Abstract
Aspartic proteases (APs) comprise a large proteolytic enzyme family widely distributed in animals, microbes, viruses, and plants. The rice genome encodes 96 APs, of which only a few have been functionally characterized. Here, the identification and characterization of a novel AP gene, OsAP65, which plays an indispensable role in pollen tube growth in rice, is reported. The T-DNA insertion line of OsAP65 caused severe segregation distortion. In the progeny derived from an individual heterozygous for the T-DNA insertion, the wild type and T-DNA-carrying heterozygote segregated at a ratio close to 1:1, while homozygotes of disrupted OsAP65 (OsAP65–/–) were not recovered. Reciprocal crosses between heterozygotes and wild-type plants demonstrated that the mutant alleles could not be transmitted through the male gamete. Examination of the anthers from heterozygous plants revealed that the mutant pollen matured normally, but did not germinate or elongate. OsAP65 was expressed in various tissues and the transcript level in heterozygous plants was about half of the amount measured in the wild-type plants. The subcellular localization showed that OsAP65 is a pre-vacuolar compartment (PVC) protein. These results indicated that OsAP65 was essential for rice pollen germination and tube growth.
Key words: Aspartic protease, Oryza sativa, pollen maturation, pollen tube growth, segregation distortion, T-DNA.
Introduction
Aspartic proteases (APs) comprise one of the four superfamilies of proteolytic enzymes and are widely distributed in all living organisms (Davies, 1990; Rawlings and Barrett, 1995). The Arabidopsis and rice genomes contain at least 69 and 96 putative AP genes, respectively (Takahashi et al., 2008; Chen et al., 2009). However, the functions of the majority of the members in this family are still unknown. Some studies have identified important roles for AP genes in development of Arabidopsis and rice. For instance, Arabidopsis CONSTITUTIVE DISEASE RESISTANCE 1 (CDR1) is involved in salicylic acid-mediated disease resistance. Overexpression of CDR1 produced dwarf plants with resistance to Pseudomonas syringae, whereas antisense CDR1 plants were more susceptible to virulent strains than the wild type (Xia et al., 2004). PROMOTION OF CELL SURVIVAL 1 (PCS1) plays an important role in cell fate determination during the reproductive process and embryonic development in Arabidopsis (Ge et al., 2005). ASPARTIC PROTEASE IN GUARD CELL1 (ASPG1) is preferentially expressed in guard cells of various aerial tissues. Overexpression of ASPG1 could confer drought avoidance via abscisic acid (ABA)-dependent signalling in Arabidopsis (Yao et al., 2012).
S5 is an important locus that regulates indica–japonica hybrid sterility by conditioning embryo-sac abortion in rice. S5 is composed of three tightly linked genes interacting in a killer–protector system in which the S5 ORF5 gene encodes an AP. S5 ORF5+ (indica type) can cause endoplasmic reticulum stress, which results in premature programmed cell death and leads to embryo-sac abortion (Chen et al., 2008; Yang et al., 2012). In addition, some other APs have also been characterized in rice. The mRNA of Oryzasin 1 was detected in leaves, roots, and seeds at 2–6 weeks after flowering and 3–5 d after germination (Asakura et al., 1995). OsAsp1 is expressed in a wide range of tissues, most abundantly in zygotic embryos 1–2 d after fertilization. However, the detailed function of OsAsp1 is still unknown (Bi et al., 2005). Ectopic expression of rice CONSTITUTIVE DISEASE RESISTANCE 1 (OsCDR1) in Arabidopsis and rice led to enhanced resistance against bacterial and fungal pathogens, indicating conservation of CDR1 function in both species (Prasad et al., 2009). OsAP25 and OsAP37 that are highly expressed in tapetal cells promote cell death in yeasts and plants (Niu et al., 2013).
In flowering plants, pollination and fertilization are crucial steps for sexual reproduction. The pollen grain germinates to form a pollen tube for transporting the male gametes to the female gametophytes during sexual reproduction (Hulskamp et al., 1995; Johnson and Preuss, 2002; Lord and Russell, 2002). Studies have shown that many genes play roles in the pollen tube growth process (de Graaf et al., 2005; Yoon et al., 2006; Deng et al., 2010; Wu et al., 2010). For example, VANGUARD1 (VGD1) encodes a pectin methylesterase (PME)-homologous protein and is expressed specifically in pollen grain and pollen tube. The vgd1 pollen tubes grow much more slowly than those of the wild type within the style and the transmitting tract. In addition, vgd1 pollen tubes are unstable, bursting more frequently than the wild-type tubes when germinated and grown in vitro (Jiang et al., 2005). To the authors’ knowledge, only two genes affecting pollen tube growth have been reported in rice. One is OsSUT1 which encodes a sucrose transporter and is expressed in various tissues of the rice plant, such as leaf blades, leaf sheaths, internodes, and developing caryopsis. OsSUT1 is essential for pollen to fertilize the ovule normally, probably through its function(s) in pollen germination and/or pollen tube growth (Hirose et al., 2010). The other is OsImpβ1 encoding a protein located in the nucleus that is specifically required for pollen tube elongation (Han et al., 2011).
In this report, a rice AP gene, OsAP65, was identified and characterized. The OsAP65 T-DNA insertion line showed segregation distortion such that an insertion homozygote could not be recovered. Genetic and phenotypic analyses indicated that OsAP65 is involved in pollen tube growth, but does not affect pollen maturation. This study provides new insight into the functional role of APs in plant development.
