Abstract
Stem cells have inherent tumor‑trophic migratory properties and can serve as vehicles for delivering effective, targeted therapy to isolated tumors and metastatic disease, making them promising anti‑cancer agents. Encapsulation of therapeutically engineered stem cells in hydrogels has been utilized to provide a physical barrier to protect the cells from hostile extrinsic factors and significantly improve the therapeutic efficacy of transplanted stem cells in different models of cancer. This review aims to discuss the potential of different stem cell types for cancer therapy, various engineered stem cell based therapies for cancer, stem cell encapsulation process and provide an in depth overview of current applications of therapeutic stem cell encapsulation in the highly malignant brain tumor, glioblastoma multiforme (GBM), as well as the prospects for their clinical translation.
Keywords: stem cells, tumors, imaging, sECM, TRAIL
Stem Cell Sources and Their Homing to Tumors
Stem cells are characterized by their capacity for self‑renewal and their ability to differentiate into specific cell types under the influence of their microenvironment. They are the natural sources of embriogenetic tissue generation and continuous regeneration throughout adult life. The embryonic stem cells originate from the inner cell mass (ICM) of the gastrula1 and form the three germ layers: endoderm, mesoderm, and ectoderm, each committed to generating specified tissues of the forming body.2 Tissue specific stem cells, such as mesenchymal stem cells (mesoderm), hematopoietic stem cells (mesoderm) and neural stem cells (ectoderm), have been identified as present and active for virtually every bodily tissue, and are hierarchically situated between their germ layer progenitors and differentiated end‑organ tissues.2 Embryonic stem cells display indefinite self‑renewal capacity due to high telomerase expression. In contrast, telomerase activity in adult stem cells seems to be lower, limiting their perpetuation capacity in the long run.3 Recently, pluripotent stem cells have been shown to be generated from murine fibroblasts4 as well as from several human organs, such as heart, skin5 and bone marrow.6 More recently, stem cells derived from dental pulp7 and menstrual blood8 have also been isolated and studied to understand their potential applications in therapy. A number of different stem cell types have been used for the delivery of therapeutics to treat various cancers. These include mesenchymal stem cells (MSC), neural stem cells (NSC), umbilical cord derived stem cells (UCB‑SC) and adipose derived stem cells (ASC). However, bone marrow derived‑MSC have been widely studied for cancer therapy.
A number of studies have shown that various stem cell types migrate to sites of injury, ischemia and tumor microenvironments; and extensive studies have shown that migration of stem cells is dependent upon the different cytokine/receptor pairs SDF‑1/CXCR4, SCF‑c‑Kit, HGF/c‑Met, VEGF/VEGFR, PDGF/PDGFr, MCP‑1/CCR2, and HMGB1/RAGE (reviewed in ref .9). SDF‑1/CXCR4 has been shown as the most prominent cytokine/receptor pair. The importance of the interaction between secreted SDF‑1 and cell surface CXCR4 for stem cell migration has been displayed by experiments in which the activity of either the receptor or the cytokine has been inhibited.10‑12 Recent studies on gene expression profiles of stem cells exposed to conditioned medium (CM) of various tumor cells, revealed the downregulation of matrix metalloproteinase‑2 (MMP‑2) and upregulation of CXCR4 in stem cells.13 This exposure to tumor cell CM enhanced migration of MSC toward tumor cells, which was further confirmed by SDF‑1 and MMP‑2 inhibition studies. Another recent study has reported the involvement of a potent pro‑inflammatory cytokine, macrophage migration inhibitory factor (MIF) in stem cell migration. An activating antibody (CD74Ab) was employed in this study to examine the effect of one MIF receptor, CD74 (major histocompatibility complex class II‑associated invariant chain), on SC motility. Targeting CD74 to regulate migration and homing potentially may be a useful strategy to improve the efficacy of a variety of SC therapies including cancers.14 A recent study suggested that bioactive lipids, sphingosine‑1 phosphate and ceramide‑1 phosphate contribute directly toward the migratory properties of stem cells and also the presence of these priming factors leads to robust response of stem cells to very low SDF‑1 gradients.15 Besides targeting the tumor main burden, different stem cell types have been shown to track tumor metastases and small intracranial microsatellite deposits of different tumor types. The stem cells have been shown to effectively treat these sites with either the factors they release, or in loco expression of tumoricidal transgenes that they have been engineered with.16‑18 These findings provide a strong rationale for the development of therapies that capitalize on the tumoritropic properties of stem cells by engineering them into carriers for anti‑tumor therapy.
