Abstract
The ability to engineer novel functionality within cells, to quantitatively control cellular circuits, and to manipulate the behaviors of populations, has many important applications in biotechnology and biomedicine. These applications are only beginning to be explored. In this review, we advocate the use of feedback control as an essential strategy for the engineering of robust homeostatic control of biological circuits and cellular populations. We also describe recent works where feedback control, implemented in silico or with biological components, was successfully employed for this purpose.
Maintaining homeostasis of metabolites and proteins requires cellular processes to continuously adjust to perturbations imposed by their fluctuating environment. Environmental deviations are often sensed by, and adapted to, using feedback control strategies. As such, feedback control is integral to many homeostatic processes in the cell. Synthetic biology, a discipline that builds novel functional circuits within cells, has sought to mimic the operation of many cellular processes. Strategies employing feedback loops have been effectively used to shape the dynamic behavior of many engineered synthetic circuits, resulting in sophisticated functionalities such as bistability and oscillations. However, the homeostatic potential of feedback control to build robustly operating circuits has remained largely untapped in most of the synthetic circuits built to date.
In this review, we examine the current understanding and implementations of feedback regulation in endogenous and engineered genetic circuits. We also highlight the use of in silico feedback, a novel technology for external control of intracellular processes.
Feedback Control Using Biological Components
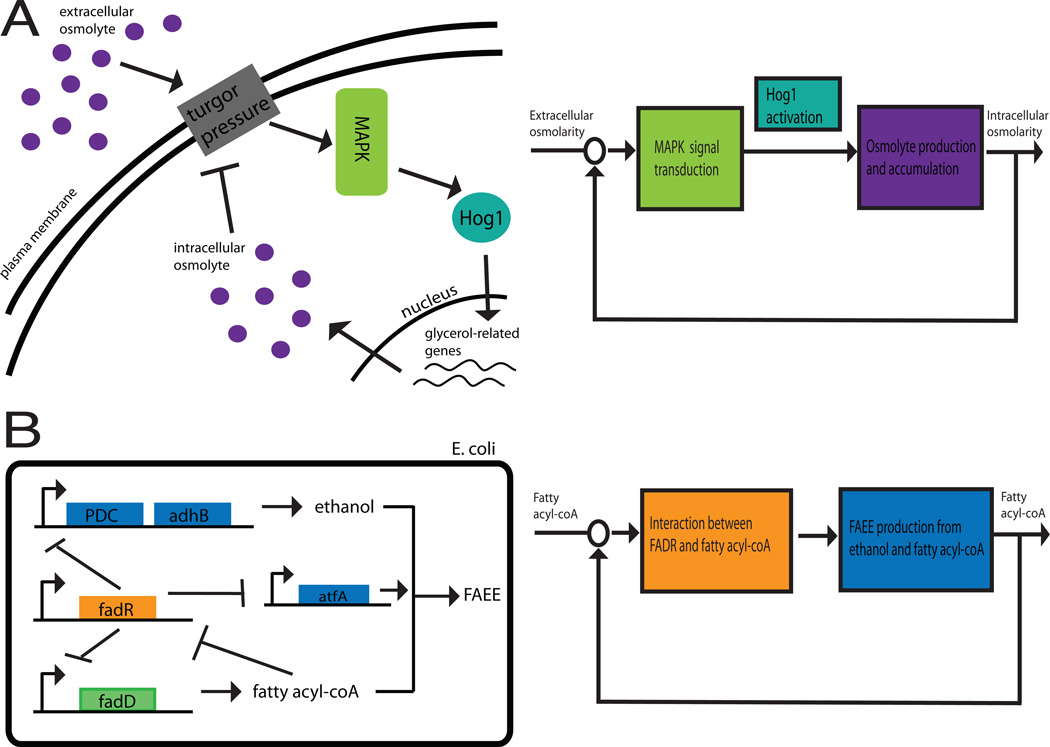
In diverse processes such as amino acid biosynthesis, chemotaxis, and stress response pathways, feedback loops ensure that any deviation from normal operation is detected and corrected1,2,3,4,5. One well-studied example of such homeostatic control occurs in the osmotic stress response, in which cells faced with external fluctuations of osmolyte concentrations actively regulate their turgor pressure. Membrane proteins are thought to sense imbalances between intracellular and external osmolarity, and activate a MAPK pathway that acts to increase the intracellular concentrations of the osmolyte glycerol. Glycerol accumulation then allows the cell to return to its resting turgor pressure, in other words to perfectly adapt (see Figure 1). Control theory analysis showed that such adaptation requires the system to implement integral feedback control6,7. Many natural biological systems, from cellular behaviors such as chemotaxis8 to physiological responses such as calcium regulation9, also exhibit adaptive feedback control. In these cases, integral control provides a general strategy that performs reliably for a wide range of perturbations and system characteristics, rather than on carefully tuned parameters. In fact, a computational search for 3-node networks capable of perfect adaptation revealed integral feedback control as one of two strategies that are necessary for this behavior10.
