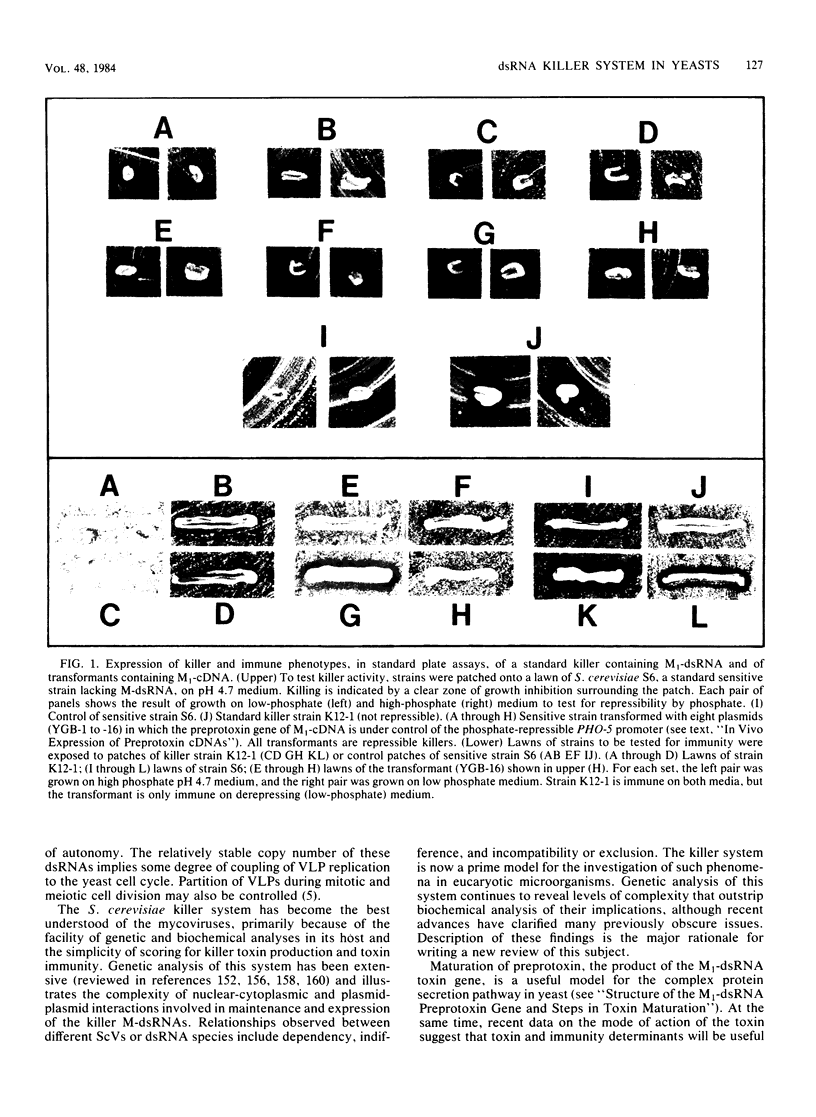
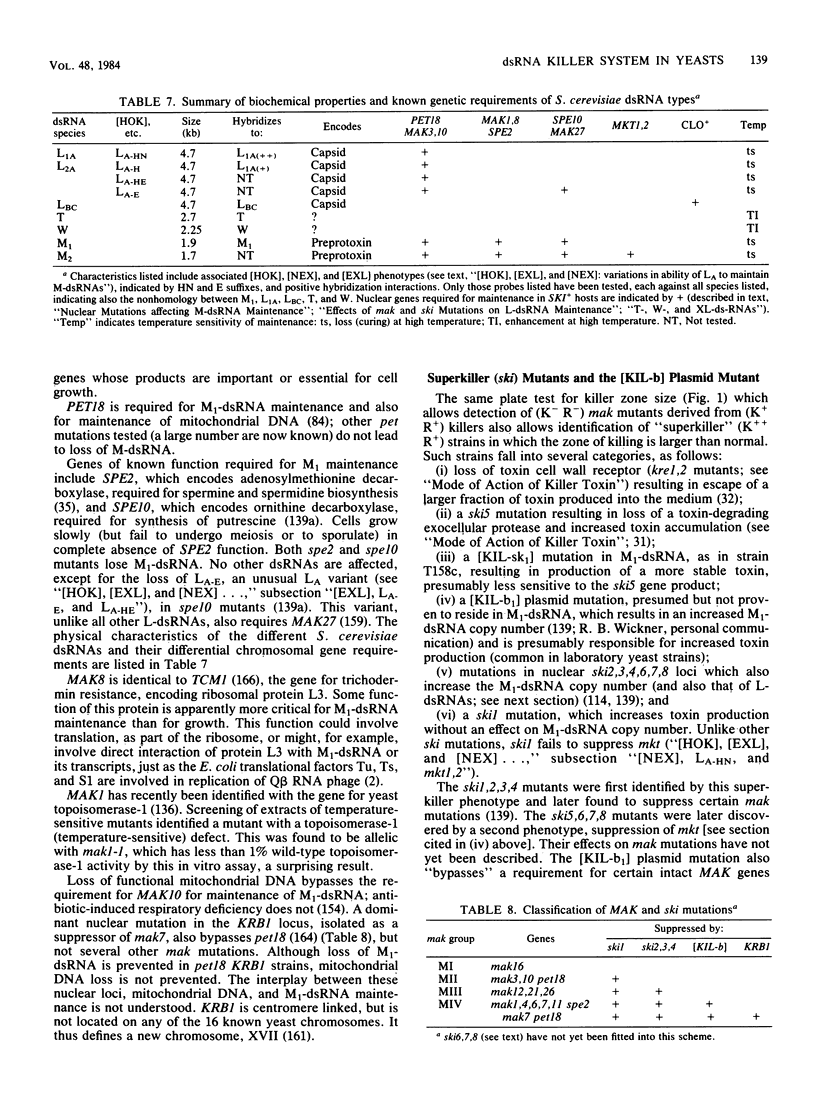
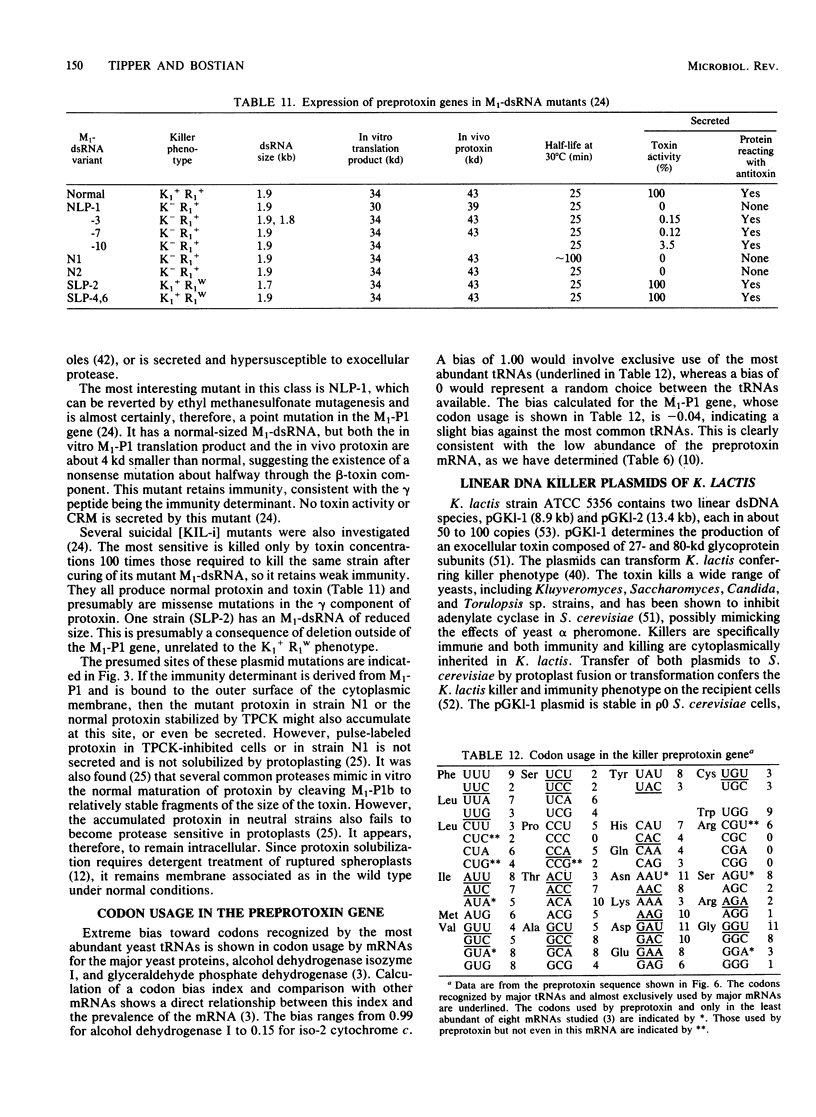
Full text
PDF




























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Aidroos K., Bussey H. Chromosomal mutants of Saccharomyces cerevisiae affecting the cell wall binding site for killer factor. Can J Microbiol. 1978 Mar;24(3):228–237. doi: 10.1139/m78-041. [DOI] [PubMed] [Google Scholar]
- Bausch J. N., Kramer F. R., Miele E. A., Dobkin C., Mills D. R. Terminal adenylation in the synthesis of RNA by Q beta replicase. J Biol Chem. 1983 Feb 10;258(3):1978–1984. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Berry E. A., Bevan E. A. A new species of double-stranded RNA from yeast. Nature. 1972 Sep 29;239(5370):279–280. doi: 10.1038/239279a0. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Somers J. M. Somatic segregation of the killer (k) and neutral (n) cytoplasmic genetic determinants in yeast. Genet Res. 1969 Aug;14(1):71–77. doi: 10.1017/s0016672300001865. [DOI] [PubMed] [Google Scholar]
- Bobek L. A., Bruenn J. A., Field L. J., Gross K. W. Cloning of cDNA to a yeast viral double-stranded RNA and comparison of three viral RNAs. Gene. 1982 Sep;19(2):225–230. doi: 10.1016/0378-1119(82)90010-5. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Burn V. E., Jayachandran S., Tipper D. J. Yeast killer dsRNA plasmids are transcribed in vivo to produce full and partial-length plus-stranded RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):1077–1097. doi: 10.1093/nar/11.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Elliott Q., Bussey H., Burn V., Smith A., Tipper D. J. Sequence of the preprotoxin dsRNA gene of type I killer yeast: multiple processing events produce a two-component toxin. Cell. 1984 Mar;36(3):741–751. doi: 10.1016/0092-8674(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan V. E., Bobek L. A., Bruenn J. A. Yeast deRNA viral transcriptase pause products: identification of the transcript strand. Nucleic Acids Res. 1981 Oct 10;9(19):5049–5059. doi: 10.1093/nar/9.19.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan V. E., Field L., Cizdziel P., Bruenn J. A. Sequences at the 3' ends of yeast viral dsRNAs: proposed transcriptase and replicase initiation sites. Nucleic Acids Res. 1981 Aug 25;9(16):4007–4021. doi: 10.1093/nar/9.16.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Preferential inclusion of extrachromosomal genetic elements in yeast meiotic spores. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5380–5384. doi: 10.1073/pnas.77.9.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzard B. L., De Kloet S. R. Reverse transcription of yeast double-stranded RNA and ribosomal RNA using synthetic oligonucleotide primers. Biochim Biophys Acta. 1983 Jan 20;739(1):122–131. doi: 10.1016/0167-4781(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Bobek L., Brennan V., Held W. Yeast viral RNA polymerase is a transcriptase. Nucleic Acids Res. 1980 Jul 11;8(13):2985–2997. doi: 10.1093/nar/8.13.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Keitz B. The 5' ends of yeast killer factor RNAs are pppGp. Nucleic Acids Res. 1976 Oct;3(10):2427–2436. doi: 10.1093/nar/3.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H. Physiology of killer factor in yeast. Adv Microb Physiol. 1981;22:93–122. doi: 10.1016/s0065-2911(08)60326-4. [DOI] [PubMed] [Google Scholar]
