Abstract
Heterochromatin protein 1 (HP1) is a conserved component of the highly compact chromatin of higher eukaryotic centromeres and telomeres. Cytogenetic experiments in Drosophila have shown that HP1 localization into this chromatin is perturbed in mutants for the origin recognition complex (ORC) 2 subunit. ORC has a multisubunit DNA-binding activity that binds origins of DNA replication where it is required for origin firing. The DNA-binding activity of ORC is also used in the recruitment of the Sir1 protein to silence nucleation sites flanking silent copies of the mating-type genes in Saccharomyces cerevisiae. A fraction of HP1 in the maternally loaded cytoplasm of the early Drosophila embryo is associated with a multiprotein complex containing Drosophila melanogaster ORC subunits. This complex appears to be poised to function in heterochromatin assembly later in embryonic development. Here we report the identification of a novel component of this complex, the HP1/ORC-associated protein. This protein contains similarity to DNA sequence-specific HMG proteins and is shown to bind specific satellite sequences and the telomere-associated sequence in vitro. The protein is shown to have heterochromatic localization in both diploid interphase and mitotic chromosomes and polytene chromosomes. Moreover, the gene encoding HP1/ORC-associated protein was found to display reciprocal dose-dependent variegation modifier phenotypes, similar to those for mutants in HP1 and the ORC 2 subunit.
INTRODUCTION
The eukaryotic nucleus undergoes dramatic morphological changes as it progresses through the cell cycle. The condensation of the chromatin upon entry into mitosis and its subsequent decondensation at the end of mitosis is one of the more stunning events. Certain regions of the chromosomes, termed heterochromatin, fail to undergo these condensation cycles but retain a compact appearance throughout the cell cycle (Heitz, 1928). Heterochromatin also has different functional properties from more typical chromosomal regions (euchromatin). It often causes silencing of euchromatic genes that are juxtaposed to it by chromosomal rearrangement, is typically replicated later in S phase than euchromatin, and usually occupies a distinct subnuclear domain along the nuclear periphery (for review, see Brown, 1966). The centromeres and telomeres of higher eukaryotic chromosomes typically have a heterochromatic organization, and this structure is known to be important for proper chromosome mechanics during mitosis and meiosis (Allshire et al., 1995; Kellum et al., 1995; Dernburg et al., 1996; Karpen et al. 1996; Fanti et al., 1998; for review, see Dobie et al., 1999).
The compact appearance of heterochromatin is likely to involve a unique nucleoprotein composition. It is known to be enriched with repetitive noncoding DNA, such as the AT-rich satellite sequences (Peacock et al., 1976, 1977; Peacock and Lohe, 1980), and to contain specific proteins. In the genetically tractable organisms of Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Drosophila (Sinclair et al., 1983; Wustmann et al., 1989; Laurenson and Rine, 1992; Allshire et al., 1995), genetic assays have been used to identify heterochromatin-associated proteins. Mutations in genes that are required to form Drosophila heterochromatin suppress the variegated expression of a euchromatic gene caused by its placement next to heterochromatin by a chromosomal rearrangement. The product of the suppressor of variegation 2-5 gene, heterochromatin protein 1 (HP1; James and Elgin, 1986; Eissenberg et al., 1990), is a heterochromatic protein that is conserved from fission yeast to humans (Saunders et al., 1993; Allshire et al., 1995; Pak et al., 1997).
Because direct DNA-binding activity had not been attributed to HP1, its localization into heterochromatin has long been thought to involve the DNA-binding activities of other proteins. Mutations in the origin recognition complex (ORC) 2 subunit of the Drosophila melanogaster ORC (DmORC) were recently shown to perturb HP1 localization (Pak et al., 1997; Huang et al., 1998). This highly conserved protein complex was purified from S. cerevisiae as in vitro binding activity for sequences that permit autonomous replication of plasmids in vivo (Bell and Stillman, 1992). It is also known to be required for replication of chromosomal DNA (Bell et al., 1993; Rowles et al., 1996; Landis et al., 1997) and to be associated with specific replicator sequences in S. cerevisiae and metazoans (Aparicio et al., 1997; Austin et al., 1999; Royzman et al., 1999). The DNA-binding activity of ORC serves a second function in S. cerevisiae in the recruitment of the Sir1 protein to ORC-binding sequences within a pair of silencing nucleation sites flanking silent copies of the mating-type genes (Bell et al., 1993; Chien et al., 1993; Fox et al., 1995; Triolo and Sternglanz, 1996). The Sir1 protein then acts in conjunction with the DNA-binding proteins repressor–activator protein 1 (Rap1) and ARS-binding factor 1 (Abf1), which also bind sequences in the silencing nucleator, to recruit other members of the Sir protein complex to the site through heterotypic and homotypic protein–protein interactions (Pillus and Rine, 1989; Kurtz and Shore, 1991; Hecht et al., 1995; Moazed et al., 1997). The observation that Drosophila ORC subunits are associated with HP1 in interphase heterochromatin and cause a perturbation in the localization of HP1 into heterochromatin when mutated suggests a similar role for ORC in Drosophila heterochromatin assembly (Pak et al., 1997; Huang et al., 1998).
It is not understood how the heterochromatin assembly function of ORC might be specified in heterochromatin, whereas its more general role in DNA replication is carried out throughout the genome. Studies in S. cerevisiae with a synthetic silencer that contains a consensus ARS sequence combined with Rap1 and Abf1 binding sites from nonsilenced chromosomal regions showed the silencing activity of an ARS sequence to be DNA context dependent (McNally and Rine, 1991). A similar set of protein-binding sequences and associated factors might also cooperate with ORC in Drosophila heterochromatin assembly. Here we report the identification of a 55-kDa protein that copurifies with an ORC-containing complex of HP1 from the cytoplasm of the early Drosophila embryo that appears to be poised for function in heterochromatin assembly later in embryonic development (Huang et al., 1998). The protein contains an HMG box and is proposed to serve a role in Drosophila heterochromatin assembly that is similar to those of the Rap1 and Abf1 proteins in budding yeast.
MATERIALS AND METHODS
Peptide Sequence Identification of HP1/ORC-associated Protein
The 55-kDa HP1/ORC-associated protein (HOAP) was coimmunoaffinity purified from a cytoplasmic extract of early Drosophila embryos as previously described (Huang et al., 1998). The Coomassie brilliant blue-stained band for a 55-kDa copurifying protein (p55) was excised from an SDS-polyacrylamide gel and subjected to in situ proteolytic cleavage and peptide sequence determination by Edman degradation at Harvard Microchemistry (Cambridge, MA). The National Center for Biotechnology Information database was searched with sequences from 2 different peptides of the protein (QMSAFLRKYLAD and TRITEEDLARPYTED), and both sequences were found to match the sequence in the hypothetical protein-coding sequence for the Drosophila anonymous fast-evolving 1G5 (anon fe 1G5) gene (Schmid and Tautz, 1997).
Antibody Preparation
Antibodies were increased against a fusion protein expressed from an anon fe 1G5 cDNA PCR amplified from an ovarian cDNA library (Stroumbakis et al., 1994) with the use of oligonucleotide primers designed from the 5′ (ATGTCGGGGACGCAAATGTCTG) and 3′ (CAATCGGGTGATGTTCTGCTTC) ends of the gene. The anon fe 1G5 cDNA was ligated into the XbaI and XhoI sites of the pET20b vector to produce the complete hypothetical anon fe 1G5 gene product with the pelB leader (19 amino acids) fused to its amino terminus and a 6-histidine tag fused to its carboxyl terminus. The tagged fusion protein was expressed in bacteria, purified by Ni-nitrile triacetic acid-agarose chromatography and PAGE, and used in rabbit immunoinjections (4 biweekly subcutaneous injections of 1 ml). The antiserum was affinity purified over an Affigel-10 column (Bio-Rad, Hercules, CA) containing the expressed anon fe 1G5 gene fusion protein.
The anti-DmORC 2 antibody was increased against a synthetic peptide of the DmORC 2 protein (KRSVDGSEQLTIPIDGALLQQFLEEQ) (Berkeley Drosophila Genome Project [BDGP] accession number AAC46955) covalently linked to keyhole lymphet hemocyanin and was affinity purified over a column containing the synthetic peptide (Research Genetics, Huntsville, AL).
The anti-HP1 antibody used in all experiments was increased against an amino-terminal peptide of HP1 (CIDNPESSAKVSDAEEE) in rabbits and immunoaffinity purified over a 6-histidine HP1 affinity chromatography column as previously described (Huang et al., 1998).
Immunoprecipitation Experiments
An antibody directed against the HP1 peptide (Huang et al., 1998) was used to immunoprecipitate the large ORC-containing HP1 complex from sized fractions of an embryonic cytoplasm extract as described by Huang et al. (1998). The antibody prepared against the anon fe 1G5 gene product was used in the reciprocal experiment of immunoprecipitating HOAP complexes from the unfractionated cytoplasmic complex. Antibodies that recognize HP1, HOAP, and Drosophila ORC subunits 2 and 6 (gifts from M. Botchan, University of California at Berkeley, Berkeley, CA) were used in immunoblot analyses of the fractions from the immunoprecipitation as described by Huang et al. (1998).
Chromatin Immunoprecipitations
Cross-linked chromatin was prepared from salt-extracted cycle 14 interphase nuclei (embryos collected 2.3–3.3 h after oviposition) with the use of a modification of the methods of Kuo and Allis (1999) and Orlando et al. (1997). Nuclei were first extracted with 50 mM HEPES, pH 7.6, containing 0.5 M KCl and 0.1% Triton-X 100 to remove the salt-sensitive fractions of HP1 that are not ORC associated (Huang et al., 1998). The salt-resistant chromatin pellet was resuspended in 50 mM HEPES, pH 7.6, and 60 mM KCl (5 mg DNA/ml), and formaldehyde, pH 7.0, was added to a final concentration of 0.9% for 10 min at room temperature. The cross-linking reaction was terminated by pelleting the chromatin and resuspending it once in ice-cold wash buffer (0.5 M EGTA and 10 mM Tris-HCl, pH 8.0) plus 10 mM EDTA and 0.25% Triton X-100, once in wash buffer plus 0.2 M NaCl and 1.0 mM EDTA, and once in wash buffer plus 1.0 mM EDTA and 1.0% Triton X-100. The chromatin pellet was then resuspended in 1% Triton X-100, 1.0 mM EDTA, 0.5 M EGTA, and 10 mM HEPES, 7.5, and sonicated (four 10-s bursts on output setting 5) to an average length of 500 bp DNA. Glycerol was added to a final concentration of 5%, and the samples were stored at −70°C. Where indicated, samples were digested with micrococcal nuclease I (Mnase I; 0.02 U/OD260 unit) for 0–10 min at room temperature after dialysis into Tris-EDTA and the addition of MgCl2 to 5 mM.