Figure 1. Feedback control in natural and engineered cellular systems.
In systems implementing feedback control, the deviation of a measured output from a desired set-point is assessed. A control algorithm uses this value to compute a corrective action, which when input into the system, regulates its output to the desired set value. Natural and engineered biological systems make use of this type of homeostatic feedback control. (A) The yeast osmoregulation system demonstrates feedback control. Disturbances to extracellular osmolarity cause a change in turgor pressure from a steady-state value. Deviation from this value activates MAPK signaling and Hog1 nuclear import, which in turn activates Hog1-dependent synthesis of glycerol. Glycerol accumulation restores the turgor pressure to its pre-perturbation value6. (B) The FAEE biosynthetic pathway has been engineered in E. coli to exhibit homeostatic feedback control. In this system, accumulation of fatty acyl-coA is sensed by fadR which adjusts the expression of enzymes, allowing for greater production of FAEE. Increased consumption of fatty acyl-coA during FAEE production reduces fatty acyl-coA levels, therefore enacting a negative feedback loop24.
In addition to their key role in homeostatic control, feedback loops also allow cells to generate useful dynamical behaviors. For example, positive feedback loops in the Xenopus Oocyte maturation circuit, when layered onto a system which contains an ultrasensitive response, can generate bistability11. By contrast, delayed negative feedback loops generate oscillations such as those observed in the Cyclin-CDK circuit that constitutes the engine of cell division cycles12.
Not surprisingly, the earliest examples of feedback loops in synthetic biology involved the construction of circuits capable of bistability and oscillations 13, 14. Elaborations on these core functionalities also made use of feedback loops to extend circuit functionality15,16,17,18,19. In one example, a synthetic circuit built in E. coli used a positive feedback loop implemented by araC auto activation and a negative feedback loop implemented by araC-mediated activation of the lacI repressor. This architecture, consisting of nested positive and negative feedback loops, allowed for robust oscillations with frequencies that are tunable by addition of the lacI inhibitor IPTG or the araC inducer arabinose18. Similar oscillatory circuits that exploit sense-anti-sense RNA expression units to build interlaced positive and negative feedback loops have also been implemented in mammalian cells 19.
In addition to building de novo functionality, synthetic positive and negative feedback loops have been used to alter the dynamic response of endogenous cellular circuits20,21. In S. cerevisiae, pheromone activates a canonical MAPK cascade that results in a dynamic program of gene expression that prepares the cells for mating. In order to introduce new dynamic responses to pheromone stimulation, feedback loops were engineered into the system by recruiting negative and positive pathway modulators to the MAPK scaffold using complementary leucine zippers20. The expression of these pathway modulators is controlled by MAPK signaling, resulting in feedback regulation. Furthermore, adjusting the binding affinity of the zippers tunes the strength of the feedback links, allowing for the creation of various dynamical behaviors such as pulse generation and ultrasensitive switching. This approach was also extended to other MAPK signaling cascades in yeast and mammalian cells, demonstrating its general applicability22.
