Abstract
An ambient method for rapid monitoring and quantitation of drugs of abuse in dried blood spots was developed using paper spray tandem mass spectrometry (PS-MS).
Drug abuse is a serious problem worldwide with an estimated 200 million users of illicit drugs.1 Many addictive drugs have valuable therapeutic effects in clinical use. For example, buprenorphine, oxycodone and heroin are useful analgesics, but abuse of these drugs has a large impact on addicts’ health as well as their social lives.2,3 Analysis of drugs in biofluids, such as urine and blood, is applied for screening drug users as well as for monitoring them undergoing therapy.4,5 In the development of therapeutic drugs for treatment of the drug abuse, dosing guidelines are established on the basis of pharmacokinetic (PK) and pharmacodynamic (PD) clinical studies; these study is dependent on the analysis of the therapeutic drugs as well as the drugs of abuse and their metabolites in blood.6 The use of drugs in therapy, such as the pain relief drug buprenorphine often used in management of opioids dependence, needs to be strictly controlled to avoid addiction as well as other side effects.7,8
Currently, immunoassays are widely used for rapid screening of drugs of abuse.9 Confirmation of positive results is achieved by liquid chromatography mass spectrometry (LC-MS) or gas chromatography mass spectrometry (GC-MS), which are the most reliable methods for chemical identification and quantitation of drugs.6,10 To achieve high accuracy and sensitivity in LC-MS and GC-MS, samples of biofluids need to be carefully prepared. For blood analysis, sample preparation includes depositing the blood on a disk to form a dried blood spot, punching out the dried blood spot, extracting the analytes, followed by purification and concentration by LC or GC.11, 12 This type of multi-step procedure works well for chemical analysis of large batches of samples. Single-step methods based on ambient ionization mass spectrometry13 have been shown to have good sensitivity for analysing target analytes in complex mixture samples.
In ambient ionization mass spectrometry, the analytes are directly ionized from a sample in its native state and transferred into the gas phase for MS analysis with little or no sample preparation. Starting with desorption electrospray ionization (DESI)14 and direct analysis in real-time (DART),15 many ambient ionization methods have been reported, such as atmospheric solids analysis probe (ASAP),16 surface sampling probe (SSP),17 extractive electrospray ionization (EESI),18 laser-ablation electrospray ionization (LAESI),19 matrix-assisted laser desorption electrospray ionization (MALDESI)20 and atmospheric-pressure thermal desorption ionization (APTDI).21 The application of ambient ionization to the analysis of dried blood spot (DBS) samples is of a significant interest, since DBS is becoming a standard method for storage and transport of blood samples.22 A very small amount (10 µL–20 µL) of blood is needed for one DBS, which allows less invasive methods for screening, such as finger prick, to be utilized when taking the sample. Collectively, these techniques potentially could provide great advantages in high-throughput screening for drugs of abuse and for development of therapies to treat abuse. The drugs and their metabolites can be quickly analyzed without extensive sample treatment. However, high accuracy and reproducibility in quantitation needs to be assured to establish the validity of these applications23 and this is the topic of this communication.
Paper spray (PS) has recently been used as an ambient ionization method in a range of problems including therapeutic drug monitoring.24 food safety,25 neonatal screening26 and tissue analysis.27, 28 To perform paper spray MS, the sample is deposited on a piece of chromatographic paper in triangle shape to form a dried sample spot. A spray solvent of small amount (10s of microliters) is applied onto the paper to extract the analytes from the sample, which are subsequently ionized in the spray produced when a high voltage is applied to the paper. When internal standards (IS) are mixed into the sample, highly precise quantitative results have been obtained for paper spray MS. Hence, paper spray is suitable for high-throughput DBS analysis at extremely low-cost and organic solvent consumption. In this manuscript, we report the development of a rapid method of screening and quantitative determination of drugs and their metabolites based on DBS analysis using paper spray MS associated for screening and therapy of drug abuse. The entire analysis process for one sample takes less than 1 min and acceptable LOQs and a linear range are achieved. Pretreatment of the paper substrate by oxidation has been found to be effective in reducing interferences due to chemicals in the paper.