Materials and methods
Plant materials and growth conditions
The OsAP65 T-DNA insertion line 4A-01549, which had the genetic background of rice variety Dongjin (Oryza sativa ssp. japonica), was obtained from the POSTECH RISD database (http://www.postech.ac.kr/life/pfg/risd/) (Jeon et al., 2000; Jeong et al., 2006). Two indica rice varieties Zhenshan 97A and Minghui 63 were used in crosses with the heterozygous OsAP65+/– plants. The rice plants were grown under normal field conditions in the rice growing season and in a greenhouse in the winter.
Genotyping the mutant plants
The genotype of each plant in the T-DNA insertion line was determined by PCR. Genomic DNA was extracted from fresh leaves of each plant using the cetyltrimethyl ammonium bromide (CTAB) method (Murray and Thompson, 1980). The amplification of genomic band was set up in a 15 μl volume system containing 30ng of DNA template, together with 1.5 μl of 2mM dNTP, 7.5 μl of 2× GC buffer I, 0.15 μl of each forward and reverse primer (both 10 μM), and 0.1 μl of 5U μl–1 rTaq polymerase (TaKaRa, Japan). The amplification of the T-DNA insertion band was in a 20 μl volume system containing 30ng of DNA template, together with 2 μl of 2mM dNTP, 2 μl of 10× PCR buffer, 0.2 μl of each forward and reverse primer (both 10 μM), and 0.2 μl of 5U μl–1 rTaq polymerase. The PCR amplifications were performed on Gene AMP PCR system 2700 or 9700 (Applied Biosystems, CA, USA), with the following profile: 94 °C for 5min, 30 cycles of 94 °C for 40 s, 58 °C for 40 s, and 72 °C for 60 s, and a final 10min extension at 72 °C. The primers for genotyping are listed in Supplementary Table S1 available at JXB online. The same PCR primers were used for genotyping the callus as used for genetic transformation.
Determining the full-length transcript
Total RNA was isolated from young rice panicles using the TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. First-strand cDNA synthesis, 5′-RACE (rapid amplification of cDNA ends), and 3′-RACE were performed using the SMART RACE cDNA Amplification Kit (Clontech, CA, USA). For 5′-RACE, the first round of PCR was performed using the primers UPM and 65-5′GSP, and the second round was performed using the primers NUPM and 65-5′NGSP. For 3′-RACE, primers UPM and 65-3′GSP were used in the first round of PCR, and NUPM and 65-3′NGSP in the second round (Supplementary Table S1 at JXB online). The UPM and NUPM primers were provided in the kit. The coding sequence of OsAP65 was amplified by reverse transcription–PCR (RT–PCR) using primers 65CDS-L and 65CDS-R2 (Supplementary Table S1), ligated into pGEM-T vector (Promega, WI, USA), and sequenced using the T7, 65-513L2, 65-1159L4, and SP6 primers (Supplementary Table S1).
RNA extraction and qPCR analysis
TRIzol reagent (Invitrogen) was used to extract the total RNA. For qPCR (quantitative real-time PCR) analysis, 3 μg of total RNA was digested using DNase I and reverse-transcribed using Superscript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. The details of the procedure for qPCR were as described previously (Yang et al., 2012). The primers for the qPCR are listed in Supplementary Table S1 at JXB online. Rice Actin1 (LOC_Os03g50885) was used as the internal control. The relative expression levels were analysed using the 2−ΔΔC T method (Livak and Schmittgen, 2001).
Genetic transformation
For genetic complementation, the full-length CDS (coding sequence) fragment of OsAP65 was amplified by PCR using primers 65CDS-KpnI-F2 and 65OE-R2 (Supplementary Table S1 at JXB online). The target fragment was digested with KpnI and BglII (TaKaRa) and directionally inserted into the modified pU2301-FLAG vector (Sun and Zhou, 2008). The empty pU2301-FLAG vector was also transformed as the negative control. The heterozygous calli generated from OsAP65 insertional heterozygous plants were used for rice transformation. The genotypes of transgenic plants and their progeny were examined by PCR amplification using gene-specific primers (Supplementary Table S1).
Microscopic observation of pollen
To examine the pollen grains, mature flowers 1 d or 2 d prior to anthesis were collected and fixed in 70% (v/v) ethanol at room temperature until use. Anthers from mature flowers were dissected and the pollen grains were stained with I2–KI staining (0.2% iodine and 2% potassium iodide). The total number of the pollen grains was counted under a bright field microscope (DM4000B, Leica, Wetzlar, Germany). Only pollen grains densely stained by the I2–KI solution were counted as mature pollen. For 4′,6-diamidino-2-phenylindole (DAPI) staining, pollen grains were fixed in EAA solution (100% ethanol:acetic acid = 3:1) for 1h at room temperature then dehydrated through an ethanol series (75, 55, and 35%). The pollen grains were stained in a 1 μg ml–1 DAPI solution for 1h at 60 °C in the dark. The DAPI solution consists of 1 μl of DAPI (1mg ml–1), 40 μl of EDTA (25mM), 1 μl of Triton X-100, and 958 μl of phosphate buffer (0.1M, pH 7.0). The stained pollen grains were observed using a microscope under UV light (DM4000B, Leica).
To evaluate the pollen germinability in vitro, pollen grains from dehisced anthers were germinated on a glass slide at 33 °C for 30min in a pollen germination medium (Han et al., 2011) where the relative humidity was maintained above 90%. The pollen grains were observed under a bright field microscope (DM4000B, Leica).
