Abstract
The mechanism by which inhaled anesthetics work is not fully understood, although they have been extensively used. Much research has been done showing the likelihood that there is more than one pathway or mechanism of action. A long-term goal of our laboratory is to decipher these mechanisms using Drosophila melanogaster, an excellent model organism for this purpose. In order to do this, we have modified and constructed an apparatus called the inebriometer to quantitatively analyze the response of flies to inhaled anesthetics. While the inebriometer is not new to the fly community, our updated design provides a relatively low-labor, high-throughput means for performing screens in search of genes involved in the anesthetic mechanism. Here we describe our construction of an airtight inebriometer that we have designed for this purpose. We also provide data that validates this apparatus and establishes a procedure for its use.
Keywords: inebriometer, construction, airtight, anesthesia, Drosophila, behavioral assay, drug response
Introduction
For more than 160 years, inhaled volatile anesthetics (VA) have been making surgical and other invasive procedures possible.1 Today, the use of anesthesia in the hospital operating room is the standard of care. It is estimated that worldwide, there are over 234 million major surgical procedures performed each year, with more than 63 million in the United States.2 General anesthesia induced by VAs is effective because it induces loss of consciousness, amnesia, analgesia, and muscle relaxation. The mechanism of how VAs work and the biochemical pathway(s) involved, however, are still not fully understood. It is generally thought that their anesthetic effects occur through multiple pathways,3,4 although there also appears to be some evidence that indicates that individual VAs may work via unique pathways.4
It is believed that VAs act upon different anatomic and molecular targets, thus providing the various behavioral responses that make general anesthesia so useful.5 It has been demonstrated that intravenous general anesthetics affect consciousness in the form of gamma-amino butyric acid type A (GABAA), opioid and α2 adrenergic receptor agonists, or N-methyl D-aspartate (NMDA) and dopamine receptor antagonists.6 It is currently hypothesized that VAs also cause their effects through GABAA and NMDA receptors as well as other receptors and ion channels.7
The Drosophila nervous system, with many of the same or similar neurotransmitters, receptors and ion channels to those of vertebrates, has already been used as a model for the human nervous system.8 It turns out that Drosophila melanogaster is an excellent model for studying the mechanism of action of anesthesia. It responds similarly to humans when exposed to inhaled anesthetics and also requires comparable concentrations for their effect.9 Much progress has been made in identifying a number of genes in mutant flies that show an altered response to VAs.10-15 In addition, the use of model organisms for identifying genes involved in the anesthetic mechanism has proven to be valuable and has uncovered a conserved mechanism between Caenorhabditis elegans and Drosophila.4 However, the mechanism of action of VAs is still poorly understood and has yet to be completely defined.
The behavioral testing apparatus called the inebriometer was first described many years ago.16-18 It was created to quantitatively perform behavioral assays on flies exposed to ethanol vapors in which total inebriation (i.e., being knocked out) is the desired endpoint. It has been used in a few studies to quantitatively compare the response of a variety of mutant strains of Drosophila to VAs.11,19-21 However this original design had flaws: It was not completely airtight and data collection was labor-intensive. We presume that these issues caused it to be discontinued in its use with VAs. Since then, others have modified the inebriometer with modern technology to make it more automated.22,23 Instructions to assemble this modified inebriometer were previously made public on a website by Dr Ulrike Heberlein (heberleinlab.ucsf.edu/).
We set out to build this apparatus, with a desire to perform genetic screens to identify specific genes within the Drosophila genome that play a role in VA function. Given the effects of anesthesia, including potential toxicity with frequent exposure, we determined to make an airtight version to avoid unnecessary VA exposure while running experiments.24 Since the inebriometer was going to be used in genetic screens, throughput and ease of use were also factors that motivated the modification of the Heberlein design in our version of the inebriometer. The purpose of this paper is to supply sufficient detail and provide ideas and options beyond the typical scientific supply sources, so that others might also construct their own airtight inebriometer, and even modify it further, if needed. Our design allowed the use of veterinary medical supplies and items from hardware supply stores, which can potentially decrease the cost of production. Although based on the Heberlein design, our inebriometer has been significantly modified. We also describe our validation of the apparatus, protocol for its use, and considerations for others wishing to build an inebriometer for their use.
