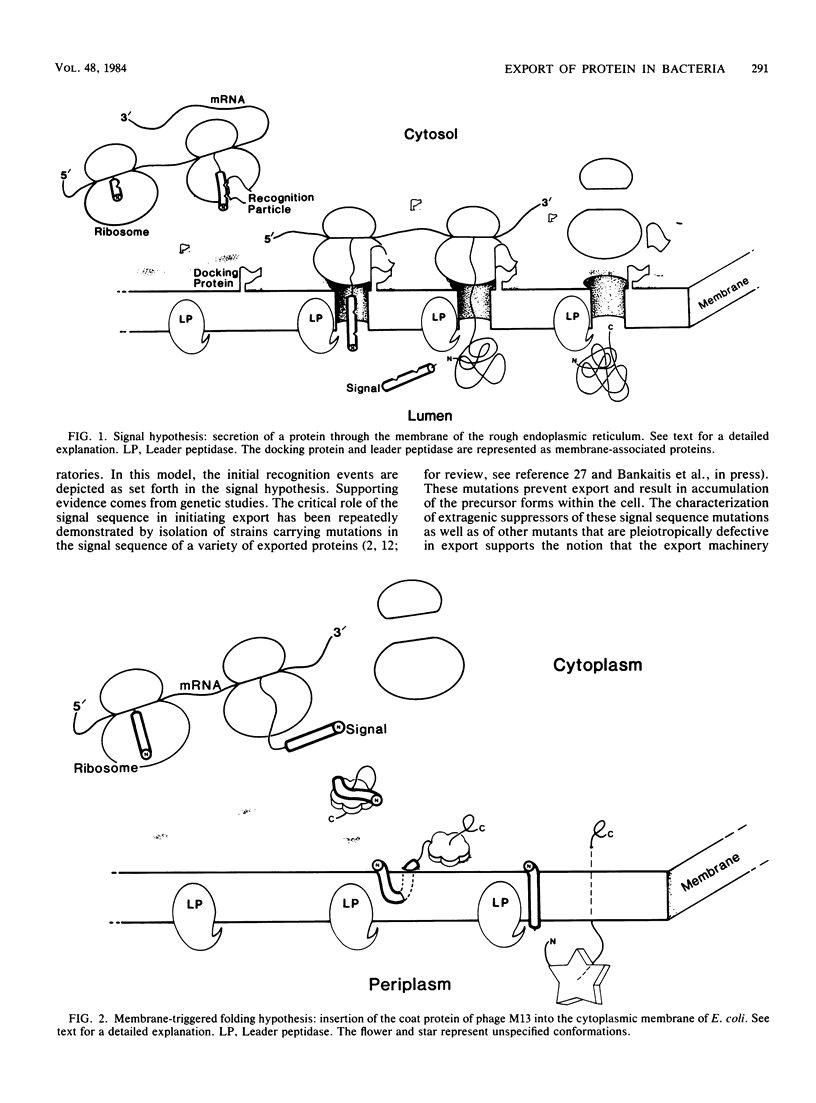
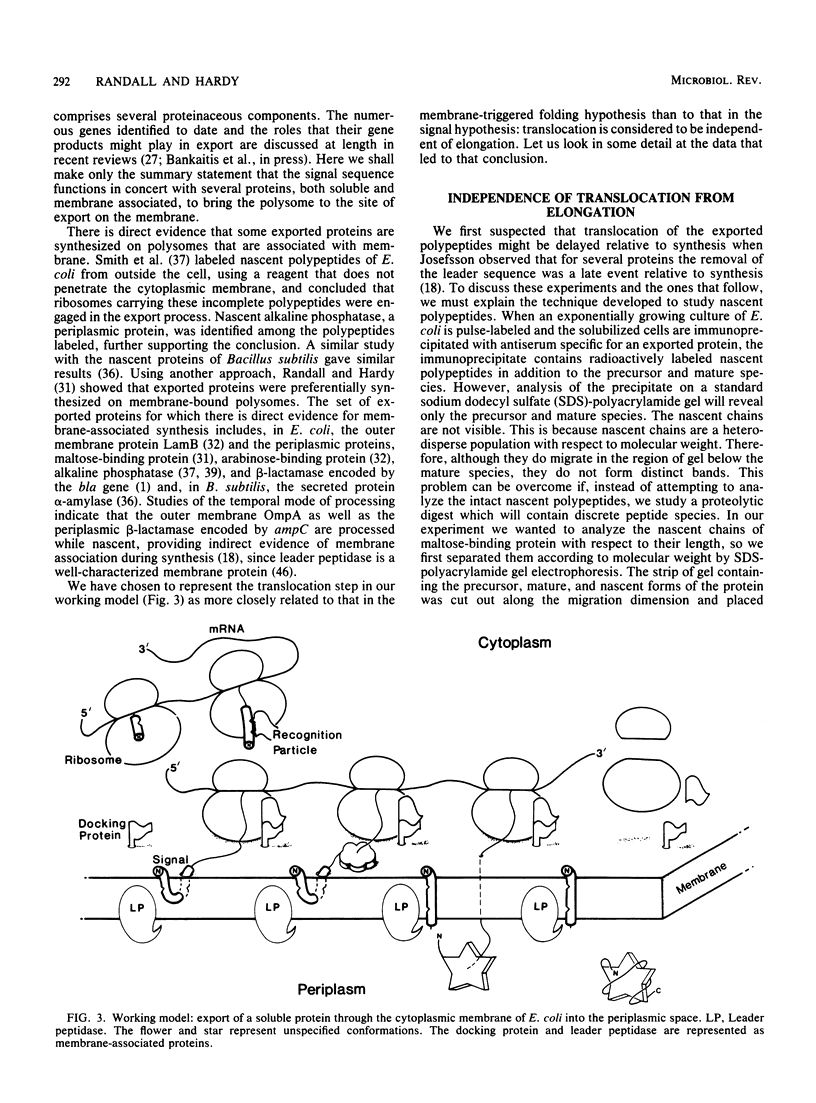
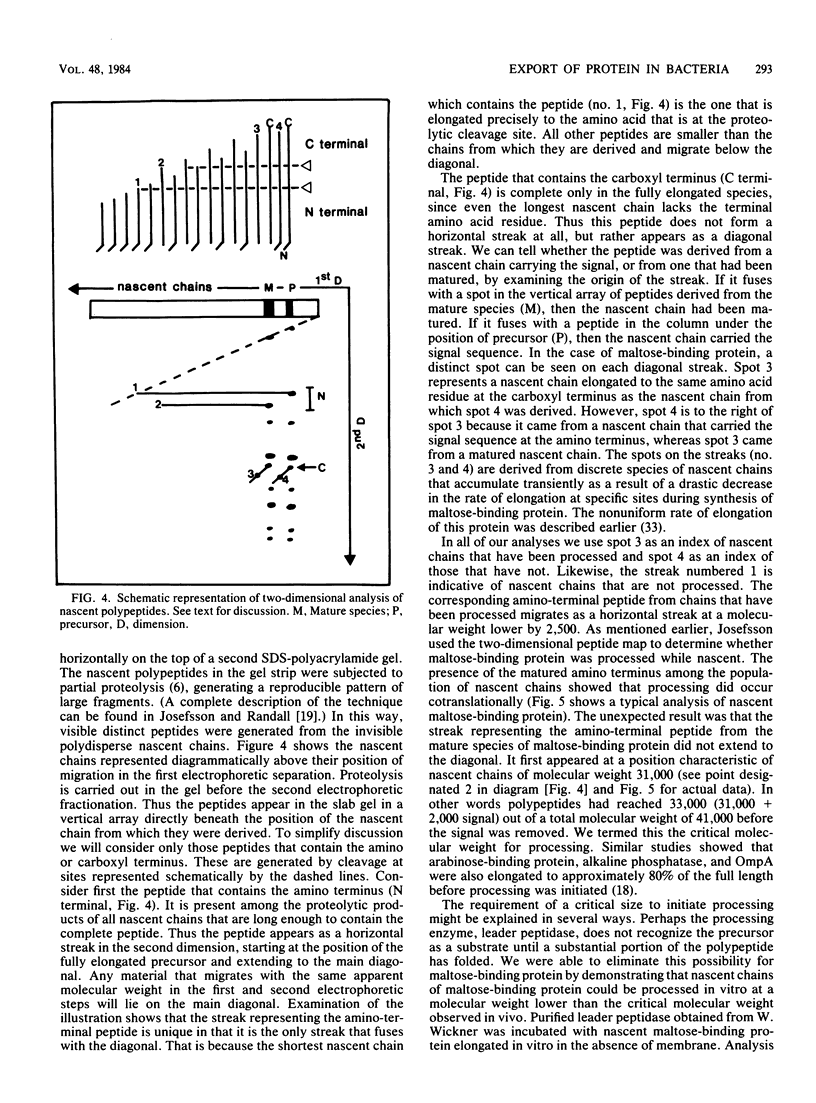
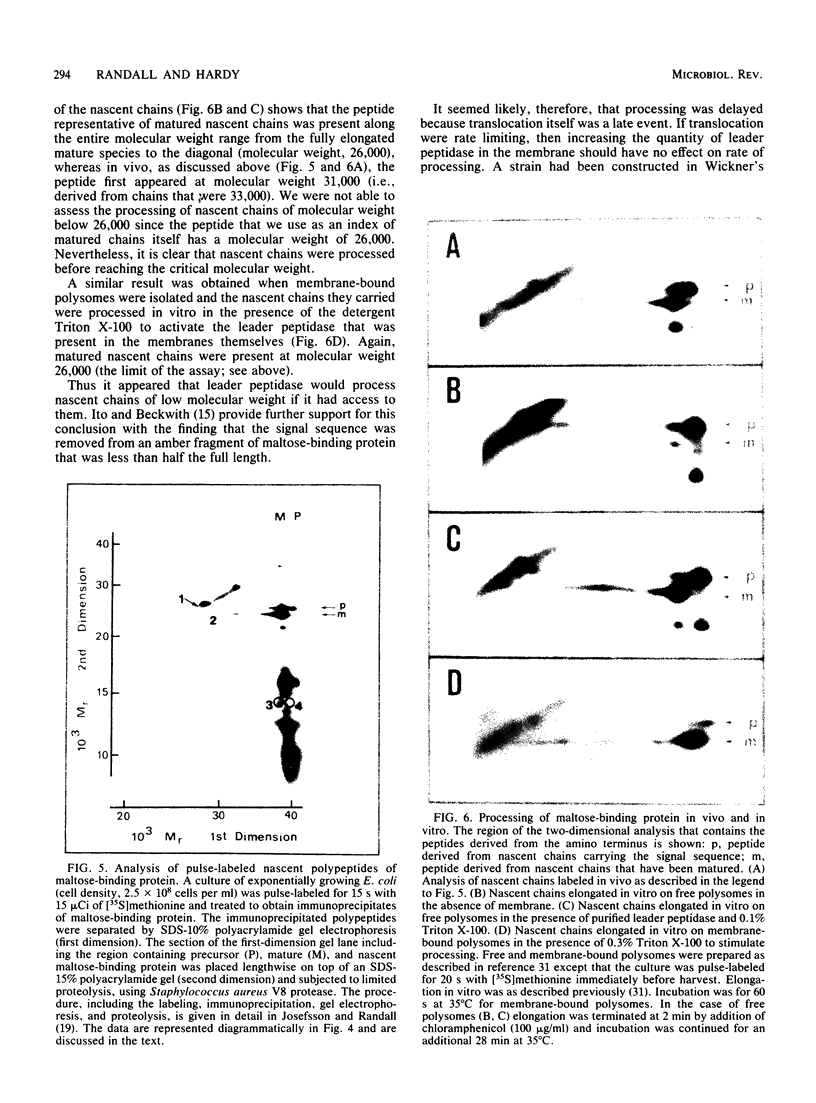
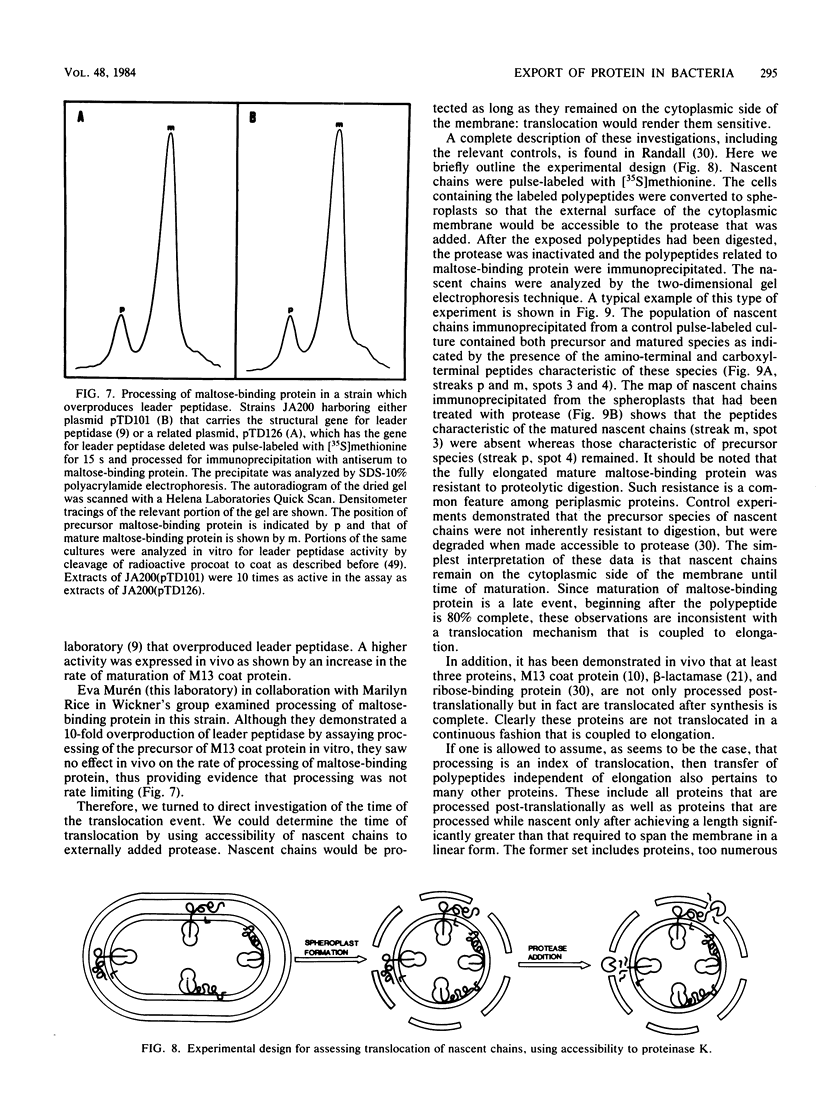
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty D., Bernadac A., Berthois Y., Lazdunski C. Synthesis and export of teml beta-lactamase in Escherichia coli. FEBS Lett. 1981 May 5;127(1):161–165. doi: 10.1016/0014-5793(81)80365-1. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Wickner W. T. Procoat, the precursor of M13 coat protein, inserts post-translationally into the membrane of cells infected by wild-type virus. J Virol. 1981 Mar;37(3):1087–1089. doi: 10.1128/jvi.37.3.1087-1089.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Wickner W. Isolation of the Escherichia coli leader peptidase gene and effects of leader peptidase overproduction in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6106–6110. doi: 10.1073/pnas.78.10.