Cross-linked chromatin samples (200 μl) were diluted 10-fold in radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100, 0.1% Na deoxycholate, 0.1% SDS, 140 mM NaCl, and 1 mM PMSF) and preincubated with protein A-agarose for 1 h before they were incubated in parallel with α-HP1, α-HOAP, and nonimmune immunoglobulin G (IgG) immunoresins for 3 h at 4°C (antibodies were covalently linked to resin with dimethylpimelimidate; Harlow and Lane, 1988). The immunoresins were washed 2 times with 10 ml RIPA buffer and 1 time with LiCl-RIPA (0.25 M LiCl, 0.5% Triton-X, 0.5% Na-deoxycholate, 1 mM Na-EDTA, and 10 mM Tris-HCl) and then boiled for 5 min in SDS loading buffer (0.25 M Tris, pH 6.8, 10% glycerol, 2% SDS, 2.5% β-mercaptoethanol, and 0.2 mM bromphenol blue) to reverse chromatin cross-links and release solubilized proteins. Antibodies that recognize HP1, HOAP, and DmORC 2 (described above), DmORC 6 (a gift from M. Botchan; Pak et al., 1997), and DmLamin (a gift from H. Saumweber and J. Sedat (Howard Hughes Medical Institute, San Francisco, CA); Saumweber et al., 1980; Frasch et al., 1988) were used to immunoblot solubilized proteins.
Immunostaining
For embryo immunostaining, Drosophila embryos were fixed in formaldehyde and immunostained as previously described (Kellum and Alberts, 1995). Larval brain squashes were prepared by dissecting larval brains from third instar larvae, incubating them in a hypotonic solution (0.5% sodium citrate) for 10 min, and then fixing them in a drop of 5 methanol:5 acetic acid:0.5 distilled water for 2 min before they were squashed under a siliconized coverslip over a microscope slide (Pimpinelli et al., 2000). Polytene chromosome squashes were prepared from salivary glands dissected from third instar larvae by the method of Alfageme et al. (1980) with the use of Cohen's nuclear medium and fixatives of 2% formaldehyde in PBS for 20 s followed by 50% acetic acid. The coverslips were removed, and the slides were immersed in cold PBS before they were immunostained with affinity-purified antibodies that recognize HP1 (1:500 dilution), HOAP (1:200), or ORC2 (1:100) antibodies (described in Antibody Preparation). In double-immunostaining experiments, the HP1 antibody was directly labeled with N-hydroxysuccinimide-rhodamine (Pierce, Rockford, IL; pamphlet 46102); HOAP and ORC 2 antibodies were indirectly labeled with a fluorescein-labeled antibody that recognizes rabbit IgG (1:1000; Jackson ImmunoResearch, West Chester, PA). Slides were incubated with antibodies for 1 h at room temperature and washed (3 times for 15 min each) between primary and secondary antibody incubations and after the secondary antibody incubation. Immunostained specimens were mounted in PBS containing 85% glycerol, 10 mM p-phenylenediamine, and 0.01 mg/ml DAPI. Images were acquired with a Leica (Nussloch, Germany) TCSNT confocal microscope (final magnification, 252×).
Electrophoretic Mobility Shift Assays
The pelB/6-histidine-tagged recombinant HOAP protein was expressed from the pET20b vector in Escherichia coli strain BL21 (DE3) and purified by Ni-NT-agarose chromatography. The column was washed with 250 mM imidizole to remove contaminating proteins and proteolytically cleaved HOAP protein before eluting the full-length fusion protein with 500 mM imidizole. The eluted protein was dialyzed into PBS and concentrated to 200 μg/ml.
Binding reactions (10 μl) were carried out with 0.5 ng (∼30 fmol, 3000 cpm) end-labeled probe and 1.2 μg 6-histidine-tagged HOAP protein (∼35 fmol) for 20 min at 30°C in a buffer of 12 mM HEPES, pH 7.9, 4 mM Tris Cl, pH 7.9, 60 mM KCl, 1 mM EDTA, 1 mM DTT, 0.2 mg/ml BSA, 2% Tween 20, 12% glycerol, and 250 ng poly[dG-C][d(G-C)] carrier DNA. The reactions were then electrophoresed through a 4% polyacrylamide gel containing Tris acetate-EDTA buffer (35 mA for 1.5 h). The labeled probes were prepared from single-stranded oligonucleotides containing 4 tandem repeats of satellite sequences AATAT, AATAG, AATAC, AAGAC, AAGAG, and AACAA and 3 tandem repeats of satellite sequences AATAAAC, AATAGAC, AAGAGAG, and AATAACATAG. Four hundred fifty-seven base pairs of the subtelomeric minisatellite telomere-associated sequence (TAS) from chromosome arm 2L (Walter et al., 1995) were used to examine binding of HOAP to the TAS sequence. The Drosophila virilis satellite sequence probes were prepared from single-stranded oligonucleotides containing 4 tandem repeats of satellite sequences ACAAACT, ATAAACT, and ACAAATT. All probes were end labeled to equivalent specific activities (6500 ± 10% cpm/ng) with the use of the filling-in activity of Klenow I. Competition experiments were performed with 50, 100, 200, and 400× excesses of cold competitor to labeled probe. A 63-bp fragment of the scs chromatin boundary element (Kellum and Schedl, 1991) was used as a nonspecific competitor DNA fragment.
Phenotypic Analyses of the anon fe 1G5 Gene
The cytological map position of the anon fe 1G5 gene was determined by in situ hybridization to polytene chromosome squashes (fee for service by the Department of Biological Sciences [E. Woloshyn]), University of Alberta, Edmonton, Alberta, Canada). The Df(3R)F89-4 deficiency stock (a gift from E. Knust and the Bloomington Stock Center at Indiana University, Bloomington, IN; Tepass and Knust, 1990) was used to determine the effect of reducing the dose of the anon fe 1G5 gene on position effect variegation. An hs-anon fe 1G5 transgene was used to determine the effect of increasing the dose of the anon fe 1G5 gene on position effect variegation. The hs-anon fe 1G5 transgenic animals were obtained by P element-mediated germ line transformation of a P{w+-CaSpeR-hs-anon fe 1G5} vector (fee for service by the Department of Biology [D. Hickey], University of Ottawa, Ottawa, Ontario, Canada).
Two different reporter genes were used to monitor the effect of the anon fe 1G5 gene on position effect variegation. The whitem4 allele, in which the white gene is juxtaposed to pericentric heterochromatin of the X chromosome, was used to monitor the effect of the Df(3R)F89-4 deficiency. To this end, wm4 females were crossed to w118/Y; Df(3R)F89-4/TM3Sb males, and the eye color phenotypes of the Df(3R)F89-4/+ progeny were compared with those of their TM3Sb/+ siblings. The eye color phenotypes of the TM3Sb/+ sibling animals were also compared with those of the wm4 animals lacking the TM3Sb chromosome, and the TM3Sb chromosome was found not to affect the variegated phenotype. The effect of the Df(3R)F89-4 deficiency and 2 additional copies of the gene from the P{w+}[hsHOAP] transgene on expression of the In(3L)BL1 hs-lacZ reporter (Lu et al., 1996) was also determined. Adults of the genotypes 1) In(3L)BL1/TM3Ser, 2) In(3L)BL1/Df(3R)f89-4, 3) P{w+}[hsHOAP]; In(3L)BL1/TM3Ser, and 4) P{w+}[hsHOAP]/CyO; In(3L)BL1/TM3Ser were first subjected to heat shock at 37°C for 30 min to induce expression of the P{w+}[hsHOAP] transgene. Embryos were then collected for 3 h at room temperature, heat shocked at 37°C for 30 min to induce expression of the In(3L)BL1 hs-lacZ reporter, and allowed to recover for 1 h at room temperature before they were stained for β-galactosidase activity as described by Lu et al. (1996). Collection and staining of embryos from all genotypes were performed side by side. β-Galactosidase enzymatic activity in each genotype was quantitated with the use of protein extracts prepared from adults (100 males and 100 females) of each genotype and chlorophenol red-β-d-galactopyranoside as substrate, as described by Simon and Lis (1987).
RESULTS
The 55-kDa Copurifying Protein Contains a Peptide Sequence from the anon fe 1G5 Gene Product
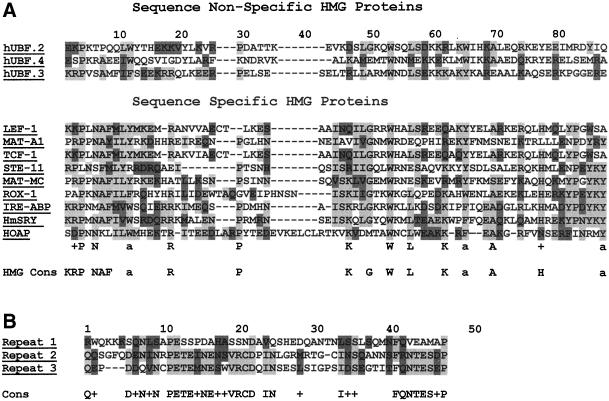
The Coomassie brilliant blue–stained band for the 55-kDa protein (p55) that copurifies with the ORC-containing cytoplasmic complex of HP1 was excised from an SDS-polyacrylamide gel and subjected to in situ proteolytic cleavage and Edman degradation peptide sequence determination by Harvard Microchemistry. The amino acid sequences of 2 different peptides from the protein were determined and used to search the National Center for Biotechnical Information protein database. Matches (100%) to both peptide sequences were found within the hypothetical protein-coding sequence of the anon fe 1G5 gene from D. melanogaster. This gene and its homologues from related Drosophila species (Drosophila simulans [86% identity and 91% similarity] and Drosophila yakuba [66% identity and 78% similarity]) were identified in a screen for fast-evolving genes in Drosophila species (Schmid and Tautz, 1997). A WU-BLASTP+BEAUTY search with the complete hypothetical coding sequence showed the amino terminus to contain similarity to the HMG domain of sex-determining region Y (SRY) proteins (24% identity and 40% similarity). This region of similarity also has 67% identity and 78% similarity to the consensus sequence shared between sequence-specific HMG proteins (Grosschedl et al., 1994; Figure 1A). The anon fe 1G5 coding sequence is most similar to the sequence-specific group of HMG proteins to which SRY belongs. Like this group of proteins, the anon fe 1G5 coding sequence contains a single HMG domain, an asparagine at amino acid position 7, and a conserved substitution for histidine at position 78 (Grosschedl et al., 1994; Figure 1A). A novel repeat sequence was also found at the carboxyl terminus of the anon fe 1G5 gene-coding sequence from D. melanogaster and its homologues in other Drosophila species (Figure 1B).
Figure 1.