- Bussey H., Sacks W., Galley D., Saville D. Yeast killer plasmid mutations affecting toxin secretion and activity and toxin immunity function. Mol Cell Biol. 1982 Apr;2(4):346–354. doi: 10.1128/mcb.2.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Saville D., Greene D., Tipper D. J., Bostian K. A. Secretion of Saccharomyces cerevisiae killer toxin: processing of the glycosylated precursor. Mol Cell Biol. 1983 Aug;3(8):1362–1370. doi: 10.1128/mcb.3.8.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Saville D., Hutchins K., Palfree R. G. Binding of yeast killer toxin to a cell wall receptor on sensitive Saccharomyces cerevisiae. J Bacteriol. 1979 Dec;140(3):888–892. doi: 10.1128/jb.140.3.888-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Sherman D., Somers J. M. Action of yeast killer factor: a resistant mutant with sensitive spheroplasts. J Bacteriol. 1973 Mar;113(3):1193–1197. doi: 10.1128/jb.113.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Sherman D. Yeast killer factor: ATP leakage and coordinate inhibition of macromolecular synthesis in sensitive cells. Biochim Biophys Acta. 1973 Apr 16;298(4):868–875. doi: 10.1016/0005-2736(73)90391-x. [DOI] [PubMed] [Google Scholar]
- Bussey H., Skipper N. Killing of Torulopsis glabrata by Saccharomyces cerevisiae killer factor. Antimicrob Agents Chemother. 1976 Feb;9(2):352–354. doi: 10.1128/aac.9.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Skipper N. Membrane-mediated killing of Saccharomyces cerevisiae by glycoproteins from Torulopsis glabrata. J Bacteriol. 1975 Oct;124(1):476–483. doi: 10.1128/jb.124.1.476-483.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare J. J., Oliver S. G. The regulation of RNA synthesis in yeast IV. Synthesis of double-stranded RNA. Mol Gen Genet. 1979 Mar 20;171(2):161–166. doi: 10.1007/BF00270002. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H., Wickner R. B. Spermidine or spermine requirement for killer double-stranded RNA plasmid replication in yeast. J Biol Chem. 1978 Aug 10;253(15):5225–5227. [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Field L. J., Bobek L. A., Brennan V. E., Reilly J. D., Bruenn J. A. There are at least two yeast viral double-stranded RNAs of the same size: an explanation for viral exclusion. Cell. 1982 Nov;31(1):193–200. doi: 10.1016/0092-8674(82)90419-6. [DOI] [PubMed] [Google Scholar]