The instrumental setup and the general analysis procedure for paper spray have been reported in previous papers.25, 29 In this study, 15 µL of blood was collected and stored in the form of a dried blood spot on a paper triangle (10 mm base width × 20 mm height). A metal clip was used to hold the paper horizontal and conduct the high voltage for paper spray ionization. The distance between the tip of the paper and the inlet to the mass spectrometer was about 5 mm. After being drenched with 25 µL of a 90% acetonitrile:10% water solution (v/v), a voltage (3500 V) was applied for spray ionization. The MS spectra were recorded using an Exactive Orbitrap mass spectrometer (Exactive, Thermo Scientific, CA). Quantitation of drugs in blood was carried out by tandem mass spectrometry (MS/MS) using a TSQ Quantum Access Max mass spectrometer (Thermo Scientific, CA) in the multiple reaction monitoring (MRM) mode with an isolation widow width of m/z 0.4. The LOQ was calculated as 10 time the standard deviation of background noise divided by the slope of the calibration curve experimentally obtained. Bovine whole blood with K2EDTA as anticoagulant was purchased from Innovative Research (Novi, MI).
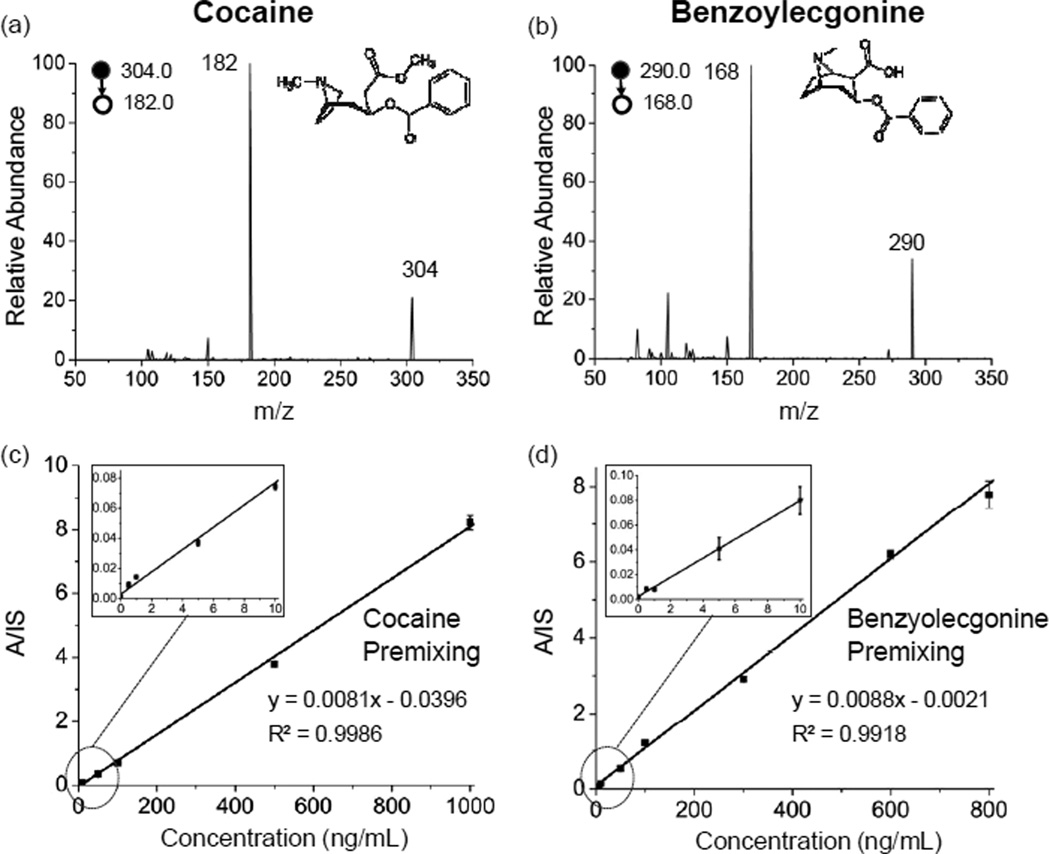
In many cases, the concentrations of a drug but also its metabolites could be used for drug abuse screening as well as in therapeutic drug monitoring, especially for those drug compounds with short metabolic half-lives. For example, cocaine has a short half-life (about 1 hr) and its metabolite benzoylecgonine is typically used in analysis. As shown in Fig. 1a and 1b, 100 ng/mL of cocaine [(M+H)+, m/z 304.0; product ion, m/z 182.0] and its metabolite benzoylecgonine [(M+H)+, m/z 290.0; product ion, m/z 168.0] were identified and confirmed using MS/MS. The high signal-to-noise (S/N) ratios in the MS/MS spectra for the analyte peaks indicate the high sensitivity of the current PS-MS method in quantitation of these abuse drugs at trace levels in blood. The parameters for MRM analysis are listed in Table S-1.