The anon fe 1G5 gene product contains sequence similarity to sequence specific HMG proteins. (A) The amino-terminal region of the anon fe 1G5 gene product contains a conserved amino acid sequence motif of sequence-specific–binding HMG proteins. (B) The carboxyl-terminal region of the anon fe 1G5 gene product contains 3 copies of a novel repeated sequence. Identical amino acids are shown in light gray; similar amino acids are shown in dark gray.
The anon fe 1G5 Gene Encodes HOAP
Analyses of expressed sequence tag (EST) cDNA clones obtained by Schmid and Tautz (1997) and Schmid et al. (1999) and the BDGP (GadFly, CG6219) predict a product of 337 amino acids encoded from the anon fe 1G5 gene. Two different versions of cDNA EST clones that differ with respect to the length of their 5′ untranslated leader sequences and the predicted translational start site are represented within the collection of BDGP EST clones. The longer cDNA contains an additional 300 nucleotides originating 736 nucleotides upstream of the shorter cDNA 5′ end, mostly consisting of an untranslated leader sequence that is predicted to encode a protein that is 8 amino acids longer than the one encoded by the shorter cDNA.
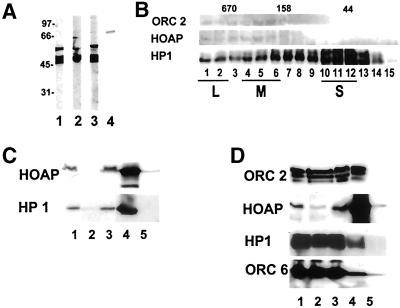
The shorter cDNA (1119 bp) encoding a 337-amino acid protein was obtained by PCR amplification and cloned in frame to the pelB leader sequence at the 5′ end and a 6-histidine tag at the 3′ end of the pET20b expression vector. The bacterially expressed anon fe 1G5 fusion protein displayed a molecular weight of 55–60 kDa by SDS-PAGE (Figure 2A, lane 2). Two lower-molecular-weight polypeptides were also observed in each affinity purification of the anon fe 1G5 fusion protein. The 55- to 60-kDa polypeptide was excised from a polyacrylamide gel, and antibodies increased against it were found to recognize the 55-kDa polypeptide as well as the 2 lower-molecular-weight polypeptides (Figure 2A, lane 1). This suggested that the lower-molecular-weight polypeptides are proteolytic cleavage products of the 55- to 60-kDa protein. Furthermore, expression of the fusion protein in a bacterial strain that is deficient for both lon(1) and ompT proteases (BL21 [DE3]) reduced the quantities of the lower-molecular-weight forms. The antiserum also recognized a 55-kDa protein and 2 lower-molecular-weight proteins in the Drosophila embryo cytoplasmic extract (Figure 2A, lane 2). A higher-molecular-weight polypeptide in the Drosophila embryo extract was also weakly recognized by this antibody (Figure 2A, lane 2) but not by subsequent batches of antibodies produced in other animals (Figure 2A, lane 3).
Figure 2.
HOAP is associated with cytoplasmic complexes containing HP1 and ORC subunits. Antibodies were increased against an anon fe 1G5 fusion protein expressed in bacteria in 2 different rabbits. Lanes 1–3, immunoblotting of the bacterially expressed anon fe 1G5 fusion protein and the endogenous anon fe 1G5 gene product in the embryo cytoplasmic extract (lane 2) with the use of antibodies produced by 1 rabbit and the endogenous anon fe 1G5 gene product in the embryo cytoplasmic extract with the use of antibodies produced by a second rabbit (lane 3). The antibody increased against the DmORC 2 peptide was used to immunoblot the endogenous DmORC 2 protein in the embryo cytoplasmic extract (lane 4). (B) Antibodies that recognize HP1, HOAP (anon fe 1G5 gene product), and DmORC 2 were used to immunoblot gel filtration fractions of the embryo cytoplasmic extract. (C) The immunoprecipitate of HP1 from the embryo cytoplasm extract was immunoblotted with antibodies that recognize the anon fe 1G5 gene product (HOAP) and HP1. Lane 1, input protein (5% total); lane 2, protein not retained on α-HP1 beads (5% total); lane 3, protein not retained on nonimmune IgG beads (5% total); lane 4, protein retained on α-HP1 beads (100% total); lane 5, protein retained on nonimmune IgG beads. (D) Immunoprecipitates of the endogenous anon fe 1G5 gene product (HOAP) from fractions of the cytoplasmic extract containing a large HP1 complex were immunoblotted with antibodies that recognize DmORC 6, HP1, the anon fe 1G5 gene product (HOAP), and DmORC 2. Lane 1, input protein (5% total); lane 2, protein not retained on α-anon fe 1G5 beads (5% total); lane 3, protein not retained on nonimmune IgG beads (5% total); lane 4, protein retained on α-anon fe 1G5 beads (100% total); lane 5, protein retained on nonimmune IgG beads.
The affinity-purified antiserum was then used to immunoblot gel filtration fractions of the cytoplasmic extract (Figure 2B). The fractionation of the endogenous anon fe 1G5 gene product was found to overlap with those of the ORC 2 subunits and the ORC-containing complexes of HP1. The large HP1 complexes were then immunoaffinity purified from the gel filtration fractions containing the HP1/ORC complexes as described by Huang et al. (1998), and antibodies increased against the anon fe 1G5 gene product were used to determine the presence of this gene product in the HP1 complexes. These analyses showed both HP1 and the endogenous anon fe 1G5 gene product to be quantitatively depleted from the input fraction (Figure 2C, lanes 1 and 2) and specifically retained on the HP1 immunoaffinity resin (Figure 2C, lanes 4 and 5).
The antiserum was then used in the reciprocal experiment of immunoprecipitating the endogenous anon fe 1G5 gene product from the unfractionated cytoplasmic extract to determine by immunoblot analyses whether HP1 and ORC subunits coimmunoprecipitate with the anon fe 1G5 gene product (Figure 2D). A comparison of the immunoblot signal for the HOAP protein in the input and unretained fractions showed that the immunoaffinity purification was carried out under near-saturating conditions (Figure 2D, compare lanes 1 and 3). HP1 and ORC proteins were also found to immunoprecipitate with the endogenous anon fe 1G5 gene product, although a comparison of the immunoblot signals for each of these proteins in the input, unretained, and retained fractions (Figure 2D, lanes 1, 2, and 4, respectively) showed that only a small fraction of each protein (2–10%) was retained in the immunoprecipitation. These data are consistent with the fraction of HP1 and ORC subunits in the cytoplasmic extract that were previously determined to exist in the large HP1 complexes (Pak et al., 1997; Huang et al., 1998) and the gel filtration profiles for HP1 and the ORC 2 subunit in comparison with that of HOAP (Figure 2B). These reciprocal immunoprecipitation data confirmed the identity of the 55-kDa copurifying HP1/ORC-associated protein as the product of the anon fe 1G5 gene; hence, we will refer to the protein as HP1/ORC-associated Protein (HOAP).
HOAP Is Associated with HP1 and ORC In Salt-resistant Chromatin
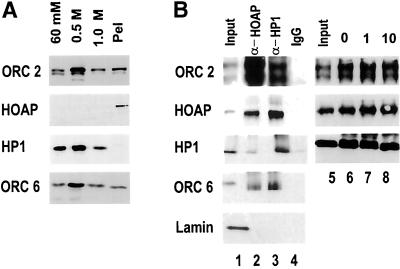
The immunoprecipitation experiments described above demonstrate an association of the anon fe 1G5 gene product (HOAP) with ORC-containing HP1 complexes in the maternally loaded cytoplasm of the early embryo. For determining whether HOAP is a nuclear protein that is associated with HP1 and ORC subunits in interphase chromatin, the antiserum increased against the anon fe 1G5 gene product was first used to immunoblot fractions of proteins that were salt extracted from interphase nuclei (Figure 3A). The interphase nuclei for these experiments were isolated from cycle 14 embryos, the stage at which a rapid series of synchronous nuclear divisions in early embryogenesis ends. During this developmental stage, the nuclei acquire a G2 phase, and the heterochromatin first becomes a distinct cytological entity. The prolonged interphase of this nuclear cycle allows one to obtain a nearly homogeneous collection of embryos with nuclei that are synchronized in interphase and contain fully formed heterochromatin. Nuclei were isolated from embryos of this stage and sequentially extracted with successively increasing concentrations of salt. It was previously shown that each differentially extracted fraction contains distinct populations of HP1 phosphoisoforms (Huang et al., 1998; Figure 3A). The fraction of HP1 that requires the highest concentration of salt to be extracted (1 M KCl) is characteristically underphosphorylated and specifically associated with Drosophila ORC subunits (Huang et al., 1998). To determine whether HOAP is a nuclear protein and the strength of its association with interphase chromatin, the HOAP antibodies were used to immunoblot the differentially extracted nuclear fractions. These analyses showed HOAP to be a nuclear protein and to be even more tightly associated with interphase chromatin than the most salt-resistant fraction of HP1 (Figure 3A, lane 4). A significant fraction of DmORC subunits 2 and 6 is also found in the salt-resistant chromatin (Figure 3A).
Figure 3.
HOAP is in a tightly bound interphase chromatin fraction containing HP1 and DmORC subunits. (A) Equal volumes of protein that were sequentially extracted from interphase nuclei with 60 mM and 0.5 and 1.0 M KCl and in the remaining pellet fraction were immunoblotted with antibodies that recognize ORC 2, HOAP, HP1, and ORC 6. (B) Formaldehyde cross-linked fractions of salt-resistant chromatin were immunoprecipitated with α-HOAP and α-HP1 antibodies and immunoblotted with antibodies that recognize DmORC 2, HOAP, HP1, DmORC 6, and DmLamin B. Lane 1, input chromatin (10% total); lane 2, chromatin retained on α-HOAP beads (50% total); lane 3, chromatin retained on α-HP1 beads (50% total); lane 4, chromatin retained on nonimmune IgG beads (50% total). The input chromatin in lanes 5–8 was treated with MNase I before immunoprecipitation with α-HP1 antibodies. Immunoprecipitated chromatin fractions were immunoblotted with antibodies that recognize DmORC 2, HOAP, and HP1. Lane 5, input chromatin treated with MNase I for 10 min (10% total); lanes 6–8, chromatin treated with MNase I for 0 (lane 6), 1 (lane 7), and 10 (lane 8) min that was retained on α-HP1 beads (50% total each).
