Abstract
Bacterial swarming is an example of dynamic self-assembly in microbiology in which the collective interaction of a population of bacterial cells leads to emergent behavior. Swarming occurs when cells interact with surfaces, reprogram their physiology and behavior, and adapt to changes in their environment by coordinating their growth and motility with other cells in the colony. This review summarizes the salient biological and biophysical features of this system and describes our current understanding of swarming motility. We have organized this review into four sections: 1) The biophysics and mechanisms of bacterial motility in fluids and its relevance to swarming. 2) The role of cell/molecule, cell/surface, and cell/cell interactions during swarming. 3) The changes in physiology and behavior that accompany swarming motility. 4) A concluding discussion of several interesting, unanswered questions that is particularly relevant to soft matter scientists.
1. Introduction
Microbiology is replete with examples of dynamic self-assembly that span subcellular, cellular, and multicellular length scales and are characterized by interactions between many components.1 Biofilms, spores, swarms, mycelia, and fruiting bodies are multicellular structures in which dynamic self-assembly controls the organization and function of populations of cells. We refer to dynamic self-assembly in the context of a system that has the following characteristics: 1) it consists of many components that interact spontaneously and reversibly via non-covalent forces; 2) it is far from equilibrium; and 3) the components form ordered structures (and patterns) only when the system is continuously dissipating energy. The emergence of collective behavior or multicellularity in bacteria arises from the dynamic self-assembly of densely packed populations of cells based on stimuli and mechanisms that are poorly understood.2, 3 This phenotype is relevant to a range of fields, including ecology, agriculture, and biomedicine and research in this area has applications in both fundamental and applied science.2, 4 The principles of emergent behavior that are uncovered in the study of microbial self-assembly may be applied to areas that extend far beyond biology and microbes into fields such as economics, weather, and population dynamics.
Eubacteria have a number of characteristics that make them an excellent experimental model for studying dynamic self-assembly. 1) Laboratory strains are relatively easy to culture and grow rapidly into high-density populations of cells in liquid and on surfaces. 2) A wealth of chemical, biochemical, and genetic data is available for different genera and make it possible to systematically perturb and study bacteria. 3) The genome of many bacterial species has been sequenced and repositories and genetic stock centers catalog, store, and distribute mutants of model organisms, including Escherichia coli (E. coli), Bacillus subtilis (B. subtilis), Salmonella enterica serovar Typhimurium (S. typhimurium), and Pseudomonas aeruginosa (P. aeruginosa). Thus it is straightforward to obtain knockouts rapidly for a reasonable fee, especially for E. coli where single and double knockouts of the entire genome have been created.5–7 4) A variety of biophysical techniques are available for manipulating individual or populations of bacterial cells on surfaces and in liquids.8, 9
One of the more interesting examples of dynamic self-assembly in microbiology is a phenotype observed in many strains of flagellated bacteria referred to as ‘swarming’.10 The introduction of the phrase ‘swarm’ was probably chosen to capture the dynamic, swirling patterns of multicellular movement reminiscent of populations of swarming bees and other insects (Movies S1–S4).11 Swarming motility occurs when planktonic cells contact surfaces and replicate to a high-density population of cells. It is unclear whether planktonic cells initially adsorb on and remain in close physical contact to surfaces or whether they are separated from surfaces by a thin layer of fluid; we discuss the latter scenario in more detail later in the review. The transition from the growth of cells in liquids to surfaces is accompanied by changes in their phenotype in many species of bacteria. Populations of swarming bacterial cells migrate collectively across surfaces at an approximate rate of 2–10 µm·sec−1 and consume nutrients in the underlying medium.12 There are three basic requirements for bacterial swarming motility: 1) cells are motile and have functional flagella; 2) cells are in contact or close proximity to surfaces; and 3) cells are in contact with other motile cells (Table 1).
Table 1.