To investigate the growth of pollen tubes in vivo, aniline blue staining of pollen tubes in pistils was performed. The spikelets were collected 2h after anthesis and fixed in FAA solution (100% ethanol:acetic acid:formalin = 14:1:2) for 16h. The fixed pistils were washed three times with distilled water and treated in softening solution of 1M NaOH for 8h. Then, the pistil tissues were washed in distilled water and stained in aniline blue solution (0.15M aniline blue in 0.1M K2HPO4 buffer, pH 8.2) for 10min in the dark. The stained pistils were observed and photographed with a Leica DM4000B fluorescence microscope.
For scanning electron microscopy (SEM) and transmission electron microscopy (TEM) observations, anthers at maturity were prepared according to previously reported methods (Dai et al., 2011; Li et al., 2011).
RNA in situ hybridization
Tissue preparation, in situ hybridization, and immunological detection were performed as described previously (Xue et al., 2008). The OsAP65 probe was PCR-amplified using the gene-specific primers 65-situ-F and 65-situ-R (Supplementary Table S1 at JXB online) and the PCR fragment was inserted into the pGEM-T vector. The sense and antisense probes were transcribed in vitro by SP6 and T7 transcriptase, respectively, using a digoxigenin RNA labelling kit (Roche, Switzerland).
Subcellular localization of the protein
The full-length CDS of OsAP65 was amplified by PCR using primers 65CDS-L and 65CDS-R2 (Supplementary Table S1 at JXB online) and directionally inserted into the modified transient expression vector pBI221 for fusion with the reporter gene GFP (green fluorescent protein). Arabidopsis mesophyll protoplast isolation and transfection were carried out as described (Yoo et al., 2007). Each time, 20 μg of the CsCl-purified plasmid DNA was transfected. After incubation at 23 °C for 12–24h, protoplasts were observed for fluorescent signal by a confocal microscopy (TCS SP2, Leica). The plasmids encoding the mitochondrial marker F1-ATPase-γ:RFP (red fluorescent protein) (Jin et al., 2003), the Golgi marker Man1–RFP (Nebenführ et al., 1999), and pre-vacuolar compartment (PVC) marker RFP–AtVSR2 (Miao et al., 2006) were as described previously.
Results
Identification of the OsAP65 T-DNA insertion line
Putative T-DNA insertion lines for 40 OsAP genes were collected from two large T-DNA tagging populations (Jeon et al., 2000; Wu et al., 2003; Jeong et al., 2006) and 24 lines had the correct T-DNA insertion sites by PCR genotyping. The rice lines were planted in a normal paddy field and some obvious changes in phenotype were observed, such as dwarf plants, curled leaves, delayed heading date, small seeds, and semi-sterility/sterility. However, these phenotypes did not co-segregate with the T-DNA insertion, presumably resulting from tissue culture or multiple copies of T-DNA insertion. Many of the lines did not show obvious phenotypic changes. One line (4A-01549) from the POSTECH RISD database has an insertion in the second exon of LOC_Os07g40260 encoding an AP and was named OsAP65 in the uniform nomenclature of the OsAP gene family (Chen et al., 2009). Although no obvious phenotypic alteration was observed under natural field conditions (Supplementary Fig. S1 at JXB online), a genetic analysis of the T-DNA insertion revealed that the progeny from self-pollinated OsAP65+/– (+ represents the wild-type allele, and – indicates the insertional mutant) plants displayed a segregation ratio of ~1:1:0 (OsAP65+/+:OsAP65+/–:OsAP65–/–), rather than the expected 1:2:1 Mendelian ratio. No OsAP65–/– homozygous plant was found in the progeny (Table 1; Supplementary Fig. S2).
Table 1.
Segregation ratio in progeny of selfed OsAP65+/– plants
| Line | Total | Progeny genotypes | P-value | ||
|---|---|---|---|---|---|
| OsAP65+/+ | OsAP65+/– | OsAP65–/– | |||
| OsAP65 | 166 | 96 | 70 | 0 | <0.001 |
T-DNA insertion in OsAP65 causes a male gametophyte defect
To determine whether the T-DNA insertion was associated with the defect in male or female gametophytes, or both, OsAP65+/– plants were used as male or female parents to cross with wild-type plants. As shown in Table 2, when the OsAP65+/– plants were used as female parents, both wild-type and heterozygous progeny were obtained. However, when OsAP65+/– plants were used as the male parents, only wild-type progeny were produced. These results indicated that pollen carrying the mutant allele was defective and the OsAP65 T-DNA insertion allele could not be transmitted through the male gamete (pollen). Thus, the inability to recover OsAP65–/– plants was due to a severe defect in the male gametophyte.
Table 2.
Genotypes of F1 progeny from OsAP65+/– crossed with wild-type plants
| Female plant | Pollen donor | Progeny containing T-DNA | P-value | ||
|---|---|---|---|---|---|
| Expected | Observed | X2 | |||
| ZS97A | OsAP65+/– | 50% | 0 (0/99) | 97 | <0.005 |
| OsAP65+/– | MH63 | 50% | 41% (47/114) | 3.17 | >0.05 |
χ2 tests were performed to evaluate the goodness-of-fit of the observed data to the predicted 1:1 ratio.
