Abstract
Despite their clinico-pathologic heterogeneity, malignant germ-cell-tumors (GCTs) share molecular abnormalities that are likely to be functionally important. In this study, we investigated the potential significance of down-regulation of the let-7 family of tumor-suppressor microRNAs in malignant-GCTs. Microarray results from pediatric and adult samples (n=45) showed that LIN28, the negative-regulator of let-7 biogenesis, was abundant in malignant-GCTs, regardless of patient age, tumor site or histologic subtype. Indeed, a strong negative-correlation existed between LIN28 and let-7 levels in specimens with matched datasets. Low let-7 levels were biologically significant, since the sequence complementary to the 2-7nt common let-7 seed ‘GAGGUA’ was enriched in the 3′untranslated regions of mRNAs up-regulated in pediatric and adult malignant-GCTs, compared with normal gonads (a mixture of germ cells and somatic cells). We identified 27 mRNA targets of let-7 that were up-regulated in malignant-GCT cells, confirming significant negative-correlations with let-7 levels. Among 16 mRNAs examined in a largely independent set of specimens by qRT-PCR, we defined negative-associations with let-7e levels for six oncogenes, including MYCN, AURKB, CCNF, RRM2, MKI67 and C12orf5 (when including normal control tissues). Importantly, LIN28 depletion in malignant-GCT cells restored let-7 levels and repressed all of these oncogenic let-7 mRNA targets, with LIN28 levels correlating with cell proliferation and MYCN levels. Conversely, ectopic expression of let-7e was sufficient to reduce proliferation and down-regulate MYCN, AURKB and LIN28, the latter via a double-negative feedback loop. We concluded that the LIN28/let-7 pathway has a critical pathobiological role in malignant-GCTs and therefore offers a promising target for therapeutic intervention.
Keywords: germ cell tumor, let-7, LIN28, microRNA
Introduction
Germ-cell-tumors (GCTs) are clinically and histopathologically complex. They present from early infancy through to late adulthood, occur at both gonadal and extragonadal sites and comprise diverse histologic subtypes (1). Benign forms show somatic differentiation and are termed teratomas, while malignant-GCTs are classified into germinomas (a collective term for testicular seminoma, ovarian dysgerminoma and extragonadal germinoma) and non-germinomatous tumors, the main types of which are yolk-sac-tumors (YSTs) and embryonal carcinoma (EC) (1).
Although most patients with malignant-GCTs have a good prognosis, some patients still have inferior outcomes and testicular germ-cell malignancy remains a leading cause of death in young men (2). Improved understanding of the molecular pathogenesis of malignant-GCTs would represent an important step towards developing novel therapeutic agents with favorable toxicity profiles, which may improve survival for patients with high-risk disease and reduce toxicity for low-risk patients. It is particularly important to identify abnormalities that are shared across the diverse spectrum of malignant-GCTs, as these are likely to be of fundamental significance in disease pathogenesis.
Using microarray profiling, we previously identified that all nine members of the lethal-7 (let-7) microRNA family were significantly under-expressed in pediatric malignant-GCTs, when compared with non-malignant control tissues (3). MicroRNAs regulate gene expression via their 5′-seed-region (nucleotides at positions 1-8; 1-8nt), which binds the corresponding seed-complementary-region (SCR) located predominantly in the 3′untranslated region (3′UTR) of mRNA targets (4). Within the seed, 2-7nt are most critical for binding-specificity (5). Importantly, all nine let-7 microRNAs share the same 2-7nt seed sequence (GAGGUA) and therefore share mRNA targets containing the 3′UTR SCR ‘TACCTC’.
Let-7 microRNAs are important tumor-suppressor genes (6) that regulate cell proliferation (7). The let-7 microRNA family is negatively regulated by the RNA-binding proteins LIN28 homolog-A (LIN28) and LIN28 homolog-B (LIN28B) (8,9), with LIN28 depletion resulting in specific increases in all let-7 family members (8,10). The LIN28 proteins bind let-7 primary-transcripts (pri-let-7) and precursor-hairpins (pre-let-7), preventing processing by Drosha and Dicer, respectively (11). Binding of pre-let-7 by LIN28 occurs through a stem-loop motif that includes ‘GGAG’ (12,13), leading to recruitment of the terminal-uridyl-transferase (TUTase) ZCCHC11 (10,12), resulting in pre-let-7 uridylation and subsequent degradation (14).
LIN28 can reprogram human somatic cells into pluripotent stem-cells, and is a putative cancer stem-cell marker (9,11,15). Of note, LIN28 is expressed at high levels in primordial-germ-cells (16), believed to be the cell of origin for malignant-GCTs (1). Previous studies used immunohistochemistry (17-19) and RNA-interference (RNAi) (20) to investigate the expression and some aspects of LIN28 function in malignant-GCTs. Here, we provide the first demonstration that low let-7 levels in malignant-GCTs are directly attributable to LIN28 expression and are likely to contribute to significant up-regulation of important cancer-associated protein-coding genes.
Materials and Methods
Tumor samples
Our study received ethical approval from Trent-MREC (ref:02/4/071) and Cambridge-LREC (ref:01/128). We analyzed the following tissue samples and datasets:
Set-1
Forty-eight samples of pediatric malignant-GCTs and non-malignant-controls, which we previously used for global microRNA profiling (3), and for global mRNA profiling of a subset of 21 cases (3). Across the sample set, the controls (n=8) represented fetal yolk-sac, fetal ovary, pre-pubertal testis, post-pubertal testis, pre-pubertal ovary and post-pubertal ovary. These samples contain germ cells, with variable representation of somatic cells. One apparent teratoma sample (MT-34) was not included in any subsequent analysis as it was a component of a mixed-malignant-GCT and clustered with malignant cases on microRNA profiling (3). The remaining 20 samples with matched microRNA and mRNA profiles comprised 17 malignant-GCTs (10 YSTs, six germinomas, one EC) and three normal gonadal controls (one pre- and one post-pubertal testis and one post-pubertal ovary) (3).
Set-2
A published dataset of global mRNA expression profiles of 25 samples from a study of adult testicular malignant-GCTs (eight YSTs; 12 germinomas) and controls (five normal adult testes, containing germ cells and somatic cells). Further details are available in the original publication (21) and our previous study (3).
Set-3
Thirty-two samples in which we measured levels of selected mRNAs and microRNAs by quantitative reverse-transcription PCR (qRT-PCR). Full details are given in Supplementary Table-S1. The malignant-GCTs represented nine YSTs, nine germinomas and three ECs, with all except three being from pediatric patients (<16y). In addition, we used six malignant-GCT cell-lines and five benign teratomas. Twenty-four of the 32 samples overlapped with set-1 and had previously been used for microRNA profiling (3). However, only six of the 32 had undergone mRNA profiling (3) (Supplementary Table-S1), enabling set-3 to be used for independent qRT-PCR validation of findings from our mRNA microarray analyses of sets-1 and -2.
When combining sets 1-3, our study encompassed a total of 81 samples, comprising 54 different malignant-GCTs (31 pediatric, 23 adult; 43 gonadal, 11 extragonadal), six malignant-GCT cell-lines and 21 control samples (eight teratomas, 13 gonads/yolk-sac).
MicroRNA and mRNA microarray expression analysis
We re-analyzed microRNA expression profiles for set-1 [obtained using the miRCURY-LNA array-v9.2 (Exiqon, Vedbaek, Denmark)] (3) and mRNA expression profiles for sets-1 and -2 [obtained using the HG-U133A GeneChip (Affymetrix, Santa Clara, CA)] (3, 21). Differential gene expression was assessed using a moderated t-statistic and p-values adjusted for multiple testing using Benjamini and Hochberg’s method (23). MicroRNAs with adjusted p-values <0.01 were considered to be significantly differentially-expressed, while mRNAs with log2 fold-change ≥1.5 and adjusted p<0.01 were considered to be over-expressed (3).