The examples above illustrate the use of feedback loops to implement increasingly sophisticated qualitative behaviors. However, the use of feedback to increase the robustness and quantitative fidelity of synthetic circuits in the face of biological noise and external perturbations is an equally important emerging application, especially in the context of metabolic engineering23. The production of industrially relevant molecules in genetically modified organisms is often limited by metabolic imbalances that lead to the accumulation of toxic product intermediates and inefficient use of feedstocks. Metabolic imbalances in endogenous metabolic pathways are corrected using feedback regulation of the enzymatic activity of the pathway, often by end-product inhibition1. Mimicking this strategy, feedback control of the metabolic intermediate fatty acyl-CoA was engineered into an E.coli strain that produced the diesel fuel replacement fatty acid acyl ester (FAEE) through condensation of acyl-CoA with ethanol24 (Figure 1). In order to maintain fatty acyl-CoA at a desired level, the authors engineered a circuit in which the fatty acid-responsive transcriptional repressor FadR regulates the expression of enzymatic components of the biosynthetic pathway. In this system, termed a “Dynamic Sensor Regulator”, build-up of fatty acyl-CoA titrates FadR from its target promoters, resulting in expression of enzymes that catalyze ethanol and FAEE production. As more fatty acyl-CoA is converted to FAEE through the action of these enzymes, FADR repression is restored, therefore implementing a negative feedback loop. This strategy led to a threefold increase in diesel fuel yield. Previous uses of feedback control of metabolic flux also include regulation of the antioxidant lycopene in E. coli through engineered responsiveness to the buildup of glucose25. Quorum sensing and a genetic toggle switch were also employed as part of a feedback control strategy to dynamically regulate protein expression in response to cell density26,27.
Monitoring and adjusting the operation of a circuit’s output (such fatty acyl-CoA) with feedback control relies on the availability of an endogenous sensor (such as FadR). This type of specialized systems is not necessarily transplantable to general applications. One strategy to circumvent this difficulty is through separating sensing from signal transduction and actuation. Recent work in this direction modularized signal transfer in bacterial two-component phosphotransfer signaling systems28. This advancement was made possible through the recruitment of a Histidine Kinase (HK) sensor to its non-native Response Regulator (RR) 29 through a synthetic scaffold. These modular components have versatile inputs and outputs and can be used, in addition to other circuits that exploit similar strategies30,31, to create portable “feedback control modules”. Additional enhancements include the use components that are orthogonal to the host cell’s endogenous pathways, therefore allowing for increased signaling specificity32.
In addition to robust intracellular regulation, feedback control has also been used to achieve inter-cellular control of population behaviors33,34,35,36,37,38,39,40,41. In one example, “quorum sensing” diffusible small molecules were co-opted to synchronize oscillations across a bacterial population33. Here, the oscillatory circuit has the same architecture as the one implemented by Stricker et al.18 but involves the production of acyl homoserine lactone (AHL), a quorum sensing small molecule from the bioluminescent bacteria vibrio fischerii that diffuses across cell membranes. Specifically, the main negative feedback loop that drives the oscillations in this circuit involves binding of AHL to luxR. This complex leads to the production of aiiA, a protein that in turns degrades AHL. Diffusion of AHL between neighboring cells allows them to synchronize their oscillations. In subsequent work, the range of synchronization was extended to the millimeter scale by using hydrogen peroxide gas as a fast diffusing signaling molecule in the system34. Similar multicellular control has been achieved in yeast cells using diffusible endogenous pathway inducers such as alpha-factor35. Orthogonal communication channels were also developed by engineering the diffusible plant hormone cytokinin isopentenyladenine (IP) into a sender yeast strain and the IP responsive cytokinin receptor AtCRE1 into a receiver yeast strain36,37. Multicellular control of this nature will be increasingly important in applications where heterogeneous populations need to synergize in order to accomplish useful functions, such as the production of biotechnologically important compounds42.
Despite these promising successes, the use of biological components to implement feedback control of synthetic circuits remains limited by our current quantitative understanding of these components43. Large uncertainties in the homeostatic controller can actually lead to amplification of the perturbations that the feedback is intended to correct. To address this problem, researchers have turned to outsourcing the control of synthetic biological circuits to man-made components. The idea here is that by converting the biological output to some experimentally measurable quantity that can be read by a computer (e.g. expression level of a fluorescent protein), an appropriate control strategy can be devised in silico and then administered in real time to the cell via an engineered input. In this way, more precise external controllers can be used to achieve robust homeostatic regulation of an underlying cellular circuit.