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hedgpeth J., Clément J. M., Silhavy T. J., Hofnung M. Sequence analysis of mutations that prevent export of lambda receptor, an Escherichia coli outer membrane protein. Nature. 1980 May 8;285(5760):82–85. doi: 10.1038/285082a0. [DOI] [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Ito K., Beckwith J. R. Role of the mature protein sequence of maltose-binding protein in its secretion across the E. coli cytoplasmic membrane. Cell. 1981 Jul;25(1):143–150. doi: 10.1016/0092-8674(81)90238-5. [DOI] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Ito K., Mandel G., Wickner W. Soluble precursor of an integral membrane protein: synthesis of procoat protein in Escherichia coli infected with bacteriophage M13. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1199–1203. doi: 10.1073/pnas.76.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Analysis of cotranslational proteolytic processing of nascent chains using two-dimensional gel electrophoresis. Methods Enzymol. 1983;97:77–85. doi: 10.1016/0076-6879(83)97121-5. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Evidence for posttranslational translocation of beta-lactamase across the bacterial inner membrane. Cell. 1982 Oct;30(3):893–902. doi: 10.1016/0092-8674(82)90294-x. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Koshland D., Sauer R. T., Botstein D. Diverse effects of mutations in the signal sequence on the secretion of beta-lactamase in Salmonella typhimurium. Cell. 1982 Oct;30(3):903–914. doi: 10.1016/0092-8674(82)90295-1. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J. E., Garwin J. L., Emr S. D., Silhavy T. J., Beckwith J. R. D-ribose metabolism in Escherichia coli K-12: genetics, regulation, and transport. J Bacteriol. 1984 May;158(2):665–673. doi: 10.1128/jb.158.2.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Müller M., Ibrahimi I., Chang C. N., Walter P., Blobel G. A bacterial secretory protein requires signal recognition particle for