Sylamer bioinformatic algorithm
Sylamer assesses enrichment and/or depletion of SCR nucleotide words of specific length in the 3′UTRs of genes within ranked lists (24). We used Sylamer to identify whether the let-7 down-regulation in malignant-GCTs was of biological significance by causing shifts in expression of mRNAs with a 3′UTR let-7 SCR (3). We performed three analyses of mRNA microarray data, examining the pediatric samples from set-1 with matched microRNA and mRNA profiles (n=20), the adult samples from set-2 (n=25) and both groups in combination (n=45). In Sylamer landscape plots, mRNA genelists were ranked on the x-axis from down-regulated (left) to up-regulated (right). The y-axis showed log10-transformed and sign-adjusted enrichment p-values for each SCR word, relative to p-values of all other words. Consequently, an SCR showing a negative y-axis deflection on the right-hand-side of each plot was enriched in up-regulated genes. As previously (3), we calculated a single summed significance score and p-value for each SCR. We only considered SCR elements that contained the core 2-7nt sequence of the microRNA seed-region, summating data for one hexamer (2-7nt), two heptamers (1-7nt, 2-8nt) and one octamer (1-8nt). P-values <0.01 were considered significant (3). We tested for enrichment in the up-regulated genes of 3′UTR SCRs corresponding to the 1-8nt seeds of all 126 microRNAs down-regulated in the 48 set-1 pediatric malignant-GCTs (3).
mRNA qRT-PCR
Relative mRNA transcript levels were measured in triplicate in clinical samples and cell-lines using QuantiTect One-Step SYBR-Green qRT-PCR (Qiagen, Crawley, UK), following reverse transcription of 1μg of total RNA using QuantiTect Reverse Transcription (Qiagen). Primers (Supplementary Table-S2) were designed using Primer3 (25), ensuring that they crossed exon-exon boundaries. Expression ratios were calculated using the comparative threshold cycle (Ct) method (26) and normalized using four housekeeping genes ACTB (Qiagen), YWHAZ, RPL13A and HMBS (27). In analyses of clinical samples, results were referenced to a pooled normal gonadal control, using total RNA from human ovaries and testes (AM6974/AM7972, Ambion, Warrington, UK). It should be noted that these tissues contain both germ cells and somatic cells. For cell-lines, results were referenced to cells treated with either non-targeting-control (NTC) siRNA, or mimic-negative-control (MNC) RNA, as appropriate.
qRT-PCR for microRNA and pri-microRNA
MicroRNA levels were quantified in triplicate using Taqman assays (Applied Biosystems, Warrington, UK), as described (3,28). Levels of pri-microRNA were determined in quadruplicate, using TURBO-DNA-free, Taqman High-Capacity RNA-to-cDNA and Taqman assays (all Applied Biosystems). Expression ratios were calculated using the comparative Ct method, with microRNAs normalized to RNU24 (3,28) and pri-microRNAs to RPLO, GUS-B and 18S. Reference samples were as for mRNA qRT-PCR. In test amplifications using 0.01nM single-stranded let-7e RNA (Sigma Aldrich, Dorset, UK) Taqman qRT-PCR for let-7b and let-7d showed no cross-reactivity with let-7e (data not shown).
Western blotting
Western blots were performed for LIN28, LIN28B, AURKB and MYCN proteins using the antibodies ab46020 (1:10,000 dilution), ab119367 (1:1000), ab2254 (1:5,000) and ab16898 (1:250), respectively. Results were normalized to beta-tubulin (ab6046; 1:10,000). All antibodies were from Abcam (Cambridge, UK). Western blot densitometry was performed using FluorChem-9900 imaging system software (Alpha-Innotech, San Leandro, CA).
GCT cell-lines
We selected four cell-lines that reflected the range of malignant-GCT histologic subtypes commonly observed in clinical practice, namely EC [2102Ep (29)], YST (GCT44 and 1411H) and germinoma/seminoma [TCam2 (30)]. Cells were cultured at 37°C in 5% CO2 in medium containing 10% fetal-calf-serum (FCS)/1% penicillin/streptomycin, and authenticated using short-tandem-repeat profiling (3).
RNA depletion and over-expression
Transcripts were depleted by RNAi in over-expressing malignant-GCT cell-lines using pools of four separate siRNAs in order to reduce any off-target-effects (31). The probes targeted LIN28 (L-018411-01), LIN28B (L-028584-01) and MYCN (L-003913-01) (all Dharmacon, Lafayette, CO). Each pool was used at 66.7nM, which represented the minimal concentration of LIN28 siRNAs that achieved >75% LIN28 transcript depletion in test experiments (data not shown). All results were normalized to cells treated with a 66.7nM pool of four NTC siRNAs (D-001810-10-05, Dharmacon) (32). We confirmed the specific effects of the LIN28-targeting pool using two independent siRNAs (Hs_LIN28_7 and Hs_LIN28_8, both Qiagen; target sequences TAAAGACTTATTGGTACGCAA and CACGCTGTGAGATCACCGCAA, respectively). Differences between experimental observations were assessed using an unpaired, two-sample t-test (two-tailed with 95% confidence-intervals). Let-7e was replenished in under-expressing malignant-GCT cell-lines using let-7e miRIDIAN double-stranded RNA-mimic (C-300479-05) at 100nM (33), normalized to cells treated with 100nM miRIDIAN MNC, (CN-001000-01, both Dharmacon).
Cell transfection and proliferation assays
Following optimization of transfection conditions (data not shown), cells were seeded in 6-well plates, with 2102Ep at 7×104 cells/well, 1411H and GCT44 at 1.0×105 cells/well and TCam2 1.5×105 cells/well. On d0, when cells were ~20% confluent, transfection was performed using Opti-MEM media and Lipofectamine-RNAiMAX (both Invitrogen, Paisley, UK). The optimal length of transfection, which maximized transfection efficiency and minimized toxicity, was 24h for 2102Ep cell-line, 6-8h for 1411H and GCT44, and 4-6h for TCam2. At least three biological replicates were performed for each treatment. For RNA and protein quantification, the replicate samples were pooled prior to further analysis. For qRT-PCR, at least three technical replicates were performed per analysis.
Cell numbers were quantified using trypan blue on a Countess automated cell counter (Invitrogen), determining the mean of two values for each of three biological replicates and then taking the mean of the resulting three values. Maximal growth rates were determined by plotting cell numbers at 24h time-points on a logarithmic scale and calculating population doubling-time from the linear section of the curve, as described (34).
Luciferase reporter assays
We studied let-7 effects on target genes using GoClone luciferase reporter plasmids containing the full-length 3′UTR for LIN28 (S813978), MYCN (S807230) or AURKB (custom-made), plus a control luciferase plasmid containing no 3′UTR (S890005) (all plasmids from SwitchGear Genomics, Menlo Park, CA). Test oligonucleotides were let-7e, non-targeting RNA (NT2; MIM9002) and a mutant let-7e (let-7e-mutant; sequence UGAGUGAGGAGGUUGUAUAGUU) in which the 2-7nt seed was mutated to ‘GAGUGA’. The latter sequence did not correspond to any known human microRNA seed, thereby avoiding seed-like off-target effects in transfected cells. All experiments were performed twice in quadruplicate in 96-well plates, using >80% confluent cells, with 50ng of plasmid/well and 100nM oligonucleotides. Luminescence was quantified on a BioTek Synergy-HT multi-mode microplate reader (BioTek Instruments-Inc, Winooski, VT). After background correction, the means for the test let-7e/let-7e-mutant oligonucleotides were normalized to values for NT2-treated cells, then referenced to cells containing the no 3′UTR control reporter that had also been treated with let-7e/let-7e-mutant, as appropriate.