Feedback Control in silico
Exerting external control to regulate the growth rate and other physiological properties of cellular populations has a long history, particularly in the context of culture growth in chemostats44. Chemostats constantly monitor culture variables such as the quantity of nutrients and use this information as inputs into an external control scheme, implemented using electronic circuits or a computer, to maintain constant growth by adjustment of culture dilution rate. This idea of external “closed loop control” of a biological system has seen renewed interest in the context of synthetic biology, specifically for the regulation of intracellular variables. Such a control scheme relies on the ability (1) to measure the appropriate variable continuously in real-time, (2) to assess the deviation of this variable from a desired value and compute a corrective action using an appropriate algorithm, and finally (3) to administer the corrective action back to the biological circuit.
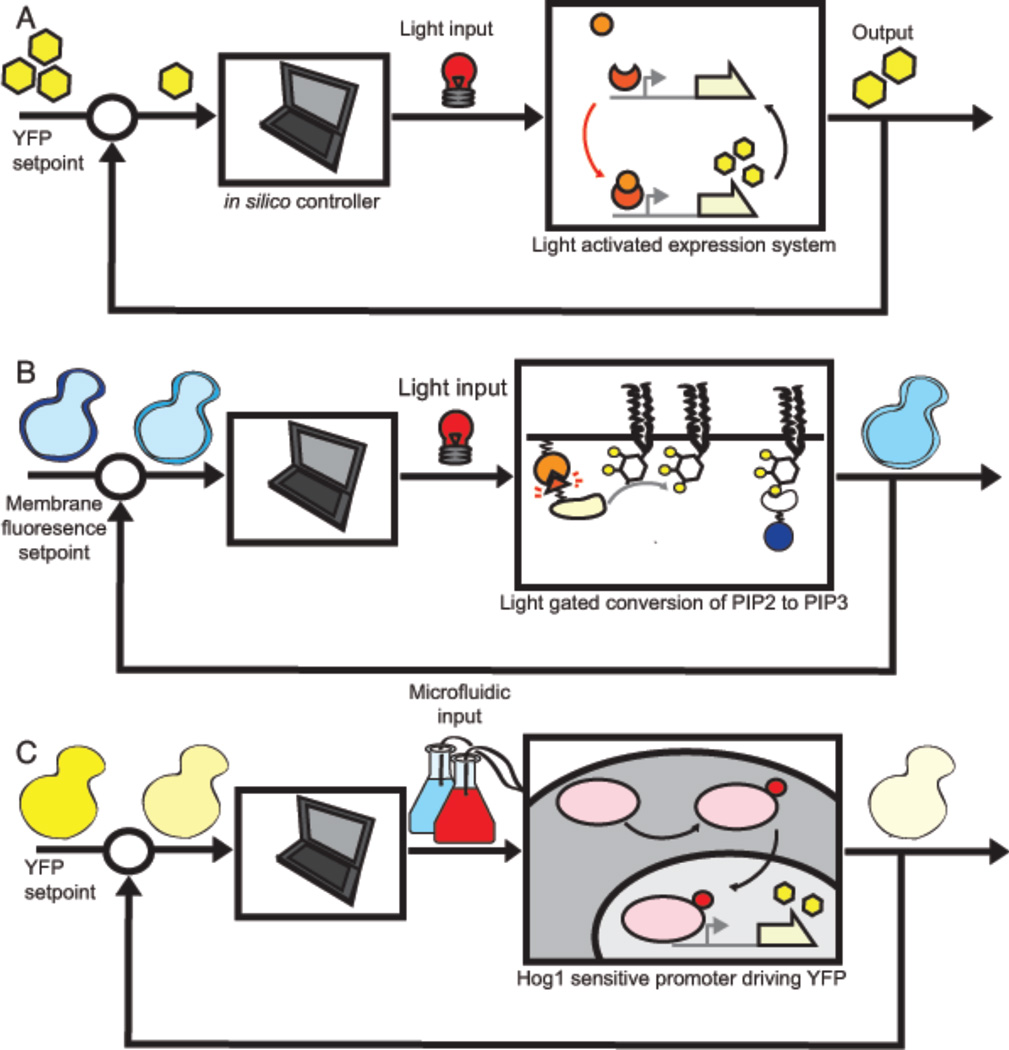
Successful implementations of external feedback control have been achieved in S. cerevisiae and mammalian cells (see figure 2). Milias-Argeitis et. al. controlled expression of a fluorescent protein (Venus) from a GAL1 promoter using this strategy45. To assess deviation from desired protein expression, fluorescence is measured using a flow cytometer and then input into a computer algorithm. The algorithm relies on a model of GAL1 gene expression and established techniques of Model Predictive Control46 to compute the corrective input. This input is then administered to the biological circuit using a light gated interaction between the photoreceptor chromoprotein PhyB and Phytochrome Interacting Factor PIF. Synthetic constructs of these two Arabidopsis Thaliana proteins were expressed in S. cerevisiae to allow light-driven regulation of the GAL1 promoter. Optogentic tools therefore allowed for real-time in silico control of this biological circuit.