Fig. 1.
Determination of drugs of abuse in DBS using 31 ET paper for paper spray. MS/MS spectra of (a) cocaine [(M+H)+, m/z 304.0; product ion, m/z 182.0] and (b) its metabolite benzoylecgonine [(M+H)+, m/z 290.0; product ion, m/z 168.0] at 100 ng/mL in whole bovine blood. Linear dynamic ranges obtained using IS premixing for (c) cocaine and (d) benzoylecgonine.
The spray solvent composition was optimized to achieve the best performance for the drug compounds in DBS. In paper spray MS, the spray solvent plays a role in both analyte extraction and electrospray at the tip of the paper substrate.30,31 Seven pure organic solvents including acetonitrile, methanol, ethanol, propanol, butanol, isopropanol and ethyl acetate, were tested. The highest signal intensity and the most accurate S/IS ratios for benzoylecgonine (measured by the intensities of product ion m/z 168) were obtained using acetonitrile (Fig. S-1a,b). We further optimized the solvent composition by adding water to acetonitrile and monitoring the analyte-to-IS (A/IS) ratios (Fig. S1c–d). The highest signal intensity occurred with 90% acetonitrile/10% water solution and decreased rapidly with increase in the percentage of water. The A/IS ratio also became less reproducible and less accurate when the percentage of water exceeded 50%. This presumably is because the increase of water percentage resulted in dissolving more water-soluble chemicals from the DBS that interfered the ionization of the analytes during the spray. This matrix effect would decrease the ionization efficiency by ion suppression during the ionization process and then lead to a lower signal intensity. The impact by the DBS size and the sample amount has also been characterized. The signal intensity increased with the increase in blood sample amount while the variation in A/IS ratio is relatively small (Fig. S1e,f). It was found that the lowest RSDs for A/IS ratio was obtained with 10 µL or 15 µL of blood sample. As a result of the optimization procedure, 10 µL 90% acetonitrile/10% water solution and DBS preparation with 15 µL blood sample were used for analysis of abused drugs.
Quantitation of cocaine and benzoylecgonine in dried blood spots was characterized with the results shown in Figure 1. Drug solutions were sequentially diluted by the bovine whole blood to make a set of samples at concentrations of 1, 10, 50, 100, 500 and 1000 ng/mL (or 800 ng/mL). The isotope-labeled internal standards (Table S-1) were added at 100 ng/mL. LOQs of 0.5 ng/mL and 1 ng/mL were achieved for cocaine and benzoylecgonine. Good linearity of calibration curve was obtained for both cocaine (R2 = 0.9986) and benzoylecgonine (R2=0.9918) over 3 orders of magnitude (Figure 1c and d), which was sufficient to cover the whole range of monitoring cocaine and benzoylecgonine for abuse screening and therapeutic purposes. An RSD smaller than 12% was obtained across the entire linear range for both cocaine and benzoylecgonine. Quantitation of other abused and therapeutic drugs as well as their metabolites in blood using paper spray MS was also characterized in similar fashion, including oxycodone, buprenorphine, methamphetamine and its metabolites morphine and 6-acetylmorphine. Limits of quantitation (LOQs) and the linear ranges of calibration obtained with both IS incorporation methods are reported in Table 1.
Table 1.