Results
Let-7 and LIN28 expression in malignant-GCTs
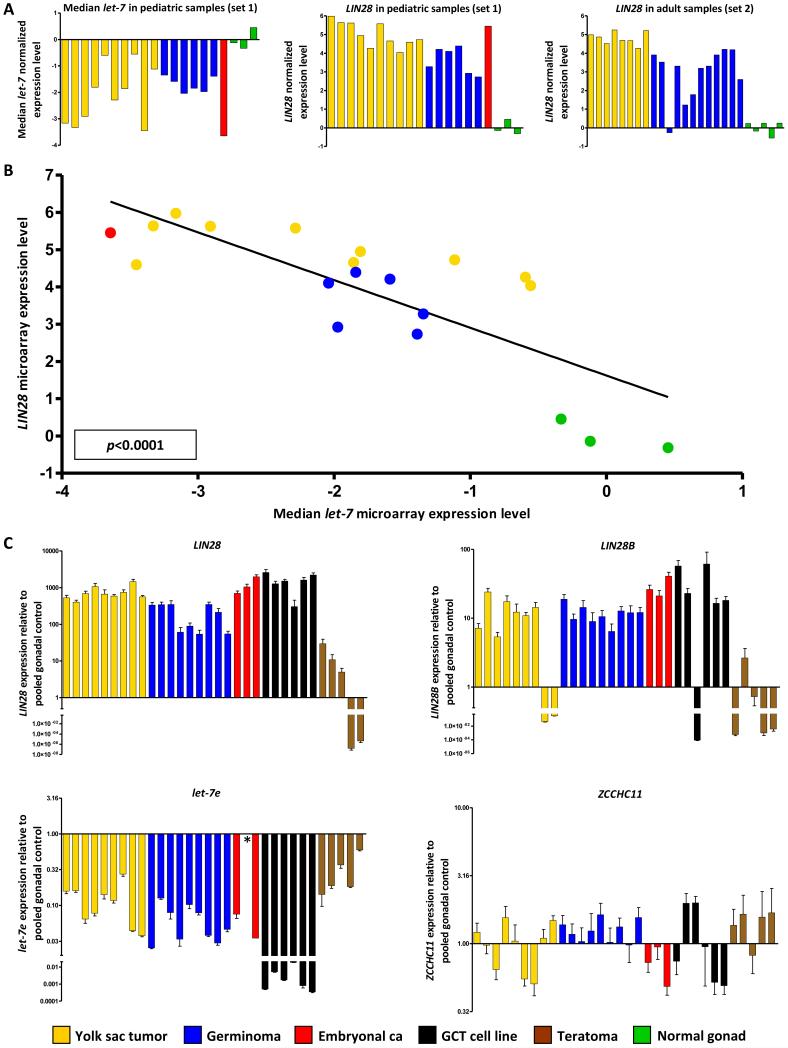
In our previous microRNA profiling study of sample set-1, we identified that all nine members of the tumor-suppressor let-7 microRNA family were significantly down-regulated in pediatric malignant-GCTs, compared with non-malignant tissues (benign GCTs and normal gonad) (3), which contain a variable representation of germ cells and somatic cells. The fold changes observed for each let-7 family member are given in Supplementary Table-S3, with let-7e being the most significantly under-expressed by p-value. For the subset of 20 pediatric samples with matched microRNA and mRNA profiles, the malignant-GCTs again showed significant reductions in let-7 microRNAs, when assessing each family member individually (Supplementary Figure-S1A), or collectively (Figure-1A).
Figure-1. Let-7 and LIN28 expression in malignant-GCTs.
A) The left and middle panels respectively show median levels of all nine let-7 family members and levels of LIN28 in the 20 samples from set-1 with matching microRNA and mRNA data. The right panel shows levels of LIN28 in sample set-2. B) Linear-regression analysis of median let-7 family levels versus LIN28 levels in the set-1 samples with matching data. In A) and B), all values are referenced to the mean of the normal gonadal samples. C) qRT-PCR validation in sample set-3, referenced to the pooled normal gonadal control sample. Error-bars= standard error of the mean (SEM). For one sample (asterisked) there was insufficient RNA for let-7e quantification. The color-code for all panels is shown in the key. For details of the normal gonadal controls, see Materials and Methods.
As individual let-7 members are transcribed from multiple genomic loci, this observation suggested the possibility of a common post-transcriptional mechanism regulating let-7 biogenesis in malignant-GCTs. We therefore sought to identify whether let-7 down-regulation was associated with over-expression of LIN28. Using all available mRNA microarray data, we found that LIN28 was highly expressed in 44/45 (97.8%) of malignant-GCTs from pediatric and adult patients (from sets-1 and -2 combined; Figure-1A), regardless of tumor site (gonadal/extragonadal) or histologic subtype. For the 20 pediatric samples with matched microRNA and mRNA data, LIN28 showed a highly significant negative-correlation with median let-7 levels (R2=0.63; p<0.0001) (Figure-1B) and with levels of each individual let-7 family member (all p<0.005) (Supplementary Figure-S1B).
We validated these microarray findings using the independent technique of qRT-PCR in a panel of 32 samples (set-3). Compared with pooled normal gonadal control RNA, all 27 of the malignant-GCT samples and cell-lines showed high LIN28 expression (Figure-1C). Twenty-four of the 27 also showed high expression of LIN28B (Figure-1C), which is located at a different chromosomal locus (6q16.3; vs. 1p36.11 for LIN28). In contrast, levels of the TUTase ZCCHC11 were not elevated either in malignant-GCT samples or teratomas (Figure-1C). Using Taqman qRT-PCR, we confirmed that let-7e (the most significantly down-regulated let-7 in our microarray analysis) showed low expression in all the malignant-GCT samples/cell-lines (Figure-1C). Linear-regression using the qRT-PCR data confirmed that let-7e levels were significantly negatively correlated with LIN28 and LIN28B (p=0.017 and p=0.036, respectively) (Supplementary Figure-S2), but not with ZCCHC11.
Biological significance of low let-7 levels in malignant-GCTs
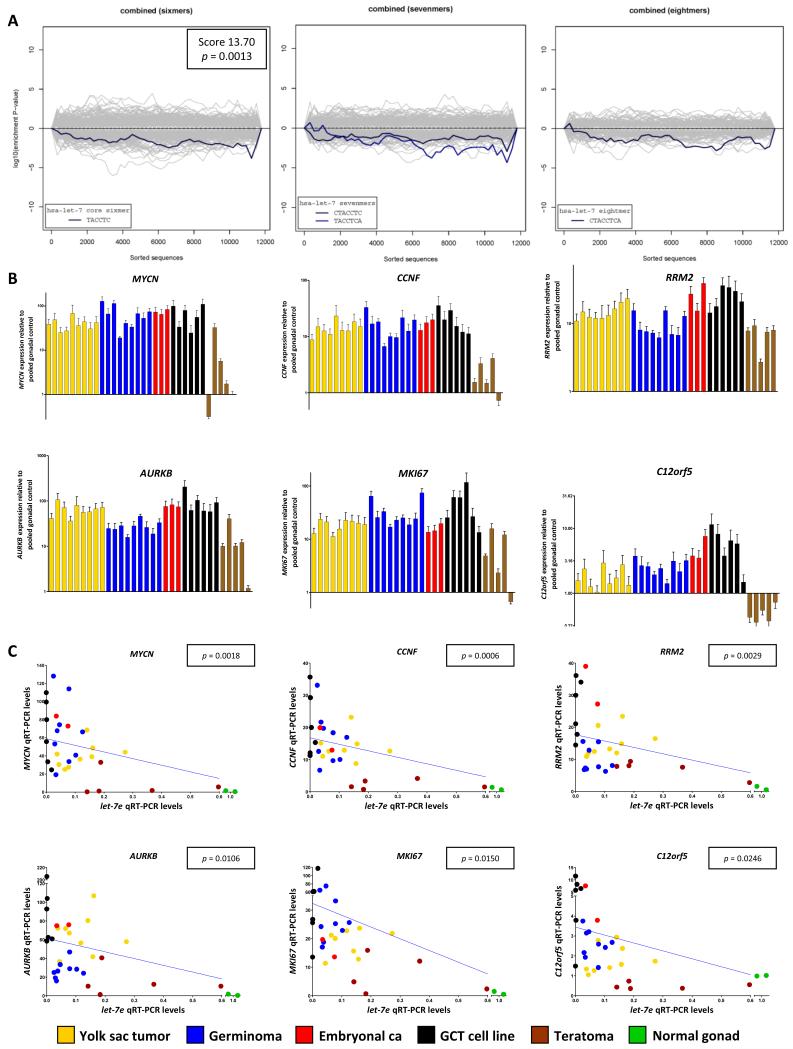
Sylamer showed that ‘TACCTC’ (complementary to the 2-7nt common let-7 seed ‘GAGGUA’) was the top-ranking SCR in mRNAs significantly up-regulated in malignant-GCTs (compared with the normal gonadal control tissues), irrespective of patient age. There were highly significant p-values for the summed significance scores of the let-7 1-8nt SCR in the datasets for the pediatric malignant-GCTs (set-1; p=0.00057), the adult malignant-GCTs (set-2; p=0.00026) (both Supplementary Figure-S3) and the combined analysis (p=0.0013) (Figure-2A). In all analyses there was no significant enrichment in the up-regulated mRNAs of SCRs for any of the other 126 microRNAs tested.
