comp Neurol. 2009;516:226–239. doi: 10.1002/cne.22123. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol. 2007;97:110–120. doi: 10.1152/jn.00414.2006. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Aoyagi Y, Stein RB, Prochazka A. Movements generated by intraspinal microstimulation in the intermediate gray matter of the anesthetized, decerebrate, and spinal cat. Can J Physiol Pharmacol. 2004;82:702–714. doi: 10.1139/y04-079. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10:68–81. doi: 10.1109/TNSRE.2002.1021588. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation of muscle groups in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Trans Rehabil Eng. 2000;8:11–21. doi: 10.1109/86.830944. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol. 2000;84:698–709. doi: 10.1152/jn.2000.84.2.698. [DOI] [PubMed] [Google Scholar]
- NSCISC. Spinal Cord Injury Facts and Figures at a Glance. Birmingham, Alabama: The National Spinal Cord Injury Statistical Center; 2012. [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D. Neuroscience: Damage to Descending Motor Pathways: The Upper Motor Neuron Syndrome. Sunderland MA: Sinauer Associates; 2001. [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Stokes BT, Noyes DH, Behrmann DL. An electromechanical spinal injury technique with dynamic sensitivity. J Neurotrauma. 1992;9:187–195. doi: 10.1089/neu.1992.9.187. [DOI] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience. 2012;200:19–30. doi: 10.1016/j.neuroscience.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res. 1999;129:401–416. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G, Heise C. In: The spinal cord: A Christopher and Dana Reeve Foundation text and atlas. Watson C, et al., editors. Academic Press; 2009. [Google Scholar]
- Yang HW, Lemon RN. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp Brain Res. 2003;149:458–469. doi: 10.1007/s00221-003-1393-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann JB, Seki K, Jackson A. Reanimating the arm and hand with intraspinal microstimulation. J Neural Eng. 2011;8:054001. doi: 10.1088/1741-2560/8/5/054001. [DOI] [PMC free article] [PubMed] [Google Scholar]