Phenotypic characterization of the OsAP65 T-DNA insertion line
To investigate how OsAP65 affects pollen development and function, the total number of pollen grains in an anther and the rate of mature pollen were examined using iodine staining. The result showed that the total number of pollen grains in an anther and the percentage of mature pollen of OsAP65+/– plants were not different from those of OsAP65+/+ plants (Fig. 1).
Fig. 1.
Pollen grains from OsAP65 T-DNA insertional mutant and wild-type plants. (A and B) Pollen grains from OsAP65+/+ and OsAP65+/– stained with I2–KI solution. (C) Total number and percentage of mature pollen grains from OsAP65+/+ and OsAP65+/– plants. The number (left) and the percentage of the matured pollen grains (right) in an anther are indicated. (This figure is available in colour at JXB online.)
DAPI staining showed that all pollen grains from both OsAP65+/– and OsAP65+/+ plants contained three nuclei in the mature pollen: two bright, intensely stained sperm nuclei and one diffuse, weakly stained vegetative nucleus (Fig. 2A, B). This analysis indicated that the mutation did not affect sperm cell development and division. SEM was used to examine the surface of the pollen grains, and no significant difference in pollen morphology could be detected between the OsAP65+/– and OsAP65+/+ plants (Fig. 2C, D, F, G). TEM scanning of pollen grains at maturity did not reveal subcellular changes between the OsAP65+/– and OsAP65+/+ plants (Fig. 2E, H). This also implies that disruption of OsAP65 does not cause a visible difference in pollen morphology. Pollen germination and pollen tube elongation were then examined in vitro and in vivo. The percentage of germinated pollen grains in vitro was found to be significantly lower in OsAP65+/– plants (56.78%) than in OsAP65+/+ plants (79.64%) (Fig. 2I, J, L). Moreover, the in vivo pollen germination rate of OsAP65+/– plants was 70.60% compared with 86.96% in OsAP65+/+ plants (Fig. 3). These results indicated that the disruption of OsAP65 might affect pollen germination or pollen tube elongation.
Fig. 2.
Morphology of pollen grains. DAPI staining of pollen grains from OsAP65+/+ (A) and OsAP65+/– (B) at maturity. Bar=50 μm. (C) SEM image of mature OsAP65+/+ pollen grains. Bar=50 μm. (D) A higher magnification image of a single pollen grain from (C). Bar=10 μm. (E) TEM image of mature OsAP65+/+ pollen grains. Bar=5 μm. (F) SEM image of mature OsAP65+/– pollen grains. Bar=50 μm. (G) A higher magnification image of a single pollen grain from (F). Bar=10 μm. (H) TEM image of mature OsAP65+/– pollen grains. Bar=5 μm. (I–K) In vitro germination of pollen from segregating wild-type OsAP65+/+, OsAP65+/–, and complementation plants, respectively. Arrows indicate the ungerminated pollen grains. (L) The germination rates of mature pollen grains from OsAP65+/+, OsAP65+/–, and complementation plants. V, vegetative nucleus; S, sperm nuclei. (This figure is available in colour at JXB online.)
Fig. 3.
In vivo pollen germination on stigma of pistils after pollination. (A and B) The pistils from OsAP65+/+ and OsAP65+/– stained with aniline blue solution. Bar=100 μm. Arrows indicate the ungerminated pollen grains. (C) The germination rates of mature pollen grains from OsAP65+/+ and OsAP65+/– plants. (This figure is available in colour at JXB online.)
Sequence analysis of OsAP65
The complete transcript of OsAP65 (1896bp) was obtained by RACE using RNA isolated from young panicles. OsAP65 is predicted to be an AP (PF00026) and the predicted protein consisted of 631 amino acids (Supplementary Fig. S3A at JXB online). A signal peptide in the N-terminus, an AP domain in the middle, and a transmembrane domain at the C-terminus were identified using SMART (http://smart.embl-heidelberg.de/) and pfam (http://pfam.sanger.ac.uk/) searches. Two active sites containing aspartate (D) residues (D109 and D305) characteristic of APs (Rawlings and Barrett, 1995) were identified with pfam analysis (Supplementary Fig. S3B). Unlike other plant APs, OsAP65 does not have the plant-specific insert (PSI) sequence (Simões and Faro, 2004) (Fig. 4).
Fig. 4.
Multiple sequence alignment of OsAP65 with some cloned aspartic proteases in plants. OsCDR1, oryzasin, OsAsp1, and S5 ORF5 are from rice. AtAP-A1, AtCDR1, and AtPCS1 are from Arabidopsis. Phytepsin is from barley. Phytepsin, oryzasin, and AtAP-A1 have the PSI domain. AtCDR1, OsCDR1, S5 ORF5, OsAsp1, and AtPCS1 do not have the PSI domain. The PSI sequence is marked with a rectangle. The two active sites of OsAP65 aspartic protease are marked with ellipses.
Genetic complementation of the OsAP65 T-DNA insertion line
The genomic sequence of the OsAP65 gene is 8322bp in length, with 12 exons and 11 introns according to the MSU Rice Genome Annotation Project Database (Release 7 of MSU RGAP; http://rice.plantbiology.msu.edu/). The T-DNA was inserted in the second exon (Supplementary Fig. S4A at JXB online). To confirm that the male defect was caused by the T-DNA interruption in OsAP65, the CDS of OsAP65 under the control of the maize ubiquitin promoter was introduced into OsAP65+/– plants (Supplementary Fig. S4B). Segregation analysis of T1 families from three independent transformants showed that the homozygous OsAP65–/– plants were recovered in all three lines (Table 3; Supplementary Fig. S5). Moreover, the percentage of germinated pollen grains of the transformants (72.23%) was recovered to the level of the OsAP65+/+ plants (79.64%) (Fig.2I, K, L). In contrast, no homozygous OsAP65–/– plants could be found in progeny of the plants transformed with the empty pU2301-FLAG vector (Table 3). This result confirmed that the male gametophyte defect is caused by the T-DNA insertion in the OsAP65 gene.