Comparison between LOQ of paper spray and regulatory cut-off level a
| Drugs | Metabolites | LOQ (ng/mL) |
Screening cut-off level (ng/mL) |
Linear range (ng/mL) |
Therapeutic range (ng/mL) |
|---|---|---|---|---|---|
| Heroin | Morphine | 5 | 20 | 5–800 | 10–100 |
| 6-acetylmorphine | 5 | 20 | 5–800 | N/A | |
| Cocaine | 0.5 | 50 | 0.5–1000 | 50–930 | |
| Benzoylecgonine | 1 | 50 | 1–800 | N/A | |
| Methamphetamine | 5 | 20 | 5–500 | 10–50 | |
| Oxycodone | 16 | 20 | 16–1000 | 10–100 | |
| Buprenorphine (Therapeutic drug) | 1.6 | 10 | 1.6–500 | 14–110 | |
Regulatory level is that set by State of Indiana
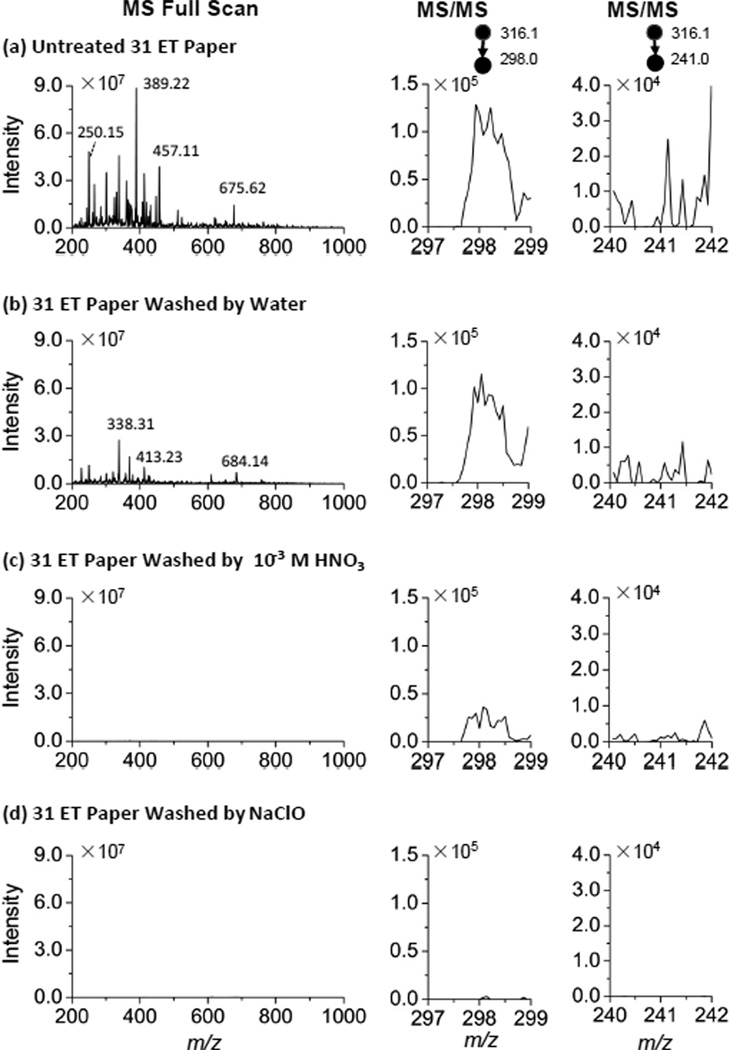
LOQs achieved for all the drugs or metabolites tested are adequate for screening and therapeutic monitoring purposes, except for oxycodone. Relatively high background signal was observed when monitoring the fragment ions m/z 298 or 241 generated in MS/MS transitions for both oxycodone and the IS oxycodone-d6. The interference is likely to be caused by the chemical compounds mixed in the paper during manufacture, which might well include oxycodone at trace levels. Various methods for treating the paper prior to use for paper spray MS were explored to eliminate the chemical interference. It was found that treating the chromatography paper with HNO3 or NaClO aqueous solution was effective. To perform this treatment, the 31 ET (Whatman, Maidstone, England) paper was immersed into 10−3 M HNO3 aqueous solution or NaClO aqueous solution (Cl% = 1.5 g/L, pH=12) in 45 °C for 3 hr and then washed using water six times. The paper was dried at 70 °C for 2 hr and stored for later use to DBS preparation and paper spray MS analysis.