Figure 2. In silico feedback enables quantitative control of cellular circuits.
In this scheme, a circuit output such as a fluorescent protein is measured by microscopy or flow cytometry. The output is compared to a desired setpoint and a regulation error is computed. This error is used to devise an appropriate control strategy in silico, which is then applied to the cellular circuit using an engineered input. A) In silico feedback regulation of gene expression through a light-activated transcription factor45. B) In silico feedback regulation of PIP3 levels in the membrane through light gated recruitment to the membrane of the enzyme that catalyzes the conversion of PIP2 to PIP347. C) In silico feedback regulation of a HOG pathway responsive promoter using a microfluidic device that administers pulses of sorbitol to the growth chamber53.
Using the same Phy/PIF light gated interaction, Toettcher et. al. were able to use a similar strategy to control fast post-translational modifications such as membrane recruitment of proteins48. In their system, the light gated interaction of Phy/PIF proteins leads to the membrane recruitment of the enzyme PIK3, which catalyzes the conversion of the phospholipid 2′ phosphoinositide (PIP2) to 3′ phosphoinositide (PIP3). A fluorescent protein fused to a PIP3 binding domain allows PIP3 membrane concentration to be measured using microscopy. Deviations from a desired PIP2 concentration value are corrected by light inputs, whose intensities are determined by an in silico proportional integral derivative (PID) controller. Importantly, this work succeeded in robustly controlling the output of a natural system both in the presence of its endogenous feedbacks and pharmacological modulators of PIP3 levels. These results, combined with advances of optogenetics technologies48, 49, 50, 51, 52, introduce the intriguing possibility of addressable control of individual cells for technological applications including the production of biofuels or small molecule drugs. These same strategies also offer exciting therapeutic opportunities, such as the possibility of using real-time external feedback control to achieve, for example, deep brain stimulation in the treatment for Parkinson’s disease or neuron de-synchronization to control epileptic seizures.
Microfluidic approaches have also been used to implement in silico control of cellular systems in the context of the yeast HOG pathway53. Expression from an osmo-responsive promoter was measured and Model Predictive Control used to compute the duration of sorbitol treatment necessary to achieve desired promoter activity. To implement this strategy, the group constructed a microfluidic device capable of changing the osmolyte concentration precisely and with high temporal resolution. When the output of a single cell was used to design the sorbitol input, the variability of this cell relative to the variability seen in the population was reduced. Microfluidic technologies have also been used to devise control strategies that do not require an underlying model of the system54. This is useful for controlling complex biological systems that contain multiple time scales and modes of endogenous regulation. Overall, advances in microfluidic technologies have increased the time periods over which cells can be studied and manipulated 41,55,56, allowing for increasingly sophisticated control of cellular populations.
CONCLUSION
Feedback control, implemented using either biological components or external in silico strategies, presents a unique opportunity to introduce robustness into the operation of synthetic circuits. In silico control of biological systems offers several advantages, such as the ability to compensate through sophisticated computational algorithms for an incomplete understanding of the underlying biology and the possibility to reprogram different behaviors in the controlled cellular circuit without the need to re-engineer it de novo. The flexibility afforded by in silico control makes it ideal for circumventing the extensive tuning often required to control the operation of synthetic circuits using only biological building blocks. However, future advances in our understanding of biological components and their rules of composition and operation might soon level this playing field.