Table 3.
The genotyping of the T1 generation from OsAP65 transgenic plants
| Lines | No. of plants | Genotype of T1 plants | ||
|---|---|---|---|---|
| OsAP65+/+ | OsAP65+/– | OsAP65–/– | ||
| OsAP65-pU2301-FLAG-2 | 45 | 14 | 17 | 14 |
| OsAP65-pU2301-FLAG-4 | 25 | 8 | 10 | 7 |
| OsAP65-pU2301-FLAG-5 | 9 | 6 | 1 | 2 |
| pU2301-FLAG (CK) | 44 | 16 | 28 | 0 |
Expression pattern of OsAP65
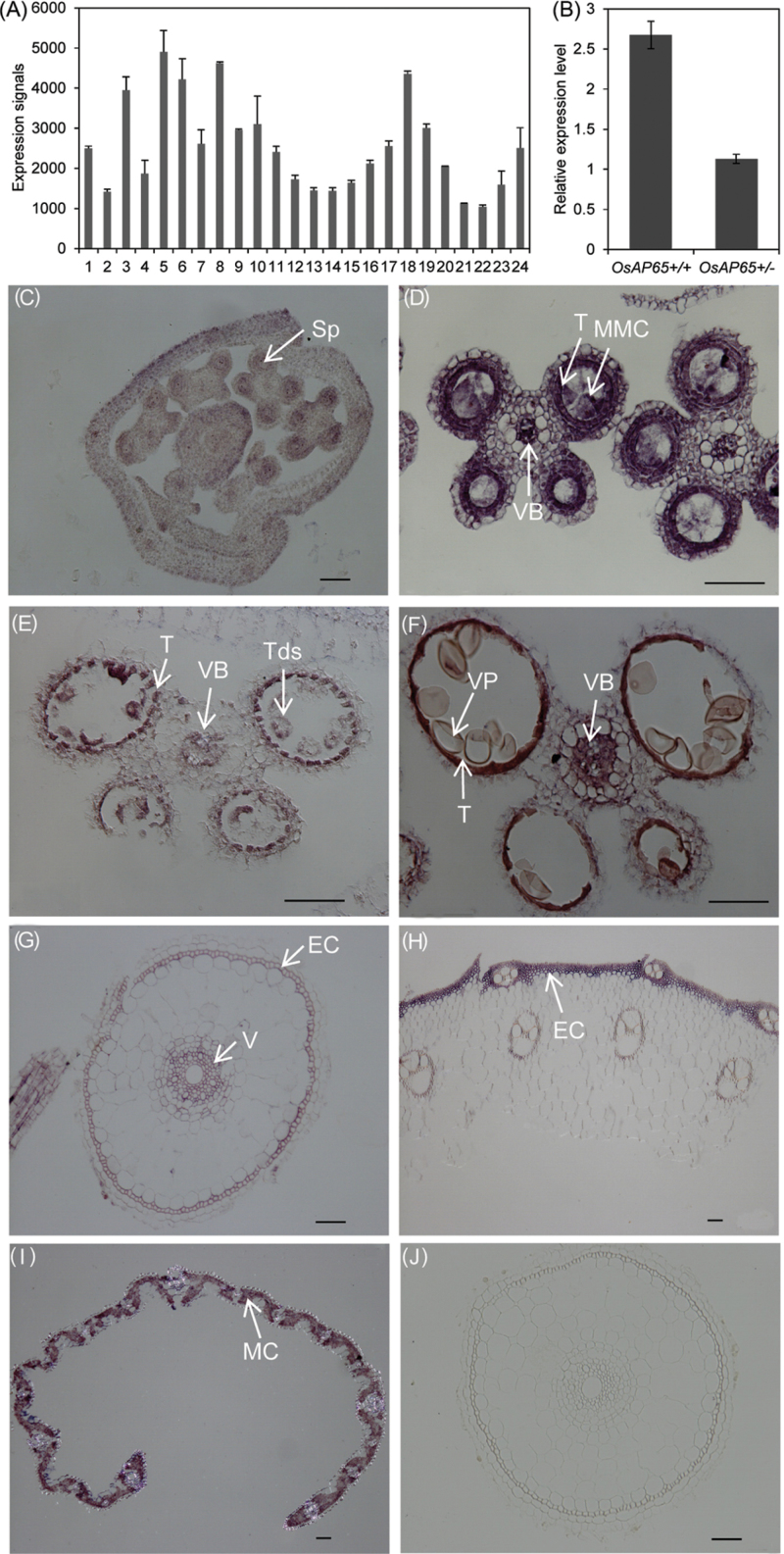
To investigate the expression pattern of OsAP65, the CREP database (http://crep.ncpgr.cn/crep-cgi/home.pl), which contains a large amount of microarray data covering the whole life cycle of the rice plant (Wang et al., 2010), was searched. OsAP65 was expressed in callus, root, stem, leaf, sheath, panicles of different developmental stages, and endosperm (Fig. 5A). A qPCR analysis showed that the transcript level in OsAP65+/– plants was about half of that measured from T-DNA negative (OsAP65+/+) plants (Fig. 5B).