The unmodified stem cells, particularly MSC, have been shown to have anti‑tumor effects both in vitro and in vivo in different mouse models of cancer. This is attributed to the factors released by MSCs that have antitumor properties; reducing the proliferation of glioma, melanoma, lung cancer, hepatoma and breast cancer cells.19‑22 Human bone marrow derived MSC injected intravenously (i.v.) in a mouse model of Kaposi's sarcoma were shown to home to sites of tumorigenesis and potently inhibit tumor growth.23 MSCs have also been shown to have anti‑angiogenic effect both in vitro and in mouse models of melanoma.24 Direct injection of MSC into subcutaneous melanoma bearing mice induced apoptosis and abrogated tumor growth.24
Stem cells based delivery of therapeutics
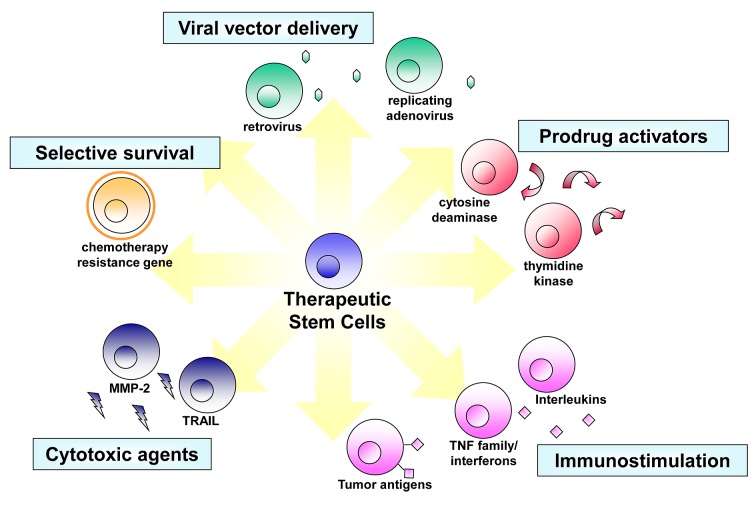
Different stem cell types have been genetically modified mainly to introduce and overexpress target exogenous genes for expression/secretion of a desired therapeutic factor for targeted treatment of different cancer types (Fig. 1).25 A number of studies have recently been published describing the successful use of stem cell based delivery of cytokines for the management of various human cancers. A few of the recent developments in this area are discussed below.
Figure 1. Transgene strategies potentiating stem cells for tumor therapy. Tailored to the specific molecular profiles associated with individual tumor types, stem cells can be engineered with a variety of different anti‑tumor agents (adapted from ref. 25 with permission).
Interleukins
Stem cells have been efficiently employed for the delivery of interleukins in order to improve the anti‑cancer immune surveillance by activating cytotoxic lymphocytes and natural killer cells. A number of previously published studies demonstrated the efficacy of stem cell delivered interleukin(IL)s such as IL‑2,20 IL‑7 [2], and IL‑1826 in various tumors. However, MSC engineered to express interleukin (IL)‑12 were recently shown to prevent metastasis into the lymph nodes and other internal organs as well as increased tumor cell apoptosis in mice bearing pre‑established metastases of melanoma, breast and hepatoma tumors.27 Furthermore,, in a separate study, human umbilical cord blood–derived mesenchymal stem cells (UCB‑MSCs) were successfully employed as delivery vehicles to deliver interleukin‑12, a therapeutic gene in the management of malignant glioma.28 In another study, human umbilical cord blood stem cells (UCBSCs) were engineered to express interleukin‑21 (IL‑21) and were shown to have therapeutic efficacy in mice bearing ovarian cancer xenografts. This study suggested that the UCBSC‑IL‑21 therapy was safe and feasible in ovarian cancer therapy, and that the method would be a promising new strategy for clinical treatment of ovarian cancer.29
Interferons
Human bone marrow MSC secreting interferon (IFN) – β has been proven to have success in diminishing a number of tumors such as melanoma,30 breast cancer31 and lung metastases.32 In a recently published study, amniotic fluid derived mesenchymal stem cells (AF‑MSCs) were isolated, investigated for their tumor tropism and capability to transport interferon‑β (IFNβ) to the region of neoplasia in a bladder tumor model. A significant inhibition of tumor growth as well as prolonged survival of mice was observed in the presence of AF‑MSC‑IFNβ, thereby demonstrating the potential of engineered AF‑MSCs as anti‑cancer vehicles.33 In another study, adipose tissue derived stem cells were engineered to secrete IFNβ and were used in combination with the chemotherapeutic agent, cisplatin to demonstrate a marked reduction in tumor growth in a mouse model of melanoma.34 Yi et al., recently demonstrated that genetically engineered stem cells are capable of migrating to and effectively eliminating lung cancer cells. Using a gene‑directed enzyme pro‑drug therapy, wherein human neural stem cells (NSC) were engineered to express the suicide gene, cytosine deaminase (CD) and also secrete IFNβ, Yi and his colleagues found a marked reduction in the growth of lung cancer cells in culture upon treatment with the prodrug for CD, 5‑flourocytosine.35
Suicide gene therapy
The suicide gene therapeutic approach is based on the conversion of non‑toxic prodrugs into active anticancer agents via introduction of non‑mammalian or mammalian enzymes. One of the earliest suicide gene therapies demonstrated is the herpes simplex virus thymidine kinase (HSV‑TK) Ganciclovir (GCV) system.36,37 HSV‑TK is a viral enzyme that catalyzes the phosphorylation of nucleotide analogs such as antiviral drug GCV.38 The phosphorylated GCV then selectively kills cancer cells through a bystander effect via apoptotic or non‑apoptotic mechanisms.39 In addition to this system, there have been several other suicide enzyme‑prodrug systems including cytosine deaminase (CD)/5‑fluorocytosine (5‑FC),40,41 cytochrome P450/cyclophosphamide,42 carboxypeptidase/chloroethyl‑mesyloxyethyl‑aminobenzoyl‑ glutamic acid (CMDA)43 and carboxylesterase (CE)/ CPT‑11.44 When human NSC line HB1.F3 carrying CD enzyme gene (F3.CD) was transplanted intracranially at distant sites from the tumor, NSC were shown to migrate through normal tissue and selectively home to the glioblastoma tumor mass, which resulted in a significant reduction in tumor volume upon administration of prodrug 5‑FC.45 In a separate study, HB1.F3 human NSCs carrying peroxin (PEX) gene, were found to surround the invading brain tumor cell population, chase down infiltrating tumor cells, and also significantly kill tumor cells as demonstrated by a reduction in tumor volume.46 Bone marrow derived MSCs expressing the suicide gene HSV‑TK, were demonstrated to potently eliminate prostate cancer cell mass both in culture and in vivo, in a study performed by Song et al.47 The different strategies that have been demonstrated so far reveal the promising potential of the combination of stem cell based therapies and suicide gene strategies to combat tumors.