Highlights.
Engineered feedback loops implement elaborate functionality in synthetic circuits.
The homeostatic potential of feedback loops is under-utilized in synthetic biology.
Feedback control of engineered or natural cellular functions can be achieved using biological components or in silico regulation.
Optogenetics and microfluidic technologies are important for implementing in silico control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Braus GH. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: a model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol Rev. 1991 Sep;55(3):349–370. doi: 10.1128/mr.55.3.349-370.1991. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levchenko A, Iglesias PA. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys. J. 2002;82:50–63. doi: 10.1016/S0006-3495(02)75373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr. Opin. Neurobiol. 2003;13:469–475. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 4.Berg HC, Brown DA. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Iglesias PA. Positive feedback may cause the biphasic response observed in the chemoattractant-induced response of Dictyostelium cells. Syst. Contr. Lett. 2006;55:329–337. doi: 10.1016/j.sysconle.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muzzey D, Gómez-Uribe CA, Mettetal JT, van Oudenaarden A. A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell. 2009;138(1):160–171. doi: 10.1016/j.cell.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin GF. Feedback Control of Dynamic Systems. 2009 [Google Scholar]
- 8.Yi TM, Huang Y, Simon MI, Doyle J. Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc Natl Acad Sci U S A. 2000;97(9):4649–4653. doi: 10.1073/pnas.97.9.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Samad H, Goff J, Khammash M. Calcium Homeostasis and Parturient Hypocalcemia: An Integral Feedback Perspective. Journal of Theoretical Biology. 2002;214(1):17–29. doi: 10.1006/jtbi.2001.2422. [DOI] [PubMed] [Google Scholar]
- 10.Ma W, Trusina A, El-Samad H, Lim WA, Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009;138(4):760–773. doi: 10.1016/j.cell.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrell JE, Pomerening JR, Kim SY, Trunnell NB, Xiong W, Huang CF, Machleder EM. Simple, realistic models of complex biological processes: Positive feedback and bistability in a cell fate switch and a cell cycle oscillator. FEBS Letters. 2009;582(3999–4005) doi: 10.1016/j.febslet.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 12.Ferrell JE, Jr, Tsai TY, Yang Q. Modeling the cell cycle: why do certain circuits oscillate? Cell. 2011;144(6):874–885. doi: 10.1016/j.cell.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403(6767):339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 14.Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403(6767):335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 15.Burrill DR, Silver PA. Making cellular memories. Cell. 2010 Jan 8;140(1):13–18. doi: 10.1016/j.cell.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Arkin AP. Sequestration-based bistability enables tuning of the switching boundaries and design of a latch. Mol Syst Biol. 2012 Oct 23;8:620. doi: 10.1038/msb.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nature Rev. Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stricker J, Cookson S, Bennett MR, Mather WH, Tsimring LS, Hasty J. A fast, robust and tunable synthetic gene oscillator. Nature. 2008;456(7221):516–519. doi: 10.1038/nature07389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tigges M, Marquez-Lago TT, Stelling J, Fussenegger M. A tunable synthetic mammalian oscillator. Nature. 2009;457(7227):309–312. doi: 10.1038/nature07616. [DOI] [PubMed] [Google Scholar]
- 20.Bashor CJ, Helman NC, Yan S, Lim WA. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science. 2008;319(5869):1539–1543. doi: 10.1126/science.1151153. [DOI] [PubMed] [Google Scholar]
- 21.Toettcher JE, Mock C, Batchelor E, Loewer A, Lahav G. A synthetic-natural hybrid oscillator in human cells. Proc Natl Acad Sci U S A. 2010 Sep 28;107(39):17047–17052. doi: 10.1073/pnas.1005615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei P, Wong WW, Park JS, Corcoran EE, Peisajovich SG, Onuffer JJ, Weiss A, Lim WA. Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells. Nature. 2012 Jul 22; doi: 10.1038/nature11259. Bacterial virulence proteins were expressed in yeast and in primary CD4+ T-cells and recruited to the MAPK scaffold to synthetically adjust signal output levels. The strategy created synthetic feedback loops to generate amplitude limiters and inducible pause switches.