Results
The inebriometer reports the number of flies dropping through the end of the column in 3 sec intervals. This allows for real-time analysis of the flies’ response to the VA (Fig. 1). This data, when plotted against time, was very similar in appearance to typical dose response curves; therefore regression analysis of the data was performed using the Hill slope equation (see Materials and Methods). This allows for determination of the number of flies eluted at a given time, or the time (number of seconds) it takes to elute a given number, or percentage, of flies. By determining the time it takes to elute 50% of the flies through the column, we obtain a statistic we term the ET50 (Elution Time for 50%), similar in concept to the commonly used ED50 (Effective Dose for 50%). This is a very useful statistic in identifying flies with different responses to VAs. However, despite the intuitive and useful nature of the ET50, this number is difficult to perform standard statistical analysis with using commonly used software such as MS Excel or even SPSS, since it is a value representing a median and not a mean. Furthermore, the large data sets (n = 100–150 flies/run with 4–12 runs/condition) makes it challenging to statistically analyze the data and not find differences. Therefore, other statistical measures were also employed in the analysis (see Materials and Methods).
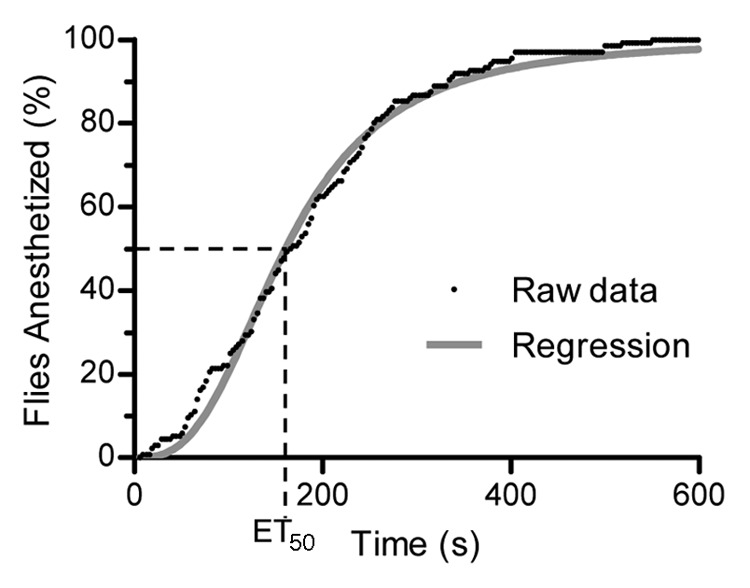
Figure 1. Observed inebriometer data and regression analysis. The raw data of flies falling out of the inebriometer, collected in 3-sec intervals, is plotted as the percent of the total number of flies anesthetized in 10 min. Regression analysis of this data are performed (as described in Materials and Methods) to allow for the calculation of the time it takes for half of the flies to be anesthetized (ET50). The data as presented here represent one run.
Initial testing of the inebriometer was performed using a bioassay with a wild-type strain, Oregon-R, to ascertain if the behavior of the flies appropriately reflected the dose of the anesthetic. A dose response experiment was performed with the commonly used VA, isoflurane (Fig. 2). This figure shows the percentage of flies that were anesthetized (i.e., had fallen through the inebriometer) at 75 sec. The 75-sec time point was chosen to maximize the amount of data obtained from the linear portion of the elution curve from all concentrations of isoflurane used. Many of the data points provided such tight responses that the error bars are virtually absent; however, all data points represent the mean ± standard error of the mean (SEM). Regression analysis of this data revealed an ED50 of 1.3% isoflurane, which is close to the median alveolar concentration of 1.15% observed in humans for this VA. This validated the use of the inebriometer and our procedure for testing VA responsiveness, as well as determining that 1% isoflurane would be used in future studies. As with all laboratory strains, the Oregon-R stock used in the dose response experiment has been maintained in our laboratory for many years. Therefore, to determine if use of our laboratory-maintained Oregon-R strain had a unique response, Oregon-R-C and Oregon-R-S strains were obtained from the Bloomington Drosophila Stock Center. No significant differences in isoflurane responsiveness were observed between the Bloomington and our laboratory strains with 1% isoflurane (Fig. S1).
