Abstract
Background and Purpose
To investigate whether the Framingham Cardiovascular Risk Profile (FCRP) and carotid artery intima-media thickness (CIMT) are associated with cortical volume and thickness.
Methods
Consecutive subjects participating in a prospective cohort study of aging and mild cognitive impairment enriched for vascular risk factors for atherosclerosis underwent structural MRI scans at 3T and 4T MRI at three sites. Freesurfer (v5.1) was used to obtain regional measures of neocortical volumes (mm3) and thickness (mm). Multiple linear regression was used to determine the association of FCRP and CIMT with cortical volume and thickness
Results
152 subjects (82 men) were aged 78 (±7) years old, 94 had a CDR of 0, 58 had a clinical dementia rating (CDR) of 0.5 and the mean mini-mental status examination (MMSE) was 28 ± 2. FCRP score was inversely associated with total gray matter (GM) volume, parietal and temporal GM volume (adjusted p<0.04). FCRP was inversely associated with parietal and total cerebral GM thickness (adjusted p<0.03). CIMT was inversely associated with thickness of parietal GM only (adjusted p=0.04). Including history of myocardial infarction or stroke and radiologic evidence of brain infarction, or apoE genotype did not alter relationships with FCRP or CIMT.
Conclusions
Increased cardiovascular risk was associated with reduced GM volume and thickness in regions also affected by Alzheimer’s disease (AD), independent of infarcts and apoE genotype. These results suggest a “double hit” toward developing dementia when someone with incipient AD also has high cardiovascular risk.
Keywords: Framingham cardiovascular risk profile, carotid intima media thickness, gray matter, cortical volume, cortical thickness, atrophy
Introduction
We have previously reported in an autopsy sample that cerebral atherosclerosis contributes to brain atrophy independent of Alzheimer pathology and cerebral infarcts1. Atrophy is a non-specific finding associated with normal aging2, Alzheimer’s disease (AD)3, stroke4, myocardial infarction (MI)5, and other neurodegenerative disorders6. Regional patterns of atrophy may differ by etiology such as in AD3, where atrophy is most prominent in the medial, inferior and lateral temporal lobes, followed by multi-modal association areas. Epidemiologic studies have shown that risk factors for atherosclerosis (e.g., hypertension, diabetes mellitus, and hyperlipidemia) increase the risk for cognitive impairment associated with both stroke and AD7-9.
Brain atrophy is associated with cognitive impairment10. The ε4 allele of the apolipoprotein E (apoE) gene, a genetic risk factor for cognitive decline in the elderly11, has demonstrated a particular phenotype of atrophy of medial temporal lobe structures12. Global brain atrophy is also seen in survivors of myocardial infarction5 and in the presence of cerebrovascular disease4, 13. Previous work focusing on the relationships between FCRP or CIMT and brain volumes in healthy older adults14 or patients with cardiovascular disease15 did not account for cognitive status, cerebrovascular disease, and cardiovascular disease. Moreover, the regional pattern of atrophy associated with atherosclerosis is relatively unknown.
The primary objective of the present study was to determine whether Framingham Cardiovascular Risk Profile (FCRP) and subclinical atherosclerosis measured as carotid artery intima media thickness (CIMT) are associated with brain atrophy independent of vascular injury and apoE. A secondary goal was to determine whether FCRP and CIMT are associated with regional patterns of brain atrophy.
In this study, FCRP16 was used to assess vascular risk and CIMT was used as a measure of subclinical atherosclerosis. If cardiovascular risk factors contribute to atherosclerosis then brain atrophy, we hypothesize that both FCRP and CIMT will be inversely associated with brain volume and cortical thickness, globally and regionally.