Pro‑apoptotic proteins
The delivery of pro‑apoptotic proteins such as TRAIL (tumor necrosis factor‑related apoptosis induced ligand) via stem cells is a relatively new approach toward tumor cell killing. TRAIL is an endogenous member of the TNF ligand family that binds to its death domain containing receptors DR4 and DR5 and induces apoptosis via activation of caspases, preferentially in cancer cells while sparing most other cell types.48 A number of studies have shown that the therapeutic efficacy of different adult stem cell types, including MSC, engineered to express TRAIL in either cell lines or mouse models of colorectal carcinoma,49 gliomas,50‑52 lung, breast, squamous and cervical cancer53 result in induction of apoptosis and a subsequent reduction of tumor cell viability. Previous work from our laboratory has focused on designing a secretable version of TRAIL that consists of fusion between the extracellular domain of TRAIL and the extracellular domain of the hFlt3 ligand which binds to the Flt3 tyrosine kinase receptor. The re‑engineered recombinant protein named ‘secretable TRAIL’ (S‑TRAIL) is efficiently secreted into the producer cell’s immediate microenvironment and exhibits higher cytotoxicity on glioma cells than the native TRAIL protein.17,54,55
Encapsulated Stem Cells for Therapy
Cell encapsulation technology refers to immobilization of cells within biocompatible, semipermeable membranes. The encapsulation of cells instead of therapeutic products, allows the delivery of molecules of interest for a longer period of time as cells release these molecules continuously. In addition, genetically engineered cells can be immobilized to express any desired protein in vivo without the modification of the host's genome.56 Encapsulation of cells presents an important advantage as compared with encapsulation of proteins, as the latter allows a sustained and controlled delivery of therapeutic molecules at a constant rate giving rise to more physiological concentrations.56 Due to their ability to provide a physiologic environment that promotes cell survival and prevent immune response while permitting easy in vivo transplantation and cell retention, biodegradable hydrogels and synthetic extracellular matrix (sECM) to encapsulate stem cells have been utilized.57,58 A number of different biomaterials such as alginate, hyaluronic acid, agarose and other polymers have been used for encapsulation.
Hyaluronic Acid‑Based Clinical Biomaterials
Hyaluronic acid (HA) is a non‑sulfated, linear polysaccharide with the repeating disaccharide, β‑1,4‑D‑glucuronic acid‑β‑1,3‑N‑acetyl‑Dglucosamine. HA is ubiquitous and highly hydrated polyanion and an essential component of the extracellular matrix (ECM); its structural and biological properties mediate cellular signaling, wound repair, morphogenesis and matrix organization.59 Although HA and its derivatives have been clinically used in the past, it has become recognized as an important building block for the creation of new biomaterials for use in cell therapy.60‑62 Chemical modification of HA alters its material and biological properties,63 and targets three functional groups: the glucuronic acid carboxylic acid, the primary and secondary hydroxyl groups, and the N‑acetyl group (following deamidation). The chemical, mechanical, and biological criteria for clinical and preclinical biomaterials are design constraints that must be incorporated into the biomaterial design.60,64 HA‑based synthetic extracellular matrices (sECMs) have been developed for use in drug evaluation and regenerative medicine.65 These sECMs were based on modification of the carboxylate groups of glycosaminoglycans (GAGs) and proteins such as gelatin using hydrazides containing disulfides.66,67 More importantly, in vivo injectable cell suspensions in the sECM macromonomers can be crosslinked with cytocompatible bifunctional polyethylene glycol (PEG) derived crosslinkers.68 The mechanical properties and rates of biodegradation could be altered by several varying parameters69: (1) molecular weight of starting HA employed; (2) percentage of thiol modification; (3) concentrations of thiolated HA and thiolated gelatin; (4) molecular weight of the crosslinker polyethylene glycol diacrylate (PEGDA); and (5) ratio of thiols to acrylates. Living hydrogels allow control of gel composition and mechanics, and permit incorporation of cells and a wide variety of small molecules, large molecules, nanoparticles, and microparticles.63
Role of HA Based Gels in Different Disease Models
Due to their ability to provide a physiologic environment that promotes stem cell survival while permitting easy in vivo transplantation and cell retention, biodegradable HA based synthetic ECMs have been utilized in a variety of rodent models. A number of previous studies have shown that biodegradable sECM increase the viability of NSC and their differentiation into neurons in vitro.70 In models of intracerebral hypoxia‑ischemia and traumatic spinal cord injury, sECM acted as the necessary biomechanical substrate for endogenous neuro‑regeneration by increasing their stem cell viability and promoting differentiation into neurons.70‑72 Subsequent studies have again highlighted the utility of biodegradable scaffolds in facilitating stem cell‑based therapy in the CNS.73,74 While sECM are ideally suited for retaining therapeutic stem cells at the site of repair as has been demonstrated by the previous studies.