MS and MS/MS spectra were recorded for the blank paper substrates using the Exactive Orbitrap and TSQ, respectively, before and after the treatments (Figure 2a,d). As shown in Figure 2a, many peaks in MS spectra and relatively high intensities at the m/z values for the fragmentation ions m/z 298 and 240 were observed for the untreated paper. Although fewer peaks were observed in the MS spectra after the paper was washed with water, the intensities at the m/z 298 and 240 in the MS/MS spectra did not decrease sufficiently (Figure 2b). After the treatments with HNO3 or NaClO aqueous solution, the chemical noise in both the MS and MS/MS spectra was significantly reduced. The comparison of chemical interference was also made with blood samples without oxycodone (Figure S2) and similar improvement has been observed with the treated paper substrates.
Fig. 2.
MS spectra (mass range: m/z 200–1000) by Orbitrap and MS/MS spectra (parent ion: m/z 316.1, mass range: 297.0–299.0 and 240.0–242.0) by TSQ for blank paper substrates subject to different treatments. (a) untreated 31 ET paper, (b) 31 ET paper washed with water, (c) 31 ET paper treated with 10−3 M HNO3 aqueous solution and (d) ET paper treated with NaClO aqueous solution (Cl% = 1.5 g/L, pH=12). Paper spray solvent: 90% acetonitrile: 10% water solution; solvent volume: 25 µL; Voltage: 3.5 kV.
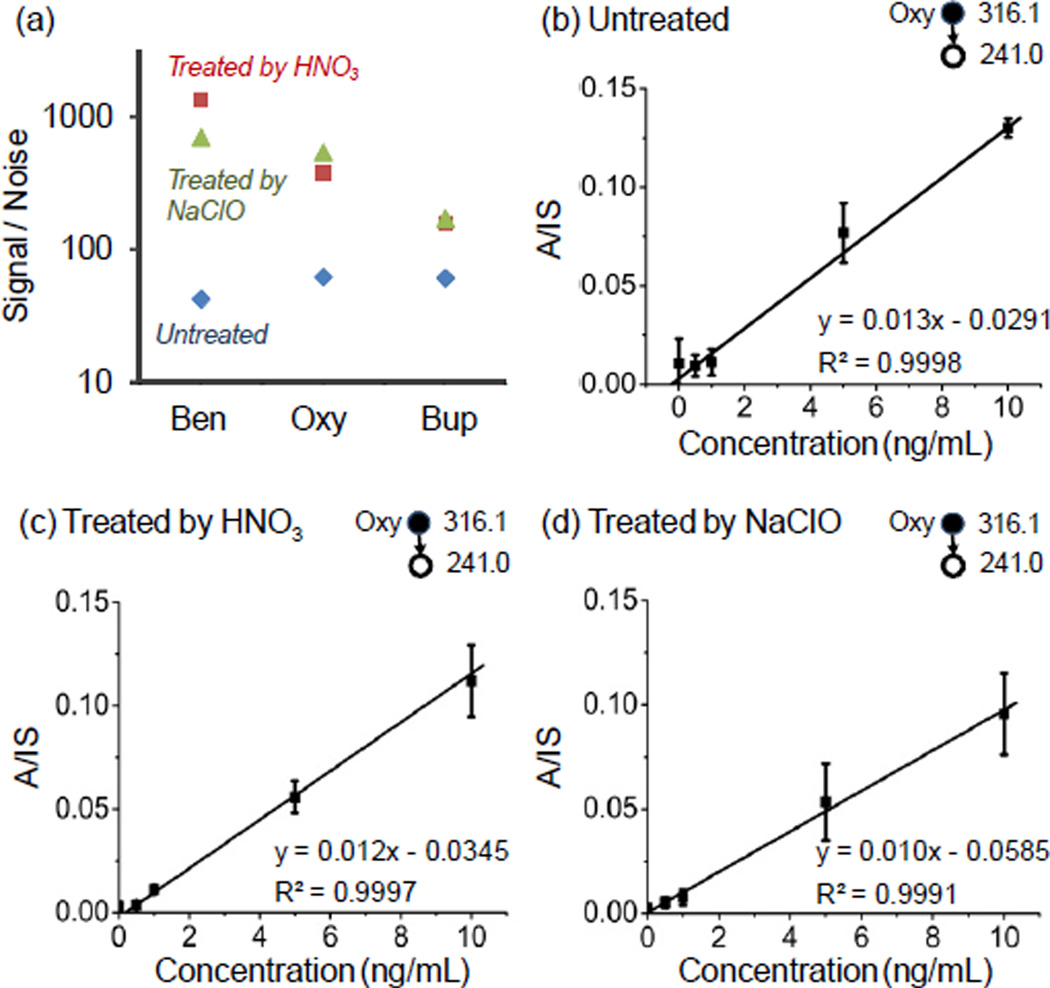
The potential improvement in quantitation of the abused drugs in blood was evaluated using oxycodone, benzoylecgonine and buprenorphine. In each case, the fragment ion intensity was measured for a DBS with 100ng/mL drug compounds and a blank DBS sample using the untreated and treated paper substrates to obtain S/N values for comparison. As shown in Figure 3a, the largest improvement was observed for benzoylecgonine, with about 16 fold increase. The MS/MS transition of m/z 316.1→241.0 was used for oxycodone and an 8 fold improvement in S/N was obtained. The improvement in quantitation with the treated paper substrates was also characterized using these three drug compounds. The lower end of the calibration curves for oxycodone are shown in Figure 3b–d for comparison. LOQs of 1.0 ng/mL and 0.9 nL were achieved with the paper substrates treated with HNO3 and NaClO, respectively. This is adequate for quantitative analysis for therapeutic monitoring purpose (Table 1).
Fig. 3.
(a) Comparison of signal to noise ratio of 100 ng/mL of benzoylecgonine (Ben), oxycodone (Oxy) and buprenorphine (Bup) before and after treatment by 10−3 M HNO3 aqueous solution or NaClO aqueous solution. Calibration curves of oxycodone in DBS on the 31 ET paper (b) without treatment, (c) treated by 10−3 M HNO3 aqueous solution and (d) treated by NaClO aqueous solution (Cl% = 1.5 g/L, pH=12).