- Field L. J., Bruenn J. A., Chang T. H., Pinhasi O., Koltin Y. Two Ustilago maydis viral dsRNAs of different size code for the same product. Nucleic Acids Res. 1983 May 11;11(9):2765–2778. doi: 10.1093/nar/11.9.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill L. K., Garger S. J. Identification and characterization of double-stranded RNA associated with cytoplasmic male sterility in Vicia faba. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7043–7046. doi: 10.1073/pnas.78.11.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry-Kopecko P., Wickner R. B. Isolation and characterization of temperature-sensitive mak mutants of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1113–1118. doi: 10.1128/jb.144.3.1113-1118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Murata K., Sakaguchi K. Transformation of Saccharomyces cerevisiae with linear DNA killer plasmids from Kluyveromyces lactis. J Bacteriol. 1982 Jul;151(1):462–464. doi: 10.1128/jb.151.1.462-464.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Tamaru A., Ozawa F., Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981 Jan;145(1):382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N. Yeast DNA plasmids. Annu Rev Microbiol. 1983;37:253–276. doi: 10.1146/annurev.mi.37.100183.001345. [DOI] [PubMed] [Google Scholar]
- Hannig E. M., Thiele D. J., Leibowitz M. J. Saccharomyces cerevisiae killer virus transcripts contain template-coded polyadenylate tracts. Mol Cell Biol. 1984 Jan;4(1):101–109. doi: 10.1128/mcb.4.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. S. Virus-like particles and double stranded RNA from killer and non-killer strains of Saccharomyces cerevisiae. Microbios. 1978;21(85-86):161–176. [PubMed] [Google Scholar]
- Hastie N. D., Brennan V., Bruenn J. A. No homology between double-stranded RNA and nuclear DNA of yeast. J Virol. 1978 Dec;28(3):1002–1005. doi: 10.1128/jvi.28.3.1002-1005.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Yeast virus-like particles possess a capsid-associated single-stranded RNA polymerase. Nature. 1977 Aug 4;268(5619):464–466. doi: 10.1038/268464a0. [DOI] [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Levine H. L., Goeddel D. V., Ammerer G., Hall B. D. Expression of a human gene for interferon in yeast. Nature. 1981 Oct 29;293(5835):717–722. doi: 10.1038/293717a0. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins K., Bussey H. Cell wall receptor for yeast killer toxin: involvement of (1 leads to 6)-beta-D-glucan. J Bacteriol. 1983 Apr;154(1):161–169. doi: 10.1128/jb.154.1.161-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Kagan B. L. Mode of action of yeast killer toxins: channel formation in lipid bilayer membranes. Nature. 1983 Apr 21;302(5910):709–711. doi: 10.1038/302709a0. [DOI] [PubMed] [Google Scholar]
- Kandel J. S., Stern T. A. Killer phenomenon in pathogenic yeast. Antimicrob Agents Chemother. 1979 Apr;15(4):568–571. doi: 10.1128/aac.15.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane W. P., Pietras D. F., Bruenn J. A. Evolution of defective-interfering double-stranded RNAs of the yeast killer virus. J Virol. 1979 Nov;32(2):692–696. doi: 10.1128/jvi.32.2.692-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Day P. R. Inheritance of killer phenotypes and double-stranded RNA in Ustilago maydis. Proc Natl Acad Sci U S A. 1976 Feb;73(2):594–598. doi: 10.1073/pnas.73.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Day P. R. Specificity of Ustilago maydis killer proteins. Appl Microbiol. 