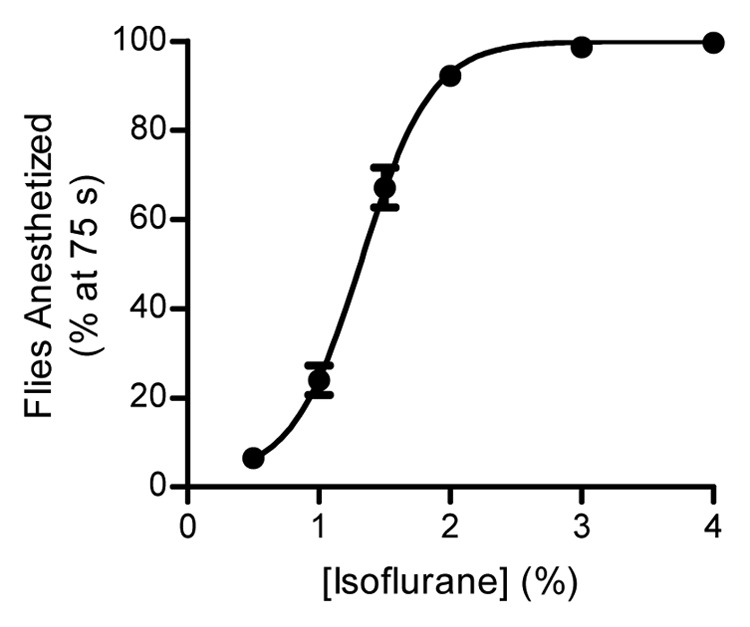
Figure 2. Response of Oregon-R flies to different doses of isoflurane. The percentage of flies anesthetized at 75 sec in relation to the total number of flies loaded in the column. The data were curve fit as described in Materials and Methods (R2 = 0.95) and the ED50 was determined to be 1.3% isoflurane. Each data point represents the mean ± SEM of at least 10 separate runs.
Previous studies that used inebriometers to study VA responsiveness did not include statistical analysis.11,19-21 Therefore, in order to determine if our newer version of the inebriometer could quantitatively determine differences of responses between strains to VAs, we first identified a suitable positive control—a previously identified mutant fly strain. This fly strain, nahar38, has a mutation in a gene coding for a sodium channel, narrow abdomen (na), which makes it resistant to halothane and other VAs—making it a perfect positive control for testing the inebriometer.11,20 Elution profiles showing the number of flies that elute in each 30 sec interval as a percentage of all flies falling in 10 min for the wild-type Oregon-R flies (which were used as a control in this experiment) and heterozygous female nahar38/FM6 flies are shown in Figure 3A. The wild-type flies exhibit a clearly right-skewed distribution (i.e., most of the flies are anesthetized early), while it is very evident that the nahar38 flies have a very different and delayed response to the anesthetic—indeed, this is a clearly left-skewed distribution, showing resistance to the anesthetic. This data was further analyzed by calculating the mean elution time (MET) for each run. The MET has been used previously to quantitatively compare responses to ethanol vapors in an inebriometer.22 The MET is a value that is similar in nature to the ET50; however, it is representative of the mean of the population, and therefore, typical statistical approaches (e.g., ANOVA, t-tests) can be used with it. The wild-type strain had an average MET of 152.8 sec, which was significantly lower than the average MET of 408.3 sec for nahar38 (p < 0.0001; Fig. 3B). The average MET was computed from at least 12 separate runs for each strain.