Fig. 5.
The expression pattern of OsAP65. (A) Expression profile of OsAP65 in various tissues covering the entire life cycle of the rice plant. Detailed information about the tissues is listed in Supplementary Table 2 at JXB online. (B) qPCR analysis of OsAP65 in segregating wild-type OsAP65+/+ and heterozygous OsAP65+/– anthers at the mature pollen stage. Actin1 was used as the control. (C–F) In situ hybridization assays of OsAP65 in anthers at stage 4, stage 6, stage 8b, and stage 10 according to the specification of rice anther development (Zhang et al., 2011), respectively. (G–I) In situ hybridization assays of OsAP65 in a transverse section of root (G), stem (H), and leaf blades (I). (J) Negative controls with the sense probe in a transverse section of root. The samples of root and leaf were collected from seedlings at the trefoil stage, and the stem from the heading stage. Bars=50 μm. Sp, sporogenous cell; MMC, microspore mother cell; T, tapetum; Tds, tetrads; VB, vascular bundle; VP, vacuolated pollen; EC, epidermal cells; V, vascular tissues; MC, mesophyll cells. (This figure is available in colour at JXB online.)
RNA in situ hybridization of OsAP65 was also performed in anthers at different developmental stages and in vegetative tissues. OsAP65 was detected in the parietal anther wall layers and microsporocyte (or microspore) in all the examined stages of developing anther (Fig. 5C–F). OsAP65 transcript was also detected in epidermal cells and vascular tissues of the roots (Fig. 5G), epidermal layer of the stems (Fig. 5H), mesophyll cells, and the vascular tissues of the leaf blades (Fig. 5I). Thus the RNA in situ hybridization results also showed that OsAP65 signals were detected in most of the tissues.
Subcellular localization of OsAP65
To investigate the subcellular localization of OsAP65 protein, a vector expressing a translational fusion of GFP and OsAP65 under the control of the Cauliflower mosaic virus (CaMV) 35S promoter was constructed and transformed into Arabidopsis protoplasts. As shown in Fig. 6, OsAP65–GFP displayed a punctate staining pattern, which presumes a distribution in the mitochondria, Golgi, or PVC. Co-expression of OsAP65–GFP and the mitochondrial marker F1-ATPase-γ: RFP showed that OsAP65 was not localized in the mitochondria (Fig. 6A–D). Some of the OsAP65–GFP green fluorescent signals overlapped with the red fluorescent signals of the Golgi marker Man1–RFP (Fig. 6E–H). However, OsAP65–GFP and the PVC marker RFP–AtVSR2 overlapped completely when co-expressed in Arabidopsis protoplasts (Fig. 6I–L). Therefore, OsAP65 is predominantly localized in the PVC, while Golgi localization is minimal.
Fig. 6.
Subcellular localization of the OsAP65 protein in Arabidopsis protoplasts. (A–D) A protoplast cell co-expressing OsAP65–GFP (A) and a mitochondrial marker F1-ATPase-γ:RFP (B), a merged image (C), and a bright-field image (D). (E–H) A protoplast cell co-expressing OsAP65–GFP (E) and a Golgi marker Man1–RFP (F), a merged image (G), and a bright-field image (H). (I–L) A protoplast cell co-expressing OsAP65–GFP (I) and a PVC marker RFP–AtVSR2 (J), a merged image (K), and a bright-field image (L). Scale bars=10 μm. (This figure is available in colour at JXB online.)
Discussion
APs have been found to play important roles in the regulation of various biological processes in different plant species, such as leaf senescence (Kato et al., 2004), immunity response (Xia et al., 2004; Prasad et al., 2009), programmed cell death (Ge et al., 2005; Niu et al., 2013), reproductive isolation (Chen et al., 2008; Yang et al., 2012), and abiotic stress (Yao et al., 2012). However, the biological functions of plant APs are poorly understood or still hypothetical. Ge et al. (2005) collected the putative knockout lines of Arabidopsis AP genes and found that the T-DNA insertion lines of PCS1 exhibited severe segregation distortion and were unable to produce any homozygous progeny. In this study, the T-DNA insertion lines were analysed for OsAP genes and it was found that the OsAP65 T-DNA insertion line also exhibited severe segregation distortion and the OsAP65–/– homozygote was not obtained among ~500 progeny individuals of OsAP65+/– plants examined. However, the reason for segregation distortion of PCS1 is different from that of OsAP65. The disruption of PCS1 affects both male gametophyte and female gametophyte transmission and embryogenesis (Ge et al., 2005), while disruption of OsAP65 does not affect female gametophyte transmission and embryogenesis, indicating that these two genes may have divergent physiological functions. OsAP65 is expressed in certain vegetative tissues including root, stem, and leaves. However, the lack of homozygous mutant plants prevented investigation of OsAP65’s role in vegetative organs.