1975 Oct;30(4):694–696. doi: 10.1128/am.30.4.694-696.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Day P. R. Suppression of the killer phenotype in Ustilago maydis. Genetics. 1976 Apr;82(4):629–637. doi: 10.1093/genetics/82.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Kandel J. S. Killer Phenomenon in USTILAGO MAYDIS: The Organization of the Viral Genome. Genetics. 1978 Feb;88(2):267–276. doi: 10.1093/genetics/88.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltin Y., Mayer I., Steinlauf R. Killer phenomenon in Ustilago maydis: mapping viral functions. Mol Gen Genet. 1978 Oct 30;166(2):181–186. doi: 10.1007/BF00285920. [DOI] [PubMed] [Google Scholar]
- Koltin Y. Virus-like particles in Ustilago maydis: mutants with partial genomes. Genetics. 1977 Jul;86(3):527–534. doi: 10.1093/genetics/86.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Bol J. F. Nucleotide sequence of the putative recognition site for coat protein in the RNAs of alfalfa mosaic virus and tobacco streak virus. Nucleic Acids Res. 1980 Aug 11;8(15):3307–3318. doi: 10.1093/nar/8.15.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., Shinmyo A., Enatsu T. Killer toxin for sake yeast: properties and effects of adenosine 5'-diphosphate and calcium ion on killing action. J Bacteriol. 1977 Feb;129(2):640–650. doi: 10.1128/jb.129.2.640-650.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. A chromosomal gene required for killer plasmid expression, mating, and spore maturation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2061–2065. doi: 10.1073/pnas.73.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. Pet18: a chromosomal gene required for cell growth and for the maintenance of mitochondrial DNA and the killer plasmid of yeast. Mol Gen Genet. 1978 Oct 4;165(2):115–121. doi: 10.1007/BF00269899. [DOI] [PubMed] [Google Scholar]
- Lemke P. A. Viruses of eucaryotic microorganisms. Annu Rev Microbiol. 1976;30:105–145. doi: 10.1146/annurev.mi.30.100176.000541. [DOI] [PubMed] [Google Scholar]
- Long M. S., Brizzard B. L., de Kloet S. R. Selective inhibition of yeast killer-specific double-stranded RNA transcription in vitro. Biochem Biophys Res Commun. 1982 Sep 16;108(1):31–35. doi: 10.1016/0006-291x(82)91827-7. [DOI] [PubMed] [Google Scholar]
- Middelbeek E. J., Crützen Q. H., Vogels G. D. Effects of potassium and sodium ions on the killing action of a Pichia kluyveri toxin in cells of Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1980 Oct;18(4):519–524. doi: 10.1128/aac.18.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbeek E. J., Hermans J. M., Stumm C., Muytjens H. L. High incidence of sensitivity to yeast killer toxins among Candida and Torulopsis isolates of human origin. Antimicrob Agents Chemother. 1980 Mar;17(3):350–354. doi: 10.1128/aac.17.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbeek E. J., Hermans J. M., Stumm C. Production, purification and properties of a Pichia kluyveri killer toxin. Antonie Van Leeuwenhoek. 1979;45(3):437–450. doi: 10.1007/BF00443282. [DOI] [PubMed] [Google Scholar]