A number of studies have been published recently, which have employed the hyaluronic acid based gels for developing novel therapies for various diseases. Ganesh et al. have developed a novel hyaluronic acid based self‑assembling nanosystems wherein they have encapsulated siRNA‑targeting CD44 in these novel hyaluronic acid‑based systems to treat solid tumors.75 In another recent study, it was demonstrated that the addition of recombinant gelatin to hyaluronic acid based gels makes them unique scaffolds that can be injected for soft tissue growth.76 Chang et al. in their recent study, have demonstrated the use of a novel hyaluronic acid‑blood biodegradable hydrogel, which offers a unique potential of transplanting stem cells into the myocardium, thereby contributing to a better cardiac function following a myocardial infarction.77 Recent in vivo studies suggest considerable potential for transplanted biodegradable scaffolds containing stem (and other neuronal) cells in models of degeneration and hypoxia‑ischemia.78 Encapsulating human MSC has been described as a novel hypo‑immunogenic platform for cellular therapy and also demonstrated this strategy to release hemopexin‑like protein (PEX) an anti‑angiogenic agent for the treatment of a glioma tumor.79
Role of HA‑Based Gels in Developing Novel Therapies for Malignant Brain Tumors
Glioblastoma (GBM) is the most common primary brain tumor in adults with a very poor prognosis.80‑82 Currently, treatment for GBM is maximal surgical tumor resection (debulking)83 followed by radiation therapy, with concomitant and adjuvant chemotherapy.84,85 However, recurrence rates of GBM and the associated patient mortality are nearly 100%. Despite the numerous pre‑clinical studies, most in vivo GBM models do not mimic the clinical scenario of surgical debulking and focus on treating solid intact intracranial tumors. Therefore, in light of the central role tumor resection plays in clinical GBM therapy, development and implementation of mouse models of GBM resection are a necessity.86 In a recent study, we have developed a mouse resection model of GBM in cranial windows using malignant GBM cells engineered with fluorescent and bioluminescent proteins, which allow real time visualization of both growth and resection of tumors in vivo thereby simulating the clinical scenario of GBM resection. While resection of the primary tumor mass has shown clinical benefit, adjuvant chemotherapy has provided limited additional benefit (Fig. 2A).80,84 One of the major impediments to the efficient delivery of many therapeutic molecules is the blood brain barrier (BBB)87 and vascular dysfunction in the tumor,88 which prevents many drugs from reaching brain tumor cells. One of the approaches to overcome the drug delivery problems to intracranial tumors is to develop onsite means to deliver novel tumor specific agents. There are a number of limitations to effectively test stem cell‑based therapeutic interventions in a mouse model of GBM resection, including developing methods to introduce stem cells into the resection cavity to prevent rapid “wash‑out” of a significant number of cells by cerebrospinal fluid (CSF). Additionally, it is critical to allow efficient secretion of anti‑GBM therapies and retain the ability of stem cells to migrate from the resection cavity into the parenchyma toward invasive tumor deposits. Due to their ability to provide a physiologic environment that promotes stem cell survival while permitting easy in vivo transplantation and cell retention, we utilized sECMs that are based on a thiol‑modified hyaluronic acid (HA) and a thiol reactive cross‑linker (polyethylene glycol diacrylate), which provides biocompatibility, physiological relevance, and customizability (Fig. 1).26
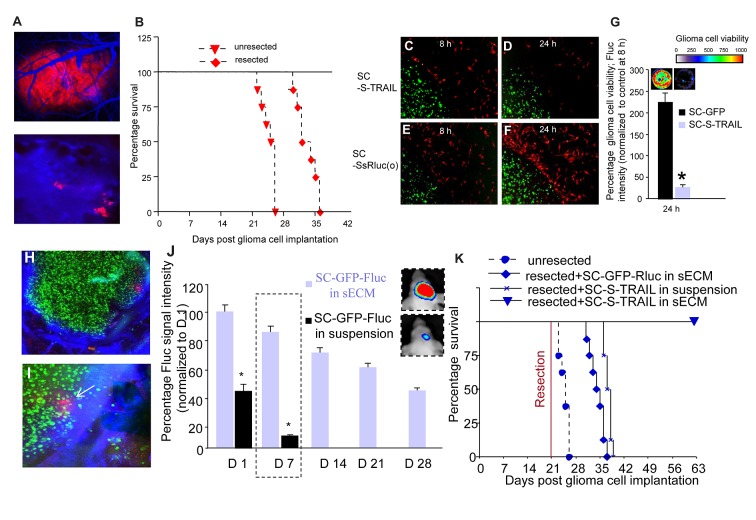