In vitro and in vivo germination assays indicated that more than half of the pollen grains from OsAP65+/– plants compared with OsAP65+/– plants were able to germinate, but the mutant allele OsAP65– could not be transmitted via the male gametes, suggesting that OsAP65 is also required for pollen function after germination. A similar phenotype has also been observed in other male gametophytic mutants; for example, SETH1 and SETH2, which encode two conserved proteins involved in the glycosylphosphatidylinositol (GPI) biosynthetic pathway, affect both pollen germination and tube growth (Lalanne et al., 2004a ). NPG1, encoding a calmodulin-binding protein in Arabidopsis, is essential for pollen germination (Golovkin and Reddy, 2003). MALE GAMETOPHYTE DEFECTIVE 2, encoding a sialyltransferase-like protein, is required for normal pollen germination and pollen tube growth in Arabidopsis (Deng et al., 2010). The pollen germination of the seth6 mutant was completely blocked, while the seth7 pollen showed both reduced pollen germination and decreased pollen tube growth (Lalanne et al., 2004b ). Despite the phenotypic similarity of OsAP65 and those genes, it still remains unclear whether OsAP65 works in the same regulatory pathway as SETH1 and SETH2 and other genes that play roles in pollen germination and pollen tube growth.
APs comprise one of the four superfamilies of proteolytic enzymes. The main function of AP is to hydrolyse substrate to support the biological processes related to growth, development, and other activities; it may be speculated that OsAP65 here degrades a specific substrate and produces some substance necessary for pollen germination and pollen tube growth. When OsAP65 was disrupted, this substrate may not be degraded in a timely manner, resulting in impaired pollen germination and pollen tube growth. However, the physiological function of OsAP65 will not be entirely clear until its substrates are identified. A recent article showed that two rice AP genes, OsAP25 and OsAP37, that were promoted by ETERNAL TAPETUM 1, trigged programmed cell death in tapetal cells in rice anthers (Niu et al., 2013). OsAP65 may participate in a molecular pathway causing male sterility in the same way as OsAP25 and OsAP37. Nevertheless, the present results demonstrate a critical role for OsAP65 in fertilization through its function in pollen tube growth, but not pollen maturation.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Characterization of the OsAP65 T-DNA insertion line.
Figure S2. PCR results for genotyping the progeny of OsAP65+/– plants.
Figure S3. Features of OsAP65 protein.
Figure S4. Schematic diagrams of the OsAP65 gene and complementation vector.
Figure S5. Genetic analyses and genotyping of the T1 generation from OsAP65 transformation plants.
Table S1. Primers for PCR analysis.
Table S2. Detailed information of rice tissues in Fig. 5A.
Acknowledgements
We thank Dr Gynheung An (POSTECH, Korea) for providing the mutants, Dr Liwen Jiang (The Chinese University of Hong Kong, Hong Kong, China) for providing the PVC marker plasmid RFP–AtVSR2 and the Golgi marker plasmid Man1–RFP, and Dr Jian Xu (Huazhong Agricultural University, China) for providing the the mitochondrial marker plasmid F1-ATPase-γ:RFP. This work was supported by grants from the National 863 Project (2012AA10A303) and the National Natural Science Foundation of China (30921091 and 31201190).
References
- Asakura T, Watanabe H, Abe K, Arai S. 1995. Rice aspartic proteinase, oryzasin, expressed during seed ripening and germination, has a gene organization distinct from those of animal and microbial aspartic proteinases. European Journal of Biochemistry 232, 77–83 [DOI] [PubMed] [Google Scholar]
- Bi X, Khush GS, Bennett J. 2005. The rice nucellin gene ortholog OsAsp1 encodes an active aspartic protease without a plant-specific insert and is strongly expressed in early embryo. Plant and Cell Physiology 46, 87–98 [DOI] [PubMed] [Google Scholar]
- Chen J, Ouyang Y, Wang L, Xie W, Zhang Q. 2009. Aspartic proteases gene family in rice: gene structure and expression, predicted protein features and phylogenetic relation. Gene 442, 108–118 [DOI] [PubMed] [Google Scholar]
- Chen J, Ding J, Ouyang Y, et al. 2008. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proceedings of the National Academy of Sciences, USA 105, 11436–11441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, You C, Chen G, Li X, Zhang Q, Wu C. 2011. OsBC1L4 encodes a COBRA-like protein that affects cellulose synthesis in rice. Plant Molecular Biology 75, 333–345 [DOI] [PubMed] [Google Scholar]
- Davies DR. 1990. The structure and function of the aspartic proteinases. Annual Review of Biophysics and Biophysical Chemistry 19, 189–215 [DOI] [PubMed] [Google Scholar]
- de Graaf BHJ, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu H-m. 2005. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. The Plant Cell 17, 2564–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wang W, Li W-Q, Xia C, Liao H-Z, Zhang X-Q, Ye D. 2010. MALE GAMETOPHYTE DEFECTIVE 2, encoding a sialyltransferase-like protein, is required for normal pollen germination and pollen tube growth in Arabidopsis . Journal of Integrative Plant Biology 52, 829–843 [DOI] [PubMed] [Google Scholar]
- Ge X, Dietrich C, Matsuno M, Li G, Berg H, Xia Y. 2005. An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Reports 6, 282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkin M, Reddy ASN. 2003. A calmodulin-binding protein from Arabidopsis has an essential role in pollen germination. Proceedings of the National Academy of Sciences, USA 100, 10558–10563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M-J, Jung K-H, Yi G, An G. 2011. Rice Importin β1 gene affects pollen tube elongation. Molecules and Cells 31, 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Zhang ZJ, Miyao A, Hirochika H, Ohsugi R, Terao T. 2010. Disruption of a gene for rice sucrose transporter, OsSUT1, impairs pollen function but pollen maturation is unaffected. Journal of Experimental Botany 61, 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulskamp M, Schneitz K, Pruitt RE. 1995. Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis . The Plant Cell 7, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Lee S, Jung KH, et al. 2000. T-DNA insertional mutagenesis for functional genomics in rice. The Plant Journal 22, 561–570 [DOI] [PubMed] [Google Scholar]