- Middelbeek E. J., Stumm C., Vogels G. D. Effects of Pichia kluyveri killer toxin on sensitive cells. Antonie Van Leeuwenhoek. 1980;46(2):205–220. doi: 10.1007/BF00444075. [DOI] [PubMed] [Google Scholar]
- Middelbeek E. J., van de Laar H. H., Hermans J. M., Stumm C., Vogels G. D. Physiological conditions affecting the sensitivity of Saccharomyces cerevisiae to a Pichia kluyveri killer toxin and energy requirement for toxin action. Antonie Van Leeuwenhoek. 1980;46(5):483–497. doi: 10.1007/BF00395829. [DOI] [PubMed] [Google Scholar]
- Mitchell D. J., Herring A. J., Bevan E. A. The genetic control of DS-RNA virus-like particles associated with Saccharomyces cerevisiae killer yeast. Heredity (Edinb) 1976 Aug;37(1):129–134. doi: 10.1038/hdy.1976.71. [DOI] [PubMed] [Google Scholar]
- Nesterova G. F., Semykina L. V., Filatov A. A. Issledovanie mutantov plazmidy "killer", poluchennykh pod vozdeistviem 5-ftoruratsila. Genetika. 1981;17(3):391–398. [PubMed] [Google Scholar]
- Newman A. M., Elliott S. G., McLaughlin C. S., Sutherland P. A., Warner R. C. Replication of double-stranded RNA of the virus-like particles in Saccharomyces cerevisiae. J Virol. 1981 Apr;38(1):263–271. doi: 10.1128/jvi.38.1.263-271.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Sakaguchi K., Gunge N. Curing of the killer deoxyribonucleic acid plasmids of Kluyveromyces lactis. J Bacteriol. 1981 Dec;148(3):988–990. doi: 10.1128/jb.148.3.988-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Isolation and properties of abrin: a toxic protein inhibiting protein synthesis. Evidence for different biological functions of its two constituent-peptide chains. Eur J Biochem. 1973 May;35(1):179–185. doi: 10.1111/j.1432-1033.1973.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Peery T., Koltin Y., Tamarkin A. Mapping the immunity function of the ustilago maydis P1 virus. Plasmid. 1982 Jan;7(1):52–58. doi: 10.1016/0147-619x(82)90026-9. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Philliskirk G., Young T. W. The occurrence of killer character in yeasts of various genera. Antonie Van Leeuwenhoek. 1975;41(2):147–151. doi: 10.1007/BF02565046. [DOI] [PubMed] [Google Scholar]
- Polonelli L., Archibusacci C., Sestito M., Morace G. Killer system: a simple method for differentiating Candida albicans strains. J Clin Microbiol. 1983 May;17(5):774–780. doi: 10.1128/jcm.17.5.774-780.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. P., Sommer S. S., Wickner R. B. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol. 1984 Apr;4(4):761–770. doi: 10.1128/mcb.4.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. P., Wickner R. B. Defective Interference in the Killer System of Saccharomyces cerevisiae. J Virol. 1983 Feb;45(2):800–812. doi: 10.1128/jvi.45.2.800-812.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. T., Saville D., Bussey H. Saccharomyces cerevisiae killer expression mutant kex2 has altered secretory proteins and glycoproteins. Biochem Biophys Res Commun. 1979 Sep 12;90(1):187–193. doi: 10.1016/0006-291x(79)91607-3. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Gomatos P. J. Replication of semliki forest virus: polyadenylate in plus-strand RNA and polyuridylate in minus-strand RNA. J Virol. 1976 Nov;20(2):446–464. doi: 10.1128/jvi.20.2.446-464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Skipper N., Bussey H. Mode of action of yeast toxins: energy requirement for Saccharomyces cerevisiae killer toxin. J Bacteriol. 1977 Feb;129(2):668–677. doi: 10.1128/jb.129.2.668-677.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper N. Synthesis of a double-stranded cDNA transcript of the killer toxin-coding region of the yeast M1 double-stranded RNA. Biochem Biophys Res Commun. 1983 Jul 29;114(2):518–525. doi: 10.1016/0006-291x(83)90811-2. [DOI] [PubMed] [Google Scholar]
- Skipper N., Thomas D. Y., Lau P. C. Cloning and sequencing of the preprotoxin-coding region of the yeast M1 double-stranded RNA. EMBO J. 1984 Jan;3(1):107–111. doi: 10.1002/j.1460-2075.1984.tb01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- Somers J. M. Isolation of Suppressive Sensitive Mutants from Killer and Neutral Strains of SACCHAROMYCES CEREVISIAE. Genetics. 1973 Aug;74(4):571–579. doi: 10.1093/genetics/74.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Co-curing of plasmids affecting killer double-stranded RNAs of Saccharomyces cerevisiae: [HOK], [NEX], and the abundance of L are related and further evidence that M1 requires L. J Bacteriol. 1982 May;150(2):545–551. doi: 10.1128/jb.150.2.545-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Stumm C., Hermans J. M., Middelbeek E. J., Croes A. F., de Vries G. J. Killer-sensitive relationships in yeasts from natural habitats. Antonie Van Leeuwenhoek. 1977;43(2):125–128. doi: 10.1007/BF00395667. [DOI] [PubMed] [Google Scholar]
- Sweeney T. K., Tate A., Fink G. R. A study of the transmission and structure of double stranded RNAs associated with the killer phenomenon in Saccharomyces cerevisiae. Genetics. 1976 Sep;84(1):27–42. doi: 10.1093/genetics/84.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Multiple L double-stranded RNA species of Saccharomyces cerevisiae: evidence for separate encapsidation. Mol Cell Biol. 1984 Jan;4(1):92–100. doi: 10.1128/mcb.4.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Leibowitz M. J. Structural and functional analysis of separated strands of killer double-stranded RNA of yeast. Nucleic Acids Res. 1982 Nov 11;10(21):6903–6918. doi: 10.1093/nar/10.21.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Wang R. W., Leibowitz M. J. Separation and sequence of the 3' termini of M double-stranded RNA from killer yeast. Nucleic Acids Res. 1982 Mar 11;10(5):1661–1678. doi: 10.1093/nar/10.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C., Voelkel K., DiNardo S., Sternglanz R. Identification of Saccharomyces cerevisiae mutants deficient in DNA topoisomerase I activity. J Biol Chem. 1984 Feb 10;259(3):1375–1377. [PubMed] [Google Scholar]