Figure 2. Stem Cells engineered to express S‑TRAIL have therapeutic efficacy in mouse tumor model of GBM resection. (A) Photomicrographs of mice bearing established U87‑mCherry‑Fluc GBM tumors in the cranial window that were injected with a blood pool agent, AngioSense‑750 before (top) and after (bottom) tumor resection. (B) Kaplan‑Meier survival curves of mice with and without resected U87‑mCherry‑Fluc tumors. (C‑G) Stem cell (SC) (green) expressing a secretable in vivo marker, Ss‑Rluc(o) or therapeutic S‑TRAIL were encapsulated in sECM and places in a culture dish containing U87‑mCherry‑Fluc tumor cells (red). Photomicrographs of SC at 8h (C,E) and 24h (D,F) and plot showing tumor cell viability (G). (H‑J) SC‑GFP‑Fluc in suspension or encapsulated in sECM were implanted intracranially in the resection cavity of the mouse model of resection, injected with Angiosense‑750 i.v. and mice were imaged by intravital microscopy and by serial imaging. Photomicrograph showing fluorescent images of sECM encapsulated SC‑GFP‑Fluc implanted in the resection cavity (H) and SC (green) targeting residual GBM cells (red) indicated by arrows in a tumor resection cavity with leaky vasculature (blue) (I). (J) Plot and representative figures of the relative mean Fluc signal intensity of SC‑GFP‑Fluc in suspension or encapsulated in sECMs placed in the GBM resection cavity. (K) SC‑S‑TRAIL or SC‑GFP‑Rluc encapsulated in sECM or SC‑S‑TRAIL in suspension were implanted intracranially in the tumor resection cavity and mice were followed for survival. Kaplan Meier survival curves are shown (adapted from ref. 86 with permission).
In our recent study, we first assessed the influence of sECMs on stem cell survival in vivo and showed that there was a significant increase in cell viability in mice bearing sECM encapsulated NSC as compared with the non‑encapsulated NSC (Fig. 2). In order to follow migration of sECM encapsulated NSC, we used intravital imaging on mice bearing GBMs in a cranial window and implanted with mNSC‑GFP‑Rluc encapsulated in sECMs 1 mm away from an established tumor. It was revealed that sECM encapsulated NSC migrate out of the sECMs and specifically home to tumors in the brain over a period of 4 d (Fig. 2). In order to assess the therapeutic potential of NSC expressing therapeutic proteins that specifically kill tumor cells, we engineered NSC to express secretable (S)‑tumor necrosis factor related apoptosis inducing ligand (TRAIL). TRAIL is a cytotoxic agent that is known to induce apoptosis in about 50% of GBM 89,90. We observed a significant reduction in GBM cell viability when NSC‑S‑TRAIL encapsulated in sECMs were placed in the culture dish containing TRAIL sensitive human GBM cells. Our in vitro studies thus revealed that sECM encapsulated engineered NSC survive longer in mice brains, migrate to tumors in the brain and induce apoptosis in cultured GBM cells (Fig. 2).
To assess survival of NSC encapsulated in sECMs in mouse model of GBM resection, we implanted NSC expressing a fusion of a fluorescent (GFP) and bioluminescent (Fluc) protein (NSC‑GFP‑Fluc) either in suspension or encapsulated in the resection cavity of U87 GBMs. sECM encapsulated NSC were retained in the tumor resection cavity at high local concentrations adjacent to the residual tumor cells and their survival in the tumor resection cavity over a period of 1 mo was significantly higher as compared with the non‑encapsulated NSC in the resection cavity (Fig. 2). When sECM encapsulated NSC‑S‑TRAIL were implanted intracranially in the tumor resection cavity to assess the therapeutic potential of sECM encapsulated NSC‑S‑TRAIL in mouse resection models of GBM, the sECM‑encapsulated NSC‑S‑TRAIL suppressed regrowth of residual tumor cells through 49 d post‑resection. Highlighting the survival benefit of this approach, 100% of mice treated with NSC‑S‑TRAIL encapsulated in sECM after GBM resection were alive 42 d post‑treatment as compared with the controls which showed a median survival of 14.5 d after GBM resection. Our studies thus revealed that sECM encapsulated therapeutic NSC are retained in the tumor resection cavity, kill residual GBM cells and result in significantly increased survival of mice. Our studies on freshly isolated primary GBM lines from GBM patients and human MSC expressing S‑TRAIL more accurately recapitulate the clinical scenario of GBMs and show that sECM encapsulated engineered human MSC have therapeutic benefits against primary patient derived GBMs. These studies clearly reveal that sECM encapsulated engineered stem cells are effective by way of increasing the concentration of therapeutic stem cells at the site of tumor resection to extend the drug exposure time to tumor cells.