- Jeong DH, An S, Park S, et al. 2006. Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. The Plant Journal 45, 123–132 [DOI] [PubMed] [Google Scholar]
- Jiang LX, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D. 2005. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. The Plant Cell 17, 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JB, Bae H, Kim SJ, Jin YH, Goh C-H, Kim DH, Lee YJ, Tse YC, Jiang L, Hwang I. 2003. The Arabidopsis dynamin-like proteins ADL1C and ADL1E play a critical role in mitochondrial morphogenesis. The Plant Cell 15, 2357–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Preuss D. 2002. Plotting a course: multiple signals guide pollen tubes to their targets. Developmental Cell 2, 273–281 [DOI] [PubMed] [Google Scholar]
- Kato Y, Murakami S, Yamamoto Y, Chatani H, Kondo Y, Nakano T, Yokota A, Sato F. 2004. The DNA-binding protease, CND41, and the degradation of ribulose-1,5-bisphosphate carboxylase/oxygenase in senescent leaves of tobacco. Planta 220, 97–104 [DOI] [PubMed] [Google Scholar]
- Lalanne E, Honys D, Johnson A, Borner GH, Lilley KS, Dupree P, Grossniklaus U, Twell D. 2004a. SETH1 and SETH2, two components of the glycosylphosphatidylinositol anchor biosynthetic pathway, are required for pollen germination and tube growth in Arabidopsis . The Plant Cell 16, 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne E, Michaelidis C, Moore JM, Gagliano W, Johnson A, Patel R, Howden R, Vielle-Calzada JP, Grossniklaus U, Twell D. 2004b. Analysis of transposon insertion mutants highlights the diversity of mechanisms underlying male progamic development in Arabidopsis . Genetics 167, 1975–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gao X, Wei Y, Deng L, Ouyang Y, Chen G, Zhang Q, Wu C. 2011. Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-box adenosine 5′-triphosphate-dependent RNA helicases regulates tapetum degeneration. The Plant Cell 23, 1416–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Lord EM, Russell SD. 2002. The mechanisms of pollination and fertilization in plants. Annual Review of Cell and Developmental Biology 18, 81–105 [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L. 2006. Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiology 142, 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research 8, 4321–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA. 1999. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant physiology 121, 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N, Liang W, Yang X, Jin W, Wilson ZA, Hu J, Zhang D. 2013. EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nature Communications 4, 1445. [DOI] [PubMed] [Google Scholar]
- Prasad BD, Creissen G, Lamb C, Chattoo BB. 2009. Overexpression of rice (Oryza sativa L.) OsCDR1 leads to constitutive activation of defense responses in rice and Arabidopsis . Molecular Plant-Microbe Interactions 22, 1635–1644 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. 1995. Families of aspartic peptidases, and those of unknown catalytic mechanism. Methods in Enzymology 248, 105–120 [DOI] [PubMed] [Google Scholar]
- Simões I, Faro C. 2004. Structure and function of plant aspartic proteinases. European Journal of Biochemistry 271, 2067–2075 [DOI] [PubMed] [Google Scholar]
- Sun Q, Zhou DX. 2008. Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proceedings of the National Academy of Sciences, USA 105, 13679–13684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Niwa H, Yokota N, Kubota K, Inoue H. 2008. Widespread tissue expression of nepenthesin-like aspartic protease genes in Arabidopsis thaliana . Plant Physiology and Biochemistry 46, 724–729 [DOI] [PubMed] [Google Scholar]
- Wang L, Xie W, Chen Y, et al. 2010. A dynamic gene expression atlas covering the entire life cycle of rice. The Plant Journal 61, 752–766 [DOI] [PubMed] [Google Scholar]
- Wu C, Li X, Yuan W, Chen G, Kilian A, Li J, Xu C, Zhou DX, Wang S, Zhang Q. 2003. Development of enhancer trap lines for functional analysis of the rice genome. The Plant Journal 35, 418–427 [DOI] [PubMed] [Google Scholar]
- Wu Y, Yan J, Zhang R, Qu X, Ren S, Chen N, Huang S. 2010. Arabidopsis FIMBRIN5, an actin bundling factor, is required for pollen germination and pollen tube growth. The Plant Cell 22, 3745–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Suzuki H, Borevitz J, Blount J, Guo Z, Patel K, Dixon RA, Lamb C. 2004. An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. EMBO Journal 23, 980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, et al. 2008. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nature Genetics 40, 761–767 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhao X, Cheng K, et al. 2012. A killer–protector system regulates both hybrid sterility and segregation distortion in rice. Science 337, 1336–1340 [DOI] [PubMed] [Google Scholar]
- Yao X, Xiong W, Ye T, Wu Y. 2012. Overexpression of the aspartic protease ASPG1 gene confers drought avoidance in Arabidopsis . Journal of Experimental Botany 63, 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y-H, Sheen J. 2007. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nature Protocols 2, 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yoon GM, Dowd PE, Gilroy S, McCubbin AG. 2006. Calcium-dependent protein kinase isoforms in petunia have distinct functions in pollen tube growth, including regulating polarity. The Plant Cell 18, 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Luo X, Zhu L. 2011. Cytological analysis and genetic control of rice anther development. Journal of Genetics and Genomics 38, 379–390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.