Abstract
Kaposi’s sarcoma–associated herpesvirus (KSHV) [or human herpesvirus 8 (HHV-8)] is the most frequent cause of malignancy among AIDS patients. KSHV and related herpesviruses have extensively pirated cellular cDNAs from the host genome, providing a unique opportunity to examine the range of viral mechanisms for controlling cell proliferation. Many of the viral regulatory homologs encode proteins that directly inhibit host adaptive and innate immunity. Other viral proteins target retinoblastoma protein and p53 control of tumor suppressor pathways, which also play key effector roles in intracellular immune responses. The immune evasion strategies employed by KSHV, by targeting tumor suppressor pathways activated during immune system signaling, may lead to inadvertent cell proliferation and tumorigenesis in susceptible hosts.
Keywords: KSHV, HHV-8, antiviral immunity, tumor virus, viral oncogenes
INTRODUCTION
The virus causing Kaposi’s sarcoma (KS) has generated considerable scientific interest as a new human (h) tumor virus. Kaposi’s sarcoma–associated herpesvirus (KSHV) [or human herpesvirus 8 (HHV-8)] is unique because of its extensive molecular piracy of critical cell regulatory genes. Functions for these genes can be deduced directly from their sequence, but in general the viral (v) proteins are modified to escape normal cellular regulation and hence behave differently from their cellular counterparts. Whereas KSHV initially appears to be unique among human tumor viruses, the opposite is actually the case because the regulatory circuits and control points targeted by KSHV are the same as those targeted by other tumor viruses. Examining the mechanisms that KSHV uses to induce cell proliferation can lead to unexpected insights into unrelated viruses and highlights important relationships between viral immune evasion and tumorigenesis.
BIOLOGY AND PATHOGENESIS
Disease Associations
KAPOSI’S SARCOMA
Chang et al. (20) discovered KSHV in 1993 using representational difference analysis during a molecular search for an infectious etiology of KS. KS consists of proliferating spindle cells that form irregular microvascular channels. These tumors are most commonly found in the dermis but also occur in viscera including lungs, liver, and intestines (Figure 1). The large majority of spindle cells are infected with virus, but because of the infiltrative nature of these lesions, uninfected tissues not harboring viral genome are intertwined with tumor cells (41).
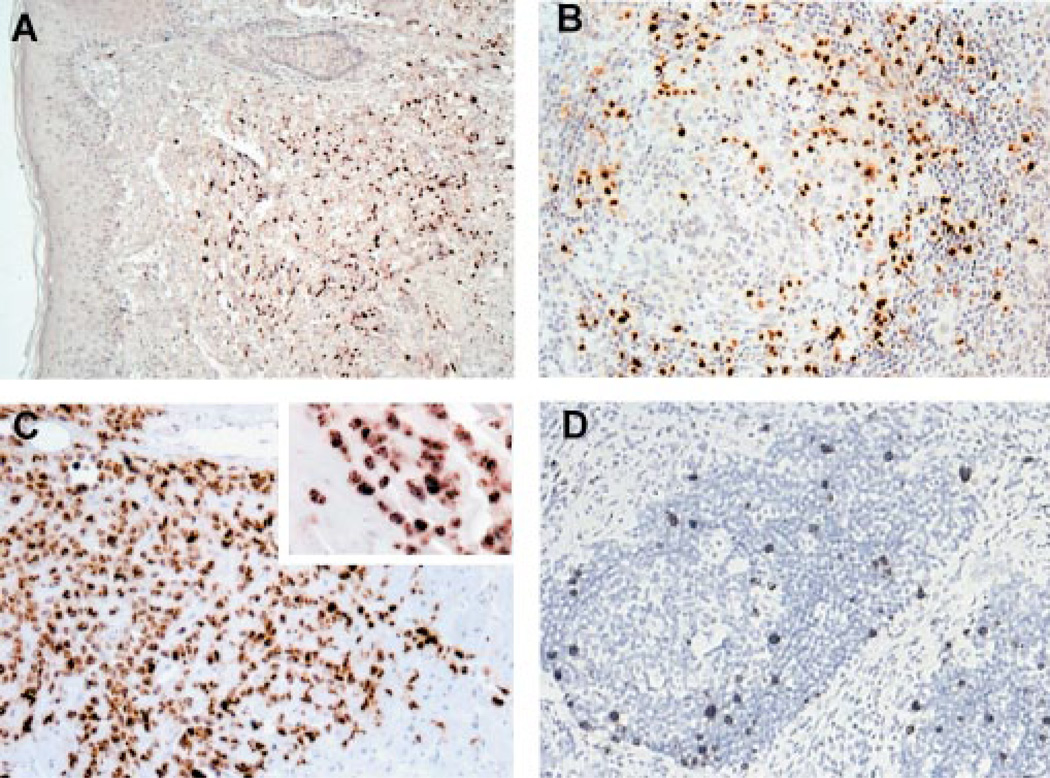
Figure 1.
Proliferative disorders associated with KSHV infection range from monoclonal neoplasia in primary effusion lymphoma (PEL) to multiclonal hyperplasia in multicentric Castleman’s disease (MCD). Kaposi’s sarcoma (KS) has both hyperplastic and neoplastic features. LANA1 immunostaining of (A) KS, (B) MCD, and (C ) PEL shows typical nuclear staining of virus-infected cells. vIL-6 expressed from (D) infected B cells in the germinal center mantle zone of MCD drives proliferation of uninfected cells.
All clinical forms of KS are infected with KSHV, a necessary but not sufficient cofactor for KS pathogenesis. The high rate of KS among AIDS patients, particularly gay men, appears to be due to both immunosuppression as well as shared sexual risk factors for infection between HIV and KSHV. To date, there is no compelling evidence to support a direct role for HIV in KS pathogenesis other than its part in causing immunosuppression. KSHV infection is uncommon overall (approximately 1%–5% of persons are infected in North America and Northern Europe), but higher infection rates occur among gay men (~40%) and Mediterranean populations (5%–20%). Sub-Saharan Africa has the highest rates of infection, with many countries experiencing seroprevalence rates exceeding 60% [for detailed descriptions of clinical and epidemiologic aspects KSHV infection, see (116)]. As could be expected from the explosion of AIDS in Africa, KS is now the most commonly reported neoplasm in many African countries and represents a largely unseen and unchallenged public health problem (4, 153).
PRIMARY EFFUSION LYMPHOMA
In addition to KS, several hematologic, predominantly B cell, disorders are associated with KSHV. The two most well characterized are primary effusion lymphomas (PELs) and a subset of multicentric Castleman’s disease (MCD). Other unusual or rare entities associated with KSHV infection have also been described including posttransplantation plasmacytic proliferations (109), posttransplantation bone marrow failure (105), MCD-associated plasmablastic lymphomas (40), and germinotropic lymphoproliferations (39). Multiple myeloma has been linked to KSHV infection in the past (137), but careful analyses show that the virus is unlikely to play a significant role in this disease (129).
PELs are monoclonal, non-Hodgkin’s B cell lymphomas that frequently lack B cell–specific surface markers (13, 19) and are commonly coinfected with Epstein-Barr virus (EBV) (84). They display immunoblastic/centroblastic morphology and postgerminal center immunophenotype (50). Cell lines established from PEL, unlike KS tumor explants, stably maintain viral episomes at high copy number (50– 150 copies per cell) and are the source of virus for most virologic and serologic studies.
MULTICENTRIC CASTLEMAN’S DISEASE
Multicentric Castleman’s disease (MCD), a B cell lymphoproliferative disorder, has a heterogeneous pathogenesis with only about 50% of Castleman’s disease tumors being infected with KSHV in HIV-negative, healthy persons. The cause of the remaining KSHV-negative MCD patients remains obscure but almost certainly involves dysregulation of endogenous interleukin (IL)-6 secretion. Nearly all MCD occurring among AIDS patients, however, is KSHV positive. MCD is a polyclonal tumor in which the bulk of the cell mass is composed of uninfected lymphocytes recruited to the site of infection by cytokines elaborated from a small proportion of B cells infected with KSHV (41, 76, 132), and displays an unusual immunoglobulin (Ig)M light chain restriction (40). There is also a high rate of secondary non-Hodgkin’s lymphoma among MCD patients, which suggests that this tumor can either predispose or evolve into a neoplastic disorder (127).
Genome Structure and Organization
The gammaherpesvirus subfamily is composed of two genera: the Lymphocryptovirus (gamma-1 herpesviruses), which includes EBV (or human herpesvirus 4), and the Rhadinovirus (gamma-2 herpesviruses), which includes KSHV and the new world monkey virus, herpesvirus saimiri (saimiirine herpesvirus 2, HVS).
CONSERVED AND UNIQUE GENE BLOCKS
Like other herpesviruses, KSHV is a large double-stranded DNA virus that replicates in the nucleus as a closed circular episome during latency but linearizes during virion packaging and replication. All identified KSHV transcripts are encoded on a continuous 145-kb long unique region. The long unique region is flanked by the 20–35 kb terminal repeat region composed of 801-bp high G+C content terminal repeat units (140).
Over 80 genes have been identified in the long unique region, and new open reading frames (ORFs) are continuously described as small gene products, alternative reading frames, and alternative splicing patterns are being investigated (Figure 2).
Figure 2.
The ~140-kb KSHV genome composed of a single long unique region with over 80 open reading frames (splice patterns not shown) flanked by G/C-rich terminal repeat units. Gene blocks containing well-conserved herpesviral genes (white) are separated by block of genes unique to KSHV and other rhadinoviruses (black). A full description of the KSHV genes is found in Reference 116. Gene names correspond to positional and sequence homologs found in HVS, which is the prototype rhadinovirus. Genes are numbered from left to right, from ORF4 to ORF75, with the first three homologs to HVS genes being absent or displaced from their corresponding positions in HVS. Genes unique to KSHV, including most of the cell regulatory homologs, are given a K prefix, e.g., ORF K1. Because several HVS genes are absent from the KSHV genome (e.g., ORF1), gene names are not necessarily consecutive.