Figure-2. Significance of let-7 down-regulation in malignant-GCTs.
A) Sylamer landscape plots for SCR words corresponding to the common seed of the nine let-7 microRNA family members in the combined analysis of pediatric (set-1) and adult (set-2) malignant-GCTs. Log10-transformed p-values for each SCR word are plotted on the y-axis, against the ranked gene list on the x-axis. A negative y-axis deflection on the right-hand side of the plot signifies SCR enrichment in up-regulated genes. The left-hand plot shows data for the hexamer complementary to the core 2-7nt component of the common seed-region, the central plot the two heptamers (1-7nt; 2-8nt), and the right-hand plot the octamer (1-8nt). The single summed significance score and p-value for all four SCR words is shown. B) Levels of the six selected let-7 mRNA targets in sample set-3, determined by qRT-PCR. Error-bars=SEM. C) Correlations between each mRNA and let-7e in set-3. P-values were determined by linear-regression.
We used Sylamer to produce a list of let-7 mRNA targets that were over-expressed in all malignant-GCTs, for further validation in clinical samples and functional investigation in vitro. We identified 198 up-regulated genes from the pediatric mRNA dataset (set-1), of which 50 (25.3%) had at least one ‘TACCTC’ 3′UTR sequence. For the adult dataset (set-2), we identified 428 up-regulated genes, of which 106 (24.8%) contained at least one 3′UTR TACCTC. These values compared with an overall frequency of 19.8% for the TACCTC sequence in the 3′UTR of all annotated genes on the array. Thirty-six let-7 mRNA targets were common to both datasets, with 27 having a significant negative-correlation with median let-7 levels in the 20 pediatric tissue samples from set-1 that had matched microRNA and mRNA microarray data (Supplementary Table-S4, Supplementary Figure-S4). We selected 16 of these 27 genes for further interrogation, based on their reported functions in human disease, including cancer. The genes were: MYCN, CCNF, RRM2, AURKB, MKI67, C12orf5, FZD5, KRAS, PGK1, SMAGP, RAB25, RAB15, MRS2, SLC2A3, LASP1 and AGL.
HMGA2, a known let-7 target in carcinoma cells (35), was included in the initial list of 36 mRNAs, as it was up-regulated in both pediatric set-1 (rank 40/50) and adult set-2 (rank 63/106). However, HMGA2 showed no significant correlation with median let-7 levels across these datasets (p=0.12). To investigate these observations further, we measured HMGA2 levels in set-3 using qRT-PCR. This showed that while HMGA2 was over-expressed in some subtypes of malignant-GCT (YSTs and EC), it showed only minimal expression changes in another major subtype, germinoma (Supplementary Figure-S5A,-S5B). The lack of overall association between HMGA2 and let-7 levels was also confirmed in this qRT-PCR analysis (p=0.12) (Supplementary Figure-S5C).
Validation of let-7 mRNA targets
By qRT-PCR analysis of the 32 samples in set-3, we confirmed over-expression of all 16 selected mRNAs in malignant-GCTs, compared with the control samples used (Figure-2B, Supplementary Figure-S6). We identified a negative-association with let-7e qRT-PCR levels for six mRNAs (MYCN, AURKB, CCNF, RRM2, MKI67, C12orf5) (Supplementary Table-S5, Figure-2C). It should be noted that the associations were only significant when including the control samples, in which there was a mixture of germ cells and somatic cells. Accordingly, these findings should be viewed with caution in the absence of follow-up functional data (see below). On the other hand, there was no significant association for the other 10 of the 16 genes, when the control samples were included. Of these other 10 mRNAs, RAB25, MRS2, PGK1, KRAS and LASP1 were over-expressed in malignant-GCTs of particular histologic subtypes (Supplementary Figure-S6), but did not show an association with let-7e levels across the whole sample set. The other five mRNAs (FZD5, SMAGP, RAB15, SLC2A3 and AGL) showed no association with let-7e qRT-PCR levels (Supplementary Figure-S6), suggesting that their expression is regulated by additional factors, which may include other microRNAs.
Depletion of LIN28 and LIN28B
We next tested the functional significance of our observations in vitro, using multiple complementary experimental approaches to minimize the possibility of non-specific observations. We tested for phenotypic and functional consistencies when: 1) depleting LIN28 or LIN28B by RNAi, using panels of four siRNAs to minimize any off-target effects (31), with separate confirmation using independent siRNAs; 2) directly over-expressing let-7e (the let-7 family member that showed the most significant under-expression in vivo; Supplementary Table-S3) using a double-stranded RNA mimic; and 3) depleting MYCN, a major let-7e target, by RNAi using a panel of four independent siRNAs. In this in vitro work, we used four representative malignant-GCT cell-lines, all of which showed LIN28 up-regulation and let-7 under-expression (Figure-1C).