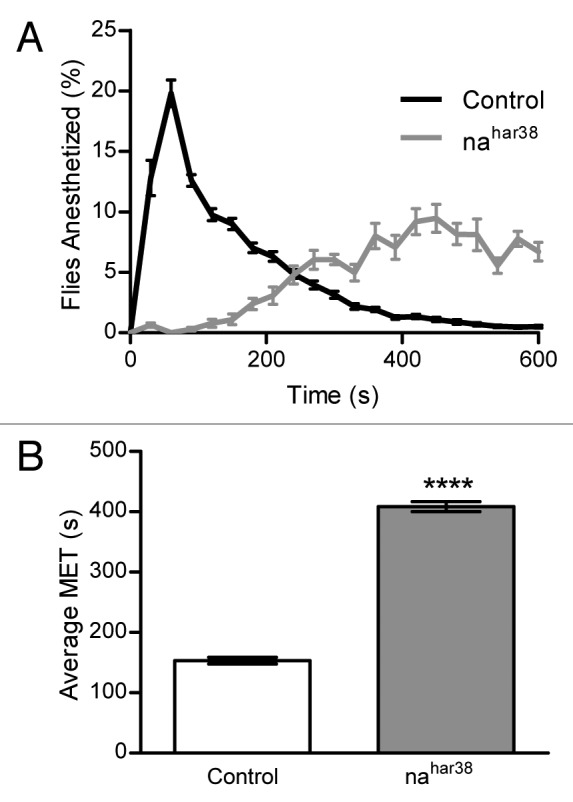
Figure 3. The inebriometer effectively quantifies differences in isoflurane responses between control and nahar38 flies. (A) Elution profiles of control (Oregon-R) and heterozygous female nahar38/FM6 (nahar38) flies in response to 1% isoflurane. Flies were counted in 30-sec intervals and divided by the total number of flies that fell in 10 min. The data points represent the mean ± SEM of the percentage of flies eluting at that time point from at least 12 runs. (B) The MET was calculated as described in Materials and Methods for the data shown in (A). The nahar38 MET is larger than that of the control flies (****p < 0.0001) as determined by a one-tailed, unpaired t-test, assuming equal variances.
A number of important considerations went into optimizing the protocol for performing a VA assay with the inebriometer. For instance, it was determined that at least 10 min of flooding the column with the anesthetic at 2 L/min per column was sufficient to equilibrate the inebriometer with the VA to provide equivalent outcomes due to anesthetic exposure (data not shown). Similarly, the flow rate of the VA vapor through the column during the experiment had no effect on MET. When the flow of 1% isoflurane was increased from 0.5 to as high as 8 L/min, no statistical differences were determined between the average MET for the Oregon-R strain (Fig. S2). Therefore, all of the inebriometer runs were performed at 0.5 L/min per column to conserve anesthetic.
In comparing the reaction of the flies to the anesthetic, a number of important considerations had to be taken into account in the protocol regarding the preparation of flies as well. As would be assumed, it became apparent that older or unhealthy flies had a reduced average MET (i.e., they showed increased sensitivity to anesthetic) when compared with younger and healthier flies (data not shown). Therefore, all flies in the experiments were collected and run prior to 20 d of age post eclosion at 25°C, while most of them were run when they were still less than 10 d old. Another variable that affected the response of the flies to VAs was prior CO2 exposure. Flies that had been previously exposed to CO2 for 5 min and allowed to recover exhibited a resistant phenotype to isoflurane (Fig. 4). When the flies recovered for 1 h after CO2 exposure, they had an increased average MET of 243.3 sec compared with 183.1 sec in flies not exposed to CO2 (p < 0.001). This was intriguing because these flies appeared to be fully alert and exhibited normal behavior. This resistant phenotype diminished to an average MET of 227.1 sec in flies that recovered for 2 h; however, they were still resistant to the isoflurane (p < 0.01). No significant differences in the average MET were observed in flies that recovered for 4 or 8 h after CO2 exposure, or were exposed to CO2 the night before. Therefore, to avoid any possible effect, the collection protocol did not expose flies to CO2 prior to an experimental run. However, when genotypes needed to be sorted or the flies counted, all CO2 exposure was done the night prior to the inebriometer run.