Prospects and Caveats on the Way to the Clinics
Encapsulating therapeutic stem cells provides numerous advantages and helps overcome a number of caveats in the current cell based therapies. Stem cells have an incredible potential in developing novel targeted therapies for various diseases including cancer. The evidence that stem cells have the potential to migrate efficiently to lesions, tumors or other sites of damage has led to the development of unique stem cell based therapeutics which are gaining significant importance in today’s scientific world. This has triggered the urge in various researchers to study and obtain a detailed understanding of these mechanisms which thereby have led to the emergence of targeted therapeutics that are robust and effective. However, in spite of significant advances in this field, the translation of such therapies from bench to bedside still remains a daunting task. The appropriate disease models that exactly mimic the clinical scenario are very crucial to demonstrate the usefulness and applicability of novel therapeutic approaches. Emerging technologies like the encapsulation strategy are much needed to supplement the cell transplantation therapeutics, and the incorporation of such strategies aid in better delivery and longer duration of the desired therapy and thereby boost the probability of the success of these proposed therapies. The use of clinically employed biodegradable hydrogels like the HA‑based gels for encapsulation of “armed” stem cells will not only have a great impact on therapeutic outcomes but will also make the translation of therapies into clinics a reality.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Deepak Bhere and Tracy Twombly for help with the preparation of this manuscript. This work was supported by NIH grants CA138922, NS071197, CA173077 (KS) and James McDonald Foundation (KS).
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/24278
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–6. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 3.Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int. 2007;7:9. doi: 10.1186/1475-2867-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–46. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 6.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–42. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Li X, Zhao H, Feng R, Zhang X, Tai D, et al. Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev. 2013;22:1147–58. doi: 10.1089/scd.2012.0428. [DOI] [PubMed] [Google Scholar]
- 8.Momin EN, Vela G, Zaidi HA, Quiñones‑Hinojosa A. The Oncogenic Potential of Mesenchymal Stem Cells in the Treatment of Cancer: Directions for Future Research. Curr Immunol Rev. 2010;6:137–48. doi: 10.2174/157339510791111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow‑derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 10.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell‑derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song C, Li G. CXCR4 and matrix metalloproteinase‑2 are involved in mesenchymal stromal cell homing and engraftment to tumors. Cytotherapy. 2011;13:549–61. doi: 10.3109/14653249.2010.542457. [DOI] [PubMed] [Google Scholar]
- 12.Barrilleaux BL, Fischer‑Valuck BW, Gilliam JK, Phinney DG, O’Connor KC. Activation of CD74 inhibits migration of human mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2010;46:566–72. doi: 10.1007/s11626-010-9279-1. [DOI] [PubMed] [Google Scholar]
- 13.Ratajczak MZ, Shin DM, Schneider G, Ratajczak J, Kucia M. Parental imprinting regulates insulin‑like growth factor signaling: a Rosetta Stone for understanding the biology of pluripotent stem cells, aging and cancerogenesis. Leukemia. 2012 doi: 10.1038/leu.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5:477–88. doi: 10.1016/S1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 15.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–7. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 18.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–7. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 19.Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk‑1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–47. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration‑dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197–205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev. 2012;64:739–48. doi: 10.1016/j.addr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Yang G, Zhang H, Prestwich GD. Evaluating dual activity LPA receptor pan‑antagonist/autotaxin inhibitors as anti‑cancer agents in vivo using engineered human tumors. Prostaglandins Other Lipid Mediat. 2009;89:140–6. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu CH, Park SH, Park SA, Kim SM, Lim JY, Jeong CH, et al. Gene therapy of intracranial glioma using interleukin 12‑secreting human umbilical cord blood‑derived mesenchymal stem cells. Hum Gene Ther. 2011;22:733–43. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 25.Hu W, Wang J, Dou J, He X, Zhao F, Jiang C, et al. Augmenting therapy of ovarian cancer efficacy by secreting IL‑21 human umbilical cord blood stem cells in nude mice. Cell Transplant. 2011;20:669–80. doi: 10.3727/096368910X536509. [DOI] [PubMed] [Google Scholar]