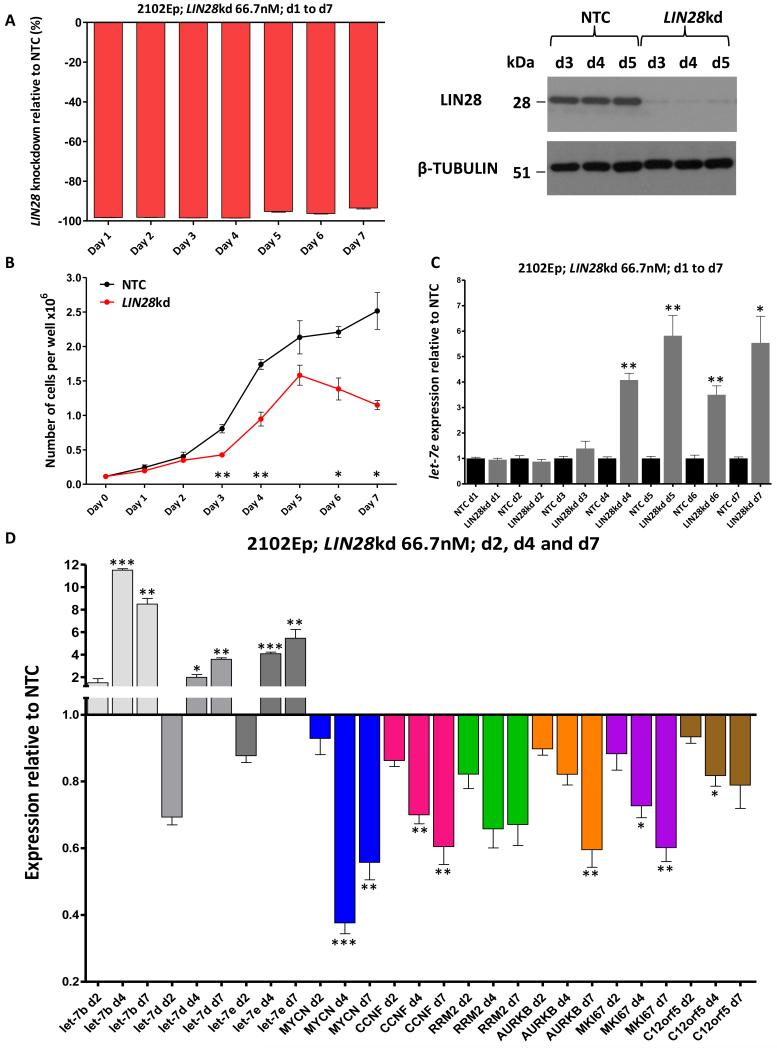
In 2102Ep cells, a single treatment with pooled siRNAs depleted LIN28 mRNA by >90% over a 7d period. There were parallel reductions in protein levels, which fell to <10% from d3 (Figure-3A and Supplementary Figure-S7A). There was no effect on LIN28B or ZCCHC11 mRNA levels on d1-d3 (data not shown). The reductions in LIN28 protein levels were mirrored by changes in cell proliferation, which fell from d3 (Figure-3B). There was a 36% increase in mean population doubling time over the 7d time-course (33.9h vs. 24.9h, respectively).
Figure-3. LIN28 depletion in 2102Ep malignant-GCT cells.
A) Depletion of LIN28, measured by qRT-PCR over d1-d7 (left) and by Western blot over d3-d5 (right). NTC=non-targeting-control siRNA, kd=knockdown. B) Cell numbers following LIN28 depletion. C) Levels of let-7e over d1-d7 following LIN28 depletion. In A) to C), statistical comparisons are versus NTC-treated cells. d=day. D) Levels of let-7 family members (let-7b, let-7d and let-7e) and the six selected let-7 mRNA targets at d2, d4 and d7 following LIN28 depletion. Expression values are referenced to NTC-treated cells. Statistical comparisons are for LIN28kd cells at d4 and d7, versus LIN28kd cells at d2. Error-bars=SEM. In panels B, C and D, *=p<0.05;**=p<0.005;***=p≤0.0001.
In keeping with these observations, levels of let-7e started increasing from d3, with significant changes from d4 (Figure-3C). The specificity of the effects of the pooled LIN28 siRNAs on cell proliferation and let-7e levels was confirmed using two independent siRNAs (Supplementary Figure-S8). The pooled LIN28 siRNAs also caused increases in other representative let-7 family members (let-7b and let-7d), when assessed at d4 and d7 (Figure-3D). There was no difference in levels of the pri-let-7e precursor at d7 (data not shown). Of the selected let-7 mRNA targets (Figure-2B), all six showed decreased levels on d4 and d7 (time-points at which growth-inhibition was observed), compared with d2 (when no growth-inhibition was seen). Most of the decreases were statistically significant (Figure-3D), with the most significant reduction being for MYCN, where transcript levels were lowered by 62% at d4 versus d2 (p<0.0001) (Figure-3D).
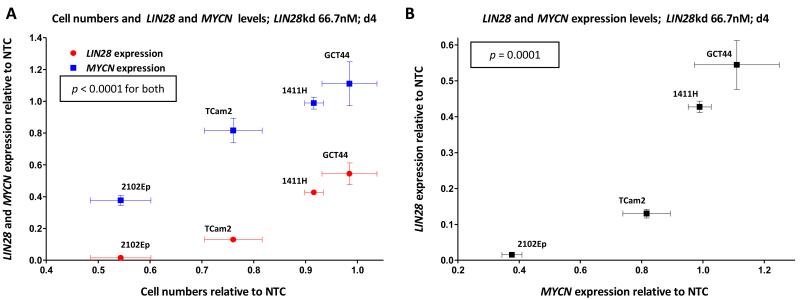
We attempted to deplete LIN28 in three other malignant-GCT cell-lines, assessing the effects at d4 post-transfection, based on our findings in 2102Ep. While we could achieve 87% transcript depletion in TCam2, cell toxicity restricted the levels of depletion achieved in 1411H and GCT44 to 57% and 46%, respectively. There were parallel reductions in protein levels, as measured at d4 following siRNA treatment (Supplementary Figure-S7B), with a significant positive correlation between LIN28 mRNA and protein levels across the four cell-lines (R2=0.88, p<0.0001) (Supplementary Figure-S7C). The LIN28 reductions observed in 1411H and GCT44 were not sufficient to affect cell numbers, compared with NTC-treated cells (Figure-4A and Supplementary Figure-7D). However, across all four cell-lines, there were significant positive-correlations between levels of LIN28 and MYCN transcripts (R2=0.78, p=0.0001) (Figure-4B) and between cell proliferation (quantifying cell numbers at d4) and transcript levels of LIN28 (R2=0.85, p<0.0001) and MYCN (R2=0.78, p<0.0001) (Figure-4A).
Figure-4. Correlations between LIN28, MYCN and cell numbers following LIN28 depletion.
The graphs show data for all four malignant-GCT cell-lines at d4 following LIN28 depletion, compared with NTC-treated cells. Panel A shows cell numbers versus the levels of LIN28 (red) and MYCN (blue), while panel B shows levels of MYCN versus LIN28. NTC=non-targeting-control siRNA, kd=knockdown. Correlation p-values were determined by linear-regression. Error-bars=SEM.
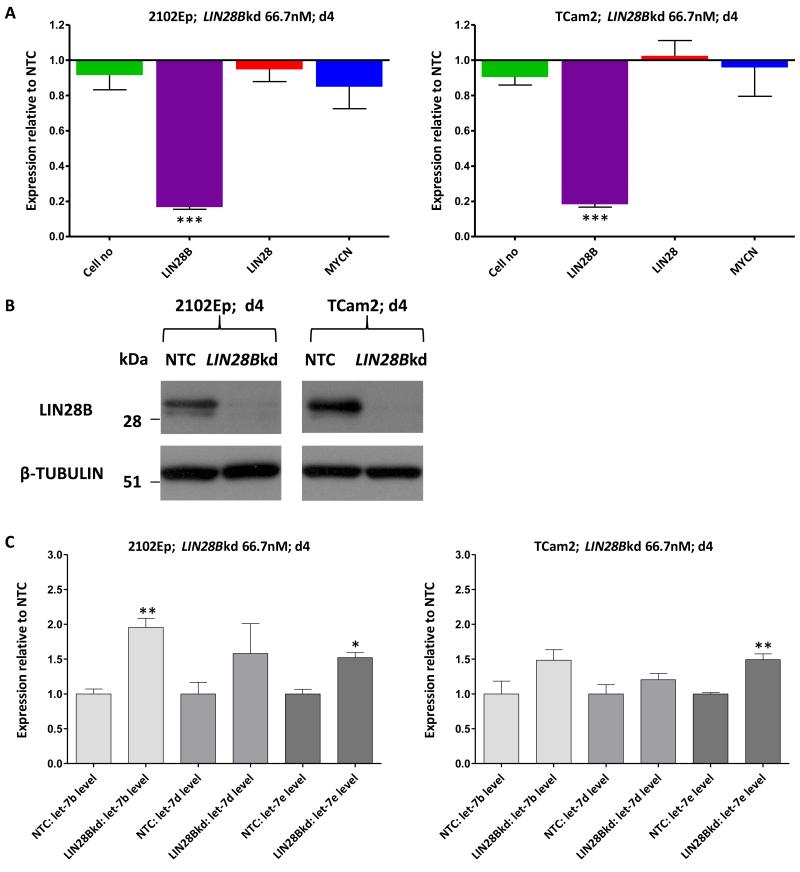
We next depleted LIN28B in 2102Ep and TCam2, achieving >80% transcript depletion and >85% protein depletion by d4 (Figure-5A,-5B). In contrast to the effects of LIN28 depletion (Figure-3D), we observed only modest increases in levels of let-7b, let-7d and let-7e (1.21-1.96-fold) (Figure-5C), with no effect on MYCN levels or cell proliferation (Figure-5A).