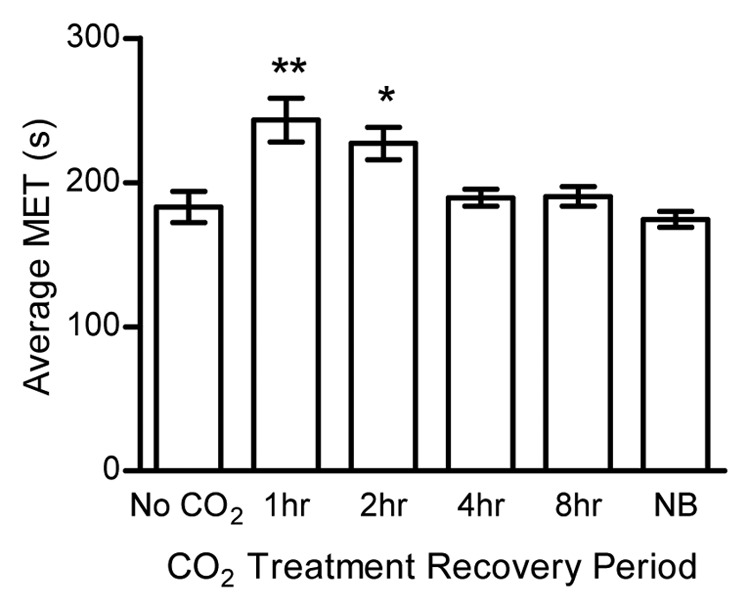
Figure 4. Prior CO2 exposure affects subsequent response to anesthesia. Flies were exposed to CO2 for 5 min and then allowed to recover for the time noted. Compared with flies that had no exposure to CO2 (No CO2) flies that recovered for 1 and 2 h had significantly longer average METs as determined by a Kruskal Wallis test with a Dunn’s multiple comparison test, post-hoc. There was no effect on the average MET in flies that had longer recovery times, including flies that were exposed the night before the run (NB). Each bar represents the average MET ± SEM from at least 8 separate runs. **p < 0.01, *p < 0.05
Discussion
Our research shows that the modifications we have made to the inebriometer help to obtain quality data for the screening of genes involved in the mechanism of anesthesia induced by VAs. The inebriometer is able to objectively compare many strains of mutant Drosophila, and through the use of these methodical steps and the Drosophila Funnel Monitor (DFM), much of the human labor, subjectivity and potential error is reduced. Another benefit of this apparatus is the ability to perform many runs back to back that allows for data to be obtained on many mutant strains in just a few hours. The vaporizer and setup chosen to be used for this apparatus have inherent variability, but this is a tradeoff made for throughput and ease of use. Additionally, by using a large population of control flies, an adequate range of normal values can be established for statistical purposes. For its use as a screening tool, we are primarily interested in identifying the strains that are different from the control population, which is something that is easily performed with the inebriometer, as seen in Figure 3.
When the variety of methods utilized to study anesthetic behavior in flies is considered, it becomes immediately apparent that it is difficult to standardize or directly compare results across studies. This is likely due to the fact that most of the methods for determining VA responses varied considerably among the studies, with many of them involving a great deal of subjectivity that could present variability among laboratories. Within this study, an example of such a source of variability is the fact that prior CO2 exposure affects the responsiveness of flies to VAs. Indeed, it was the variability observed with flies exposed to CO2 that led to our understanding of this phenomenon. While this might be an obvious point of consideration to a scientist trained in physiology, this might be overlooked by fly geneticists not trained in physiology when performing varying VA assays, leading to different results between studies. Indeed, a review of the Drosophila VA literature clearly shows that some laboratories understood that this was an important enough point to mention that CO2 exposure was not performed prior to their VA assay, while there is no mention of it in other papers.