Methods
Subjects
Consecutive subjects were identified from an ongoing, longitudinal, multi-institutional Aging Brain program project that recruits subjects with normal cognition to mild cognitive impairment, representing a spectrum of low to high vascular risk17. Most participants were acquired through community-based recruitment using a protocol designed to obtain a demographically diverse cohort, or through sources such as stroke clinics and support groups attended by people with high vascular risk factors. All participants gave written informed consent in accordance with the policies of each institutional review board. Inclusion criteria include age 60 or older, with cognitive function in the normal to mild cognitive impairment range (Clinical Dementia Rating [CDR] score of 0 or 0.5) 18. Persons with history of multiple vascular risk factors, coronary or carotid disease, myocardial infarction, or ischemic stroke were targeted for inclusion, although patients with very large strokes that interfered with estimation of cortical volume and thickness were excluded. Exclusion criteria included evidence of alcohol or substance abuse, head trauma with loss of consciousness lasting longer than 15 minutes, factors contraindicating MRI, and severe medical illness, neurologic or psychiatric disorders unrelated to AD or vascular dementia that could significantly affect brain structure (e.g., schizophrenia and other psychotic disorders, bipolar disorder, current major depression, post-traumatic stress disorder, obsessive-compulsive disorder, liver disease, multiple sclerosis, amyotrophic lateral sclerosis). Participant demographics by CDR are shown in Table 1.
Table 1.
Participant demographics by cognitive status.
| CDR=0 | CDR=0.5 | |
|---|---|---|
| N=94 | N=58 | |
| Age (yrs) | 77±7 | 79±7 |
| Education (yrs) | 16±3 | 16±3 |
| MMSE | 29.0±1.3 | 27.6±2.3* |
| % men | 50 | 62 |
| % stroke history | 19 | 31 |
| % radiologically identified brain infarcts | 19 | 33 |
| % myocardial infarction | 12 | 16 |
| % coronary artery bypass or angioplasty | 17 | 17 |
| % carotid endarterectomy | 2 | 5 |
| % coronary, carotid or other artery stent | 12 | 17 |
| % taking medication to reduce blood pressure | 76 | 67 |
| % taking medication to reduce cholesterol | 64 | 67 |
| apoE4 allele (pos, neg) | 17, 55 | 6, 24 |
| %apoE4 allele | 24 | 20 |
| FCRP (% risk) | 12 ± 7 | 14 ± 8 |
| CIMT (mm) | 0.92 ± 0.14 | 0.95 ± 0.14 |
p<0.05
continuous variables are summarized as mean ± SD
continuous variables compared with t-test, proportions compared with Fisher’s exact test
CDR=clinical dementia rating; MMSE=mini-mental status examination; apoE4=apolipoprotein E epsilon 4; FCRP=Framingham cardiovascular risk profile; CIMT=common carotid artery intima-media thickness
Measures of cardiovascular risk and carotid atherosclerosis
The FCRP uses empirically-derived age- and gender-adjusted weighting of categorical variables to predict the 10-year risk of coronary heart disease and is a weighted sum of: age, gender, active smoking, diabetes, systolic blood pressure (and/or use of hypertensive medications) and total cholesterol and high-density lipoprotein cholesterol levels16. Higher scores indicate greater coronary risk.
CIMT was used as a measure of subclinical atherosclerosis. CIMT is a measures of the thickness of the inner two layers of the carotid artery; higher CIMT indicates greater atherosclerosis burden. High-resolution B-mode ultrasound images of the right and left common carotid arteries were obtained with a 7.5-MHz linear array transducer attached to an ATL Apogee ultrasound system (Bothell, WA). CIMT was determined as the average of 70 to 100 measurements between the intima-lumen and media-adventitia interfaces along a 1 cm length just proximal to the carotid artery bulb at the same point of the cardiac cycle using comperterized automated edge detection. Right and left CIMT were measured in each individual whenever possible. For individuals with CIMT measurements from both sides, the maximum of these two quantities was used in subsequent statistical analyses.
Measure of AD risk
Blood was drawn with the subject’s consent for apolipoprotein E genotyping. Genotyping was completed for 102 participants. Subjects with 3/4 or 4/4 combined alleles were classified as apoE ε4 positive, and those with 3/3 alleles as apoE ε4 negative. Because the 2/4 combined allele is associated with a lower risk of AD19, these subjects were not included in the APOE ε4 positive group.