Figure-5. LIN28B depletion in malignant-GCT cells.
A) Cell numbers and qRT-PCR expression levels of LIN28B, LIN28 and MYCN on d4 following LIN28B depletion in 2102Ep (left) and TCam2 (right). B) Western blots showing expression of LIN28B on d4 following LIN28B depletion in 2102Ep (left) and TCam2 (right), compared with NTC-treated cells. C) Levels of representative let-7 family members (let-7b, let-7d and let-7e) on d4 following LIN28B depletion in 2102Ep (left) and TCam2 (right). All values are referenced to NTC-treated cells. NTC=non-targeting-control siRNA, kd=knockdown, error-bars=SEM. *=p<0.05;**=p<0.005;***=p≤0.0001.
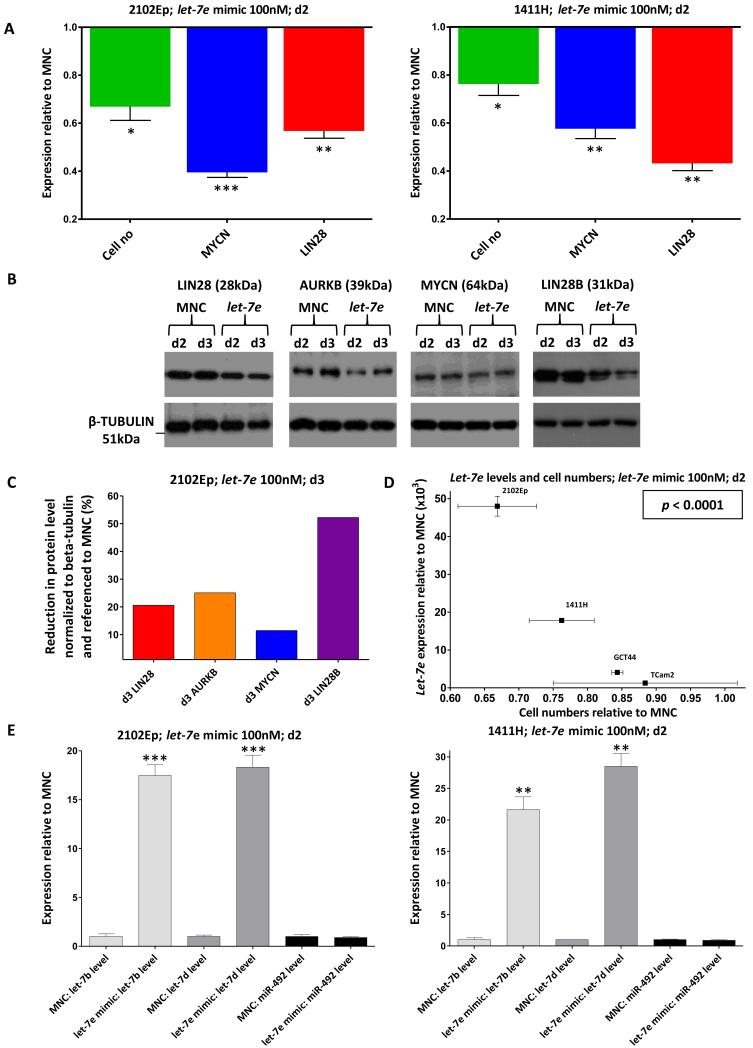
Restoration of let-7 levels
Transfection with let-7e mimic produced the greatest increases in let-7e levels in 2102Ep and 1411H (Figure-6), which were selected for further investigations. In both cell-lines, let-7e transfection resulted in significant reductions in mRNA levels of MYCN and LIN28 at d2, when compared with MNC-treated cells (all p≤0.001) (Figure-6A). Over a 3d time-course in 2102Ep, we observed reduced levels of MYCN, AURKB, LIN28 and LIN28B transcripts (Supplementary Figure-S9A), with a significant negative correlation between mean transcript depletion over d1-d3 and the number of 3′UTR let-7 SCRs (R2=0.97, p=0.0145) (Supplementary Figure-S9B). Levels of all four proteins were reduced when assessed on d2 and d3 (Figure-6B,-6C). Overall, across the four malignant-GCT cell-lines examined, there was a significant negative-correlation between let-7e levels obtained following let-7e mimic transfection and cell proliferation (R2=0.94; p<0.0001) (Figure-6D).
Figure-6. Effects of let-7e mimic in malignant-GCT cells.
A) Cell numbers and expression levels of MYCN and LIN28 in 2102Ep (left) and 1411H (right), at d2 post-transfection of let-7e mimic, relative to cells treated with mimic-negative-control (MNC) RNA. B) Western blots showing expression of LIN28, AURKB, MYCN and LIN28B proteins at d2 and d3 following let-7e mimic transfection of 2102Ep cells, corresponding to Supplementary Figure-S9A. The lower row shows the beta-tubulin loading-control. C) The graph shows protein levels at d3, as determined by densitometry of the western blots shown in B), normalized to beta-tubulin and referenced to MNC-treated cells. D) Cell numbers versus let-7e levels at d2 in four different malignant-GCT cell-lines, compared to MNC-treated cells. The p-value was determined by linear-regression. E) Levels of other representative let-7 family members (let-7b and let-7d) and control miR-492, at d2 following let-7e transfection in 2102Ep (left) and 1411H (right), relative to MNC-treated cells.
The LIN28 and LIN28B depletion (Figure-6A, Supplementary Figure-S9A) were explained by the presence in 3′UTRs of the ‘TACCTC’ SCR for the common 2-7nt let-7 seed, with one copy in LIN28 (Supplementary Figure-S9C) and five copies in LIN28B (data not shown). In keeping with the reduced LIN28 levels, we observed ~15-30 fold increases in other let-7 family members examined (let-7b and let-7d) in both 2102Ep (p=0.0001 for both) and 1411H (p=0.0006 and p=0.0002, respectively) (Figure-6E). There was no increase in levels of a control microRNA (miR-492) lacking the LIN28 ‘GGAG’ binding site in its stem-loop (Figure-6E).