A chromatin immunoprecipitation assay was then used to determine whether the anon fe 1G5 gene product is present in chromatin fragments containing HP1 and ORC (Figure 3B). The chromatin samples used for these experiments were prepared from interphase nuclei of cycle 14 embryos that had first been extracted with 0.5 M KCl to remove all but the most salt-resistant fraction of HP1 that is associated with ORC subunits. The chromatin was cross-linked by treatment with formaldehyde and then sonicated to an average length of 500 bp. Aliquots of the chromatin fragments were then incubated in parallel with α-HP1, α-HOAP, and nonimmune IgG immunoresins. After an extensive wash, each immunoresin was resuspended in SDS loading buffer and boiled for 5 min to reverse the cross-links in the retained chromatin. The protein released from each immunoresin was then immunoblotted with antibodies that recognize HP1, HOAP, and DmORC subunits 2 and 6 (Figure 3B). The immunoblot analyses showed each protein to be present in the α-HP1 chromatin immunoprecipitate (Figure 3B, lane 3) as well as the α-HOAP chromatin immunoprecipitate (Figure 3B, lane 2). In contrast, Drosophila lamin, which was also present in the salt-resistant chromatin fraction, did not coimmunoprecipitate with either HP1 or HOAP (Figure 3B, lanes 1–3, Lamin). Visual comparisons of the immunoblot signal for each protein in the input (Figure 3B, lane 1, 10% total) and the immunoresin-retained fraction (Figure 3B, lane 3, 50% total) indicated that HP1, HOAP, and the 2 DmORC subunits were largely depleted from the input fraction in the α-HP1 immunoprecipitation. The DmORC subunits and HOAP were also depleted from the input chromatin in the HOAP immunoprecipitation; however; only a small fraction (∼10%) of HP1 appeared to be associated with HOAP in this fraction of chromatin (Figure 3B, compare lanes 1 and 2). The exact nature of the associations among HP1, HOAP, and DmORC subunits in this chromatin fraction is not clear, although the ability of HOAP antibodies to immunodeplete the ORC subunits suggests a close association among these proteins in this fraction. To further examine the possibility that HP1, HOAP, and ORC proteins coprecipitate in chromatin as a result of protein–protein interactions between different chromatin fragments or the mere proximity of their binding sites on DNA rather than their being in a complex on chromatin, the cross-linked chromatin was treated with Mnase I before immunoprecipitation. As shown in Figure 3B, lanes 5–8, the results of the chromatin immunoprecipitation with HP1 antibodies were unaffected by this treatment.
HOAP Colocalizes with Subfractions of HP1 and ORC in Heterochromatin
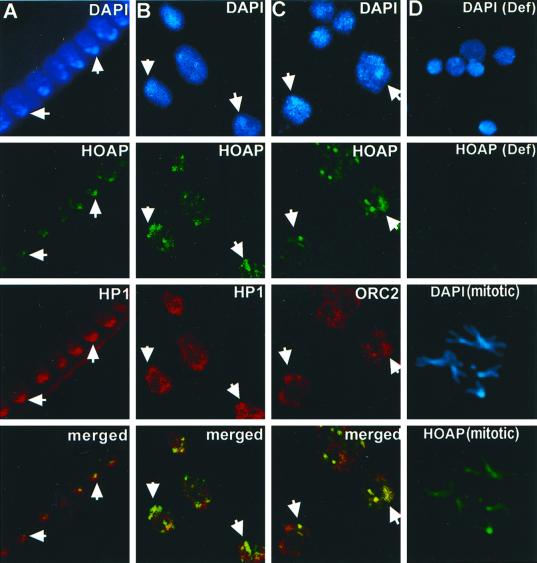
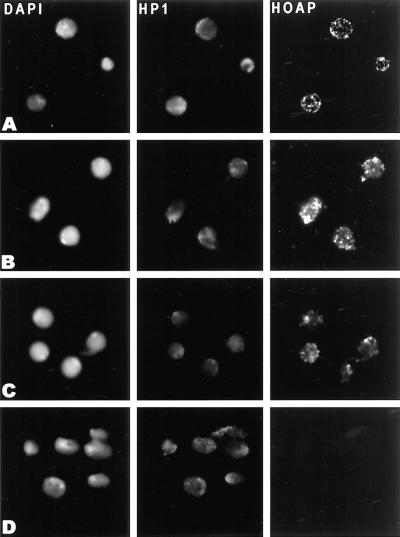
Immunostaining experiments were then used to determine where in interphase chromatin the HOAP protein is located. The antibody increased against the anon fe 1G5 gene product was first used to immunostain cycle 14 Drosophila embryos. Pericentric heterochromatin is known to occupy a distinct domain at the nuclear apical surface in embryos of this developmental stage (Foe and Alberts, 1983). HP1 is enriched approximately fourfold in this domain (James et al., 1989; Kellum et al., 1995). A side view of the nuclei of an embryo of this stage shows HOAP localized in a pattern of brightly stained spots at the apical surface where the intensely DAPI-stained sequences are clustered (Figure 4A). The embryos were coimmunostained with α-HP1 antibodies, and the spots of HOAP immunostaining were found to lie within the larger domain of enriched HP1 staining in these nuclei (Figure 4A, HOAP [green] and HP1 [red], merged).
Figure 4.
HOAP colocalizes with subfractions of HP1 and DmORC2 in interphase nuclei. (A) Immunolocalization of HOAP (green), HP1 (red), and HOAP (green) and HP1 (red) merged in DAPI-stained interphase nuclei (blue) of a cycle 14 Drosophila embryo (side view). (B) Immunolocalization of HOAP (green), HP1 (red), and HOAP (green) and HP1 (red) merged in DAPI-stained interphase nuclei (blue) of a larval brain squash. (C) Immunolocalization of HOAP (green), DmORC2 subunit (red), and HOAP (green) and DmORC2 (red) merged in DAPI-stained interphase nuclei (blue) of a larval brain squash. (D) Immunolocalization of HOAP [HOAP (Def)] in DAPI-stained interphase nuclei [DAPI (Def)] of a brain squash from homozygous Df(3R)crb-F89-4 larvae and HOAP [HOAP (mitotic)] in DAPI-stained metaphase chromosomes [DAPI (mitotic)] of a wild-type larval brain squash.
A similar punctate pattern of HOAP immunostaining was also observed in the diploid nuclei of wild-type larval brain squashes (Figure 4, B and C) but absent in squashes from larvae that were homozygous for a deficiency removing the anon fe 1G5 gene [Figure 4D, (Def)]. A double-immunostaining experiment was carried out with α-HOAP and α-HP1 antibodies to determine whether the spots of HOAP immunostaining overlap with the domain of HP1 enrichment in heterochromatin of these nuclei. As had been observed in embryos, the spots of HOAP immunostaining were clustered within the domain of enriched HP1 overlapping the intensely DAPI-stained satellite sequences of these nuclei [Figure 4B, (DAPI)]. HOAP was also diffusely localized throughout the mitotic chromosomes of larval brain squashes with a slight enrichment throughout pericentric heterochromatin [Figure 4D, (mitotic)].
We also wished to examine the localization of HOAP relative to that of the DmORC 2 subunit in heterochromatin. To this end, a double-immunolocalization experiment was undertaken with HOAP antibodies and antibodies increased against a peptide of the DmORC 2 subunit (Figure 4C). The DmORC 2 antibody recognizes a single polypeptide of the molecular weight expected for the DmORC 2 subunit in the embryonic cytoplasm extract (Figure 2A, lane 3). When these antibodies were used in conjunction with antibodies that recognize HOAP in larval brain squash immunostaining experiments, HOAP was found to overlap or to be closely apposed to spots of enriched ORC 2 immunostaining in the heterochromatic domain of the nucleus (Figure 4C). These spots were also located near the intensely DAPI-stained satellite sequences in heterochromatin [Figure 4C, (DAPI)], which were shown in separate experiments to lie within the domain of enriched HP1 in heterochromatin as described by Pak et al. (1997) (our unpublished results).
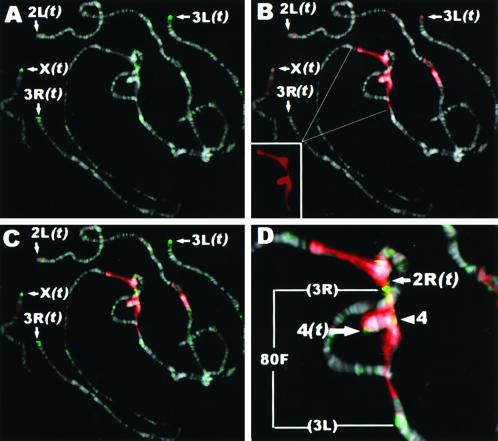
HOAP Is Localized Predominantly at Telomeres and Diffusely throughout Regions of Pericentric Heterochromatin of Salivary Gland Polytene Chromosomes
To examine the distribution of HOAP protein at higher resolution, polytene chromosomes from D. melanogaster salivary glands were coimmunostained with α-HOAP and α-HP1 antibodies (Figure 5). These experiments showed a prominent HOAP localization at the telomere of each chromosome [Figure 5A, arrows, (t)]. Staining at the end of 2L was weaker and observed less consistently than at other chromosomes. HP1 has also been frequently observed at the telomeres of polytene chromosomes, most consistently at the ends of the X, 3R, and 2R chromosomes (James et al., 1989; Fanti et al., 1998). More diffuse HOAP immunostaining was also observed throughout a region of pericentric heterochromatin that stains intensely with the α-HP1 antibody (Figure 5, B [HP1] and C [HP1 and HOAP merged]) on the left and right arms of the third chromosome. The HOAP immunostaining in the pericentric heterochromatin of the third chromosome was flanked by prominent bands of staining at 80F at the bases of 3R and 3L (Figure 5D, bracket 80F). It is of interest that prominent DmORC 2 immunostaining at the chromocenter of polytene chromosomes is also restricted to the fourth chromosome and the base of 3R (Pak et al., 1997). Diffuse staining was also consistently found throughout the pericentric heterochromatin at the base of the fourth chromosome (Figure 5D, arrowhead 4). The localization pattern for HOAP resembles that reported for the TAS element, which is found predominantly at the telomeres of 2R and 3R, but weaker staining is also observed throughout the chromocenter of polytene chromosomes (Karpen and Spradling, 1992).
Figure 5.
HOAP is localized predominantly at telomeres and weakly throughout regions of pericentric heterochromatin of polytene chromosomes. Shown are immunostaining of HOAP (green; A), HP1 (red; B), and HOAP (green) and HP1 (red) merged (C) and an enlargement of HOAP (green) and HP1 (red) merged at the chromocenter (D). The HP1 signal appears faded when superimposed over the bright signal from the intensely DAPI-stained chromocenter. (B) Inset, HP1 signal alone.