KSHV shares structural and biological features with the human tumor virus EBV but possesses none of its latency genes involved in cell immortalization and transformation (140). Although KSHV latency genes have no evolutionary homology to EBV latency genes, there is a clear genetic correspondence between the two viruses (116). In general, cellular genes that are induced by EBV proteins have been captured and modified by KSHV during its evolution. This is not unexpected because both viruses use B lymphocytes as reservoirs during latency and face similar biological challenges in establishing persistent infections in a B cell environment. KSHV also infects endothelial cells, as occurs in KS tumors, and possibly monocytes and epithelial cells (at least in transit during initial infection).
The origin of the pirated cDNAs is unknown. No clear mechanism has been shown which incorporates cellular cDNAs into the viral genomes of not only KSHV and related rhadinoviruses, but also other viruses including poxviruses, which also have extensively captured cellular cDNAs.
KSHV GENE EXPRESSION
KSHV gene expression depends on a variety of factors including whether the virus is latent or lytic, the type of host cell infected, and the host cell environment. When induced into lytic replication, the virus genome replicates through a rolling circle mechanism with individual viral genomes being cleaved in the terminal repeat region and packaged as linear molecules into viral capsids.
A salient feature of the genome reveals itself when the functions and expression patterns of the genes are examined: Structural genes and highly conserved genes involved in lytic replication tend to cluster in islands separated by novel genes, including many of the cDNA homologs of cellular regulatory genes (144). When KSHV enters lytic replication, the clusters of lytic replication genes are induced in an orderly cascade. As shown by Sun et al. and Zhu et al. (161, 179), the progression of lytic gene activation follows an ordered pattern similar to that of other herpesviruses. Unique gene clusters have more complicated expression patterns, and many genes are expressed at low levels during latency but are induced during lytic replication, a pattern referred to as class II transcription, distinguishing it from constitutive (class I) or lytic (class III) expression (144).
Differentiating gene expression during lytic and latent replication has been useful for classifying KSHV genes, but it is evident that mutually exclusive latent and lytic gene categories are too simplistic to adequately describe the biology of KSHV. For example, ORF K10.5 [latency-associated nuclear antigen (LANA2)] is only constitutive in hematopoietic cells but not in KS tumors (138), and even the constitutive genes encoding vFLIP (FLICE-inhibitory protein), vCYC (cyclin), and LANA1 at the major latency locus are expressed in a G1/S cell cycle–dependent pattern (147). vIL-6 is induced during lytic replication (114) but is also activated by interferon (IFN) signaling independent of replication cycle (22). Phorbol ester treatment may directly activate some genes, such as ORF K5 [modulators of immune response (MIR2)] (128), further complicating whether these viral genes are solely activated during lytic replication. Two genes, ORF K12 (kaposin) and ORF K7 (PAN, polyadenylated nuclear RNA), which are highly expressed and commonly used as markers for latent and lytic virus replication, respectively, are induced during lytic replication in PEL cells (141, 144). This complexity is not unexpected because KSHV is a large virus with the capacity to respond in complex ways to its cellular environment.
One rationale for classifying viral genes into latent and lytic replication categories is that latency is generally assumed to be the state leading to cell proliferation because lytic replication results in cell death and is therefore antitumorigenic. Even this assumption, however, is increasingly being called into question and does not apply to all types of virus-induced proliferation. The viralGprotein–coupled receptor (ORF74), for example, has transcriptional kinetics resembling that of an early lytic gene (27, 83), but evidence suggests that it may play an important paracrine role in KS pathogenesis (2, 177). In contrast, latent KSHV gene expression together with host cell mutations leads to PEL cell transformation and monoclonal proliferation.
Examining viral gene expression directly in tumors is necessary to determine genes likely to be involved in disease pathogenesis. This has been undertaken for a number of different viral proteins using antibodies and in situ hybridization (27, 75, 76, 131, 132, 138, 157, 160, 174). These studies show that the three major proliferative syndromes associated with KSHV have different virus expression programs that contribute to the tumor phenotype.
To better understand the roles that pirated genes play in the biology of KSHV, they are discussed under the broad categories of immune evasion, apoptosis inhibition, and regulation of the cell cycle. These are artificial divisions and some KSHV proteins, such as vIL-6, have roles in all three biologic functions. The overlap between these areas reflects not only the incomplete information on viral protein functions, but also the interconnected nature of these broad areas of cell biology.
FUNCTIONS OF KSHV HOMOLOG GENES
Immune Evasion by KSHV Immunomodulatory Proteins
Active viral infections rely on highly efficient replication to outpace the development of an effective adaptive immune response. Viruses causing chronic infections must instead evade innate immunity as well as adaptive immune responses so infection can be immediately established to maintain long-term infection. Latency itself, in which nonessential viral protein expression is repressed to limit foreign antigen presentation, is a fundamental viral immune evasion strategy. KSHV employs a broad repertoire of immune evasion proteins during both latency and lytic replication—perhaps more than any other virus described to date (Table 1) (Figure 3). Although individual mechanisms may be unique to KSHV and related viruses, a wide range of viruses employ similar strategies.
TABLE 1.
KSHV genes with immune modulatory functions
| KSHV protein |
KSHV gene |
Features and functions | Related proteins from other viruses |
|---|---|---|---|
| vKCP | ORF4 | Homolog of cellular complement control regulators; inhibits C3 deposition on cell surface |
HVS-ORF4, HSVl-gC, Vaccinia-vCP/C21L, Cowpox-IMP, Vaccinia-SPICE |
| KIS | ORFK1 | Constitutively active membrane protein containing ITAM motif; interacts with mu chains of B cell receptor complex to block surface transport; transforms rodent fibroblasts and primary T cells; activates Syk signaling pathway; variable ectodomain used for KSHV strain analysis |
HVS-STP |
| MIR1/MIR2 | ORFK3/K5 | Enhanced endocytosis of surface MHO via ubiquitination; MIR2 also ubiquitinates and downregulates ICAM-1 and B7.2 |
MHV 68-K3, CMV-US2 |
| vCCL-2 (vMIP-II) |
ORFK4 | Binds to CCR3 and induces angiogenesis in ovo; induces eosinophil and Th2 chemotaxis; CCR8 and vGPCR antagonist. |
Molluscum-MC148 |
| vCCL-3 (vMIP-III) |
ORFK4.1 | Agonist for CCR4 and induces Th2 chemotaxis; induces angiogenesis in ovo; protein expressed in KS lesions (by Western blot) |
|
| vCCL-1 (vMIP-I) |
ORFK6 | Bids CCR5 and induces angiogenesis in ovo; CCR8 agonist; induces Th2 chemotaxis |
Molluscum-MC148 |
| vIRF-1 | ORFK9 | Inhibits IFN signaling; binds CBP/p300; transforms rodent fibroblasts; protein expressed in MCD and PEL cell lines, not detectable in KS or PEL |
EBV-EBNA2, Adeno-EIA, EBV-EBNA2, Adeno-EIA, SV40-LT, HTLV-Tax |
| vFLIP | ORFK13 | Homolog of cellular apoptosis inhibitor (FLIP); transcribed on major latency transcripts (LT)1 and LT2; posttranscriptional regulation of protein expression in vivo |
HSV-ORF71 |
| ORF45 | ORF45 | Binds to and inhibits IRF7 nuclear translocation | Vaccinia-E3L |
Figure 3.
The range of immune regulatory pathways in a B cell inhibited by specific KSHV immune evasive proteins. KSHV proteins that have been experimentally shown to induce cell transformation and proliferation include KIS, vIL-6, vFLIP, and vIRF1.
ADAPTIVE IMMUNE EVASION STRATEGIES: THE MIR PROTEINS, vFLIP, AND VIRAL CHEMOKINES
Viral antigen processing and presentation through major histocom-patibility complex (MHC) I is a critical step in initiating an effective antiviral cell-mediated immune response. Burgert et al. (16) first identified viral inhibition of MHC I function by adenovirus E3 protein, and Ploegh and colleagues have elegantly described mechanisms to prevent antigen presentation for herpes simplex virus, cytomegalovirus (CMV), and other viruses [for review see (166)]. Building on this work, Coscoy & Ganem (30) systematically tested each unique KSHV K protein for the ability to downregulate MHC I cell surface expression. Two transmembrane proteins, MIR1 and MIR2, encoded by ORFs K3 and K5, respectively, efficiently inhibit MHC I surface expression through a novel mechanism not found in other viruses. These results were quickly confirmed and extended by others (72, 158).
MIR1 and MIR2 remove MHC I from the plasma membrane through enhanced endocytosis, which results in lysosomal targeting and degradation of MHC molecules (30). The endocytic, lysosomal trafficking and protein targeting functions of MIR1 and MIR2 are genetically separable and have been examined through domain swapping studies (110, 142). MIR1 and MIR2 possess N-terminal C4HC3 zinc-finger domains, called plant homeodomain motifs, that are characteristic for a class of E3 ubiquitin ligases (32), and ubiquitinate plasma membrane-bound MHC I resulting in its endocytosis. While protein ubiquitination generally results in proteosomal degradation, it also serves to signal for protein trafficking. The K3 homolog of the mouse gammaherpesvirus MHV68 possesses a similar MHC ubiquitination function but prevents egress of MHC from the endoplasmic reticulum (103). MIR2 ubiquitinates and downregulates the accessory immune receptors ICAM-1 and B7.2 in addition to MHC I (31, 71). A newly recognized family of cellular and viral MIR1/2-like proteins exist, whose functions are beginning to be explored. For example, a poxvirus homolog of K3 has been described to ubiquitinate and downregulate surface expression of CD4 (49, 107).
Downregulation of MHC I and its accessory immune receptors poses the risk of initiating a natural killer (NK) cell response (122). NK cells interrogate cells for MHC I expression and initiate receptor-activated apoptosis through Fas (CD95/Apo-1) in cells lacking appropriate MHC I expression. Human and murine CMVs avoid this by presenting virus-encoded MHC-like molecules to thwart NK cell recognition (166). KSHV and related gammaherpesviruses inhibit NK-mediated killing through expression of v-FLICE-inhibitory proteins (vFLIPs) (165).