- Toh-E A., Wickner R. B. "Superkiller" mutations suppress chromosomal mutations affecting double-stranded RNA killer plasmid replication in saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jan;77(1):527–530. doi: 10.1073/pnas.77.1.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Wickner R. B. A mutant killer plasmid whose replication depends on a chromosomal "superkiller" mutation. Genetics. 1979 Apr;91(4):673–682. doi: 10.1093/genetics/91.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Inouye S., Oshima Y. Structure and function of the PHO82-pho4 locus controlling the synthesis of repressible acid phosphatase of Saccharomyces cerevisiae. J Bacteriol. 1981 Jan;145(1):221–232. doi: 10.1128/jb.145.1.221-232.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi A. K., Wickner R. B., Tabor C. W., Tabor H. Specificity of polyamine requirements for the replication and maintenance of different double-stranded RNA plasmids in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1149–1153. doi: 10.1073/pnas.81.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Fink G. R. A nucleic acid associated with a killer strain of yeast. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1069–1072. doi: 10.1073/pnas.70.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. Induction of yeast killer factor mutations. J Bacteriol. 1977 Oct;132(1):346–348. doi: 10.1128/jb.132.1.346-348.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D., Leibowitz M. J. Transcription of killer virion double-stranded RNA in vitro. Nucleic Acids Res. 1980 Jun 11;8(11):2365–2375. doi: 10.1093/nar/8.11.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J. Localization of genes for the double-stranded RNA killer virus of yeast. Proc Natl Acad Sci U S A. 1982 Feb;79(3):786–789. doi: 10.1073/pnas.79.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. D., Leibowitz M. J., Wickner R. B. Virion DNA-independent RNA polymerase from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Jun 11;8(11):2349–2363. doi: 10.1093/nar/8.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Wickner R. B. Two new double-stranded RNA molecules showing non-mendelian inheritance and heat inducibility in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):181–187. doi: 10.1128/mcb.4.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. "Killer character" of Saccharomyces cerevisiae: curing by growth at elevated temperature. J Bacteriol. 1974 Mar;117(3):1356–1357. doi: 10.1128/jb.117.3.1356-1357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Boutelet F., Hilger F. Evidence for a new chromosome in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Mar;3(3):415–420. doi: 10.1128/mcb.3.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Chromosomal and nonchromosomal mutations affecting the "killer character" of Saccharomyces cerevisiae. Genetics. 1974 Mar;76(3):423–432. doi: 10.1093/genetics/76.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Deletion of mitochondrial DNA bypassing a chromosomal gene needed for maintenance of the killer plasmid of yeast. Genetics. 1977 Nov;87(3):441–452. doi: 10.1093/genetics/87.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Genetic control of replication of the double-stranded RNA segments of the killer systems in Saccharomyces cerevisiae. Arch Biochem Biophys. 