MRI: acquisition
Structural T1-weighted MRI scans for participants were collected on 3T and 4T MRI systems. Forty-three participants were scanned at the University of Southern California using a 3T General Electric Signal HDx system with an 8-channel head coil. Acquired images included a T1-weighted volumetric SPGR (TR = 7 ms, TE = 2.9 ms, TI= 650 ms, 1 mm3 isotropic resolution). Fifty-four participants were scanned at the University of California, Davis research center. Nine participants were scanned using a 3T Siemens Magnetom Trio Syngo System with an 8-channel head coil. Forty-five were scanned using a 3T Siemens Magnetom TrioTim system with an 8-channel head coil. Acquired images for all 54 participants included a T1-weighted volumetric MP-RAGE (TR = 2500, TE = 2.98, TI = 1100, 1 mm3 isotropic resolution). Thirty-three participants were scanned at the San Francisco Veterans Administration Medical Center using a 4T Siemens MedSpec Syngo System with an 8-channel head coil. A T1-weighted volumetric MP-RAGE scan (TR = 2300, TE = 2.84, TI = 950, 1 mm3 isotropic resolution) was acquired. Twenty-two participants were scanned at the University of California, San Francisco Neuroscience Imaging Center using a 3T Siemens Magnetom TrioTim system with a 12-channel head coil. Acquired images included a T1-weighted volumetric MP-RAGE (TR = 2500, TE = 2.98, TI = 1100, 1 mm3 isotropic resolution).
MRI: processing
The publicly available Freesurfer v5.1 (http://surfer.nmr.mgh.harvard.edu/) volumetric segmentation and cortical surface reconstruction methods were used to obtain regional measures of neocortical volumes (mm3) and thickness (mm). The reconstructed cortical surface models for each participant were manually inspected to ensure segmentation accuracy; regions with poor segmentation accuracy due to poor image quality or misregistration were excluded from further statistical analyses. Cortical surfaces were automatically parcellated20 and combined to create average cortical thickness and volume for total GM and for frontal, temporal, parietal, and occipital lobar regions. Region of interest volumes and thicknesses by cognitive status are shown in Table 2.
Table 2.
Relationships of FCRP to measures of cortical volume and thickness, reported as B (SE); B=unstandardized regression coefficient, SE=standard error.
| Measure | Region | Intercept B | FCRP B | Age B | Male Gender B | 3T Field strength B | CDR=0 B | ICV B |
|---|---|---|---|---|---|---|---|---|
| Volume (mm3) | Total gray matter | 368694 (32485) | -836 (360) | -1388 (372) | 1749 (6897) | 4262 (4262) | 4203 (4944) | 0.088 (0.015) |
| Frontal | 138026 (13393) | -225 (155) | -337 (152) | 2609 (2854) | 1584 (2522) | 473 (2083) | 0.035 (0.006) | |
| Parietal | 100417 (9502) | -236 (109) | -307 (105) | 1664 (1995) | -538 (1747) | 1773 (1474) | 0.020 (0.004) | |
| Temporal | 114547 (9770) | -304 (108) | -589 (110) | 666 (2021) | 4422 (1786) | 2627* (1466) | 0.026 (0.004) | |
| Occipital | 30424 (3849) | -67 (44) | -130 (42) | 421 (806) | 75 (707) | 898 (589) | 0.007 (0.002) | |
| Average Thickness (mm) | Total gray matter | 2.596 (0.086) | -0.003 (0.001) | -0.003 (0.001) | -0.062 (0.017) | 0.130 (0.018) | 0.048 (0.015) | NA |
| Frontal | 2.349 (0.092) | -0.001 (0.001) | -0.0001 (0.001) | -0.049 (0.018) | 0.139 (0.020) | 0.041 (0.016) | NA | |
| Parietal | 2.467 (0.093) | -0.003* (0.001) | -0.004 (0.001) | -0.063 (0.018) | 0.080 (0.020) | 0.059 (0.017) | NA | |
| Temporal | 3.159 (0.114) | -0.004 (0.001) | -0.008 (0.001) | -0.066 (0.022) | 0.172 (0.024) | 0.072 (0.020) | NA | |
| Occipital | 2.040 (0.099) | -0.003* (0.001) | -0.003 (0.001) | -0.045 (0.020) | 0.029 (0.021) | 0.044 (0.018) | NA |
Bold: adjusted p<0.05;
0.05<adjusted p<0.10.