KSHV vFLIP encoded by ORF K13 (ORF71) possesses two death effector domains and acts as a dominant-negative inhibitor of receptor-activated apoptosis by binding to Fas-associated death domain protein and caspase 8 (FLICE) (6). This prevents activated caspase recruitment into the death-inducing signaling complex [reviewed in detail elsewhere (87)]. The discovery of vFLIPs in gammaherpesviruses and poxviruses by database searches for death effector domain containing proteins (9, 68, 165) led to the subsequent discovery of cellular FLIPs existing in multiple splice forms (cFLIPL and cFLIPs) that have similar functions (87).
Because of its constitutive expression and role in inhibiting receptor-mediated apoptosis, vFLIP is a candidate contributor to KSHV-induced cell transformation and tumorigenesis. Transduction studies using vFLIP show that it enhances tumorigenicity of mouse B lymphoma cells in immunocompetent mice strains (38). KSHV vFLIP shares with cFLIPs the ability to activate NF-κB through IκB kinase activation, which may also contribute to B cell proliferation (23, 77, 102). NF-κB is a pro-proliferative transcription factor for most B cell tumors, and use of specific NF-κB inhibitors induces apoptosis in PEL cell lines (80). vFLIP is expressed constitutively on two latent transcripts (LT1 and LT2) as a bi- or tricistronic message translated from an internal ribosomal entry site (IRES) located in the 3′ region of the vCYC gene ORF72 (11, 60, 104). IRES-regulated translation of cellular anti-apoptotic transcripts maintains cap-independent translation during cellular stress (63, 67).
Recruitment of helper T cell subtype 2 (Th2) rather than Th1 CD4+ lymphocytes to the site of infection polarizes immune responses toward an antibody-predominant Th2 immune reaction (119). KSHV inhibits effective cell-mediated immune responses through secretion of virus-encoded chemoattractant cytokines (chemokines) to enhance Th2 polarization. Patients with active KS can have antibody titers against LANA1 exceeding 1:100,000, indicating that specific antibody responses are robust but ineffectual in clearing infection (53, 153). KSHV encodes three secreted chemokines, vCCL1 (ORF K6), vCCL2 (ORF K4), and vCCL3 (ORF K4.1), formerly known as vMIP-I/MIP-1a, vMIP-II/MIP-1b, and vMIP-III/BCK, respectively, that act on receptors involved in Th2 chemotactic immune responses.
All three chemokines have dicysteine motifs but have different receptor specificities. vCCL2 initiates a strong chemotactic response through CCR3 activation (12), while vCCL1 and vCCL3 activate CCR8 (35, 43) and CCR4 receptors (159), respectively. Unlike cellular chemokines, the viral chemokines have broad antagonistic activities for CC and CXC receptors to inhibit chemotaxis of Th1 and NK lymphocytes (12, 24). Weber et al. (173) demonstrated that vCCL2 effectively blocks RANTES-mediated chemotaxis of Th1-like lymphocytes and initiates firm arrest of cells on human microvascular endothelium under flow conditions (173). The immune-inhibiting properties of vCCL2 were also demonstrated by experiments in which mismatched cardiac allograft survival was increased, and CTL infiltration diminished, by viral chemokine expression in rodents (36). Furthermore, administration of vCCL2 inhibits fractalkine-mediated inflammatory glomerulonephritis in a mouse model (24). While vCCL1 and vCCL2 expression is generally limited to lytic replication, vCCL3 is found in KS tumors and may contribute to its pathogenesis (159). The KSHV chemokines are pro-proliferative for some cell types and induce neoangiogenesis, in part through induction of vascular endothelial growth factor (12, 101, 159). Chemokines and cytokines encoded by EBV, CMV, murine CMV, and poxviruses also have Th2 activity, which suggests that this is a common viral strategy for blunting adaptive immune responses (119).
KSHV encodes a neural cell adhesion molecule (NCAM)-like adhesin (ORF K14, vOX-2/vAdh) homologous to CD200 (OX-2) that promotes Th2 polarization and/or foreign antigen presentation (58, 66). Initial studies suggest, however, that it activates rather than inhibits inflammatory macrophage responses (29). Homologs of this protein are found among poxviruses as well.
INNATE IMMUNE EVASION STRATEGIES: KCP, KIS, vIL-6, ORF45 PROTEIN, AND vIRF1
In addition to inhibiting adaptive immune responses through the MIR proteins, vFLIP, and the viral chemokines, KSHV possesses means to block innate immune responses as well. These mechanisms include complement binding, downregulation of the B cell receptor (BCR), and inhibition of IFN initiation and signaling.
Complement control and the B cell receptor
The KSHV ORF4 encodes a protein designated KSHV complement control protein (KCP), which has homology to human complement regulators (140). In addition to an unspliced, full-length mRNA of 1679 bp, at least two alternatively, internally spliced transcripts have also been described that are translated into 175-, 82-, and 62-kDa proteins detectable only in induced KSHV-infected PEL cell cultures. These three proteins retain C-terminal transmembrane domains and four N-terminal complement control protein SUSHI domains required for membrane attachment and complement regulation, respectively. Stable expression in cells exposed to human serum shows that all three KCP isoforms regulate complement activation by inhibiting C3 deposition on the cell surface (156). Some viruses including poxviruses and other members of herpesvirus family (HVS and MHV-68) have developed similar strategies to circumvent the complement component of the host immune response, which plays an important role in limiting virus infection (74). Other viruses including HCMV, HIV, and human T cell lymphotropic virus (HTLV)-1 upregulate host cell surface expression of complement regulators or acquire these proteins in their envelopes on egress from the cell.
The BCR, interacting with the complement-binding proteins CD19 and CD21, regulates B cell development and response to antigen. ORF K1 encodes a small transmembrane, immunoglobulin-like, glycoprotein called KIS (K ITAM-signaling), which possesses a cytoplasmic immunoreceptor tyrosine activation motif (ITAM) similar to that of the BCR (57). The KIS protein ITAM is tyrosine-phosphorylated in situ, allowing recruitment of SH2 domain proteins, Syk pathway activation, and intracellular calcium mobilization (88, 92). Jung and colleagues found that overexpression of KIS causes transformation of Rat-1 fibroblasts and that when KIS is substituted for the STP oncogene in recombinant HVS virus, it can immortalize primary T lymphocytes and induce lymphomas in marmoset monkeys (93). KIS protein resembles the BCR signal transduction subunits Igα and Igβ in its ability to induce signaling and to interact with mu chains of the BCR. However, unlike Igα and Igβ, which interact with mu chains to direct BCR complexes to the cell surface, KIS interacts with mu chains to block the intracellular transport of BCR complexes to the cell surface (90).
Viral hijacking of BCR signaling results in cell transformation, but it is unclear how this promotes virus survival. The specific downregulation of BCR by KIS implies an importance in controlling B cell viral infections. The BCR interacts with complement receptors CD19 and CD21; it remains to be determined what, if any, relationship KIS and ORF4 proteins have to each other in the infected cell.
Interferon inhibition
Interferons (IFNs) are central to antiviral innate immunity, particularly in limiting initial viral replication prior to development of a specific immune response (70). IFN activation, one of the first immune responses to virus infection, can effectively limit establishment of infection so that subsequent humoral and cellular immunities are not overwhelmed by viral load. The importance of the IFN response to KSHV is shown by the multiple mechanisms it employs to subvert IFN signaling. This involves inhibition of IFN-α signaling, antagonism of IFN-initiated gene transcription, and blockade of interferon regulatory factors (IRF) 3 and 7.
ANTAGONISM OF INTERFERON RECEPTOR SIGNALING BY vIL-6
KSHV has evolved a complex mechanism for IFN-α receptor inhibition involving autocrine signaling through the virus-encoded IL-6 cytokine. IL-6 is mitogenic for B cells and is involved in orchestrating Th2-type inflammatory responses (168, 169). vIL-6 is 26% identical to cellular IL-6, and extensive analyses demonstrate that it has nearly identical signaling patterns (15, 64, 114, 124, 130). Both cytokines activate STAT1 and STAT3 phosphorylation, as well as additional IL-6 response pathways involving MAP-kinase and other serine/threonine kinases (130). vIL-6 substitutes for hIL-6 in maintaining cell proliferation of mouse and human B cell lines dependent on IL-6 signaling (15, 114, 124).
vIL-6 expression is limited to a subpopulation of cells in KSHV-associated hematolymphoid disorders (Figure 1D). In MCD, vIL-6 is primarily localized to scattered B cells in the mantle zone, and the bulk of each tumor is caused by vIL-6-induced proliferation of uninfected B cells. vIL-6 expression is also common in PEL tumors and PEL cell lines (14, 131), where it is expressed in a portion of cells through two alternative transcripts that are differentially regulated (37). Studies of PEL cell lines demonstrate that they are autocrine dependent (xenocrine-dependence) on vIL-6, together with hIL-10, but not on hIL-6 (73).
Despite similarities, hIL-6 and vIL-6 differ in their receptor engagement and utilization. hIL-6 signals at the plasma membrane by first binding gp80 (IL-6Rα), which complexes to gp130, the molecule responsible for signal transduction across the membrane, at its cytokine—homology–binding region composed of domains 1 and 2 (D1D2). Subsequent recruitment of gp130 D3 domain fully activates gp130 dimerization and transmembrane signaling. Crystallographic studies reveal that vIL-6 directly interacts with D1D2D3 epitopes through hydrophobic interactions to activate gp130 in the absence of the IL-6Rα receptor (28). This provides structural evidence supporting the experimental finding (112) that vIL-6 directly activates gp130 independent of gp80.