The HOAP Localization Pattern Is Conserved in Drosophila Species Containing Similar Satellite Sequence Compositions
The punctate pattern of localization for HOAP in the intensely DAPI-stained regions of the nucleus suggests that it may be associated with 1 or more repetitive DNA sequences enriched in these regions. One approach used to investigate which satellite sequence might serve as the binding site for a heterochromatin protein has been to compare its distribution in Drosophila species containing similar satellite compositions with that in species containing dissimilar compositions (Raff et al., 1994; Platero et al., 1998). The anon fe 1G5 gene was identified in a molecular evolutionary study aimed at identifying fast evolving genes that are conserved in species that are closely related to D. melanogaster (D. simulans, Drosophila mauritiana, and D. yakuba) but are absent from the more distantly related D. virilis. Interestingly, the closely related species containing an anon fe 1G5 gene homologue are also known to have similar satellite sequence compositions (Lohe and Roberts, 1988), whereas the more distantly related D. virilis lacks an anon fe 1G5 gene homologue and has a very different satellite sequence composition from that of D. melanogaster and its close relatives (Gall and Atherton, 1974).
It has been suggested that the conservation of heterochromatin-binding proteins is driven by the sequence bias of heterochromatin DNA repeats in that species (Csink and Henikoff, 1998). According to this view, the spots of HOAP staining observed in D. melanogaster might also be expected in its closely related species. A similar punctate pattern of staining in these species would also support binding of HOAP to satellite sequences that are shared between the species. To each of these ends, HOAP immunostaining experiments were carried out on larval brain squashes from 2 closely related species (D. simulans and D. mauritiana) and from D. virilis (Figure 6). Spots of HOAP immunostaining were observed in the region of intensely DAPI-stained satellite sequences in interphase nuclei from each closely related species (Figure 6, A–C, HOAP) but not in the nuclei from D. virilis (Figure 6D, HOAP). Coimmunostaining of these nuclei with HP1 antibodies showed the spots of HOAP immunostaining to be located within or closely juxtaposed to the larger domain of HP1 immunostaining (Figure 6, A–C).
Figure 6.
Punctate localization of HOAP in heterochromatin is conserved in closely related Drosophila species that contain an anon fe 1G5 gene and similar satellite sequence compositions. Larval brain squashes from D. melanogaster (A), D. simulans (B), D. mauritiana (C), and D. virilis (D) were stained with DAPI and α-HP1 and α-HOAP antibodies.
HOAP Protein Binds Specific D. melanogaster Satellite- and Telomere-associated Sequences In Vitro
The similarity of the anon fe 1G5 protein-coding sequence to that of HMG proteins suggests that it possesses DNA-binding activity. The immunostaining experiments described above indicate a binding specificity for 1 or more repetitive DNA found in heterochromatin. Gel mobility shift assays were used to determine whether HOAP is capable of binding to each of 10 different satellite DNA sequences enriched in D. melanogaster heterochromatin (Brutlag, 1980) and to the TAS (Karpen and Spradling, 1992; Walter et al., 1995).
A bacterially expressed hexahistidine-tagged recombinant form of the HOAP protein was used in the gel mobility shift assays. The recombinant protein was affinity purified over a Ni-NT agarose column with the use of an elution profile that yielded >90% full-length recombinant HOAP protein (Figure 7A). The DNA probes for the experiments were prepared by annealing single-stranded oligonucleotides of each D. melanogaster satellite sequence. The 5-bp satellite sequences (AATAT, AATAG, AATAC, AAGAC, AAGAG, and AACAA) were repeated in tandem 4 times, whereas the 7- and 10-bp satellite sequences (AATAAAC, AATAGAC, AAGAGAG, and AATAACATAG) were repeated in tandem 3 times. The double-stranded oligonucleotides and the 457-bp TAS isolated from the telomere of 2L (Walter et al., 1995) were then labeled to equivalent specific activities with the use of Klenow I to fill in a single-stranded overhang.
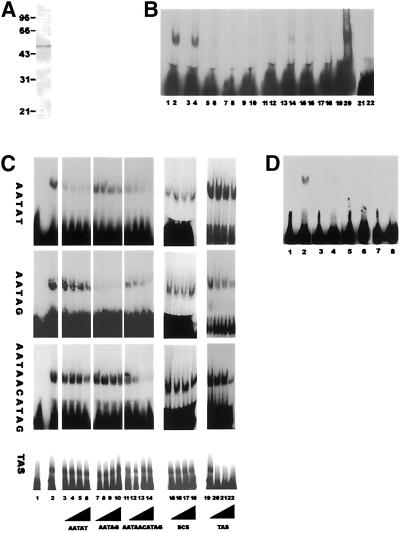
Figure 7.
HOAP binds D. melanogaster satellite and TAS in vitro. (A) Coomassie brilliant blue staining of the bacterially expressed hexahistidine-tagged recombinant HOAP protein used in the electrophoretic mobility shift assays. (B) Electrophoretic mobility shift assays were carried out with the recombinant HOAP protein and Drosophila satellite sequences AATAT (lanes 1 and 2), AATAG (lanes 3 and 4), AATAC (lanes 5 and 6), AAGAC (lanes 7 and 8), AAGAG (lanes 9 and 10), AACAA (lanes 11 and 12), AATAAAC (lanes 13 and 14), AATAGAC (lanes 15 and 16), AAGAGAG (lanes 17 and 18), and AATACATAG (lanes 19 and 20). For each pair of lanes, the odd-numbered lane contains a given satellite sequence free probe, and the even-numbered lane contains that same probe in the presence of HOAP protein. (C) Competitive binding studies with recombinant HOAP protein, satellite sequences AATAT, AATAG, and AATAACATAG, and TAS element. For each labeled probe (AATAT, AATAG, AATAACATAG, or TAS, as indicated on the left) lanes are as follows: lane 1, free labeled probe; lane 2, labeled probe in the presence of HOAP protein; lanes 3–22, binding of HOAP protein to the probe indicated to the left competed with an increasing (50, 100, 200, and 400×) molar excess of cold AATAT competitor (lanes 3–6), AATAG competitor (lanes 7–10), AATAACATAG competitor (lanes 11–14), TAS competitor (100, 200, and 300× molar excess; lanes 15–18), and nonspecific DNA competitor (scs sequence; lanes 19–22). (D) Electrophoretic mobility shift assays were carried out with the labeled D. melanogaster satellite sequence AATAACATAG (lanes 1 and 2) and D. virilis satellite sequences ACAAACT (lanes 3 and 4), ATAAACT (lanes 5 and 6), and ACAAATT (lanes 7 and 8) in the absence and presence of HOAP protein, respectively.
In the gel mobility shift assays that were then used to monitor binding of the recombinant HOAP protein to each labeled satellite sequence and to a nonspecific DNA fragment, the HOAP protein displayed the strongest binding affinity for 3 specific satellite sequences (AATAT, AATAG, and AATAACATAG; Figure 7B), although minimal binding to the other sequences could also be observed with prolonged autoradiographic exposures (our unpublished results). The recombinant HOAP protein was also able to bind the 457-bp TAS isolated from the telomere of 2L (Walter et al., 1995) in a gel mobility shift assay (Figure 7C). To determine which of the satellite sequences HOAP binds with the greatest affinity, competitive binding assays were performed with the 3 strongest binding satellite sequences and the TAS element (Figure 7C). Each sequence was used as a competitor to binding of HOAP to itself and to each of the other binding sequences. In these experiments, the binding of HOAP to each sequence was most effectively competed by a cold excess of that same sequence (e.g., cold AATAT with labeled AATAT [AATAT; Figure 7C, lanes 3–6], cold AATAG with labeled AATAG [AATAG; lanes 7–10], cold AATAACATAG with labeled AATAACATAG [AATAACATAG; lanes 11–14], and cold TAS with labeled TAS [TAS, lanes 15–18]). The binding of HOAP to the AATAT probe could also be effectively competed by an excess of cold AATAG (AATAT; Figure 7C, lanes 7–10), because binding to the AATAG probe could be competed with an excess of cold AATAT (AATAG; Figure 7C, lanes, 3–6). A cold excess of the 10-bp repeat sequence (AATAACATAG) was able to compete with HOAP binding to itself and to the two 5-bp sequences almost as effectively as each 5-bp sequence to itself (Figure 7C, lanes 11–14). In contrast, the two 5-bp sequences were only able to compete for binding of HOAP to AATAACATAG at the highest molar excess of cold competitor used (AATAACATAG; Figure 7C, lanes 3–6 and 7–10). HOAP binding to the TAS element could be competed by itself but not by any of the 3 satellite sequences (even at a 400-fold molar excess). However, HOAP binding to each of the 3 satellite sequences could be competed by a 300-fold molar excess of the TAS element, possibly indicating a preference for HOAP binding to the TAS element. An unrelated nonspecific DNA sequence was found to be an ineffective competitor for HOAP binding to the 3 satellite sequences or the TAS element even at a 400-fold molar excess (Figure 7C, lanes 15–19).
The punctate heterochromatic localization of HOAP is conserved in closely related Drosophila species with similar satellite sequence compositions [(RRN)m(RN)n rule]. The more distantly related D. virilis lacks an anon fe 1G5 gene and also contains satellite sequences that follow a different rule [(AAN)m(NA)n; Gall and Atherton, 1974; Lohe and Roberts, 1988]. It has been suggested that the conservation of heterochromatin-binding proteins might be driven by the sequence bias of satellite sequence repeats present in a given species (Csink and Henikoff, 1998). To test this idea with regard to conservation of the anon fe 1G5 gene, each of the 3 different D. virilis satellite sequences (ACAAACT, ATAAACT, and ACAAATT) was tested as a binding site for the HOAP protein. HOAP displayed minimal binding to each of the D. virilis sequences in comparison with binding to the D. melanogaster AATAACATAG satellite repeat labeled to equivalent specific activity (Figure 7D).
The anon fe 1G5 Gene Displays a Reciprocal Modifier of Variegation Phenotypes
The immunoprecipitation and immunostaining experiments described above demonstrate an association between HOAP and the salt-resistant fractions of HP1 and ORC in interphase nuclei. Mutants for the DmORC 2 subunit display a Suppressor of variegation [Su(var)] phenotype (Pak et al., 1997) and also exhibit defects in HP1 localization into heterochromatin (Huang et al., 1998). To determine whether HOAP also functions in heterochromatin assembly, we undertook genetic analyses of a Drosophila mutant that is deficient for the chromosomal region containing the anon fe 1G5 gene encoding HOAP.
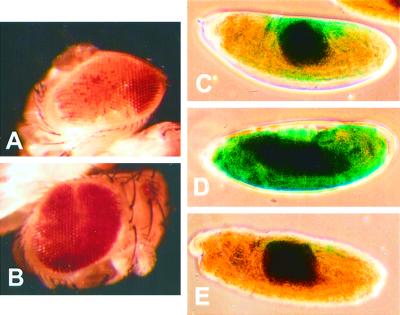
The cytological position of the anon fe 1G5 gene was determined by DNA in situ hybridizations to polytene chromosomes from larval salivary glands (our unpublished results). The cytological location determined by these experiments (95D–E) was later confirmed by a DNA sequence from the BDGP (unpublished results, accession number AC008203) and by in situ hybridizations in the independent study of Schmid et al. (1999). A mutant stock in which the chromosomal interval from 95D7-11-95F15 (Df(3R)crb-F89-4) has been removed was obtained through the Bloomington Stock Center. This stock was used to determine the effect of removing 1 copy of the anon fe 1G5 gene on the position effect–variegated phenotype of the whitem4 allele, in which the white gene has undergone a chromosomal rearrangement juxtaposing it to centric heterochromatin. The variegated phenotype associated with the whitem4 rearrangement (Figure 8A) was found to be suppressed by the Df(3R)crb-F89-4 chromosome (Figure 8B).