CDR=clinical dementia rating; FCRP=Framingham cardiovascular risk profile; ICV=intracranial volume
Vascular injury
History of vascular injury
Data regarding clinical history of stroke or myocardial infarction were obtained from medical history.
Radiologic evidence of vascular brain injury
Infarcts were identified by an experienced neurologist (NS) blind to any other participant data using the T1-weighted and FLAIR MRI images. Infarcts were categorized according to structures involved, size (small: 3-10mm, large: >10mm), and severity (cystic, not cystic). For the current analysis, infarcts were then labeled: cortical gray matter (affecting any cortical region), white matter (affecting any subcortical white matter region, internal capsule, corpus callosum), subcortical gray matter (affecting basal ganglia, thalamus, amygdala, or hippocampus), and other (affecting midbrain, pons, medulla, or cerebellum). The number of infarcts for each participant (range 0-4) was used in subsequent statistical models.
Statistical analysis
Multiple linear regressions were used to test the association of cardiovascular risk or atherosclerosis with measures of brain volume and cortical thickness. Analyses were adjusted for age, sex, magnet strength (3T vs. 4T), CDR, and intracranial volume (volume analyses only). For volume and thickness models, P-values for FCRP or CIMT were adjusted for multiple comparisons according to the number of ROIs (5 ROIs: total GM, frontal, temporal, parietal and occipital GM) and the average intercorrelations among the ROIs21. Average intercorrelations were r = 0.813 for volumes, r = 0.713 for thickness. A 2-sided adjusted P<0.05 was considered statistically significant.
Results
There were 152 consecutive subjects with mean age 78 (range 62-92), 45% women, mean years of education 15.7 (range 9-24), and mean MMSE 28.3 (range 20-30). The cognitive status groups were very similar, with CDR=0.5 having significantly lower MMSE score and thinner cortex in all regions. All other measures were equivalent, as shown in Table S1 (please see http://stroke.ahajournals.org).
Thirty-four participants had radiologically identified brain infarcts. Of these participants, 3 had cortical, 15 had subcortical gray, 14 had white matter, and 9 had an infarct in another location. These numbers do not sum to 34 because eight individuals had more than one infarct and many infarcts affected multiple regions. Cortical infarcts were located in the frontal and occipital lobes. Of the 34 people with MRI-identified infarct, 18 (53%) had a clinical history of stroke. Of the 118 with no MRI-identified infarct, 18 (15%) had a clinical history of stroke. Fifty-one participants had a clinical history of stroke or myocardial infarction.
Table 2 shows the relationships between FCRP and measures of cortical volume and thickness. All fits were significant (all model p<0.0001, 0.19<R2<0.42). Significant inverse relationships we observed between FCRP and total GM volume and volume of the parietal and temporal lobes. FCRP was significantly inversely associated with total and temporal GM thickness, with a trend for parietal and occipital thickness.
Table 3 shows the relationships between CIMT and measures of cortical volume and thickness. All fits were significant (all model p<0.0002, 0.19<R2<0.50). There were no significant associations between CIMT and brain volume. CIMT was inversely associated with thickness of the parietal lobe. GM volume and thickness relationships are illustrated in Figures S1 and S2 (please see http://stroke.ahajournals.org).
Table 3.