Autocrine-dependence of PEL cells on vIL-6 but not hIL-6 led Chatterjee et al. to examine the effects of vIL-6 on IFN-α signaling because IFN-α induces cell arrest through induction of the p21CIP cyclin-dependent kinase inhibitor (22) (Figure 4). Cell cycle arrest by IFN is an effective and reversible means to establish an antiviral state by limiting nucleic acid precursors available to the virus. vIL-6 inhibits Tyk2 and STAT2 phosphorylation by IFN-αR, effectively blocking downstream signaling (M. Chatterjee, P.S. Moore & Y. Chang, unpublished observation) and IFN induction of p21CIP. The vIL-6 promoter itself possesses IFN-stimulated response element sequences inducible by IFN-α, providing a novel negative feedback mechanism in which the virus senses and regulates IFN signaling. hIL-6 cannot reproduce this effect because IFN-α downregulates surface expression of IL-6Rα receptor, preventing gp130 signal transduction. Because vIL-6 interacts directly with gp130, it bypasses this regulatory mechanism, making PEL cell lines autocrine dependent on this virus-derived cytokine. This strategy blocks IFN from inducing an antiviral cellular state but at the price of increased cell proliferation for infected and nearby uninfected B cells.
Figure 4.
Autocrine mechanism of vIL-6 action to inhibit IFN-induced cell cycle arrest. The vIL-6 promoter is activated by IFN-α signaling, acting as a sensor of IFN signaling activity. vIL-6 directly activates the IL-6 gp130 signal transducer molecule, whereas hIL-6 requires gp80, but this is downregulated by IFN. Reprinted with permission (22).
INHIBITION OF INTERFERON ACTIVATION AND TRANSCRIPTION
KSHV also interferes with initiation and continued transcription of the IFN response. Yeast two-hybrid screening of ORF45 protein, a protein with an immediate-early expression pattern (179), revealed binding to cellular IRF7 and blocking of its phosphorylation after virus infection (180). This prevents translocation of this transcription factor to the nucleus, an effect previously seen with the poxvirus E3L protein (154). The significance of IRF7 inhibition is that IRF7, together with IRF3, initiates an IFN response against viral infection by activation of IFN gene promoters (3). Once IFN secretion begins, it further activates its own promoter through induction of the positive IFN transcription factor IRF1 [for review, see (163)].
KSHV vIRF1, encoded by ORF K9 with homology to the IRF family of proteins, performs a function similar to that of the ORF45 protein but does so through direct inhibition of IFN-induced transcription. vIRF1 lacks the complete cellular IRF DNA-binding motif and does not directly bind IFN-stimulated response element; instead, it interacts with cellular IRF3, which provides promoter specificity for this repressor protein (99). vIRF1 prevents IRF3 recruitment of p300 and CBP (CREB-binding protein) histone acetyltransferase coactivators into the IFN transcriptional complex (94, 172). Sequestration of p300/CBP by vIRF1 is the presumed mechanism for its broad inhibition of both class I and class II IFN transcription (46, 52, 95, 181), which is similar to the IFN inhibition reported for the adenovirus E1A protein (10). The effect of vIRF1 on histone acetylation is profound, producing global chromatin condensation that can be measured by differential staining with DNA intercalating dyes (94). vIRF1 acts as a transactivator (139), as well as a repressor protein, and has been shown by Li et al. (95) to induce the vIL-6 gene. When vIRF1 is expressed in NIH3T3 fibroblasts, it causes full cell transformation similar to that of the IRF2 repressor protein (52). Two related proteins, vIRF2 (ORF K11.1) and LANA2 (ORF K10.5), are encoded by spliced genes that probably arose from gene duplication of vIRF1 and have low homology to the cellular IRFs. vIRF2 is expressed at low levels constitutively in PEL cell lines and activates genes possessing multimeric NF-κB elements (94). Preliminary evidence suggests that it binds and inactivates dsRNA-activated protein kinase (PKR), thereby preventing initial activation of IFN through this pathway (17).
The repertoire of KSHV immune inhibitory proteins is at first glance bewildering. But the proteins are expressed in different viral stages and cell types and hence may have specialized functions. vIRF1, for example, is primarily expressed in KS tumor, but not in PELs. Other proteins, such as vFLIP and vIL-6, may act constitutively in tumors and are likely to play a critical role in tumorigenesis. More importantly, these viral defenses against the host immune system illustrate the overlapping nature of immune evasion and cell transformation (Figure 5).
Figure 5.
Examples of immune activation of tumor suppressor checkpoints. Both cell cycle arrest and apoptosis can be initiated by immune recognition of a virus-infected cell. KSHV vIL-6, vIRF1, and vFLIP, which are putative oncogenes, inhibit these responses.
The Antiapoptotic Proteins: vBCL-2, vIAP, LANA1, LANA2, and vIRF1
It is now widely accepted that a cellular response limiting viral infections includes apoptosis (8, 111, 115), although programmed cell death is not universal for all viruses and some viruses may capitalize on cell dissolution during apoptosis to enhance replication (164). The importance of apoptosis regulation to the virus can be seen by the variety of KSHV factors inhibiting this process (Table 2). KSHV encodes proteins targeting downstream apoptotic signaling at the mitochondria, such as vBCL-2 and viral inhibitor of apoptosis protein (vIAP), as well as upstream p53 apoptotic signaling through proteins such as vIRF, LANA1, and LANA2. Inhibition of receptor-mediated apoptosis by vFLIP has already been described and future studies may more clearly link the remaining antiapoptotic proteins to immune evasion functions.
TABLE 2.
KSHV genes with antiapoptotic functions
| KSHV protein |
KSHV gene |
Features and functions | Functional homologs of other viruses |
|---|---|---|---|
| vFLIP | ORF K13 | Homolog of cellular apoptosis inhibitor (FLIP); transcribed on major latency transcripts (LT)1 and LT2; posttranscriptional regulation of protein expression in vivo |
HVS ORF71 |
| vBCL-2 | ORF16 | Inhibits bax-mediated apoptosis | EBV BHRF-1 |
| vIL-6 | ORF K2 | B cell proliferation factor; prevents apoptosis in IL-6-dependent plasmacytoma cells; binds to gp130 subunit of the IL-6 receptor, does not require IL6Ra subunit; induces angiogenesis and VEGF in rodent fibroblasts; protein expressed only in subpopulation of infected hematopoietic cells |
EBV gp350/220 and LMP1 induces cellular IL-6 |
| LANA2 | ORF10.5 | Inhibits p53-mediated transcription in hematopoietic cells |
Mechanism of inhibition unknown |
| vIAP | ORF K7 | Homolog of survivin-DeltaEx3; glycoprotein which can inhibit apoptosis; binds to Bcl2 via BH2 domain; inhibits caspase-3 activity via BIR motif |
|
| vIRF-1 | ORF K9 | Inhibits p53; binds CBP/p300; protein expressed in MCD and PEL cell lines |
Adeno E1A, Adeno E1A, SV40 LT, EBV EBNA2, HTLV Tax |
INHIBITION OF MITOCHONDRIAL APOPTOTIC SIGNALING
KSHV like many other DNA viruses (34) encodes a homolog to the cellular BCL-2 antiapoptotic protein (146). BCL-2 family proteins have both pro- and antiapoptotic functions determined in part by their ability to heterodimerize and regulate release of mitochondrial apoptotic components. vBCL-2 encoded by ORF16 was the first KSHV protein to be investigated for its apoptosis-inhibitory properties (25, 146) and possesses BCL-2 homology (BH)1 and BH2 domains characteristic for this family of proteins. Although no sequence similarity to BH3 and BH4 domains involved in heterodimerization and antiapoptotic functions are present in vBCL-2, solution structure studies reveal strong structural similarities to these domains (69), allowing the viral protein to tightly bind proapoptotic Bak and Bax peptides, as suggested through two-hybrid heterodimerization studies (146). Whereas cellular BCL-2 can be cleaved by caspase proteolysis and converted to a proapoptotic version, KSHV vBCL-2 lacks this cleavage site and escapes cellular regulation (7).
vBCL-2 in PEL cell culture is primarily expressed as an early gene during lytic replication (146, 161) and allows optimal virion production (34, 117, 145). Immunostaining of KS tissues shows vBCL-2 production in a minority of infected spindle cells of advanced nodular lesions consistent with a role primarily in delaying lytic apoptosis (174). Functionally, however, it can inhibit apoptosis owing to latent vCYC-CDK6 complex overexpression, whereas cellular BCL-2 is phosphorylated and inactivated by vCYC-CDK6 (126). Despite its presumed lytic expression pattern, studies from a related murine gammaherpesvirus suggest that vBCL-2 plays a critical role in maintaining chronic viral infection (51).
Another recently described KSHV antiapoptotic factor acting at the mitochondrial membrane is the vIAP encoded by ORF K7, which has structural similarity to cellular survivin protein (45, 171). vIAP, like vBCL-2, is a glycoprotein that localizes to mitochondria and possesses a BH2 domain (171). Mitochondrial membrane permeabilization, Ca+2 depolarization, and release of cytochrome C are early events in the activation of apoptotic caspase cascades. vIAP appears to stabilize Ca+2 mitochondrial flux during cell stress, in part by binding to the calcium-modulating cyclophilin ligand, and can inhibit apoptosis caused by a variety of agents, including Fas, thapsigargin, and staurosporine (45).
INHIBITION OF p53-MEDIATED APOPTOSIS
p53, a transcriptional regulator of cell cycle arrest and apoptosis, is a second major target of antiapoptotic KSHV proteins. LANA1, the large multifunctional protein encoded by ORF73 (78, 136) first identified as a serologic antigen expressed constitutively in all infected cells (118), was subsequently shown by Friborg et al. (48) to bind p53. LANA1 efficiently inhibits p53 activation of a promoter containing the multimerized p53 element from the p21CIP promoter and prevents apoptosis owing to p53 overexpression, whereas a truncated mutant possessing only the first 440 amino acids has no activity. Transcribed at low abundance together with vCYC and vFLIP mRNAs, LANA1 protein is highly stable and is easy to identify on immunostaining by its characteristic speckled, nuclear pattern (53, 79). In interphase nuclei, LANA1 associates with heterochromatin through an amino-terminal region (98) and directly interacts with chromatin and methylated DNA-binding proteins, including histone H1 and MeCP1 (33, 85).
The mechanism and consequences of p53-LANA1 interaction have not been fully examined. It is an attractive candidate oncoprotein because it may antagonize vCYC-induced apoptosis during viral latency. LANA1 has broad transcriptional repressor activity (54, 86, 149) and acts on cell cycle regulation as well as on p53. A second latency-expressed nuclear antigen, LANA2, is expressed as a spliced transcript from ORF K10.5 (44, 138). Less is known about the properties of LANA2, although it has sequence similarity to cellular IRF4 and is expressed only in infected B cells. Like LANA1, LANA2 inhibits p53-mediated transcription and apoptosis, but direct binding to p53 has not been found.