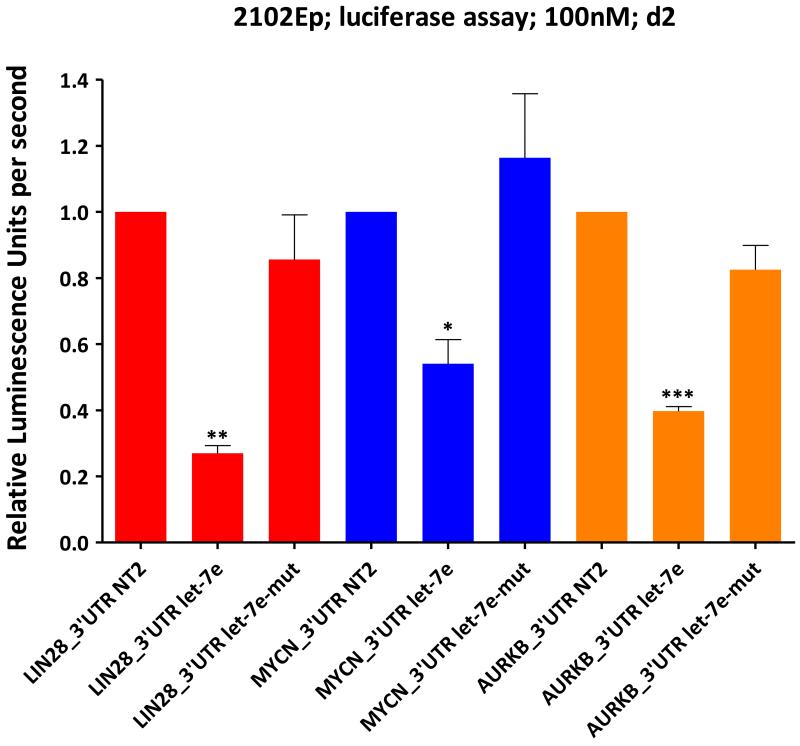
We confirmed let-7e effects on LIN28, MYCN and AURKB using quantitative luciferase reporter assays. Let-7e produced a significant reduction in luminescence relative to non-targeting oligonucleotides, in cells containing the 3′UTR for LIN28 (p=0.0003), MYCN (p=0.011) or AURKB (p<0.0001), while there were no reductions with mutant let-7e (Figure-7). These findings are supported by evidence from other cell types showing direct targeting of MYCN by let-7e (36) and of AURKB by let-7b (37), which has an identical 2-7nt seed to that of let-7e.
Figure-7. Luciferase assay confirmation of let-7 targets in malignant-GCT cells.
Luciferase assay data at d2 for 2102Ep cells transfected with a reporter containing the full-length 3′UTR for LIN28 (red), MYCN (blue) or AURKB (orange). Cells were also transfected with either let-7e or let-7e-mutant (let-7e-mut). Luminescence values were normalized to cells treated with non-targeting oligonucleotides (NT2), then referenced to cells containing a no 3′UTR control reporter and treated with let-7e/let-7e-mutant, as appropriate. Error-bars=SEM. All correlation p-values were determined by linear-regression. *=p<0.05;**=p<0.005;***=p≤0.0001.
Effects of MYCN depletion
We tested whether LIN28/LIN28B levels in malignant-GCTs might be regulated by MYCN and the related protein CMYC, similar to findings in other tumor types (38,39). Our microarray data showed up-regulation of MYCN in both pediatric (set-1; n=20) and adult (set-2; n=25) malignant-GCTs, compared with the controls used. There was a significant positive correlation with LIN28 levels in both sets (p<0.0001 and p=0.001, respectively) (Supplementary Figure-S10). In contrast, CMYC levels showed no elevation and no positive correlation with LIN28 in either set (Supplementary Figure-S10). As LIN28B was not represented on the microarray used to profile mRNAs in the pediatric malignant-GCTs, we used qRT-PCR analysis of set-3 (n=32) to show significant positive correlations between MYCN and both LIN28 and LIN28B in malignant-GCTs (p=0.0018 and p=0.0121, respectively). While depleting MYCN in 2102Ep and TCam2 reduced cell numbers, it had no consistent effects on levels of LIN28 or LIN28B (Supplementary Figure-S11). Together, these data supported our evidence that MYCN is an important up-regulated let-7 target in malignant-GCT cells, but argued against a significant effect of MYCN or CMYC upstream of LIN28/LIN28B.
Discussion
This study demonstrates that LIN28-homolog A (LIN28) is abundantly expressed in all malignant-GCTs, regardless of patient age, histologic type or anatomic site, thereby extending published reports describing predominantly or exclusively tumors of adults (17-20). Importantly, we identify the functional significance of the observed LIN28 expression, which results in let-7 family down-regulation (Supplementary Figure-S12). Our qRT-PCR analysis of tissue samples suggested that let-7 under-expression may contribute to increased expression of let-7 protein-coding-gene targets, a possibility that was supported by our functional data from LIN28 depletion and let-7e over-expression experiments. The potential let-7 targets in malignant-GCTs have known pro-malignant effects, such as increased proliferation and reduced apoptosis (Supplementary Table-S5). While LIN28B is also highly expressed in malignant-GCTs, our data do not indicate an important role for the protein in regulating let-7 microRNAs.
A previous study showed that LIN28 depletion in malignant-GCT cells led to down-regulation of stem-cell markers (e.g. OCT4/POU5F1 and NANOG) and induction of differentiation, although effects on let-7 expression were not assessed (20). In our LIN28 depletion experiments, protein levels fell to <10% from d3, a change that coincided with reduced cell growth from d3 and increased let-7 levels from d4. These findings are consistent with the observation that LIN28 depletion in carcinoma cells in vitro was not associated with significant increases in let-7 levels until d4 after transfection (40). In addition, the let-7e targets identified in the present study resonate with those seen in other malignancies, suggesting molecular parallels between disparate tumor types (40,41). Post-transcriptional effects of LIN28/let-7 deregulation on MYCN levels (36) would explain the observations that MYCN is frequently over-expressed in malignant-GCTs (42) but shows copy-number-gain (at 2p23.4) in only ~1/3 of adult tumors (43) and <1/5 of pediatric cases (44). Interestingly, we found that LIN28 depletion led to increased levels of mature let-7 without reducing levels of pri-let-7. It is likely that levels of pri-let-7 are low even in the presence of abundant LIN28, for example due to degradation after LIN28 binding.
As well as down-regulation of let-7 by LIN28, we observed a reciprocal effect, with down-regulation of LIN28 by let-7e, via a let-7 SCR in the LIN28 3′UTR (45,46). In malignant-GCT cells, let-7e-mediated down-regulation of LIN28 produced specific effects, by increasing other let-7 family targets of LIN28 rather than producing a more generalized effect on microRNA biogenesis. Other microRNAs known to down-regulate LIN28 in embryonic stem-cells and cancer cells through 3′UTR SCRs include miR-9 (47), miR-30 family (47), miR-125 (47, 48) and miR-181 (49). Interestingly, all four were identified in our previous profiling study as being universally under-expressed in malignant-GCTs, compared to the control tissues used (3). As copy-number-gain at the LIN28 locus (1p36.11) is not a feature of malignant-GCTs (44), down-regulation of these microRNAs is likely to be an important further contributor to LIN28 over-expression in vivo.
Our data suggest that LIN28/let-7 interactions are promising targets for novel therapies in malignant-GCTs. As well as directly depleting LIN28, it may also be possible to overcome the effects of over-expressed LIN28 on microRNA maturation, for example, by protective small molecule targeting of pre-let-7 stem-loop binding motifs, inhibition of the TUTase ZCCHC11 or induction of the stem-loop binding protein KSRP, which promotes maturation of a subset of microRNAs that includes let-7 (50). These indirect interventions would not counteract LIN28 effects on primary transcript processing and may not restore adequate levels of mature let-7 molecules if used in isolation. An alternative strategy is direct replacement of let-7 using mature let-7 mimics. Our data indicate that administering a single member of the let-7 family should restore levels of other family members in malignant-GCTs by targeting LIN28. The other let-7 members would lead to further reinforcement of LIN28 down-regulation, providing a molecular ‘switch’ effect that should result in a sustained reversion of cell phenotype.
Supplementary Material
Acknowledgements
We would like to thank Mr Alex Byford for technical assistance.
Financial support:
Cancer Research-UK programme grant (NC), Medical Research Council Fellowship (MJM) and Addenbrooke’s Charitable Trust (MJM).