- 23.Holtz WJ, Keasling JD. Engineering static and dynamic control of synthetic pathways. Cell. 2010 Jan 8;140(1):19–23. doi: 10.1016/j.cell.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 24. Zhang F, Carothers JM, Keasling JD. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nature Biotechnology. 2012;30(4):354–359. doi: 10.1038/nbt.2149. In a synthetic metabolic circuit engineered into E. coli, the authors created a feedback loop that increased the output of the biodiesel FAEE by linking the concentration of a key pathway intermediate and enzymes that increased the processing of that intermediate. This approach illustrates the use of feedback to dynamically adjust a systems output using biological components.
- 25.Farmer WR, Liao JC. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotechnol. 2000;18:533–537. doi: 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Kaern M, Araki M, Chung K, Gardner TS, Cantor CR, Collins JJ. Programmable cells: interfacing natural and engineered gene networks. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22) doi: 10.1073/pnas.0402940101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolaos A, Cluett WR, Mahadevan R. Dynamic metabolic engineering for increasing bioprocess productivity. Metabolic Engineering. 2008;10(5):255–266. doi: 10.1016/j.ymben.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 29. Whitaker WR, Davis SA, Arkin AP, Dueber JE. Engineering robust control of twocomponent system phosphotransfer using modular scaffolds. Proc Natl Acad Sci USA. 2012;109(44):18090–18095. doi: 10.1073/pnas.1209230109. The authors re-wired bacterial two component systems to mix and match Histone Kinase sensor domains to Response Regulator domains. The ability to decouple input sensing from downstream signaling is an important step toward building specific, modular feedback into biological circuits.
- 30.Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KLJ, Keasling JD. Synthetic protein scaffolds provide modular control over flux through an engineered metabolic pathway. Nature Biotechnology 2009. 2009 Aug 2;27:753–759. doi: 10.1038/nbt.1557. [DOI] [PubMed] [Google Scholar]
- 31.Conrado RJ, Wu GC, Boock JT, Xu H, Chen SY, Lebar T, Turnsek J, Tomsic N, Avbelj M, Gaber R, Koprivnjak T, Mori J, Glavnik V, Vovk I, Bencina M, Hodnik V, Anderluh G, Dueber JE, Jerala R, DeLisa MP. DNA-guided assembly of biosynthetic pathways promotes improved catalytic efficiency. Nucleic Acids Research. 2011;40(4):1879–1889. doi: 10.1093/nar/gkr888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon TS, Lou C, Tamsir A, Stanton BC, Voigt CA. Genetic programs constructed from layered logic gates in single cells. Nature. 2012;491(7423):249–253. doi: 10.1038/nature11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danino T, Mondragon-Palomino O, Tsimring L, Hasty J. A synchronized quorum of genetic clocks. Nature. 2010;426:326–330. doi: 10.1038/nature08753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prindle A, Samayoa P, Razinko I, Danino T, Tsimring L, Hasty J. A sensing array of radically coupled genetic ‘biopixels’. Nature. 2012;481:39–44. doi: 10.1038/nature10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regot S, Macia J, Conde N, Furukawa K, Kjellen J, Peeters T, Hohmann S, de Nada E, Posas F, Sole R. Distributed biological computation with multicellular engineered networks. Nature. 2011;469(7329):207–211. doi: 10.1038/nature09679. [DOI] [PubMed] [Google Scholar]
- 36.Chen MT, Weiss R. Artificial cell-cell communication in yeast Saccharomyces cerevisiae using signaling elements from Arabidopsis thaliana. Nat Biotechno. 2005;23(12):1551–1555. doi: 10.1038/nbt1162. [DOI] [PubMed] [Google Scholar]
- 37.Bacchus W, Lang M, El-Baba MD, Weber W, Stelling J, Fussenegger M. Synthetic twoway communication between mammalian cells. Nat Biotechnol. 2012;30(10):991–996. doi: 10.1038/nbt.2351. [DOI] [PubMed] [Google Scholar]
- 38.You L, Cox RS, 3rd, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing. Nature. 2004 Apr 22;428(6985):868–871. doi: 10.1038/nature02491. [DOI] [PubMed] [Google Scholar]