The IRF homolog vIRF1, in addition to its IFN-inhibiting properties, binds and inactivates p53 (120, 151). This effect may in part be mediated by sequestration of p300, which is a p53 transcription coactivator and a p53 acetylator (61, 97, 150, 155). vIRF1 effectively blocks apoptosis from DNA-damaging agents and Fas and fully transforms NIH3T3 cells to cause tumors in severe combined immunodeficiency (SCID) mice. Although the IFN-inhibition function of vIRF1 has also been reported to inhibit the transcription of FasL (82), it is unclear what significance this has for the virus because it does not generally infect immune effector T cells. Other KSHV proteins, such as the highly spliced transmembrane K15 protein (55), may also regulate apoptotic responses but are in early stages of investigation (152).
Regulation of the Cell Cycle: vCYC, LANA1, and K-bZIP/RAP
A third cellular function targeted by KSHV regulatory proteins is control of the cell cycle (Table 3) (Figure 6). The retinoblastoma protein pRB acts at the G1/S cell cycle checkpoint to prevent unscheduled entry into S phase. Acting as a transcriptional repressor, pRB inactivates E2F transcription factors and complexes with E2F to bind E2F-responsive promoter elements in promoters for genes involved in DNA synthesis and chromosomal replication (167). Histone deacety lase and methyl-transferase recruitment by pRB shuts off transcription of E2F-responsive genes. pRB itself is regulated and phosphorylated by cyclins that complex with CDKs.
TABLE 3.
KSHV genes with mitogenic or cell cycle regulatory functions
| KSHV protein |
KSHV gene |
Features and functions | Related proteins from other viruses |
|---|---|---|---|
| LANA1 | ORF73 | Inhibits p53; binds to ori-P and maintains viral episome; protein expressed in all KSHV-infected cells; major latency associated nuclear antigen used in serologic assays |
SV40-LT, HPV-E6, Adeno-E1B, EBV-LMP1; SV40-LT, EBV-EBNA-1 |
| vCYC | ORF72 | Associates with cdk6 to phosphorylate RB and histone H1; resistant to inhibition by p16, p21, and p27; phosphorylates and downregulates p27; latent mRNA expressed in cell cycle–dependent manner |
SV40 LT, HPV-E7, Adeno-E1A |
| vGPCR | ORF74 | Chemokine receptor homolog; induces transformation, angiogenesis, and VEGF in rodent fibroblast; signals through heterotrimeric G proteins, JNK/Sapk, p38/HOG; signaling inhibited by IFN γ –inducible protein-10 (IP-10) and vMIP-II |
HVS-ECRF3; EBV-EBNA-2 activates Burkitt lymphoma receptor-2 (BRL-2/EBI1), a cellular GPCR |
| vIL-6 | ORF K2 | Prevents IFN induction of p21waf1 | EBV gp350/220 and LMP1 induces cellular IL-6 |
| vIRF-1 | ORF K9 | Inhibits IFN signaling; binds CBP/p300; transforms rodent fibroblasts; protein expressed in MCD and PEL cell lines, not detectable in KS or PEL |
Adeno-E1A; Adeno-E1A, SV40 LT, EBV-EBNA2 |
| K-bZIP/ RAP |
ORF K8 | Inhibits G2/M transition | EBV-BZLF1 |
Figure 6.
vCYC inhibits the pRB G1/S checkpoint by cyclin-dependent kinase phosphorylation and induces apoptosis when overexpressed alone. vCYC is resistant to cellular cyclin-dependent kinase inhibitors and may also act at additional points in the cell cycle. vCYC-induced apoptosis is p53 dependent but does not occur through E2F–p14ARF activation. KSHV inhibitors of p53, such as LANA1 and LANA2, are potential candidates to prevent vCYC-induced apoptosis during latent virus infection.
Small DNA tumor viruses possess proteins that directly inhibit pRB by binding to the pRB HDAC-interaction domain through LXCXE-containing motifs (91). The KSHV vCYC protein achieves a similar effect, but does so by mimicking the action of cellular D-type cyclins, which act at the G1/S transition to phosphorylate and inactivate pRB by partnering with CDK4 and 6 (21, 56, 96). The cellular cyclin–CDK complex itself is activated by a cyclin-activating kinase and inhibited by cyclin-dependent kinase inhibitors (CDKIs),including p21CIP and p27KIP. vCYC complexes with only CDK6 and is resistant to inactivation by CDKIs (162) either through structural features to prevent CDKI binding (148) or, in the case of p27KIP, by phosphorylating and inactivating p27KIP (42, 106). Although vCYC functions as a D cyclin that escapes cell regulatory control, it has a broader range of substrates than D cyclins do. Similar to cyclin A or E-CDK2 complexes, vCYC and CDK6 phosphorylate histone H1 and ORC1, triggering DNA synthesis in isolated late G1-phase nuclei (89).
Although vCYC dysregulates the pRB G1/S tumor suppressor checkpoint, direct evidence of vCYC playing a role in cell transformation has been scant until recently. This is in part due to apoptosis resulting from vCYC overexpression in cell lines (125, 126). Activation of E2F after pRB inhibition results in induction of the p14ARF tumor suppressor (p19ARF in the mouse), which in turn increases p53 stability through inhibition of its E3 ubiquitin ligase, MDM2 (5). This feedback control to induce apoptosis serves as a check to prevent tumor formation in the event of isolated loss of pRB function. PEL cell lines are frequently null for p16INK4a, suggesting a possible role in resistance to KSHV-induced apoptosis (133).
Using primary cell lines, Verschuren et al. (170) unexpectedly found that vCYC induces p53-dependent apoptosis that is independent of E2F or p14ARF. Instead, apoptosis occurs from unscheduled DNA synthesis without cytokinesis. When transgenic mice expressing vCYC under control of a B cell promoter were crossed with p53-null mice, all animals developed B cell lymphomas. In addition to vCYC, LANA1 also binds pRB in the pocket region and together with H-Ras can transform rodent embryonic fibroblasts (135). Thus, like SV40 T antigen, LANA1 targets both pRB and p53 checkpoints and serves as a viral episome maintenance protein.
KSHV affects cell cycle regulation through mitogenic pathways as well. Viral G protein–coupled receptor encoded by ORF74 is a constitutively active CXC receptor expressed during lytic replication (27, 83). It activates MAPK, p38, Akt, and NF-κB pathways, resulting in expression of angiogenic factors, such as VEGF, and in cell transformation (2, 18, 108, 113, 134). Another interesting mechanism for KSHV-induced mitogenesis occurs during virus binding to cells. The gB virion glycoprotein expressed on the KSHV envelope binds α 3β1 integrin as the KSHV receptor through an RGD-containing peptide (1). Virus cross-linking of α 3β1 results in activation of focal adhesion kinase and of the MEK-ERK pathway through PI3-kinase and protein kinase C activation (121). It is postulated that mitogenic signaling by KSHV generates a suitable intracellular environment for maintaining virus infection.
One KSHV cell cycle regulator has activity opposing that mentioned for the previous KSHV proteins. The ORF K8 gene product, called K-bZIP or RAP (replication associated protein), is homologous to the EBV transactivator BZLF1 protein (59, 100). Unlike its EBV counterpart, K-bZIP/RAP is not the primary activator of lytic virus replication but instead acts to inhibit cell cycle progression by inducing p21CIP through CCAAT/enhancer-binding protein(C/EBP)-α (176), a finding which has been replicated for the EBV BZLF1 protein as well (175). This may activate the G2/M checkpoint during lytic replication to prevent mitosis, thereby “fixing” the cell at a point in the cell cycle to maximize viral DNA synthesis.
KSHV AS A MODEL TUMOR VIRUS: MOLECULAR PIRACY OF CELLULAR REGULATORY GENES AND IMMUNE EVASION
Ease in identifying and characterizing potential KSHV oncoproteins has made the rapid development of KSHV as a tumor virus model possible. Studies examining KSHV-induced cell proliferation have already generated insights into novel mechanisms applicable to other tumor viruses. This is particularly true for mechanisms inhibiting both innate immune and tumor suppressor signaling pathways (115).
Much of what is currently known about viral carcinogenesis is derived from studies of small DNA viruses and acutely transforming retroviruses. In general, tumor viruses do not cause tumors as part of their natural life cycles: Viral carcinogenesis occurs in nonnative or immunocompromised hosts, or through adventitious host and viral mutagenesis. Nonetheless, viral oncogenes are highly conserved through convergent evolution, indicating their important roles in enhancing viral replication fitness. The presumed function of tumor virus oncogenes based on small tumor virus models is to inhibit G1/S checkpoints during lytic replication. This allows the virus to enhance available replication resources through illicit S phase entry to generate large amounts of viral DNA during lytic replication (123). There is ample evidence for this, including data from KSHV.
An alternative explanation expands on the limited-resource hypothesis to include cellular responses to virus infection and is illustrated by the wealth of immune evasion genes encoded by KSHV (115). The latent proteins LANA1, vFLIP, and vCYC inhibit tumor suppressor pathways and appear to contribute to the transformed PEL phenotype (21, 48, 135, 165, 170). But there is little obvious need to overcome the G1/S checkpoint or inhibit apoptosis during latency because the viral episome replicates in tandem with the cell. If the cell senses latent viral infection and activates G1/S checkpoint arrest and apoptotic programs, then there would be a clear benefit to the virus in inhibiting these innate immune mechanisms.
This assumes that there is activation of tumor suppressor checkpoints during innate immunity, which increasing evidence suggests is the case. Unicellular yeast possess programmed death routines, presumably to limit colony viral infections (47), providing evidence for a primordial innate immune system. Similarly, IFN causes cell cycle checkpoint activation and arrest in a wide variety of cell types (26, 62, 65, 143). The overlap between KSHV proteins having immune evasion functions (e.g., KIS, vFLIP, vIRF1, and vIL-6) and the ability to transform cells or induce cell proliferation gives strong support for this notion. Other pro-proliferative proteins including vCYC, LANA1, vIAP, and vBCL-2 may also play a role in protecting the virus from host immune signaling.