1983 Apr 1;222(1):1–11. doi: 10.1016/0003-9861(83)90496-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Killer of Saccharomyces cerevisiae: a double-stranded ribonucleic acid plasmid. Bacteriol Rev. 1976 Sep;40(3):757–773. doi: 10.1128/br.40.3.757-773.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Killer systems in Saccharomyces cerevisiae: three distinct modes of exclusion of M2 double-stranded RNA by three species of double-stranded RNA, M1, L-A-E, and L-A-HN. Mol Cell Biol. 1983 Apr;3(4):654–661. doi: 10.1128/mcb.3.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Dominant chromosomal mutation bypassing chromosomal genes needed for killer RNA plasmid replication in yeast. Genetics. 1977 Nov;87(3):453–469. doi: 10.1093/genetics/87.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Mak mutants of yeast: mapping and characterization. J Bacteriol. 1979 Oct;140(1):154–160. doi: 10.1128/jb.140.1.154-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of the killer plasmid of Saccharomyces cerevisiae dependent on chromosomal diploidy for expression and maintenance. Genetics. 1976 Feb;82(2):273–285. doi: 10.1093/genetics/82.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Ridley S. P., Fried H. M., Ball S. G. Ribosomal protein L3 is involved in replication or maintenance of the killer double-stranded RNA genome of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4706–4708. doi: 10.1073/pnas.79.15.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. The killer double-stranded RNA plasmids of yeast. Plasmid. 1979 Jul;2(3):303–322. doi: 10.1016/0147-619x(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Toh-e A. [HOK], a new yeast non-Mendelian trait, enables a replication-defective killer plasmid to be maintained. Genetics. 1982 Feb;100(2):159–174. doi: 10.1093/genetics/100.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. R., Bevan E. A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J Gen Microbiol. 1968 Apr;51(1):115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]
- Young T. W., Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Van Leeuwenhoek. 1978;44(1):59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Wagner D. W., Fangman W. L. Yeast L double-stranded ribonucleic acid is synthesized during the G1 phase but not the S phase of the cell cycle. Mol Cell Biol. 1981 Aug;1(8):673–679. doi: 10.1128/mcb.1.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Louvencourt L., Fukuhara H., Heslot H., Wesolowski M. Transformation of Kluyveromyces lactis by killer plasmid DNA. J Bacteriol. 1983 May;154(2):737–742. doi: 10.1128/jb.154.2.737-742.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña P., Barros F., Gascón S., Lazo P. S., Ramos S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 25;256(20):10420–10425. [PubMed] [Google Scholar]
- de la Peña P., Barros F., Gascón S., Ramos S., Lazo P. S. Primary effects of yeast killer toxin. Biochem Biophys Res Commun. 1980 Sep 30;96(2):544–550. doi: 10.1016/0006-291x(80)91390-x. [DOI] [PubMed] [Google Scholar]