In conclusion, a simple method for both fast screening and quantitative determination of abused drugs and therapeutic drugs in DBS was developed by paper spray mass spectrometry. Drugs can be directly extracted, identified and quantified without any pre-treatment or separation. The composition of solvent and the size of the blood spot were found to be important in determining signal intensity in paper spray. Internal standards were used in the quantitation of all the drugs of abuse and the approach to loading internal standard significantly affects the quantitation result. The ranges of quantitation are sufficient to cover the screening range of abused drugs and metabolites with good linearity (R2> 0.99) and acceptable reproducibility (RSD ~ 12%). In addition, a simple treatment of the paper using HNO3 or NaClO was developed to improve quantitative performance by eliminating the matrix effect due to small molecules in the paper. The signal to noise ratios of benzoylecgonine, oxycodone and buprenorphine were increased by 32 times, 8 times and a factor of two after the treatment, respectively. The limits of quantitation were also obviously improved to be lower than the cut-off level of the confirmation screening of oxycodone. Paper spray mass spectrometry has the potential to be an alternative method in quick monitoring of drug abuse.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation (CHE 0847205), National Center for Research Resources (5R21RR031246-02) and the National Institute of General Medical Sciences (8R21GM103454) from the National Institutes of Health.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Degenhardt L, Hall W. The Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 3.Dasgupta A. Beating Drug Tests and Defending Positive Results: A General Overview. Springer; 2010. [Google Scholar]
- 4.Crowe AH, Reeves R. Treatment for alcohol and other drug abuse: Opportunities for coordination. TAP 11. 1994 [Google Scholar]
- 5.Kleiman MA. Drug addiction and drug policy: The struggle to control dependence. 2001;13:168. [Google Scholar]
- 6.Wolff K, Farrell M, Marsden J, Monteiro M, Ali R, Welch S, Strang J. Addiction. 1999;94:1279–1298. doi: 10.1046/j.1360-0443.1999.94912792.x. [DOI] [PubMed] [Google Scholar]
- 7.Tracqui A, Kintz P, Ludes B. J. Anal. Toxicol. 1998;22:430–434. doi: 10.1093/jat/22.6.430. [DOI] [PubMed] [Google Scholar]
- 8.Kintz P. Forensic Sci. Int. 2001;121:65–69. doi: 10.1016/s0379-0738(01)00454-6. [DOI] [PubMed] [Google Scholar]
- 9.Rockville M. Center for Substance Abuse Treatment. 2009 [Google Scholar]
- 10.Hubbard L, Marsden E, Racholl V. Drug abuse treatment. Chapel Hill: The Univ. of North Carolina press; 1989. [Google Scholar]