Relationships of common carotid artery intima-media thickness (CIMT) to measures of cortical volume and thickness, reported as B (SE); B=unstandardized regression coefficient, SE=standard error.
| Measure | Region | Intercept B | cIMT B | Age B | Male Gender B | 3T Field strength B | CDR=0 B | ICV B |
|---|---|---|---|---|---|---|---|---|
| Volume (mm3) | Total gray matter | 361903 (38012) | -14216 (20095) | -1488 (435) | -8245 (6947) | 8675 (6421) | 5988 (5545) | 0.100 (0.016) |
| Frontal | 136575 (15246) | -12561 (7885) | -306* (166) | 633 (2779) | 2690 (2604) | 2394 (2238) | 0.040 (0.007) | |
| Parietal | 104097 (11285) | - 10782* (5840) | -305 (121) | 136 (2032) | 492 (1906) | 1935 (1655) | 0.023 (0.005) | |
| Temporal | 111427 (11220) | 1296 (5930) | -672 (127) | -3146 (2027) | 6163 (1893) | 3162* (1613) | 0.029 (0.005) | |
| Occipital | 29937 (4345) | -1578 (2234) | -128 (46) | -307 (786) | 552 (738) | 1178* (628) | 0.007 (0.002) | |
| Average Thickness (mm) | Total gray matter | 2.584 (0.099) | -0.041 (0.059) | -0.003 (0.001) | -0.084 (0.016) | 0.145 (0.016) | 0.039 (0.019) | NA |
| Frontal | 2.345 (0.106) | -0.049 (0.061) | 0.0003 (0.001) | -0.061 (0.017) | 0.149 (0.017) | 0.037 (0.020) | NA | |
| Parietal | 2.534 (0.108) | -0.144 (0.063) | -0.003 (0.001) | -0.075 (0.018) | 0.093 (0.018) | 0.051 (0.021) | NA | |
| Temporal | 3.110 (0.136) | -0.002 (0.082) | -0.008 (0.002) | -0.099 (0.023) | 0.190 (0.022) | 0.067 (0.026) | NA | |
| Occipital | 2.034 (0.117) | -0.077 (0.069) | -0.002 (0.001) | -0.065 (0.019) | 0.050* (0.019) | 0.037* (0.023) | NA |
Bold: adjusted p<0.05;
0.05<adjusted p<0.10.
CDR=clinical dementia rating; FCRP=Framingham cardiovascular risk profile; ICV=intracranial volume
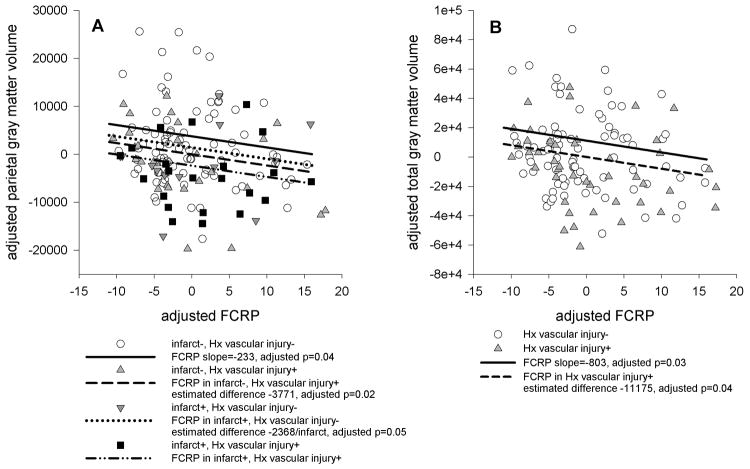
Models were re-run with two additional independent variables adjusting for history and evidence of vascular injury. The number of brain infarcts had a negative effect on frontal and parietal volumes (adjusted p<0.05), and history of vascular injury had a negative effect on frontal, parietal, and occipital volume (adjusted p<0.04). The number of brain infarcts did not affect cortical thickness, and history of vascular injury had a negative effect on occipital thickness only (adjusted p=0.02). The inverse relationship of FCRP or CIMT with volume and thickness were preserved in models accounting for history and evidence of vascular injury, and the regression coefficients were essentially unchanged. There was no evidence for a mediating effect of vascular injury on either volume or thickness. Moreover, as FCRP and infarcts were uncorrelated (r=-0.05, p=0.57), there was no evidence that the presence of infarcts was obscuring a relationship between FCRP and frontal volume or thickness. Figure 1 illustrates these results, demonstrating that the FCRP/volume relationships were preserved even when history and/or evidence of vascular injury significantly affected GM volume.