Figure 8.
The anon fe 1G5 gene displays a reciprocal modifier of variegation phenotypes. The variegated phenotype of whitem4 was observed in a wild-type genetic background (A) and a Df(3R)F89-4/TM3Ser genetic background (B). Variegated expression of the In(3L)BL hs-LacZ reporter was observed in a wild-type genetic background (C), a Df(3R)F89-4/TM3Ser genetic background (D), and the presence of 2 copies of a heat shock–induced hs-anon fe 1G5 transgene (E).
The chromosomal interval removed in the Df (3R)crb-F89-4 stock is rather large; information from BDGP GadFly indicates that ∼60 genes in addition to anon fe 1G5 are removed. We therefore wished to determine whether the reduced dose of the anon fe 1G5 gene in the Df(3R)crb-F89-4 stock was at least partially responsible for this Su(var) phenotype. To this end, we determined whether expression of the anon fe 1G5 gene from a transgene in the Df(3R)crb-F89-4 stock could suppress the Su(var) phenotype of the deficiency stock. The ability of the anon fe 1G5 transgene to suppress the Su(var) phenotype would be supportive of the idea that loss of the anon fe 1G5 gene in this deficiency is at least partly responsible for the Su(var) phenotype. Because the white gene served as the reporter for identifying anon fe 1G5 transgenic animals for the transformation vector we were using, it was necessary to use a different assay that is unrelated to eye pigmentation to monitor rescue of the Su(var) phenotype by the anon fe 1G5 transgene. We chose to use the In(3L)BL1 stock of Lu et al. (1996), which carries a variegating hsp70-LacZ transgene juxtaposed to pericentric heterochromatin by a chromosomal rearrangement. Embryos were collected from the In(3L)BL1 stock carrying 2 wild-type copies of the anon fe 1G5 gene and compared with those collected simultaneously from the same stock carrying either the Df(3R)crb-F89-4 deficiency chromosome or 2 additional copies of the anon fe 1G5 gene as a transgene. Each parental genotype was simultaneously subjected to heat shock to induce expression of the hs-anon fe 1G5 transgene in the transgenic stock. This treatment also induces the hs-lacZ reporter in each parental genotype, having the effect of neutralizing rather than enhancing anon fe 1G5 transgene rescue. Embryos collected from each genotype were then heat shocked to induce expression of the In(3L)BL1hs-lacZ reporter (as well as the hs-anon fe 1G5 transgene) before they were fixed and stained for β-galactosidase activity from expression of the hs-lacZ reporter (Figure 8, C–E). Embryos from animals carrying 2 copies of the endogenous anon fe 1G5 gene displayed moderate levels of variegated staining (Figure 8C). In contrast, embryos from animals carrying the Df(3R)crb-F89-4 chromosome contained high levels of β-galactosidase staining throughout the embryo (Figure 8D). The reciprocal phenotype was observed in embryos collected from animals with either 1 or 2 copies of the anon fe 1G5 transgene. The majority of embryos collected from these animals lacked β-galactosidase staining altogether or contained reduced levels of variegated staining (Figure 8E).
Quantitative measurements of β-galactosidase activities in adults of each genotype were also obtained with the use of chlorophenol red-β-d-galactopyranoside as substrate (Simon and Lis, 1987). The level of β-galactosidase activity in extracts from animals carrying the Df(3R)crb-F89-4 deficiency chromosome (Table 1, Df(3R)F89-4) was approximately twofold higher than that measured in extracts from wild-type animals (Table 1, wild-type). The Su(var) phenotype associated with the Df(3R)crb-F89-4 was partially reversed by expression of the anon fe 1G5 transgene from a heat shock–inducible promoter (Table 1, Df(3R)F89-4; P{hsHOAP}). The level of β-galactosidase activity in extracts from animals carrying 2 copies of the anon fe 1G5 transgene in addition to the 2 endogenous copies of the gene (Table 1, P{hsHOAP}/P{hsHOAP}) was almost twofold lower than that measured in extracts from wild-type animals. A more moderate reduction in activity was measured in animals carrying a single extra copy of the gene as a transgene (Table 1, P{hsHOAP}/CyO).
Table 1.
Quantitation of β-galactosidase activities from In(3L)BL1hs-lacZ reporter in HOAP-overexpressing and -underexpressing lines
| A574a | Mut/wtb | |
|---|---|---|
| Wild type | 0.869 | 1.00 |
| Df(3R)F89-4 | 2.02 | 2.32 |
| Df(3R)F89-4; P{hsHOAP} | 1.10 | 1.27 |
| P{hsHOAP}/CyO | 0.696 | 0.616 |
| P{hsHOAP}/P{hsHOAP} | 0.489 | 0.563 |
A574 units measured upon conversion of CPRG (chlorophenol red-β-D-galactoside) substrate to product (A574).
Mut, mutant; wt, wild type.
DISCUSSION
Interphase nuclei contain multiple fractions of HP1, containing different levels of phosphorylation and having different strengths of chromatin association. The most tightly bound chromatin fraction is underphosphorylated and specifically associated with Drosophila ORC subunits (Huang et al., 1998). A specific fraction of the protein in the maternally loaded cytoplasm of the early embryo is also underphosphorylated and specifically associated with a multiprotein complex containing DmORC subunits. This cytoplasmic fraction is thought to be poised for assembly into the tightly bound chromatin fraction during heterochromatin assembly later in embryonic development. Mutants for the ORC2 subunit display a perturbation in HP1 localization into Drosophila heterochromatin. This observation has led to the postulation that ORC plays a role in the recruitment of HP1 into Drosophila heterochromatin that may be analogous to its role in recruiting the SIR1 protein to silencing nucleation sites in S. cerevisiae (Huang et al., 1998). Here we report the identification of an unknown protein component (p55) of the HP1/ORC complex of the maternally loaded cytoplasm. The peptide sequence from this protein matches the sequence from the hypothetical protein product of the Drosophila anon fe 1G5 gene (Schmid and Tautz, 1997). Antibodies increased against a recombinant protein produced from this gene were used in reciprocal immunoprecipitation experiments to show that the HOAP p55 protein is the product of this gene.
HOAP Contains a Region of Similarity to HMG Proteins
The amino terminus of the anon fe 1G5 gene-coding sequence contains similarity to the HMG domain of the SRY protein and the group of DNA sequence-specific HMG proteins to which it belongs. This similarity to HMG proteins is of interest in view of reports of interactions of a human homologue of HP1 with the SP100-HMG protein (Lehming et al. 1998; Seeler et al., 1998). This HMG protein displays a punctate localization similar to that of HOAP in normal cells that becomes disrupted in some cancer cells (Szostecki et al., 1990; Ascoli and Maul, 1991). The HMG sequence motif in the HOAP protein suggested that it may possess DNA-binding activity. We have, indeed, demonstrated sequence-specific binding of HOAP to 3 D. melanogaster satellite DNA sequences (AATAT, AATAG, and AATAACATAG) and to the TAS.
The protein was also found to have a punctate pattern of distribution in the region where pericentric satellite sequences are clustered in interphase nuclei. The spots of HOAP staining lie within the domain of HP1 enrichment and are closely apposed to sites of enriched ORC 2 immunostaining in this domain. This enrichment of HOAP in pericentric heterochromatin was also observed in mitotic chromosomes of diploid cells. The distribution of HOAP in polytene chromosomes was also heterochromatic; it was predominantly found at telomeres, but weaker staining was also observed throughout regions of pericentric heterochromatin. These results indicate an association of HOAP with 1 or more repeated sequences in telomeric and pericentric heterochromatin. The TAS is 1 repetitive sequence that is found in both regions (Karpen and Spradling, 1992). HOAP was able to bind this sequence in vitro; however, further studies will be required to determine the actual in vivo binding site for the HOAP protein.
The pattern of distribution for HOAP in diploid and polytene tissues and its dynamics during the cell cycle differ from those of any previously characterized heterochromatin-associated protein in Drosophila. HP1 displays prominent enrichment throughout pericentric heterochromatin and at each telomere of salivary gland polytene chromosomes (James et al., 1989; Fanti et al., 1998). The association of HP1 with pericentric heterochromatin, however, appears to be less stable during mitosis (Kellum et al., 1995; Platero et al., 1998). Two other heterochromatin-associated proteins (GAGA factor and Prod) have prominent binding sites in both heterochromatin and euchromatin. Binding to heterochromatic satellite repeat sequences (AAGAG and AATAACATAG, respectively) is observed in mitotic chromosome spreads, but both proteins become redistributed to sites of euchromatic gene regulation during interphase (Raff et al., 1994; Torok et al., 1997; Platero et al., 1998). This redistribution and differences in the representation of satellite sequences in polytene and diploid tissues (Miklos and Cotsell, 1990) contribute to a euchromatic pattern of distribution for both proteins in interphase polytene chromosomes. HOAP, by contrast, is found predominantly at heterochromatic regions of both diploid and polytene nuclei and appears to retain its association with pericentric heterochromatin throughout the diploid cell cycle. Its localization predominantly at telomeres of polytene chromosomes but in pericentric regions of diploid chromosomes probably reflects differences in the representation of particular heterochromatic sequences in the 2 cell types. These distinct characteristics of HOAP distribution in heterochromatin are likely to reflect a unique role for it in these regions.
A Role for HOAP in Heterochromatin Assembly
Genetic screens in Drosophila have been used to identify chromosomal regions that modify the position effect–variegated phenotype of the whitemot4 allele when either duplicated or deleted (Locke et al., 1988; Wustmann et al., 1989). The proteins encoded by these genes are thought to play a role in heterochromatin assembly. We have used a deficiency for the chromosomal region containing the anon fe 1G5 gene [Df(3R)F89-4] to determine whether the HOAP protein has a role in heterochromatin assembly. This deficiency caused a suppression of the position effect–variegated phenotype of the whitem4 allele as well as the hs-lacZ reporter of the In(3L)BL1 chromosomal rearrangement (Lu et al., 1996). Suppression of the Su(var) phenotype with an anon fe 1G5 transgene suggests that loss of the anon fe 1G5 gene is at least partially responsible for the Su(var) phenotype associated with this deficiency.