- 26.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow‑derived mesenchymal stem cells as vehicles for interferon‑beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 27.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira‑Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted‑delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 28.Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon‑alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–8. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitsika V, Roubelakis MG, Zagoura D, Trohatou O, Makridakis M, Pappa KI, et al. Human amniotic fluid‑derived mesenchymal stem cells as therapeutic vehicles: a novel approach for the treatment of bladder cancer. Stem Cells Dev. 2012;21:1097–111. doi: 10.1089/scd.2011.0151. [DOI] [PubMed] [Google Scholar]
- 30.Seo KW, Lee HW, Oh YI, Ahn JO, Koh YR, Oh SH, et al. Anti‑tumor effects of canine adipose tissue‑derived mesenchymal stromal cell‑based interferon‑β gene therapy and cisplatin in a mouse melanoma model. Cytotherapy. 2011;13:944–55. doi: 10.3109/14653249.2011.584864. [DOI] [PubMed] [Google Scholar]
- 31.Yi BR, O SN, Kang NH, Hwang KA, Kim SU, Jeung EB, et al. Genetically engineered stem cells expressing cytosine deaminase and interferon‑β migrate to human lung cancer cells and have potentially therapeutic anti‑tumor effects. Int J Oncol. 2011;39:833–9. doi: 10.3892/ijo.2011.1126. [DOI] [PubMed] [Google Scholar]
- 32.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector‑producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–2. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 33.Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990;82:297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- 34.Hamel W, Magnelli L, Chiarugi VP, Israel MA. Herpes simplex virus thymidine kinase/ganciclovir‑mediated apoptotic death of bystander cells. Cancer Res. 1996;56:2697–702. [PubMed] [Google Scholar]
- 35.Fillat C, Carrió M, Cascante A, Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase gene/Ganciclovir system: fifteen years of application. Curr Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 36.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5‑fluorocytosine to 5‑fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91:8302–6. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Shanmugam N, Katayose D, Huber B, Srivastava S, Cowan K, et al. Enzyme/prodrug gene therapy approach for breast cancer using a recombinant adenovirus expressing Escherichia coli cytosine deaminase. Cancer Gene Ther. 1997;4:113–7. [PubMed] [Google Scholar]
- 38.Wei MX, Tamiya T, Rhee RJ, Breakefield XO, Chiocca EA. Diffusible cytotoxic metabolites contribute to the in vitro bystander effect associated with the cyclophosphamide/cytochrome P450 2B1 cancer gene therapy paradigm. Clin Cancer Res. 1995;1:1171–7. [PubMed] [Google Scholar]
- 39.Marais R, Spooner RA, Light Y, Martin J, Springer CJ. Gene‑directed enzyme prodrug therapy with a mustard prodrug/carboxypeptidase G2 combination. Cancer Res. 1996;56:4735–42. [PubMed] [Google Scholar]
- 40.Danks MK, Morton CL, Pawlik CA, Potter PM. Overexpression of a rabbit liver carboxylesterase sensitizes human tumor cells to CPT‑11. Cancer Res. 1998;58:20–2. [PubMed] [Google Scholar]
- 41.Kim SU, Jeung EB, Kim YB, Cho MH, Choi KC. Potential tumor‑tropic effect of genetically engineered stem cells expressing suicide enzymes to selectively target invasive cancer in animal models. Anticancer Res. 2011;31:1249–58. [PubMed] [Google Scholar]
- 42.Kim SK, Cargioli TG, Machluf M, Yang W, Sun Y, Al‑Hashem R, et al. PEX‑producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11:5965–70. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 43.Song C, Xiang J, Tang J, Hirst DG, Zhou J, Chan KM, et al. Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Hum Gene Ther. 2011;22:439–49. doi: 10.1089/hum.2010.116. [DOI] [PubMed] [Google Scholar]
- 44.Walczak H, Bouchon A, Stahl H, Krammer PH. Tumor necrosis factor‑related apoptosis‑inducing ligand retains its apoptosis‑inducing capacity on Bcl‑2‑ or Bcl‑xL‑overexpressing chemotherapy‑resistant tumor cells. Cancer Res. 2000;60:3051–7. [PubMed] [Google Scholar]
- 45.Mueller LP, Luetzkendorf J, Widder M, Nerger K, Caysa H, Mueller T. TRAIL‑transduced multipotent mesenchymal stromal cells (TRAIL‑MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011;18:229–39. doi: 10.1038/cgt.2010.68. [DOI] [PubMed] [Google Scholar]
- 46.Kim KU, Seo SY, Heo KY, Yoo YH, Kim HJ, Lee HS, et al. Antitumor activity of TRAIL recombinant adenovirus in human malignant glioma cells. J Korean Med Sci. 2005;20:1046–52. doi: 10.3346/jkms.2005.20.6.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12‑secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–63. [PubMed] [Google Scholar]
- 48.Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, et al. Induction of glioblastoma apoptosis using neural stem cell‑mediated delivery of tumor necrosis factor‑related apoptosis‑inducing ligand. Cancer Res. 2002;62:7170–4. [PubMed] [Google Scholar]
- 49.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–42. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah K, Tung CH, Yang K, Weissleder R, Breakefield XO. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer Res. 2004;64:3236–42. doi: 10.1158/0008-5472.CAN-03-3516. [DOI] [PubMed] [Google Scholar]
- 51.Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S‑TRAIL‑mediated tumor regression and apoptosis. Mol Ther. 2005;11:926–31. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Murua A, Portero A, Orive G, Hernández RM, de Castro M, Pedraz JL. Cell microencapsulation technology: towards clinical application. J Control Release. 2008;132:76–83. doi: 10.1016/j.jconrel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Ríhová B. Immunocompatibility and biocompatibility of cell delivery systems. Adv Drug Deliv Rev. 2000;42:65–80. doi: 10.1016/S0169-409X(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 54.Morris PJ. Immunoprotection of therapeutic cell transplants by encapsulation. Trends Biotechnol. 1996;14:163–7. doi: 10.1016/0167-7799(96)10020-2. [DOI] [PubMed] [Google Scholar]