Combining these ideas, one can speculate that cells sense lytic virus replication and respond to it by initiating tumor suppressor checkpoint activation. Lytic viral proteins inhibit this response by targeting tumor suppressor checkpoints and allowing virion replication. Obviously, this does not lead to cancers because the virus only postpones cellular apoptosis during lytic replication, helping to explain the presence of proto-oncogenes in nontumor viruses as diverse as baculovirus and CMV. What kind of cellular sensor can perform this function? An interesting possibility is the DNA-damage response, which activates p53-mediated apoptosis and cell cycle arrest. Linear viral chromosome replication occurring during lytic replication should trigger DNA-damage responses. In line with this, DNA damage activates phosphorylation of IRF3 (81, 178), an initial signaling event for the IFN cascade. Similar mechanisms can be proposed for a latent episome-sensing function. Investigations of how cells initially sense and respond to viral infection will ultimately determine to what degree these hypothetical mechanisms are valid.
CONCLUSIONS
KSHV illustrates that viral immune evasion is intimately intertwined with viral oncogenesis. A large fraction of the nonstructural regulatory homologs encoded by KSHV induce cell proliferation but also target pathways leading to development of innate and adaptive immunity. Immune system and tumor suppressor signaling are only partially overlapping, and other viruses capable of persistent infection without tumorigenesis may have successfully evolved means of inhibiting immunity without abrogating tumor suppressor checkpoints. While the methods used by KSHV and related rhadinoviruses to target cell regulatory pathways are unique, the lessons learned from these viruses can be applied to understanding unrelated viruses, which face the challenge of infecting the hostile environment of the eukaryotic cell.
ACKNOWLDEGMENTS
We would like to thank Rusung Tan for comments and review of the manuscript. This effort was in part supported by NIH NCI grants CA83485, CA67391, and CA87661.
Contributor Information
Patrick S. Moore, Email: psm9@pitt.edu.
Yuan Chang, Email: yc70@pitt.edu.
LITERATURE CITED
- 1.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/ HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 2.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, et al. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 3.Barnes B, Lubyova B, Pitha PM. Review: on the role of IRF in host defense. J. Interferon Cytokine Res. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- 4.Bassett MT, Chokunonga E, Mauchaza B, Levy L, Ferlay J, Parkin DM. Cancer in the African population of Harare, Zimbabwe, 1990–1992. Int. J. Cancer. 1995;63:29–36. doi: 10.1002/ijc.2910630107. [DOI] [PubMed] [Google Scholar]
- 5.Bates S, Phillips AC, Clark PA, Stott F, Peters G, et al. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–25. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 6.Belanger C, Gravel A, Tomoiu A, Janelle ME, Gosselin J, et al. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J. Hum. Virol. 2001;4:62–73. [PubMed] [Google Scholar]
- 7.Bellows DS, Chau BN, Lee P, Lazebnik Y, Burns WH, Hardwick JM. Antiapoptotic herpesvirus bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J. Virol. 2000;74:5024–5031. doi: 10.1128/jvi.74.11.5024-5031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 9.Bertin J, Armstrong RC, Ottilie S, Martin DA, Wang Y, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, et al. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- 11.Bieleski L, Talbot SJ. Kaposi’s sarcoma-associated herpesvirus vCyclin open reading frame contains an internal ribosome entry site. J. Virol. 2001;75:1864–1869. doi: 10.1128/JVI.75.4.1864-1869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, et al. Angiogenic and HIV inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 13.Boshoff C, Gao SJ, Healy LE, Matthews S, Thomas AJ, et al. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood. 1998;91:1671–1679. [PubMed] [Google Scholar]
- 14.Brousset P, Cesarman E, Meggetto F, Lamant L, Delsol G. Colocalization of the viral interleukin-6 with latent nuclear antigen-1 of human herpesvirus-8 in endothelial spindle cells of Kaposi’s sarcoma and lymphoid cells of multicentric Castleman’s disease. Hum. Pathol. 2001;32:95–100. doi: 10.1053/hupa.2001.21131. [DOI] [PubMed] [Google Scholar]
- 15.Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, et al. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–1863. [PubMed] [Google Scholar]
- 16.Burgert HG, Maryanski JL, Kvist S. “E3/19K” protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc. Natl. Acad. Sci. USA. 1987;84:1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burysek L, Pitha PM. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J. Virol. 2001;75:2345–2352. doi: 10.1128/JVI.75.5.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon M, Philpott NJ, Cesarman E. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 2003;77:57–67. doi: 10.1128/JVI.77.1.57-67.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 20.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;265:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, et al. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298:1432–1435. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Bacon KB, Li L, Garcia GE, Xia Y, et al. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J. Exp. Med. 1998;188:193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng EH, Nicholas J, Bellows DS, Hayward GS, Guo HG, et al. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 27.Chiou CJ, Poole LJ, Kim PS, Ciufo DM, Cannon JS, et al. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2002;76:3421–3439. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow D-c, He X-l, Snow AL, Rose-John S, Garcia KC. Structure of an extracellular gp130 cytokine receptor signaling complex. Science. 2001;291:2150–2155. doi: 10.1126/science.1058308. [DOI] [PubMed] [Google Scholar]
- 29.Chung YH, Means RE, Choi JK, Lee BS, Jung JU. Kaposi’s sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production. J. Virol. 2002;76:4688–4698. doi: 10.1128/JVI.76.10.4688-4698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coscoy L, Ganem D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coscoy L, Ganem D. Aviral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Invest. 2001;107:1599–1606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 2001;155:1265–1273. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter MA, 2nd, Robertson ES. The latency-associated nuclear antigen tethers the Kaposi’s sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264:254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 34.Cuconati A, White E. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- 35.Dairaghi DJ, Fan RA, McMaster BE, Hanley MR, Schall TJ. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J. Biol. Chem. 1999;274:21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- 36.DeBruyne LA, Li K, Bishop DK, Bromberg JS. Gene transfer of virally encoded chemokine antagonists vMIP-II and MC148 prolongs cardiac allograft survival and inhibits donor-specific immunity. Gene Ther. 2000;7:575–582. doi: 10.1038/sj.gt.3301128. [DOI] [PubMed] [Google Scholar]
- 37.Deng H, Song MJ, Chu JT, Sun R. Transcriptional regulation of the interleukin-6 gene of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) J. Virol. 2002;76:8252–8264. doi: 10.1128/JVI.76.16.8252-8264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du MQ, Diss TC, Liu H, Ye H, Hamoudi RA, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 40.Du MQ, Liu H, Diss TC, Ye H, Hamoudi RA, et al. Kaposi sarcoma-associated herpesvirus infects monotypic (IgMlambda) but polyclonal naive B cells in Castleman disease and associated lympho proliferative disorders. Blood. 2001;97:2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 41.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis M, Chew YP, Fallis L, Freddersdorf S, Boshoff C, et al. Degradation of p27(Kip) cdk inhibitor triggered by Kaposi’s sarcoma virus cyclin-cdk6 complex. EMBO J. 1999;18:644–653. doi: 10.1093/emboj/18.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. The Kaposi’s sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 1999;189:1993–1998. doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fakhari FD, Dittmer DP. Charting latency transcripts in Kaposi’s sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 2002;76:6213–6223. doi: 10.1128/JVI.76.12.6213-6223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng P, Park J, Lee BS, Lee SH, Bram RJ, Jung JU. Kaposi’s sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J. Virol. 2002;76:11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flowers C, Flowers S, Nabel G. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor confers resistance to the antiproliferative effect of interferon-alpha. Mol. Med. 1998;4:402–412. [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser A, James C. Fermenting debate: Do yeast undergo apoptosis? Trends Cell Biol. 1998;8:219–221. doi: 10.1016/s0962-8924(98)01275-6. [DOI] [PubMed] [Google Scholar]
- 48.Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 49.Fruh K, Bartee E, Gouveia K, Mansouri M. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Res. 2002;88:55–69. doi: 10.1016/s0168-1702(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 50.Gaidano G, Capello D, Cilia AM, Gloghini A, Perin T, et al. Genetic characterization of HHV-8/KSHV-positive primary effusion lymphoma reveals frequent mutations of BCL6: implications for disease pathogenesis and histogenesis. Genes Chromosomes Cancer. 1999;24:16–23. doi: 10.1002/(sici)1098-2264(199901)24:1<16::aid-gcc3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 51.Gangappa S, van Dyk LF, Jewett TJ, Speck SH, Virgin HW. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 2002;195:931–940. doi: 10.1084/jem.20011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. KSHV ORF K9 (vIRF) is an oncogene that inhibits the interferon signaling pathway. Oncogene. 1997;15:1979–1986. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- 53.Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat. Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 54.Garber AC, Shu MA, Hu J, Renne R. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2001;75:7882–7892. doi: 10.1128/JVI.75.17.7882-7892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glenn M, Rainbow L, Aurad F, Davison A, Schulz TF. Identification of a spliced gene from Kaposi’s sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J. Virol. 1999;73:6953–6963. doi: 10.1128/jvi.73.8.6953-6963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, et al. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gold MR. To make antibodies or not: signaling by the B-cell antigen receptor. Trends Pharmacol. Sci. 2002;23:316–324. doi: 10.1016/s0165-6147(02)02045-x. [DOI] [PubMed] [Google Scholar]
- 58.Gorczynski RM, Yu K, Clark D. Receptor engagement on cells expressing a ligand for the tolerance-inducing molecule OX2 induces an immunoregulatory population that inhibits alloreactivity in vitro and in vivo. J. Immunol. 2000;165:4854–4860. doi: 10.4049/jimmunol.165.9.4854. [DOI] [PubMed] [Google Scholar]