The chromatin immunoprecipitation experiments with HP1 and HOAP antibodies showed HOAP to be located in close proximity to HP1 and ORC proteins in the salt-resistant fraction of interphase chromatin. We speculate that this fraction of chromatin contains binding sequences for ORC and HOAP from which heterochromatin assembly may be nucleated by a process resembling the recruitment of the Sir complex to silencing nucleation sites in S. cerevisiae. The demonstration that HOAP binds specific satellite sequences suggests that it may play a role in Drosophila heterochromatin that is analogous to the roles of the DNA-binding Rap1 and Abf1 proteins in budding yeast silencing. Indeed, the limited degree of overlap between the HP1-containing chromatin fractions and those containing ORC or HOAP in the chromatin immunoprecipitation experiments may be indicative of a heterochromatin assembly nucleation activity for HOAP rather than as a bona fide structural component of heterochromatin.
A repeated DNA sequence of any type has been shown, in some cases, to induce heterochromatin formation in euchromatic regions of Drosophila chromosomes (Dorer and Henikoff, 1994). It has been proposed that heterochromatin assembly is induced in these situations as a result of heterochromatin proteins recognizing unusual folded structures at sites of paired repeats. HMG proteins constitute 1 class of protein that is likely to play a role in such a mechanism; the sequence recognition motif of even the sequence-nonspecific class of HMG proteins involves recognition of an altered DNA secondary structure (Lilley, 1988; Pohler et al., 1998). However, HOAP was found to display preferred binding to specific satellite repeats as well as to the mini-satellite TAS. Interestingly, TASs have been found flanking a number of variegating transgenes inserted into both telomeres (of X, 2R, and 3R) and pericentric heterochromatin (of 2R and 2L; Karpen and Spradling, 1992; Zhang and Spradling, 1995; Cryderman et al., 1998). They have also been implicated in the mediation of the P cytotype by a single strongly repressed autonomous P element [Lk-P(1A)] inserted next to a block of TAS repeats in the telomere of the X chromosome (Ronsseray et al., 1996; Roche and Rio, 1998). The variegated expression of telomeric transgenes flanked by TAS elements has been found to be unaffected by a reduced dose of the HP1 gene [Su(var)2-5] (Cryderman et al., 1998), although the ability of Lk-P(1A) to repress P dysgenesis is strongly reduced (Ronsseray et al., 1996, 1998).
HOAP Is Encoded by a Fast-evolving Gene
The anon fe 1G5 gene was identified in a screen for fast-evolving Drosophila genes on the basis of their conservation in species closely related to D. melanogaster (D. simulans, D. yakuba, and D. mauritiana) but not in the more distantly related D. virilis (Schmid and Tautz, 1997). It is of interest that an anon fe 1G5 gene homologue is present in each of the closely related species that contain satellite sequences constituting in vitro HOAP binding sites but not in the distantly related D. virilis containing different satellite sequences that do not serve as in vitro binding sites for the HOAP protein (Gall and Atherton, 1974; Brutlag, 1980; Schmid et al., 1999). One of these satellite sequences (AATAT) is even known to have a conserved chromosomal location in each of the species containing an anon fe 1G5 gene (Lohe and Roberts, 1988). HOAP immunostaining in interphase and mitotic diploid cells of D. melanogaster is consistent with an association of HOAP with 1 or more of these sequences in vivo. Moreover, the punctate pattern of HOAP distribution in the heterochromatic regions is conserved in the closely related species. The localization pattern of HOAP on polytene chromosome also suggests a relationship to the TAS first described by Karpen and Spradling (1992). It is not currently known whether TASs are present in other Drosophila species, but a different subtelomeric satellite has been found in D. virilis that is not yet known to exist in D. melanogaster (Biessmann et al., 2000).
The conservation of an anon fe 1G5 gene only in species containing HOAP-binding sequences is consistent with the ideas put forth by Csink and Henikoff (1998) of a relationship between conservation of heterochromatin-binding proteins and the sequence bias of repetitive heterochromatin sequences in a given species. Csink and Henikoff (1998) also speculated that the enrichment of satellite sequences at centromeres might play a passive role in centromere formation by providing a mechanism for excluding efficient origins of replication, and, thereby, might determine these regions as centromeres as a consequence of their late replication. ORC 2 immunostaining experiments indicate that ORC is enriched rather than excluded from pericentric heterochromatin in Drosophila (Pak et al., 1997). Moreover, ORC binding does not appear to play the critical role in determining the firing time of an origin; rather, other factors such as chromatin organization and Cdc45p binding are thought to be important factors (Wintersberger, 2000). ORC proteins may function in concert with HP1 and DNA-binding proteins such as HOAP to establish a heterochromatin structure in pericentric regions that then determines the late-replicating properties of those regions. According to this model, the repetitive sequences in heterochromatin play an active rather than a passive role in centromere formation. Although a conserved feature of pericentric heterochromatin is its repetitive sequence composition, there is little conservation at the level of the primary DNA sequence. The collection of DNA-binding proteins participating in heterochromatin formation in a given species is, therefore, likely to be determined by the satellite sequence composition of that species.
A number of fast-evolving genes, including those of SRY proteins, are known to have roles in sexual selection or species-specific differentiation (Whitfield et al., 1993; Schmid and Tautz, 1997). Functions for heterochromatin in organizing nuclear architecture, chromosome pairing, and centromere function (Allshire et al., 1995; Kellum et al., 1995; Dernburg et al., 1996; Karpen et al. 1996; Fanti et al., 1998; for review, see Dobie et al. 1999) are likely to be relevant to processes that drive species differentiation. As such, it may not be surprising that a heterochromatin sequence-binding protein might play a role in evolutionary processes driving species differentiation.
ACKNOWLEDGMENTS
We thank M. Botchan for the DmORC 2 and 6 antibodies, H. Saumweber and J. Sedat for the DmLamin antibodies, J. Eissenberg for the In(3L)BL1 stock, E. Knust for the Df(3R)89-4 stock, L. Wallrath and H. Biessmann for the TAS sequence clone, and P. Tolias for the ovarian gt22A cDNA library. This work was supported by grants from the Canadian Medical Research Council and National Institutes of Health.
REFERENCES
- Alfageme CR, Rudkin GT, Cohen LH. Isolation, properties, and cellular distribution of D1, a chromosomal protein of Drosophila. Chromosoma. 1980;78:1–31. doi: 10.1007/BF00291907. [DOI] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Weinstein DM, Bell SP. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- Ascoli CA, Maul GG. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin RJ, Orr-Weaver TL, Bell SP. DrosophilaORC specifically binds to ACE3, an origin of DNA replication control element. Genes Dev. 1999;13:2639–2649. doi: 10.1101/gad.13.20.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SP, Kobayashi R, Stillman B. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- Bell SP, Stillman B. ATP-dependent recognition of eucaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- Biessmann H, Zurovcova M, Yao JG, Lozovskaya E, Walter MF. A telomeric satellite in Drosophila virilisand its sibling species. Chromosoma. 2000;109:372–380. doi: 10.1007/s004120000094. [DOI] [PubMed] [Google Scholar]
- Brown SW. Heterochromatin. Science. 1966;151:417–425. doi: 10.1126/science.151.3709.417. [DOI] [PubMed] [Google Scholar]
- Brutlag DL. Molecular arrangement and evolution of heterochromatic DNA. Annu Rev Genet. 1980;14:121–144. doi: 10.1146/annurev.ge.14.120180.001005. [DOI] [PubMed] [Google Scholar]
- Chien CT, Buck S, Sternglanz R, Shore D. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell. 1993;75:531–541. doi: 10.1016/0092-8674(93)90387-6. [DOI] [PubMed] [Google Scholar]
- Cryderman DE, Cuaycong MH, Elgin SCR, Wallrath LL. Characterization of sequences associated with position-effect variegation at pericentric sites in Drosophilaheterochromatin. Chromosoma. 1998;107:277–285. doi: 10.1007/s004120050309. [DOI] [PubMed] [Google Scholar]
- Csink AK, Henikoff S. Something from nothing: the evolution and utility of satellite repeats. Trends Genet. 1998;14:200–204. doi: 10.1016/s0168-9525(98)01444-9. [DOI] [PubMed] [Google Scholar]
- Dernburg AF, Sedat JW, Hawley RS. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell. 1996;86:135–146. doi: 10.1016/s0092-8674(00)80084-7. [DOI] [PubMed] [Google Scholar]
- Dobie KW, Hari KL, Maggert KA, Karpen GH. Centromere proteins and chromosome inheritance: a complex affair. Curr Opin Genet Dev. 1999;9:206–217. doi: 10.1016/S0959-437X(99)80031-8. [DOI] [PubMed] [Google Scholar]
- Dorer DR, Henikoff S. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell. 1994;77:993–1002. doi: 10.1016/0092-8674(94)90439-1. [DOI] [PubMed] [Google Scholar]
- Eissenberg JC, James TC, Foster-Hartnett DM, Hartnett T, Ngan V, Elgin SCR. Mutation in a heterochromatin-specific protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci USA. 1990;87:9923–9927. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti L, Giovinazzo G, Berloco M, Pimpinelli S. The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol Cell. 1998;2:527–538. doi: 10.1016/s1097-2765(00)80152-5. [DOI] [PubMed] [Google Scholar]
- Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behavior during the five mitotic cycles that precede gastrulation in Drosophilaembryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Fox CA, Loo S, Dillin A, Rine J. The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes Dev. 1995;9:911–924. doi: 10.1101/gad.9.8.911. [DOI] [PubMed] [Google Scholar]
- Frasch M, Paddy M, Saumweber H. Developmental and mitotic behavior of two novel groups of nuclear envelope antigens of Drosophila melanogaster. J Cell Sci. 1988;90:247–263. doi: 10.1242/jcs.90.2.247. [DOI] [PubMed] [Google Scholar]
- Gall JG, Atherton DD. Satellite DNA sequences in Drosophila virilis. J Mol Biol. 1974;85:633–664. doi: 10.1016/0022-2836(74)90321-0. [DOI] [PubMed] [Google Scholar]
- Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. Storing and purifying antibodies. , 726. [Google Scholar]
- Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- Heitz E. Das Heterochromatin der Moose. I Jahrb Wissensch Bot. 1928;69:762–818. [Google Scholar]