- 55.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 56.Prestwich GD. Engineering a clinically‑useful matrix for cell therapy. Organogenesis. 2008;4:42–7. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allison DD, Grande‑Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–40. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 59.Prestwich GD. Hyaluronic acid‑based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–9. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prestwich GD. Simplifying the extracellular matrix for 3‑D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem. 2007;101:1370–83. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 61.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–48. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 62.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide‑crosslinked hyaluronan‑gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–34. doi: 10.1016/S0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 63.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross‑linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–11. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 64.Vanderhooft JL, Mann BK, Prestwich GD. Synthesis and characterization of novel thiol‑reactive poly(ethylene glycol) cross‑linkers for extracellular‑matrix‑mimetic biomaterials. Biomacromolecules. 2007;8:2883–9. doi: 10.1021/bm0703564. [DOI] [PubMed] [Google Scholar]
- 65.Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological properties of cross‑linked hyaluronan‑gelatin hydrogels for tissue engineering. Macromol Biosci. 2009;9:20–8. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan L, Ren Y, Cui F, Xu Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J Neurosci Res. 2009;87:3207–20. doi: 10.1002/jnr.22142. [DOI] [PubMed] [Google Scholar]
- 67.Sayyar B, Dodd M, Wen J, Ma S, Marquez‑Curtis L, Janowska‑Wieczorek A, et al. Encapsulation of factor IX‑engineered mesenchymal stem cells in fibrinogen‑alginate microcapsules enhances their viability and transgene secretion. J Tissue Eng. 2012;3:2041731412462018. doi: 10.1177/2041731412462018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KS, Auguste KI, et al. Global gene and cell replacement strategies via stem cells. Gene Ther. 2002;9:613–24. doi: 10.1038/sj.gt.3301721. [DOI] [PubMed] [Google Scholar]
- 69.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell‑based therapy. Front Biosci. 2008;13:806–21. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- 71.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self‑assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuin A, Zandstra J, Kluijtmans SG, Bouwstra JB, Harmsen MC, Van Luyn MJ. Hyaluronic acid‑recombinant gelatin gels as a scaffold for soft tissue regeneration. Eur Cell Mater. 2012;24:320–30. doi: 10.22203/ecm.v024a23. [DOI] [PubMed] [Google Scholar]
- 73.Chang CY, Chan AT, Armstrong PA, Luo HC, Higuchi T, Strehin IA, et al. Hyaluronic acid‑human blood hydrogels for stem cell transplantation. Biomaterials. 2012;33:8026–33. doi: 10.1016/j.biomaterials.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10:682–92. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 75.Goren A, Dahan N, Goren E, Baruch L, Machluf M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long‑term cellular therapy. FASEB J. 2010;24:22–31. doi: 10.1096/fj.09-131888. [DOI] [PubMed] [Google Scholar]
- 76.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 77.Affronti ML, Heery CR, Herndon JE, 2nd, Rich JN, Reardon DA, Desjardins A, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115:3501–11. doi: 10.1002/cncr.24398. [DOI] [PubMed] [Google Scholar]
- 78.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–83. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 79.Asthagiri AR, Pouratian N, Sherman J, Ahmed G, Shaffrey ME. Advances in brain tumor surgery. Neurol Clin. 2007;25:975–1003, viii‑ix. doi: 10.1016/j.ncl.2007.07.006. [viii‑ix.] [DOI] [PubMed] [Google Scholar]
- 80.Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 81.Erpolat OP, Akmansu M, Goksel F, Bora H, Yaman E, Büyükberber S. Outcome of newly diagnosed glioblastoma patients treated by radiotherapy plus concomitant and adjuvant temozolomide: a long‑term analysis. Tumori. 2009;95:191–7. doi: 10.1177/030089160909500210. [DOI] [PubMed] [Google Scholar]
- 82.Kauer TM, Figueiredo JL, Hingtgen S, Shah K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat Neurosci. 2012;15:197–204. doi: 10.1038/nn.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 84.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 85.Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998;427:124–8. doi: 10.1016/S0014-5793(98)00409-8. [DOI] [PubMed] [Google Scholar]
- 86.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–23. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 88.Son BR, Marquez‑Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal‑derived factor‑1‑CXCR4 and hepatocyte growth factor‑c‑met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 89.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106:4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma W, Fitzgerald W, Liu QY, O’Shaughnessy TJ, Maric D, Lin HJ, et al. CNS stem and progenitor cell differentiation into functional neuronal circuits in three‑dimensional collagen gels. Exp Neurol. 2004;190:276–88. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]