Figure 1.
Scatterplots showing the relationships between Freesurfer regional volumes and FCRP. FCRP and volumes were regressed on age, gender, magnet strength, intracranial volume, and cognitive status, and the residuals were plotted against each other. The slope of the line of best fit is the same as the regression coefficient for FCRP in the linear models that included history and evidence of vascular injury. When there are significant differences due to evidence and/or history of vascular injury, regression lines offset by the estimated difference are plotted. A: FCRP and parietal gray matter volume, illustrating effect of history of vascular injury, evidence of vascular injury, and combined effect of history and evidence of vascular injury. B: FCRP and total gray matter volume, illustrating effect of history of vascular injury.
Models with significant relationships between FCRP or CIMT and brain measures were also rerun adjusting for the presence of apoE4 genotype, to determine whether the associations were independent of the genetic risk for AD. In these models our sample size was substantially reduced, because apoE genotyping was only available in 68% of the participants. No volume or thickness measure was associated with apoE4 genotype. All FCRS and CIMT regression coefficients were of similar magnitude and direction as in the model without apoE.
Tables 2 and 3 also show the association of the other covariates on brain volume and cortical thickness. Higher age was significantly associated with decreased brain volume and thickness in all regions, even in the very restricted age range studied. Men had significantly thinner cortex in all regions. CDR=0.5 participants had thinner cortices in all regions compared to CDR=0 participants.
Discussion
Greater cardiovascular risk was associated with global and regional cortical atrophy and thinning. CIMT was associated with cortical thinning of the parietal lobe. History of vascular cardiac or brain injury affected total and parietal GM volume and occipital GM volume and thickness. Radiologic evidence of vascular brain injury (i.e., number of infarcts) was associated with lower frontal and parietal GM volume. However, the FCRP and CIMT effects were independent of those of vascular injury. Together the findings suggest that cardiovascular risk and atherosclerosis may lead to cortical loss via mechanisms other than, or in addition to, frank vascular brain injury, and that FCRP and CIMT are measuring different aspects of vascular injury.
Given the well-established association between cardiovascular risk factors and atherosclerosis, it is perhaps surprising that we found no relationship between CIMT and most measures of brain volume and cortical thickness. One consideration is that CIMT is an imperfect measure of atherosclerosis. CIMT is essentially a point measure of a widely distributed process that varies from location to location. Changes in the thickness of the carotid wall reflect both hypertrophic and inflammatory processes, and because CIMT avoids areas of significant atherosclerotic plaque it is not a measure of stenosis. Thus, the lack of association we observed does not rule out the possibility that atherosclerosis has an effect on brain structure.
The pattern of associations with regional atrophy measures may be informative. The hypothesis that cardiovascular risk leads to cortical changes via subclinical small vessel cerebrovascular disease would suggest that relationships between FCRP and atrophy would be especially strong in frontal lobe. In fact, correlations with frontal lobe were notably absent. Instead, temporal and parietal lobe measures were consistently associated with both FCRP and CIMT.