- Huang DW, Fanti L, Pak DTS, Botchan MR, Pimpinelli S, Kellum R. Distinct cytoplasmic and nuclear fractions of Drosophilaheterochromatin protein 1: their phosphorylation levels and associations with origin recognition complex proteins. J Cell Biol. 1998;142:307–318. doi: 10.1083/jcb.142.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TC, Eissenberg JC, Craig C, Dietrich V, Hobson A, Elgin SCR. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol. 1989;50:170–180. [PubMed] [Google Scholar]
- James TC, Elgin SCR. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogasterand its gene. Mol Cell Biol. 1986;6:3862–3872. doi: 10.1128/mcb.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen GH, Le MH, Le H. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophilafemale meiosis. Science. 1996;273:118–122. doi: 10.1126/science.273.5271.118. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Spradling AC. Analysis of subtelomeric heterochromatin in the Drosophilaminichromosome Dp1187 by single P element insertional mutagenesis. Genetics. 1992;132:737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R, Alberts BM. Heterochromatin protein 1 is required for correct chromosome segregation in Drosophilaembryos. J Cell Sci. 1995;108:1419–1431. doi: 10.1242/jcs.108.4.1419. [DOI] [PubMed] [Google Scholar]
- Kellum R, Raff J, Alberts BM. Heterochromatin protein 1 distribution during development and during the cell cycle in Drosophilaembryos. J Cell Sci. 1995;108:1407–1418. doi: 10.1242/jcs.108.4.1407. [DOI] [PubMed] [Google Scholar]
- Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- Kuo M-H, Allis CD. In vivo cross-linking and immunoprecipitation for studying dynamic protein: DNA associations in a chromatin environment. Methods. 1999;19:425–433. doi: 10.1006/meth.1999.0879. [DOI] [PubMed] [Google Scholar]
- Kurtz S, Shore D. RAP1 protein activates and silences transcription of mating-type genes in yeast. Genes Dev. 1991;5:616–628. doi: 10.1101/gad.5.4.616. [DOI] [PubMed] [Google Scholar]
- Landis G, Kelley R, Spradling AC, Tower J. The k43 gene, required for chorion gene amplification and diploid cell chromosome replication, encodes the Drosophilahomolog of yeast origin recognition complex subunit 2. Proc Natl Acad Sci USA. 1997;94:3888–3892. doi: 10.1073/pnas.94.8.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenson P, Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N, Le Saux A, Schuller J, Ptashne M. Chromatin components as part of a putative transcriptional repressing complex. Proc Natl Acad Sci USA. 1998;95:7322–7326. doi: 10.1073/pnas.95.13.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley DM. DNA opens up—supercoiling and heavy breathing. Trends Genet. 1988;4:111–114. doi: 10.1016/0168-9525(88)90099-6. [DOI] [PubMed] [Google Scholar]
- Locke J, Kotarski MA, Tartof KD. Dosage-dependent modifiers of position effect variegation in Drosophilaand a mass action model that explains their effect. Genetics. 1988;120:181–198. doi: 10.1093/genetics/120.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe A, Roberts P. Evolution of satellite DNA sequences in Drosophila. In: Verma RS, editor. Heterochromatin: Molecular and Structural Aspects. Cambridge, United Kingdom: Cambridge University Press; 1988. pp. 148–186. [Google Scholar]
- Lu BY, Bishop CP, Eissenberg JC. Developmental timing and tissue specificity of heterochromatin-mediated silencing. EMBO J. 1996;15:1323–1332. [PMC free article] [PubMed] [Google Scholar]
- McNally FJ, Rine J. A synthetic silencer mediates SIR-dependent functions in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5648–5659. doi: 10.1128/mcb.11.11.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D, Kistler A, Axelrod A, Rine J, Johnson AD. Silent information regulator protein complexes in Saccharomyces cerevisiae: a SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci USA. 1997;94:2186–2191. doi: 10.1073/pnas.94.6.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos GL, Cotsell JN. Chromosome structure at interfaces between major chromatin types: alpha- and beta-heterochromatin. Bioessays. 1990;12:1–6. doi: 10.1002/bies.950120102. [DOI] [PubMed] [Google Scholar]
- Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- Pak DTS, Pflumm M, Chesnokov I, Huang DW, Kellum R, Marr J, Romanowski P, Botchan MR. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell. 1997;91:311–323. doi: 10.1016/s0092-8674(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Peacock WJ, Appels R, Dunsmuir P, Lohe AR, Gerlach WL. Highly repeated DNA sequences: chromosomal location and evolutionary conservation. In: Brinkley BR, Porter KR, editors. International Cell Biology. New York: Rockefeller University Press; 1976. pp. 494–506. [Google Scholar]
- Peacock WJ, Lohe AR. Repeated sequences in the chromosomes of Drosophila. Symp R Entomol Soc Lond. 1980;10:1–12. [Google Scholar]
- Peacock WJ, Lohe AR, Gerlach WL, Dunsmuir P, Dennis ES, Appels R. Fine structure and evolution of DNA in heterochromatin. Cold Spring Harbor Symp Quant Biol. 1977;42:1121–1135. doi: 10.1101/sqb.1978.042.01.113. [DOI] [PubMed] [Google Scholar]
- Pillus L, Rine J. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell. 1989;59:637–647. doi: 10.1016/0092-8674(89)90009-3. [DOI] [PubMed] [Google Scholar]
- Pimpinelli S, Bonaccorsi S, Fanti L, Gatti M. Preparation and analyses of Drosophilamitotic chromosomes. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 3–23. [Google Scholar]
- Platero JS, Csink AK, Quintanilla A, Henikoff S. Changes in chromosomal localization of heterochromatin-binding proteins during the cell cycle in Drosophila. J Cell Biol. 1998;140:1297–1306. doi: 10.1083/jcb.140.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohler JR, Norman DG, Bramham J, Bianchi ME, Lilley DM. HMG box proteins bind to four-way DNA junctions in their open conformation. EMBO J. 1998;17:817–826. doi: 10.1093/emboj/17.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff JR, Kellum R, Alberts B. The DrosophilaGAGA transcription factor is associated with specific regions of heterochromatin throughout the cell cycle. EMBO J. 1994;13:5977–5983. doi: 10.1002/j.1460-2075.1994.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche SE, Rio DC. Trans-silencing of P elements in subtelomeric heterochromatin involves the Drosophilapolycomb group gene, enhancer of zeste. Genetics. 1998;149:1839–1855. doi: 10.1093/genetics/149.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S, Lehmann M, Nouaud D, Anxolabehere D. The regulatory properties of autonomous subtelomeric P elements are sensitive to a suppressor of variegation in Drosophila melanogaster. Genetics. 1996;143:1663–1674. doi: 10.1093/genetics/143.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S, Marin L, Lehmann M, Anxolabehere D. Repression of hybrid dysgenesis in Drosophila melanogasterby combinations of telomeric P-element reporters and naturally occurring P elements. Genetics. 1998;149:1857–1866. doi: 10.1093/genetics/149.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles A, Chong JP, Brown L, Howell M, Evan GI, Blow JJ. Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- Royzman I, Austin RJ, Bosco G, Bell SP, Orr-Weaver TL. ORC localization in Drosophila follicle cells and the effects of mutations in dE2F and dDP. Genes Dev. 1999;13:827–840. doi: 10.1101/gad.13.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumweber H, Symmons P, Kabisch R, Will H, Bonhoeffer F. Monoclonal antibodies against chromosomal proteins of Drosophila melanogaster: establishment of antibody producing cell lines and partial characterization of corresponding antigens. Chromosoma. 1980;80:253–275. doi: 10.1007/BF00292684. [DOI] [PubMed] [Google Scholar]
- Saunders WS, Chue C, Goebl M, Craig C, Clark RF, Powers JA, Eissenberg JC, Elgin SCR, Rothfield NF, Earnshaw WC. Molecular cloning of a human homolog of Drosophilaheterochromatin protein HP1 using anti-centromere autoantibodies with anti-chromo specificity. J Cell Sci. 1993;104:573–582. doi: 10.1242/jcs.104.2.573. [DOI] [PubMed] [Google Scholar]
- Schmid KJ, Nigro L, Aquadro CF, Tautz D. Large number of replacement polymorphisms in rapidly evolving genes of Drosophila. Implications for genome-wide surveys of DNA polymorphism. Genetics. 1999;153:1717–1729. doi: 10.1093/genetics/153.4.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid KJ, Tautz D. A screen for fast evolving genes from Drosophila. Proc Natl Acad Sci USA. 1997;94:9746–9750. doi: 10.1073/pnas.94.18.9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler J-S, Marchio A, Sitterlin D, Transby C, Dejean A. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc Natl Acad Sci USA. 1998;95:7316–7321. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Lis JT. A germline transformation analysis reveals flexibility in the organization of heat shock consensus elements. Nucleic Acids Res. 1987;15:2971–2988. doi: 10.1093/nar/15.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DAR, Mottus RC, Grigliatti TA. Genes which suppress position-effect variegation in Drosophila melanogasterare clustered. Mol Gen Genet. 1983;191:326–333. [Google Scholar]
- Stroumbakis ND, Li Z, Tolias PP. RNA- and single-stranded DNA-binding (SSB) proteins expressed during Drosophila melanogasteroogenesis: a homolog of bacterial and eukaryotic mitochondrial SSBs. Gene. 1994;143:171–177. doi: 10.1016/0378-1119(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Szostecki C, Guldner HH, Netter HJ, Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J Immunol. 1990;145:4338–4347. [PubMed] [Google Scholar]
- Tepass U, Knust E. Phenotypic and developmental analysis of mutations at the crumbs locus, a gene required for the development of epithelia in Drosophila melanogaster. Roux Arch Dev Biol. 1990;199:189–206. doi: 10.1007/BF01682078. [DOI] [PubMed] [Google Scholar]
- Torok T, Harvie PD, Buratovich M, Bryant PJ. The product of proliferation disrupter is concentrated at centromeres and required for mitotic chromosome condensation and cell proliferation in Drosophila. Genes Dev. 1997;11:213–226. doi: 10.1101/gad.11.2.213. [DOI] [PubMed] [Google Scholar]
- Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- Walter MF, Jang C, Kasravi B, Donath J, Mechler BM, Mason JM, Biessmann H. DNA organization and polymorphism of a wild-type Drosophilatelomere region. Chromosoma. 1995;104:229–241. doi: 10.1007/BF00352254. [DOI] [PubMed] [Google Scholar]
- Whitfield LS, Lovell-Badge R, Goodfellow PN. Rapid sequence evolution of the mammalian sex-determining gene SRY. Nature. 1993;364:713–715. doi: 10.1038/364713a0. [DOI] [PubMed] [Google Scholar]
- Wintersberger E. Why is there late replication? Chromosoma. 2000;109:300–307. doi: 10.1007/s004120000080. [DOI] [PubMed] [Google Scholar]
- Wustmann G, Szidonya J, Taubert H, Reuter G. The genetics of position-effect variegation modifying loci in Drosophila melanogaster. Mol Gen Genet. 1989;217:520–527. doi: 10.1007/BF02464926. [DOI] [PubMed] [Google Scholar]
- Zhang P, Spradling AC. The Drosophilasalivary gland chromocenter contains highly polytenized subdomains of mitotic heterochromatin. Genetics. 1995;139:659–670. doi: 10.1093/genetics/139.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]