- 59.Gruffat H, Portes-Sentis S, Sergeant A, Manet E. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) encodes a homologue of the Epstein-Barr virus bZip protein EB1. J. Gen. Virol. 1999;80:557–561. doi: 10.1099/0022-1317-80-3-557. [DOI] [PubMed] [Google Scholar]
- 60.Grundhoff A, Ganem D. Mechanisms governing expression of the v-FLIP gene of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2001;75:1857–1863. doi: 10.1128/JVI.75.4.1857-1863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 62.Harvat BL, Jetten AM. Gamma-interferon induces an irreversible growth arrest in mid-G1 in mammary epithelial cells which correlates with a block in hyperphosphorylation of retinoblastoma. Cell Growth Differ. 1996;7:289–300. [PubMed] [Google Scholar]
- 63.Henis-Korenblit S, Shani G, Sines T, Marash L, Shohat G, Kimchi A. The caspase-cleaved DAP5 protein supports internal ribosome entry site-mediated translation of death proteins. Proc. Natl. Acad. Sci. USA. 2002;99:5400–5405. doi: 10.1073/pnas.082102499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hideshima T, Chauhan D, Teoh G, Raje N, Treon SP, et al. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clin. Cancer Res. 2000;6:1180–1189. [PubMed] [Google Scholar]
- 65.Hobeika AC, Subramaniam PS, Johnson HM. IFNα induces the expression of the cyclin-dependent kinase inhibitor p21 in human prostate cancer cells. Oncogene. 1997;14:1165–1170. doi: 10.1038/sj.onc.1200939. [DOI] [PubMed] [Google Scholar]
- 66.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, et al. Downregulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 67.Holcik M, Sonenberg N, Korneluk RG. Internal ribosome initiation of translation and the control of cell death. Trends Genet. 2000;16:469–473. doi: 10.1016/s0168-9525(00)02106-5. [DOI] [PubMed] [Google Scholar]
- 68.Hu S, Vincenz C, Buller M, Dixit VM. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J. Biol. Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 69.Huang Q, Petros AM, Virgin HW, Fesik SW, Olejniczak ET. Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus. Proc. Natl. Acad. Sci. USA. 2002;99:3428–3433. doi: 10.1073/pnas.062525799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, et al. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13:365–374. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 72.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 2000;74:5300–5309. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. [PubMed] [Google Scholar]
- 74.Kapadia SB, Levine B, Speck SH, Virgin HW. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity. 2002;17:143–155. doi: 10.1016/s1074-7613(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 75.Katano H, Sato Y, Itoh H, Sata T. Expression of human herpesvirus 8 (HHV-8)-encoded immediate early protein, open reading frame 50, in HHV-8-associated diseases. J. Hum. Virol. 2001;4:96–102. [PubMed] [Google Scholar]
- 76.Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology. 2000;269:335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 77.Kataoka T, Budd RC, Holler N, Thome M, Martinon F, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr. Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 78.Kedes DH, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J. Clin. Invest. 1997;100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepi-demiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 80.Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- 81.Kim T, Kim TY, Song YH, Min IM, Yim J, Kim TK. Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J. Biol. Chem. 1999;274:30686–30689. doi: 10.1074/jbc.274.43.30686. [DOI] [PubMed] [Google Scholar]
- 82.Kirchhoff S, Sebens T, Baumann S, Krueger A, Zawatzky R, et al. Viral IFN-regulatory factors inhibit activation-induced cell death via two positive regulatory IFN-regulatory factor 1-dependent domains in the CD95 ligand promoter. J. Immunol. 2002;168:1226–1234. doi: 10.4049/jimmunol.168.3.1226. [DOI] [PubMed] [Google Scholar]
- 83.Kirshner JR, Staskus K, Haase A, Lagunoff M, Ganem D. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi’s sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 1999;73:6006–6014. doi: 10.1128/jvi.73.7.6006-6014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83a.Knipe DM, Howley PM, Griffin D, Lamb R, Martin M, Straus S, editors. Fields Virology. Philadelphia: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- 84.Komanduri KV, Luce JA, McGrath MS, Herndier BG, Ng VL. The natural history and molecular heterogeneity of HIV-associated primary malignant lym-phomatous effusions. J. Acquir. Immun. Defic. Syndr. Hum. Retrovirol. 1996;13:215–226. doi: 10.1097/00042560-199611010-00003. [DOI] [PubMed] [Google Scholar]
- 85.Krithivas A, Fujimuro M, Weidner M, Young DB, Hayward SD. Protein interactions targeting the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 2002;76:11596–11604. doi: 10.1128/JVI.76.22.11596-11604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krithivas A, Young DB, Liao G, Greene D, Hayward SD. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 2000;74:9637–9645. doi: 10.1128/jvi.74.20.9637-9645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Biol. Cell. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi’s sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA. 1999;96:5704–5709. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laman H, Coverley D, Krude T, Laskey R, Jones N. Viral cyclin-cyclin-dependent kinase 6 complexes initiate nuclear DNA replication. Mol. Cell Biol. 2001;21:624–635. doi: 10.1128/MCB.21.2.624-635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee BS, Alvarez X, Ishido S, Lackner AA, Jung JU. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi’s sarcoma-associated herpesvirus K1. J. Exp. Med. 2000;192:11–21. doi: 10.1084/jem.192.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee C, Cho Y. Interactions of SV40 large T antigen and other viral proteins with retinoblastoma tumour suppressor. Rev. Med. Virol. 2002;12:81–92. doi: 10.1002/rmv.340. [DOI] [PubMed] [Google Scholar]
- 92.Lee H, Guo J, Li M, Choi JK, DeMaria M, et al. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi’s sarcoma-associated herpesvirus. Mol. Cell Biol. 1998;18:5219–5228. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee H, Veazey R, Williams K, Li M, Guo J, et al. Deregulation of cell growth by the K1 gene of Kaposi’s sarcoma-associated herpesvirus. Nat. Med. 1998;4:435–440. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 94.Li M, Damania B, Alvarez X, Ogryzko V, Ozato K, Jung JU. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol. Cell Biol. 2000;20:8254–8263. doi: 10.1128/mcb.20.21.8254-8263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li M, Lee H, Guo J, Neipel F, Fleckenstein B, et al. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 1998;72:5433–5440. doi: 10.1128/jvi.72.7.5433-5440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, et al. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lill NL, Grossman SR, Ginsberg D, De-Caprio J, Livingston DM. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 98.Lim C, Sohn H, Lee D, Gwack Y, Choe J. Functional dissection of latency-associated nuclear antigen 1 of Kaposi’s sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 2002;76:10320–10331. doi: 10.1128/JVI.76.20.10320-10331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, et al. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene. 2001;20:800–811. doi: 10.1038/sj.onc.1204163. [DOI] [PubMed] [Google Scholar]
- 100.Lin SF, Robinson DR, Miller G, Kung HJ. Kaposi’s sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus. J. Virol. 1999;73:1909–1917. doi: 10.1128/jvi.73.3.1909-1917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C, Okruzhnov Y, Li H, Nicholas J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 2001;75:10933–10940. doi: 10.1128/JVI.75.22.10933-10940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J. Biol. Chem. 2002;277:13745–13751. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- 103.Lorenzo ME, Jung JU, Ploegh HL. Kaposi’s sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J. Virol. 2002;76:5522–5531. doi: 10.1128/JVI.76.11.5522-5531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Low W, Harries M, Ye H, Du MQ, Boshoff C, Collins M. Internal ribosome entry site regulates translation of Kaposi’s sarcoma-associated herpesvirus FLICE inhibitory protein. J. Virol. 2001;75:2938–2945. doi: 10.1128/JVI.75.6.2938-2945.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luppi M, Barozzi P, Schulz TF, Setti G, Staskus K, et al. Bone marrow failure associated with human herpesvirus 8 infection after transplantation. N. Engl. J. Med. 2000;343:1378–1385. doi: 10.1056/NEJM200011093431905. [DOI] [PubMed] [Google Scholar]
- 106.Mann DJ, Child ES, Swanton C, Laman H, Jones N. Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi’s sarcoma-associated herpesvirus. EMBO J. 1999;18:654–663. doi: 10.1093/emboj/18.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mansouri M, Bartee E, Gouveia K, Hovey Nerenberg BT, Barrett J, et al. The PHD/LAP-domain protein M153R of myxomavirus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J. Virol. 2003;77:1427–1440. doi: 10.1128/JVI.77.2.1427-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masood R, Cesarman E, Smith DL, Gill PS, Flore O. Human herpesvirus-8-transformed endothelial cells have functionally activated vascular endothelial growth factor/vascular endothelial growth factor receptor. Am. J. Pathol. 2002;160:23–29. doi: 10.1016/S0002-9440(10)64344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsushima AY, Strauchen JA, Lee G, Scigliano E, Hale EE, et al. Posttransplantation plasmacytic proliferations related to Kaposi’s sarcoma-associated herpesvirus. Am. J. Surg. Pathol. 1999;23:1393–1400. doi: 10.1097/00000478-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 110.Means RE, Ishido S, Alvarez X, Jung JU. Multiple endocytic trafficking pathways of MHC class I molecules induced by a herpesvirus protein. EMBO J. 2002;21:1638–1649. doi: 10.1093/emboj/21.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meinl E, Fickenscher H, Thome M, Tschopp J, Fleckenstein B. Anti-apoptotic strategies of lymphotropic viruses. Immunol. Today. 1998;19:474–479. doi: 10.1016/s0167-5699(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 112.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 1997;272:19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- 113.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–2648. [PubMed] [Google Scholar]
- 114.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 115.Moore PS, Chang Y. Antiviral activity of tumor-suppressor pathways: clues from molecular piracy by KSHV. Trends Genet. 1998;14:144–150. doi: 10.1016/s0168-9525(98)01408-5. [DOI] [PubMed] [Google Scholar]