The number of infarcts was related to frontal and parietal GM volume, independent of the effects of FCRP or CIMT. Moreover, cortical infarctions were located in the frontal and occipital lobes, and the vast majority (12/15) of white matter infarcts were located in the frontal lobe. Therefore, although infarct was related to GM atrophy, particularly in the frontal lobe, it does not appear to be the mechanism by which risk leads to GM atrophy in the temporal and parietal regions. The temporal and parietal regions are selectively vulnerable to Alzheimer’s disease. A recent report, based on a subset of these same subjects, found that elevated levels of cerebral amyloid deposition, as measured by [11C]Pittsburgh compound B positron emission tomography (PIB PET), was correlated with greater FCRP17 but not with greater burden of abnormal white matter22. This is consistent with previous findings reporting that atherosclerosis may be associated with the pathology of AD23, 24. However, in the current study, we found no association between GM volume or thickness with the apoE ε4 allele. Cross-sectional MRI studies of individuals with apoE-ε4 compared with non-ε4 subjects have reported smaller bilateral hippocampus in AD patients and healthy elderly12. Given these reports, the lack of significant volume or thickness reductions in the apoE ε4 carriers for the temporal lobe was surprising. Segmentation inaccuracies may contribute to our failure to detect an effect of apoE in the temporal lobe25. We also had relatively few subjects with apoE4 ε4 allele (overall 23%) suggesting that our sample was relatively enriched for cerebrovascular disease, rather than AD. Limitations of this study include the relatively small number of participants with high FCRP scores. In this study, 41% of participants were low risk (FCRP <10%), 41% were intermediate risk (10%<FCRP<20%), and 18% were high risk (FCRP>20%). Most participants took medication to control their blood pressure (73%) and cholesterol (65%). This may have reduced the number of participants with FCRP scores >20%, despite the fact that a substantial number have already experienced stroke or myocardial infarction and have relatively high CIMT measures. Hypertensive and statin therapy may also reduce CIMT and confound the relationship between CIMT and GM volume or thickness. In addition, we did not control for carotid stenosis, which is reportedly associated with cortical atrophy26, 27, the possible presence of which may have confounded relationships between cardiovascular risk and brain measures.
The study included images acquired from five different MRI scanners and two different magnetic field strengths, introducing a significant technical issue. In our examination of images, we ascertained that the images from all 3T magnets were comparable, but that the 4T images showed greater magnetic field inhomogeneity. To address this, all image processing outputs were manually inspected and we statistically covaried for magnet strength in analyses. A potential limitation is that carotid atherosclerosis was assessed using common carotid artery IMT. A previous report suggests that internal carotid artery IMT is a better marker for cognitive impairment than the common carotid28, and by extension might also be a better marker of brain volume and thickness to the degree that these underlie cognition. Lastly, apoE genotyping was only available on a subset of participants, so our interpretations that the associations between brain volume and cardiovascular risk are independent of a genetic marker for AD should be viewed with caution.
Conclusions
Increased cardiovascular risk as measured by the FCRP was associated with reduced volumes of total, parietal and temporal GM. Increased FCRP scores were also significantly associated with reduced thickness for temporal and total GM. Notably, FCRP was not associated with frontal GM volume or thickness. Increased carotid atherosclerosis, indexed by CIMT, was associated with reduced parietal cortical thickness. These results suggest that FCRP and CIMT are measuring different aspects of vascular brain injury. The brain regions associated with increased cardiovascular risk are also affected by AD, and this may partially explain why hypertension and diabetes were found to be risk factors for clinically-diagnosed AD in epidemiological studies7, 8. GM atrophy of the temporal lobe, especially the hippocampus and ERC, is a structural hallmark of AD and its clinical precursor, mild cognitive impairment10, 29. With AD progression, atrophy in the parietal regions, particularly the posterior cingulate and precuneus regions, is also observed30, 31. These results suggest a “double hit” toward developing dementia when someone with incipient AD also has high cardiovascular risk.
Supplementary Material
Acknowledgments
We thank the staff at the Center for the Imaging of Neurodegenerative Diseases at the San Francisco VA and the imaging facilities at USC, UC Davis, and UCSF for help with data acquisition and preparation. We thank the recruiters of the Aging Brain Program Project.
Sources of funding
This work was partially supported by the National Center for Aging, the National Center for Research Resources and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through grants P41EB015904 and P01AG12435, which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. The work was also supported by P30AG10129.
Footnotes
Disclosures
None.