It is more intuitive to think that prior anesthesia would actually lead to subsequent increased sensitivity to anesthetics. Therefore, it is intriguing to find that one form of anesthesia, like CO2, would actually make a fly more resistant to subsequent anesthesia by another chemical. It has been proposed that CO2 in its hydrate form, H2CO3, can affect integral membrane proteins.25-27 Additionally, a recent study revealed that CO2 exposure results in important metabolic changes and altered energetic metabolism that last for hours following CO2 exposure.28 Both of these situations might be a possible explanation for this CO2-pretreatment resistant phenotype if the CO2 treatment can decrease the function of VA target proteins or decrease mitochondrial energy production. The latter possibility is intriguing given that unpublished results from our laboratory indicate that mutations in electron transport chain genes generally yield VA resistant phenotypes. Whether this change in responsiveness is a characteristic of CO2 exposure, or any anesthetic is unknown. For the purposes of this techniques and methods paper, however, it is good to know that the assay in our study was sensitive enough to detect this effect. All the initial tests and trials have convinced us of the soundness of our apparatus and protocol, and that they are capable of reliably performing the necessary behavioral assay for identifying mutant strains with different anesthetic responsiveness. Therefore, the inebriometer, as described here, along with the accompanying protocol, can act as standard in this area of research, allowing researchers to build, use and compare their data directly with those of others.
Materials and Methods
Inebriometer assembly
As this is a methods and technical advances paper, all the necessary information regarding the construction, assembly and materials and methods for the inebriometer has been provided in brief here. For further details, we refer the reader to the supplementary online material to allow for accurate reproduction of the apparatus. Four four-foot long glass columns were each fitted with 17 internal mesh baffles, evenly spaced and alternating in their orientation by 180° (Figs. S3–S6). These allow flies the opportunity to catch themselves if only partially anesthetized and potentially move upward. At the top of the column, a clear PVC pipe with an angled upper edge was attached (Fig. S8). An acrylic sheet with a cut centrifuge tube inserted into its center was attached to the angled surface of the pipe to allow for inserting the flies via a modified syringe (Figs. S8, S9, and S17). Following insertion, the flies enter the column through a funnel that inserts through a mesh barrier, which prevents the flies from leaving the column (Fig. S6).
A glass funnel connects the base of the column to a collection box, with a DFM (TriKinetics Inc.) in-between to count the flies (Fig. S7). After the glass funnel neck goes through the DFM, it then inserts through a hole in the center of the top of an airtight collection box (Fig. S13). An outlet was installed into the posterior side of the collection box through which the VA could exhaust to the laboratory hood ventilation system (Fig. S12). An inlet port for the VA gas mixture was installed into the posterior of the insertion chamber at the top of the column. Airtight seals were established at all junctions within and between sections of the inebriometer by using silicone sealant, stopcock grease and O-rings as necessary. A low decibel compressor, P50TC AL Panther Silent Compressor (Werther International Inc.) was used to provide air at 50 to 55 pounds/inch2 to an air flowmeter that then attached to an Ohmeda Tec style isoflurane vaporizer, which is commonly used in veterinary medicine (Fig. S3). One vaporizer is able to run all four columns simultaneously. The vaporizer can be set to the desired concentration (i.e., 0.5%, 1%, 2%, etc.) of anesthetic with a ± 15% possible variation from the stated concentration. The vaporizer is certified annually to ensure appropriate dosages and is recalibrated every 3 to 5 y. The vaporizer was filled with medical grade isoflurane obtained from the veterinarian in our animal facility.
From the vaporizer, the anesthetic vapor output was distributed through tubing containing two Y-connectors in a series, making four equal length distribution lines. Ball-valves were placed in each line prior to the column to accommodate turning on/off individual columns (Figs. S5 and S11). The DFM at the base of the column connects into a Power Supply Interface Unit (TriKinetics Inc.) and from there into a computer that records the data through a USB connection. Trikinetics provides a free program called the Drosophila Activity Monitoring (DAM) System that is able to format the data into a text document.
Operation of the inebriometer
The columns are initially flooded with the isoflurane gas at a flow rate of 2 L/min/column for at least 10 min prior to inserting the flies. Following the flooding, the flow rate of the gas is reduced to 0.5 L/min/column. The DAM software is turned on to capture data in 3-sec intervals and the flies are injected into the top of the inebriometer. Data collection continues for at least 10 min. Results are then analyzed (in Excel) to curve-fit the data and determine the ET50 and MET values.