- 116.Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus. 2001:2803–2833. doi: 10.1098/rstb.2000.0777. See Ref. 83a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moore PS, Chang Y. Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Philos. Trans. R. Soc. London B Biol. Sci. 2001;356:499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, et al. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J. Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murphy PM. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2001;2:116–122. doi: 10.1038/84214. [DOI] [PubMed] [Google Scholar]
- 120.Nakamura H, Li M, Zarycki J, Jung JU. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 2001;75:7572–7582. doi: 10.1128/JVI.75.16.7572-7582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-{zeta}-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 2002;77:1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, non-self discrimination. Annu. Rev. Immunol. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 123.Nevins J. Cell transformation by viruses. 2001:245–275. See Ref. 83a. [Google Scholar]
- 124.Nicholas J, Ruvolo VR, Burns WH, Sand-ford G, Wan X, et al. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 125.Ojala PM, Tiainen M, Salven P, Veikkola T, Castanos-Velez E, et al. Kaposi’s sarcoma-associated herpesvirus-encoded v-cyclin triggers apoptosis in cells with high levels of cyclin-dependent kinase 6. Cancer Res. 1999;59:4984–4989. [PubMed] [Google Scholar]
- 126.Ojala PM, Yamamoto K, Castanos-Velez E, Biberfeld P, Korsmeyer SJ, Makela TP. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat. Cell Biol. 2000;2:819–925. doi: 10.1038/35041064. [DOI] [PubMed] [Google Scholar]
- 127.Oksenhendler E, Boulanger E, Galicier L, Du MQ, Dupin N, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99:2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 128.Okuno T, Jiang YB, Ueda K, Nishimura K, Tamura T, Yamanishi K. Activation of human herpesvirus 8 open reading frame K5 independent of ORF50 expression. Virus Res. 2002;90:77–89. doi: 10.1016/s0168-1702(02)00142-9. [DOI] [PubMed] [Google Scholar]
- 129.Olsen SJ, Tarte K, Sherman W, Hale EE, Weisse MT, et al. Evidence against KSHV infection in the pathogenesis of multiple myeloma. Virus Res. 1998;57:197–202. doi: 10.1016/s0168-1702(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 130.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum. Immunol. 1999;60:921–927. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 131.Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, et al. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am. J. Pathol. 2000;156:743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parravicini C, Corbellino M, Paulli M, Magrini U, Lazzarino M, et al. Expression of a virus-derived cytokine, KSHVvIL-6,in HIV-seronegative Castleman’s disease. Am. J. Pathol. 1997;151:1517–1522. [PMC free article] [PubMed] [Google Scholar]
- 133.Platt G, Carbone A, Mittnacht S. p16INK4a loss and sensitivity in KSHV associated primary effusion lymphoma. Oncogene. 2002;21:1823–1831. doi: 10.1038/sj.onc.1205360. [DOI] [PubMed] [Google Scholar]
- 134.Polson AG, Wang D, DeRisi J, Ganem D. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2002;62:4525–4530. [PubMed] [Google Scholar]
- 135.Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene hras transforms primary rat cells. Nat. Med. 2000;6:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 136.Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, et al. The 222-to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rettig MB, Ma HJ, Vescio RA, Pold M, Schiller G, et al. Kaposi’s sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]
- 138.Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 2001;75:429–438. doi: 10.1128/JVI.75.1.429-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roan F, Zimring JC, Goodbourn S, Offermann MK. Transcriptional activation by the human herpesvirus-8-encoded interferon regulatory factor. J. Gen. Virol. 1999;80:2205–2209. doi: 10.1099/0022-1317-80-8-2205. [DOI] [PubMed] [Google Scholar]
- 140.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc. Natl. Acad. Sci. USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sadler R, Wu L, Forghani B, Renne R, Zhong W, et al. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1999;73:5722–5730. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanchez DJ, Coscoy L, Ganem D. Functional organization of MIR2, a novel viral regulator of selective endocytosis. J. Biol. Chem. 2002;277:6124–6130. doi: 10.1074/jbc.M110265200. [DOI] [PubMed] [Google Scholar]
- 143.Sangfelt O, Erickson S, Einhorn S, Grander D. Induction of Cip/Kip and Ink4 cyclin dependent kinase in-ibitors by interferon-alpha in hematopoietic cell lines. Oncogene. 1997;14:415–423. doi: 10.1038/sj.onc.1200832. [DOI] [PubMed] [Google Scholar]
- 144.Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J. Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarid R, Olsen SJ, Moore PS. Kaposi’s sarcoma-associated herpesvirus: epidemiology, virology and molecular biology. Adv. Virus Res. 1999;52:139–232. doi: 10.1016/s0065-3527(08)60299-7. [DOI] [PubMed] [Google Scholar]
- 146.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 147.Sarid R, Wiezorek JS, Moore PS, Chang Y. Characterization and cell cycle regulation of the major Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 1999;73:1438–1446. doi: 10.1128/jvi.73.2.1438-1446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schulze-Gahmen U, Jung JU, Kim SH. Crystal structure of a viral cyclin, a positive regulator of cyclin-dependent kinase 6. Structure. 1999;7:245–254. doi: 10.1016/s0969-2126(99)80035-5. [DOI] [PubMed] [Google Scholar]
- 149.Schwam DR, Luciano RL, Mahajan SS, Wong L, Wilson AC. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 2000;74:8532–8540. doi: 10.1128/jvi.74.18.8532-8540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Scolnick DM, Chehab NH, Stavridi ES, Lien MC, Caruso L, et al. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 151.Seo T, Park J, Lee D, Hwang SG, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 2001;75:6193–6198. doi: 10.1128/JVI.75.13.6193-6198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sharp TV, Wang HW, Koumi A, Hollyman D, Endo Y, et al. K15 protein of Kaposi’s sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 2002;76:802–816. doi: 10.1128/JVI.76.2.802-816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sitas F, Carrara H, Beral V, Newton R, Reeves G, et al. Antibodies against human herpesvirus 8 in black South African patients with cancer. N. Engl. J. Med. 1999;340:1863–1871. doi: 10.1056/NEJM199906173402403. [DOI] [PubMed] [Google Scholar]
- 154.Smith EJ, Marie I, Prakash A, Garcia-Sastre A, Levy DE. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by Vaccinia virus E3L protein. J. Biol. Chem. 2001;276:8951–8957. doi: 10.1074/jbc.M008717200. [DOI] [PubMed] [Google Scholar]
- 155.Somasundaram K, El-Deiry WS. Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene. 1997;14:1047–1057. doi: 10.1038/sj.onc.1201002. [DOI] [PubMed] [Google Scholar]
- 156.Spiller OB, Robinson M, O’Donnell E, Milligan S, Morgan BP, et al. Complement regulation by Kaposi’s sarcoma-associated herpesvirus ORF4 protein. J. Virol. 2003;77:592–599. doi: 10.1128/JVI.77.1.592-599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, et al. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stevenson PG, Efstathiou S, Doherty PC, Lehner PJ. Inhibition of MHC class I-restricted antigen presentation by gamma2-herpesviruses. Proc. Natl. Acad. Sci. USA. 2000;97:8455–8460. doi: 10.1073/pnas.150240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stine JT, Wood C, Hill M, Epp A, Raport CJ, et al. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–1157. [PubMed] [Google Scholar]
- 160.Sturzl M, Wunderlich A, Ascherl G, Hohenadl C, Monini P, et al. Human herpesvirus-8 (HHV-8) gene expression in Kaposi’s sarcoma (KS) primary lesions: an in situ hybridization study. Leukemia. 1999;13(Suppl. 1):S110–S112. doi: 10.1038/sj.leu.2401323. [DOI] [PubMed] [Google Scholar]
- 161.Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, et al. Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J. Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Swanton C, Mann DJ, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 163.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 164.Teodoro JG, Branton PE. Regulation of apoptosis by viral gene products. J. Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thome M, Schneider P, Hofman K, Fickenscher H, Meinl E, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 166.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 167.Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- 168.Urashima M, Ogata A, Chauhan D, Vidriales MB, Teoh G, et al. Interleukin-6 promotes multiple myeloma cell growth via phosphorylation of retinoblastoma protein. Blood. 1996;88:2219–2227. [PubMed] [Google Scholar]
- 169.Urashima M, Teoh G, Chauhan D, Hoshi Y, Ogata A, et al. Interleukin-6 overcomes p21WAF1 upregulation and G1 growth arrest induced by dexamethasone and interferon-gamma in multiple myeloma cells. Blood. 1997;90:279–289. [PubMed] [Google Scholar]
- 170.Verschuren EW, Klefstrom J, Evan GI, Jones N. The oncogenic potential of Kaposi’s sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell. 2002;2:229–241. doi: 10.1016/s1535-6108(02)00123-x. [DOI] [PubMed] [Google Scholar]
- 171.Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602–2615. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weaver BK, Kumar KP, Reich NC. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell Biol. 1998;18:1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weber KS, Grone HJ, Rocken M, Klier C, Gu S, et al. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur J. Immunol. 2001;31:2458–2466. doi: 10.1002/1521-4141(200108)31:8<2458::aid-immu2458>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 174.Widmer I, Wernli M, Bachmann F, Gudat F, Cathomas G, Erb P. Differential expression of viral Bcl-2 encoded by Kaposi’s sarcoma-associated herpesvirus and human Bcl-2 in primary effusion lymphoma cells and Kaposi’s sarcoma lesions. J. Virol. 2002;76:2551–2556. doi: 10.1128/jvi.76.5.2551-2556.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu FY, Chen H, Wang SE, apRhys CMJ, Liao G, et al. CCAAT/enhancer binding protein {alpha} interacts with ZTA and mediates ZTA-induced p21CIP-1 accumulation and G1 cell cycle arrest during the Epstein-Barr virus lytic cycle. J. Virol. 2002;77:1481–1500. doi: 10.1128/JVI.77.2.1481-1500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu FY, Tang QQ, Chen H, apRhys C, Farrell C, et al. Lytic replication-associated protein (RAP) encoded by Kaposi sarcoma-associated herpesvirus causes p21CIP-1-mediated G1 cell cycle arrest through CCAAT/enhancer-binding protein-alpha. Proc. Natl. Acad. Sci. USA. 2002;99:10683–10688. doi: 10.1073/pnas.162352299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang BT, Chen SC, Leach MW, Manfra D, Homey B, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angio-proliferative disease resembling Kaposi’s sarcoma. J. Exp. Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhu FX, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA. 2002;99:5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zimring JC, Goodbourn S, Offermann MK. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J. Virol. 1998;72:701–707. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]