Footnotes
Conflict-of-interest: none
References
- 1.Murray MJ, Nicholson JC. Germ Cell Tumours in Children and Adolescents. Paediatrics and Child Health. 2010;20:109–16. [Google Scholar]
- 2.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003;170:5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- 3.Palmer RD, Murray MJ, Saini HK, van Dongen S, Abreu-Goodger C, Muralidhar B, et al. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 2010;70:2911–23. doi: 10.1158/0008-5472.CAN-09-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 5.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–8. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol. 2009;16:1021–5. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–9. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138:696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Loughlin FE, Gebert LF, Towbin H, Brunschweiger A, Hall J, Allain FH. Structural basis of pre-let-7 miRNA recognition by the zinc knuckles of pluripotency factor Lin28. Nat Struct Mol Biol. 2012;19:84–9. doi: 10.1038/nsmb.2202. [DOI] [PubMed] [Google Scholar]
- 14.Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–84. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2010;29:2153–9. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 16.West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–13. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao D, Allan RW, Cheng L, Peng Y, Guo CC, Dahiya N, et al. RNA-binding protein LIN28 is a marker for testicular germ cell tumors. Hum Pathol. 2011;42:710–8. doi: 10.1016/j.humpath.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Cao D, Liu A, Wang F, Allan RW, Mei K, Peng Y, et al. RNA-binding protein LIN28 is a marker for primary extragonadal germ cell tumors: an immunohistochemical study of 131 cases. Mod Pathol. 2011;24:288–96. doi: 10.1038/modpathol.2010.195. [DOI] [PubMed] [Google Scholar]
- 19.Xue D, Peng Y, Wang F, Allan RW, Cao D. RNA-binding protein LIN28 is a sensitive marker of ovarian primitive germ cell tumours. Histopathology. 2011;59:452–9. doi: 10.1111/j.1365-2559.2011.03949.x. [DOI] [PubMed] [Google Scholar]
- 20.Gillis AJ, Stoop H, Biermann K, van Gurp RJ, Swartzman E, Cribbes S, et al. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Androl. 2011;34:e160–74. doi: 10.1111/j.1365-2605.2011.01148.x. [DOI] [PubMed] [Google Scholar]
- 21.Korkola JE, Houldsworth J, Chadalavada RS, Olshen AB, Dobrzynski D, Reuter VE, et al. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer Res. 2006;66:820–7. doi: 10.1158/0008-5472.CAN-05-2445. [DOI] [PubMed] [Google Scholar]
- 22.Mills SE. Sternbergs Histology for Pathologists. Lippincott, Williams and Wilkins; New York: 2006. 3rd revised ed. [Google Scholar]
- 23.Benjamini YHY. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc Ser B-Methodol. 1995;57:289–300. [Google Scholar]
- 24.van Dongen S, Abreu-Goodger C, Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods. 2008;5:1023–5. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primer 3.[cited 26th August 2012]; Available from: http://frodo.wi.mit.edu/primer3/
- 26.Herdman MT, Pett MR, Roberts I, Alazawi WO, Teschendorff AE, Zhang XY, et al. Interferon-beta treatment of cervical keratinocytes naturally infected with human papillomavirus 16 episomes promotes rapid reduction in episome numbers and emergence of latent integrants. Carcinogenesis. 2006;27:2341–53. doi: 10.1093/carcin/bgl172. [DOI] [PubMed] [Google Scholar]
- 27.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray MJ, Saini HK, van Dongen S, Palmer RD, Muralidhar B, Pett MR, et al. The two most common histological subtypes of malignant germ cell tumour are distinguished by global microRNA profiles, associated with differential transcription factor expression. Mol Cancer. 2010;9:290. doi: 10.1186/1476-4598-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damjanov I, Andrews PW. Ultrastructural differentiation of a clonal human embryonal carcinoma cell line in vitro. Cancer Res. 1983;43:2190–8. [PubMed] [Google Scholar]
- 30.de Jong J, Stoop H, Gillis AJ, Hersmus R, van Gurp RJ, van de Geijn GJ, et al. Further characterization of the first seminoma cell line TCam-2. Genes Chromosomes Cancer. 2008;47:185–96. doi: 10.1002/gcc.20520. [DOI] [PubMed] [Google Scholar]
- 31.Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- 32.Muralidhar B, Winder D, Murray M, Palmer R, Barbosa-Morais N, Saini H, et al. Functional evidence that Drosha overexpression in cervical squamous cell carcinoma affects cell phenotype and microRNA profiles. J Pathol. 2011;224:496–507. doi: 10.1002/path.2898. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Wu F, Brant SR, Kwon JH. IL-23 receptor regulation by Let-7f in human CD4+ memory T cells. J Immunol. 2011;186:6182–90. doi: 10.4049/jimmunol.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pett MR, Alazawi WO, Roberts I, Dowen S, Smith DI, Stanley MA, et al. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004;64:1359–68. doi: 10.1158/0008-5472.can-03-3214. [DOI] [PubMed] [Google Scholar]
- 35.Boyerinas B, Park SM, Shomron N, Hedegaard MM, Vinther J, Andersen JS, et al. Identification of let-7-regulated oncofetal genes. Cancer Res. 2008;68:2587–91. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 36.Buechner J, Tomte E, Haug BH, Henriksen JR, Lokke C, Flaegstad T, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105:296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 38.Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A. 2009;106:3384–9. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cotterman R, Knoepfler PS. N-Myc regulates expression of pluripotency genes in neuroblastoma including lif, klf2, klf4, and lin28b. PLoS One. 2009;4:e5799. doi: 10.1371/journal.pone.0005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helland A, Anglesio MS, George J, Cowin PA, Johnstone CN, House CM, et al. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS One. 2011;6:e18064. doi: 10.1371/journal.pone.0018064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molenaar JJ, Domingo-Fernandez R, Ebus ME, Lindner S, Koster J, Drabek K, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012 doi: 10.1038/ng.2436. In Press. [DOI] [PubMed] [Google Scholar]
- 42.Alagaratnam S, Lind GE, Kraggerud SM, Lothe RA, Skotheim RI. The testicular germ cell tumour transcriptome. Int J Androl. 2011;34:e133–50. doi: 10.1111/j.1365-2605.2011.01169.x. discussion e50-1. [DOI] [PubMed] [Google Scholar]
- 43.Kraggerud SM, Skotheim RI, Szymanska J, Eknaes M, Fossa SD, Stenwig AE, et al. Genome profiles of familial/bilateral and sporadic testicular germ cell tumors. Genes Chromosomes Cancer. 2002;34:168–74. doi: 10.1002/gcc.10058. [DOI] [PubMed] [Google Scholar]
- 44.Palmer RD, Foster NA, Vowler SL, Roberts I, Thornton CM, Hale JP, et al. Malignant germ cell tumours of childhood: new associations of genomic imbalance. Br J Cancer. 2007;96:667–76. doi: 10.1038/sj.bjc.6603602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen AX, Yu KD, Fan L, Li JY, Yang C, Huang AJ, et al. Germline genetic variants disturbing the Let-7/LIN28 double-negative feedback loop alter breast cancer susceptibility. PLoS Genet. 2011;7:e1002259. doi: 10.1371/journal.pgen.1002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Lin X, Zhong X, Kaur S, Li N, Liang S, et al. Double-negative feedback loop between reprogramming factor LIN28 and microRNA let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res. 2010;70:9463–72. doi: 10.1158/0008-5472.CAN-10-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong X, Li N, Liang S, Huang Q, Coukos G, Zhang L. Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells. J Biol Chem. 2010;285:41961–71. doi: 10.1074/jbc.M110.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol Cell Biol. 2005;25:9198–208. doi: 10.1128/MCB.25.21.9198-9208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Zhang J, Gao L, McClellan S, Finan MA, Butler TW, et al. MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell Death Differ. 2012;19:378–86. doi: 10.1038/cdd.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010–4. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.