Fly stocks and handling
Flies are inserted into the inebriometer, exposed to the isoflurane gas, counted using the DFM and collected in a soapy water morgue (alcohol was specifically avoided) in the collection box (Fig. S13). An Oregon-R stock maintained in our laboratory for many years is the main strain used in the experiments. Other stocks, nahar38/FM6 (FBst0026704), Ore-R-C (FBst0000005) and Ore-R-S (FBst0004269) were obtained from the Bloomington Drosophila Stock Center. The flies are raised on standard cornmeal molasses agar food and maintained at 25°C. About 100–150 flies are used in each column for each run. If sorting or counting is required, CO2 is used the night before the inebriometer run, unless noted otherwise in the experiment. Flies are collected and run before they reach 20 d post eclosion.
Data analysis
In all the experiments performed, the raw fly elution data coming from the DFM was only collected for 10 min. We found that this time frame was sufficient to identify differences in anesthetic responses. Typically, the raw fly elution data was obtained and curve fit in Excel using the Solver plugin. The formula used for regression analysis was:
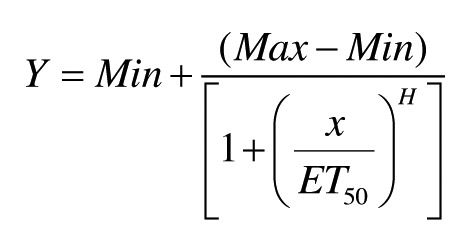 |
where H = Hill slope coefficient, and the ET50 is the time at which half of the flies are eluted. The H and ET50 values are determined by iteration, while Min and Max are constrained to 0 and 100, respectively. In general, the curve fit excellently (as seen in Fig. 1) with typical R2 values being > 0.95. In order to create a typical dose response curve, the percentage of flies anesthetized at 75 sec was determined from each of the individually curve-fit experiments. The average ± SEM of these numbers were plotted vs. the concentration of isoflurane used and these data points were fit with the above formula to determine the ED50 using GraphPad Prism software. MET has been used and described previously.22 Briefly, weighted elution rates are calculated for all 30-sec intervals for the entire 10-min period by taking the number of flies eluted within that interval multiplied by the time of elution (maximum value of that interval in sec) divided by the total number of flies eluted in the 10-min time frame. The MET is the sum of these individual weighted rate values. Thus, the MET is computed in the same manner as the arithmetic mean would be computed from a frequency distribution. The METs from multiple runs are averaged appropriately (weighted) and this value is statistically analyzed as noted in each figure legend using the GraphPad Prism software. On average, each experiment was repeated 13 times, with the minimum number of replicates being four separate runs.
Supplementary Material
Acknowledgments
We thank Drs Weber and Heberlein for their contribution to our construction of the inebriometer. This work was performed with startup funds from Midwestern University and a generous donation from the Charity Fidelity Gift Fund. We thank Daniel Dawson for the photography required for describing the construction of this apparatus. We also would like to acknowledge Krista Pearman for her excellent technical assistance throughout the project.