translocation across mammalian endoplasmic reticulum. J Biol Chem. 1982 Oct 25;257(20):11860–11863. [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Josefsson L. G., Hardy S. J. Novel intermediates in the synthesis of maltose-binding protein in Escherichia coli. Eur J Biochem. 1980 Jun;107(2):375–379. doi: 10.1111/j.1432-1033.1980.tb06039.x. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Roggenkamp R., Kustermann-Kuhn B., Hollenberg C. P. Expression and processing of bacterial beta-lactamase in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4466–4470. doi: 10.1073/pnas.78.7.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Extracellular labeling of growing secreted polypeptide chains in Bacillus subtilis with diazoiodosulfanilic acid. Biochemistry. 1979 Jan 9;18(1):198–202. doi: 10.1021/bi00568a030. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Stahl S., Gilbert W. Eukaryotic signal sequence transports insulin antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3369–3373. doi: 10.1073/pnas.77.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenne S., Piovant M., Pagès J. M., Lazdunski C. Evidence for synthesis of alkaline phosphatase on membrane-bound polysomes in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):603–606. doi: 10.1111/j.1432-1033.1978.tb12344.x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Huth A., Rapoport T. A. Xenopus oocytes can secrete bacterial beta-lactamase. Nature. 1984 Jun 14;309(5969):637–639. doi: 10.1038/309637a0. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Zimmermann R., Watts C., Wickner W. The biosynthesis of membrane-bound M13 coat protein. Energetics and assembly intermediates. J Biol Chem. 1982 Jun 10;257(11):6529–6536. [PubMed] [Google Scholar]
- Zimmermann R., Wickner W. Energetics and intermediates of the assembly of Protein OmpA into the outer membrane of Escherichia coli. J Biol Chem. 1983 Mar 25;258(6):3920–3925. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]