- 11.Moeller MR, Steinmeyer S, Kraemer T. J. Chromatogr. B. 1998;713:91–109. doi: 10.1016/s0378-4347(97)00573-2. [DOI] [PubMed] [Google Scholar]
- 12.Maurer HH. Anal. Bioanal. Chem. 2005;381:110–118. doi: 10.1007/s00216-004-2774-z. [DOI] [PubMed] [Google Scholar]
- 13.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 14.Takats Z, Wiseman JM, Gologan B, Cooks RG. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 15.Cody RB, Laramée JA, Durst HD. Anal. Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 16.McEwen CN, McKay RG, Larsen BS. Anal. Chem. 2005;77:7826–7831. doi: 10.1021/ac051470k. [DOI] [PubMed] [Google Scholar]
- 17.Ford MJ, Van Berkel GJ. Rapid Commun. Mass Spectrom. 2004;18:1303–1309. doi: 10.1002/rcm.1486. [DOI] [PubMed] [Google Scholar]
- 18.Chen HW, Venter A, Cooks RG. Chem. Commun. 2006;0:2042–2044. doi: 10.1039/b602614a. [DOI] [PubMed] [Google Scholar]
- 19.Nemes P, Vertes A. Anal. Chem. 2007;79:8098–8106. doi: 10.1021/ac071181r. [DOI] [PubMed] [Google Scholar]
- 20.Sampson JS, Hawkridge AM, Muddiman DC. J. Am. Soc. Mass. Spectrom. 2006;17:1712–1716. doi: 10.1016/j.jasms.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Ouyang Z, Cooks RG. Angew. Chem. Int. Ed. 2006;45:3656–3660. doi: 10.1002/anie.200600660. [DOI] [PubMed] [Google Scholar]
- 22.Spooner N, Lad R, Barfield M. Anal. Chem. 2009;81:1557–1563. doi: 10.1021/ac8022839. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman JM, Evans CA, Bowen CL, Kennedy JH. Analyst. 2010;135:720–725. doi: 10.1039/b922329k. [DOI] [PubMed] [Google Scholar]
- 24.Manicke NE, Yang Q, Wang H, Oradu S, Ouyang Z, Cooks RG. Int. J. Mass spectrom. 2011;300:123–129. doi: 10.1016/j.ijms.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZP, Cooks RG, Ouyang Z. Analyst. 2012;137:2556–2558. doi: 10.1039/c2an35196j. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Manicke NE, Wang H, Petucci C, Cooks RG, Ouyang Z. Anal. Bioanal. Chem. 2012;404:1389–1397. doi: 10.1007/s00216-012-6211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Manicke NE, Yang Q, Zheng L, Shi RY, Cooks RG, Ouyang Z. Anal. Chem. 2011;83:1197–1201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li AY, Wang H, Ouyang Z, Cooks RG. Chem. Commun. 2011;47:2811–2813. doi: 10.1039/c0cc05513a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Liu JJ, Cooks RG, Ouyang Zheng. Angew. Chem. Int. Ed. 2010;122:889–892. [Google Scholar]
- 30.Ren Y, Wang H, Liu JJ, Zhang ZP, McLuckey MN, Ouyang Z. Chromatographia. 2013:1–8. doi: 10.1007/s10337-013-2458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang ZP, Xu W, Manicke NE, Cooks RG, Ouyang Z. Anal. Chem. 2012;84:931–938. doi: 10.1021/ac202058w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.