Glossary
Abbreviations:
- VA
volatile anesthetic
- GABAA
gamma-amino butyric acid type A
- NMDA
N-methyl D-aspartate
- ET50
elution time for 50% of flies
- ED50
50% effective dose
- DFM
Drosophila funnel monitor
- DAM
Drosophila activity monitoring
- na
narrow abdomen
- MET
mean elution time
- SEM
standard error of the mean
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental material may be found here: http://www.landesbioscience.com/journals/fly/article/24142
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/24142
References
- 1.Antkowiak B. How do general anaesthetics work? Naturwissenschaften. 2001;88:201–13. doi: 10.1007/s001140100230. [DOI] [PubMed] [Google Scholar]
- 2.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 3.Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr., Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–48. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, et al. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol. 2007;17:624–9. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 5.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 6.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmings HC., Jr. Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anaesth. 2009;103:61–9. doi: 10.1093/bja/aep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci. 2010;11:514–22. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allada R, Nash HA. Drosophila melanogaster as a model for study of general anesthesia: the quantitative response to clinical anesthetics and alkanes. Anesth Analg. 1993;77:19–26. doi: 10.1213/00000539-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Gamo S, Dodo K, Matakatsu H, Tanaka Y. Molecular genetical analysis of Drosophila ether sensitive mutants. Toxicol Lett. 1998;100-101:329–37. doi: 10.1016/S0378-4274(98)00203-3. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan KS, Nash HA. A genetic study of the anesthetic response: mutants of Drosophila melanogaster altered in sensitivity to halothane. Proc Natl Acad Sci U S A. 1990;87:8632–6. doi: 10.1073/pnas.87.21.8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholz H, Franz M, Heberlein U. The hangover gene defines a stress pathway required for ethanol tolerance development. Nature. 2005;436:845–7. doi: 10.1038/nature03864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walcourt A, Nash HA. Genetic effects on an anesthetic-sensitive pathway in the brain of Drosophila. J Neurobiol. 2000;42:69–78. doi: 10.1002/(SICI)1097-4695(200001)42:1<69::AID-NEU7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Walcourt A, Scott RL, Nash HA. Blockage of one class of potassium channel alters the effectiveness of halothane in a brain circuit of Drosophila. Anesth Analg. 2001;92:535–41. doi: 10.1213/00000539-200102000-00047. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Hu ZY, Ye QQ, Dai SH. Development of three Drosophila melanogaster strains with different sensitivity to volatile anesthetics. Chin Med J (Engl) 2009;122:561–5. [English Edition] [PubMed] [Google Scholar]
- 16.Cohan FM, Graf JD. Latitudinal cline in Drosophila melanogaster for knockdown resistance to ethanol fumes and for rates of response to selection for further resistance. Evolution. 1985;39:278–93. doi: 10.2307/2408362. [DOI] [PubMed] [Google Scholar]
- 17.Weber KE. An Apparatus for Measurement of Resistance to Gas-phase Reagents. Drosoph Inf Serv. 1988;67:90–2. [Google Scholar]
- 18.Cohan FM, Hoffmann AA. Genetic divergence under uniform selection. II. Different responses to selection for knockdown resistance to ethanol among Drosophila melanogaster populations and their replicate lines. Genetics. 1986;114:145–64. doi: 10.1093/genetics/114.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibovitch BA, Campbell DB, Krishnan KS, Nash HA. Mutations that affect ion channels change the sensitivity of Drosophila melanogaster to volatile anesthetics. J Neurogenet. 1995;10:1–13. doi: 10.3109/01677069509083455. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DB, Nash HA. Use of Drosophila mutants to distinguish among volatile general anesthetics. Proc Natl Acad Sci U S A. 1994;91:2135–9. doi: 10.1073/pnas.91.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash HA, Campbell DB, Krishnan KS. New mutants of Drosophila that are resistant to the anesthetic effects of halothane. Ann N Y Acad Sci. 1991;625:540–4. doi: 10.1111/j.1749-6632.1991.tb33885.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/S0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 23.Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2000;24:1127–36. doi: 10.1111/j.1530-0277.2000.tb02075.x. [DOI] [PubMed] [Google Scholar]
- 24.Carney FM, Van Dyke RA. Halothane hepatitis: a critical review. Anesth Analg. 1972;51:135–60. doi: 10.1213/00000539-197201000-00036. [DOI] [PubMed] [Google Scholar]
- 25.Miller SL. A theory of gaseous anesthetics. Proc Natl Acad Sci U S A. 1961;47:1515–24. doi: 10.1073/pnas.47.9.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauling L. A molecular theory of general anesthesia. Science. 1961;134:15–21. doi: 10.1126/science.134.3471.15. [DOI] [PubMed] [Google Scholar]
- 27.Sillans D, Biston J. Studies on the anesthetic mechanism of carbon dioxide by using Bombyx mori larvae. Biochimie. 1979;61:153–6. doi: 10.1016/S0300-9084(79)80063-2. [DOI] [PubMed] [Google Scholar]
- 28.Colinet H, Renault D. Metabolic effects of CO(2) anaesthesia in Drosophila melanogaster. Biol Lett. 2012;8:1050–4. doi: 10.1098/rsbl.2012.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