- 39.You L, Cox RS, 3rd, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing. Nature. 2004 Apr; doi: 10.1038/nature02491. [DOI] [PubMed] [Google Scholar]
- 40.Maharbiz MM. Synthetic multicellularity. Trends Cell Biol. 2012 Dec;22(12):617–623. doi: 10.1016/j.tcb.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Balagaddé FK, Song H, Ozaki J, Collins CH, Barnet M, Arnold FH, Quake SR, You L. A synthetic Escherichia coli predator-prey ecosystem. Mol Syst Biol. 2008;4:187. doi: 10.1038/msb.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace W, Beshear S, Williams D, Hospadar S, Owens M. Perchlorate reduction by a mixed culture in an up-.ow anaerobic .xed bed reactor. Journal of Industrial Microbiology & Biotechnology. 1998;20:126–131. [Google Scholar]
- 43.Jordan A, Bagh S, Ingalls BP, McMillen DR. Considerations for using integral feedback control to construct a perfectly adapting synthetic gene network. Journal of Theoretical Biology. 2010;266(4):723–738. doi: 10.1016/j.jtbi.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 44.De Leenheer P, Smith H. Feedback control for chemostat models. Journal of Mathematical Biology. 2003;46(1):48–70. doi: 10.1007/s00285-002-0170-x. [DOI] [PubMed] [Google Scholar]
- 45. Milias-Argeitis A, Summers S, Stewart-Ornstein J, Zuleta I, Pincus D, El- Samad H, Khammash M, Lygeros J. In silico feedback for in vivo regulation of a gene expression circuit. Nat Biotechnol. 2011;29(12):1114–1116. doi: 10.1038/nbt.2018. The authors achieved in silico feedback control of gene expression to a desired level in yeast using optogenetics.
- 46.James Rawlings, David Mayne. Model Predictive Control Theory and Design. Nob Hill Publishers; 2009. [Google Scholar]
- 47. Toettcher JE, Gong D, Lim WA, Weiner OD. Light-based feedback for controlling intracellular signaling dynamics. Nat Methods. 2011;8(10):837–839. doi: 10.1038/nmeth.1700. The authors were able to control PIP3 membrane concentration by using light gated membrane recruitment of an enzyme that catalyzes the conversion of PIP2 to PIP3. This work demonstrated that in silico control is also possible in the presence of endogenous feedbacks operating within the system.
- 48.Shimizu-Sato S, Huq E, Tepperman JM, Quail PH. A light-switchable gene promoter system. Nature Biotechnol. 2002;20:1041–1044. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Chen X, Yang Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nature methods. 2012;9(3):266–269. doi: 10.1038/nmeth.1892. [DOI] [PubMed] [Google Scholar]
- 50.Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, Sosnick TR, et al. tuliPs : tunable, light-controlled interacting protein tags for cell biology. Nature. 2012;9(4) doi: 10.1038/nmeth.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen Ja, Nonneman RJ, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63(1):27–39. doi: 10.1016/j.neuron.2009.06.014. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE. Induction of protein-protein interactions in live cells using light. Nature biotechnology. 2009;27(10):941–945. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- 53. Uhlendorf J, Miermont A, Delaveau T, Charvin G, Fages F, Bottani S, Batt G, Hersen P. Long-term model predictive control of gene expression at the population and single-cell levels. PNAS. 2012;109(35):14271–14276. doi: 10.1073/pnas.1206810109. The authors used a microfluidic device capable of delivering pulses of sorbitol to growing yeast to control gene expression from a HOG responsive promoter. This work demonstrates the feasibility of in silico feedback control of native systems.
- 54.Menolascina F, Di Bernardo M, Di Bernardo D. Analysis, design and implementation of a novel scheme for in-vivo control of synthetic gene regulatory networks. Automatica. 2011;47(6):1265–1270. [Google Scholar]
- 55.Balagaddé FK, You L, Hansen CL, Arnold FH, Quake SR. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science. 2005 Jul 1;309(5731):137–140. doi: 10.1126/science.1109173. [DOI] [PubMed] [Google Scholar]
- 56.Balagadde FK. The new role of the microchemostat in the bioengineering revolution. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1064–1066. doi: 10.1109/IEMBS.2009.5335037. [DOI] [PubMed] [Google Scholar]