Bibliography
- 1.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, et al. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr, Holland D, et al. Regional rates of neocortical atrophy from normal aging to early alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grau-Olivares M, Bartres-Faz D, Arboix A, Soliva JC, Rovira M, Targa C, et al. Mild cognitive impairment after lacunar infarction: Voxel-based morphometry and neuropsychological assessment. Cerebrovasc Dis. 2007;23:353–361. doi: 10.1159/000099134. [DOI] [PubMed] [Google Scholar]
- 5.Grubb NR, Fox KA, Smith K, Best J, Blane A, Ebmeier KP, et al. Memory impairment in out-of-hospital cardiac arrest survivors is associated with global reduction in brain volume, not focal hippocampal injury. Stroke. 2000;31:1509–1514. doi: 10.1161/01.str.31.7.1509. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to ad, vascular dementia, and cognitive function. Neurology. 2002;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- 8.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 9.Jagust W. What can imaging reveal about obesity and the brain? Current Alzheimer research. 2007;4:135–139. doi: 10.2174/156720507780362146. [DOI] [PubMed] [Google Scholar]
- 10.Chao LL, Mueller SG, Buckley ST, Peek K, Raptentsetseng S, Elman J, et al. Evidence of neurodegeneration in brains of older adults who do not yet fulfill mci criteria. Neurobiol Aging. 2010;31:368–377. doi: 10.1016/j.neurobiolaging.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson AS, Easteal S, Jorm AF, Mackinnon AJ, Korten A, Christensen H, et al. Apolipoprotein e allele epsilon 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387–1390. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- 12.Lehtovirta M, Laakso MP, Frisoni GB, Soininen H. How does the apolipoprotein e genotype modulate the brain in aging and in alzheimer’s disease? A review of neuroimaging studies. Neurobiol Aging. 2000;21:293–300. doi: 10.1016/s0197-4580(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 13.Du AT, Schuff N, Chao LL, Kornak J, Ezekiel F, Jagust WJ, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging. 2005;26:553–559. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Sojkova J, Najjar SS, Beason-Held LL, Metter EJ, Davatzikos C, Kraut MA, et al. Intima-media thickness and regional cerebral blood flow in older adults. Stroke. 2010;41:273–279. doi: 10.1161/STROKEAHA.109.566810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen RA, Poppas A, Forman DE, Hoth KF, Haley AP, Gunstad J, et al. Vascular and cognitive functions associated with cardiovascular disease in the elderly. J Clin Exp Neuropsychol. 2009;31:96–110. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Reed BR, Marchant NL, Jagust WJ, Decarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. [November 30, 2011];Neurobiol Aging. 2011 Nov 9; doi: 10.1016/j.neurobiolaging.2011.10.002. [published online ahead of print November 10, 2011] http://www.neurobiologyofaging.org/ [DOI] [PMC free article] [PubMed]
- 18.Morris JC. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–176. doi: 10.1017/s1041610297004870. discussion 177-178. [DOI] [PubMed] [Google Scholar]
- 19.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, et al. Protective effect of apolipoprotein e type 2 allele for late onset alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 21.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Marchant NL, Reed BR, DeCarli CS, Madison CM, Weiner MW, Chui HC, et al. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol Aging. 2012;33:1006.e25–1006.e36. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honig LS, Kukull W, Mayeux R. Atherosclerosis and ad: Analysis of data from the us national alzheimer’s coordinating center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 24.Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of willis atherosclerosis: Association with alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 25.Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (freesurfer and ibaspm) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- 26.Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI. Carotid atherosclerosis and progression of brain atrophy: The smart-mr study. Ann Neurol. 2011;70:237–244. doi: 10.1002/ana.22392. [DOI] [PubMed] [Google Scholar]
- 27.Fierstra J, Poublanc J, Han JS, Silver F, Tymianski M, Crawley AP, et al. Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatry. 2010;81:290–293. doi: 10.1136/jnnp.2009.188078. [DOI] [PubMed] [Google Scholar]
- 28.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, et al. Carotid artery atherosclerosis, mri indices of brain ischemia, aging, and cognitive impairment: The framingham study. Stroke. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and alzheimer’s disease. Hum Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choo IH, Lee DY, Oh JS, Lee JS, Lee DS, Song IC, et al. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and alzheimer’s disease. Neurobiol Aging. 2010;31:772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, et al. Global and local gray matter loss in mild cognitive impairment and alzheimer’s disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.