A comparison of the characteristics of planktonic and swarmer cell for several swarming genera of Eubacteria. Abbreviations: lipopolysaccharide (LPS); 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs); peritrichous (Per.); polar (Pol.). Question marks indicate unknown or suggested factors or characteristics of swarming motility. The percentage of agar that is required to induce swarming in E. coli refers to Eiken agar; E. coli K12 strains no not swarm on the same percentage of Difco agar.
| Planktonic cell | Swarmer cell | |||||||
|---|---|---|---|---|---|---|---|---|
| Organism | Cell Length (µm) |
Number of flagella |
Cell length (µm) |
Increase in flagella relative to planktonic |
Rate of surface migration (µm min−1) |
Secreted surfactant or wetting agent |
Agar % (w/v) that Supports swarming |
Mechanisms that affect swarmer cell development |
|
B. subtilis (undomesticated strains) |
1–2.548 | 5–10 (Per.) |
3–648 | 10×163 | 167–23348 | Surfactin48 | 0.5–0.748 | Extracellular serine protease (Epr);91 ComX pheromone164 |
| E. coli K12 | 2–457 | 4–7 (Per.)23 |
5–2057 | 2–3×57 | 120–60057 | LPS; O-antigen?70, 76 | 0.45–0.5 (Eiken)57 | Chemotaxis system144 |
| P. mirabilis | 1–279 | 6–10 (Per.)165 | 10–8079 | 10–50×13 | 950;13 70010 | Colony migration factor78 | 1.5–313 | Inhibition of flagellar rotation;14 Glutamine;143 Putrescine166 |
| P. aeruginosa | 1–249 | 1 (Pol.)162 |
2–349 | ≥ 2× (Pol.)49 | ? | Rhamnolipids (Mono/Di); HAAs;97–99 Exopolysaccharide (alginate)49 |
0.45–0.5;49 0.5–0.7162 | Nitrogen limitation162; Quorum sensing97 |
| V. parahaemolyticus | 1.5–2121 | 1 (Pol.)121 |
30–4014, 121 | 10–50×121, 127 | 12513 | ? | 1.5–214 | Inhibition of flagellar rotation;122 Iron limitation;126 Chemotaxis system14 |
| S. typhimurium | 2–457 | 6–10 (Per.)22 | 5–2057 | 2–3×57 | 120–60057 | LPS;70 FlhE?167 | 0.5–0.857 | Chemotaxis system147 |
|
S. marcescens/ S. liquefaciens |
0.5–260 | 1–2 (Per.)60 |
5–3014, 60 | 2–3×59, 83 | 8813 | Serrawettin85 | 0.6–0.813 | Inhibition of flagellar rotation;14 Quorum sensing;85 Chemotaxis system14 |
Much of our understanding of this field was pioneered by Belas, Harshey, Henrichsen, Hughes, Shapiro, Williams, and others and is based on studying the genetics and biochemistry of swarming.3, 4, 10–17 In this review we focus on introducing scientists and engineers that study soft condensed matter to a system of dynamic bacterial self-assembly that will benefit greatly from the introduction of polymeric materials and the physical techniques of materials science and engineering. We have divided this review into four sections that focus on the following areas: 1) the biophysical components of bacteria cell motility in fluids and its relevance to swarming; 2) the role of cell/molecule, cell/surface, and cell/cell interactions during swarming; 3) the changes in physiology and behavior that accompany swarming motility; and 4) a concluding discussion of several interesting, unanswered questions that is particularly relevant to soft matter scientists.
2. An introduction to bacterial motility
Many genera of bacteria use flagella for their motility, which are actuated by the cell and perform work on the surrounding fluid.18 Our current understanding of motility in bacteria indicates that the mechanisms used by planktonic cells to translate through bulk fluids are closely related to or are identical to the mechanisms used by swarming cells. To introduce the reader to this area, we provide a description of the motility system in bacteria and the physics of fluids at the length scale of bacterial cells that plays a fundamental role in their motility. We focus on E. coli and S. typhimurium as these model organisms were used to dissect much of what we currently know about the role of flagella in bacterial motility.
a. Expression of flagellar proteins
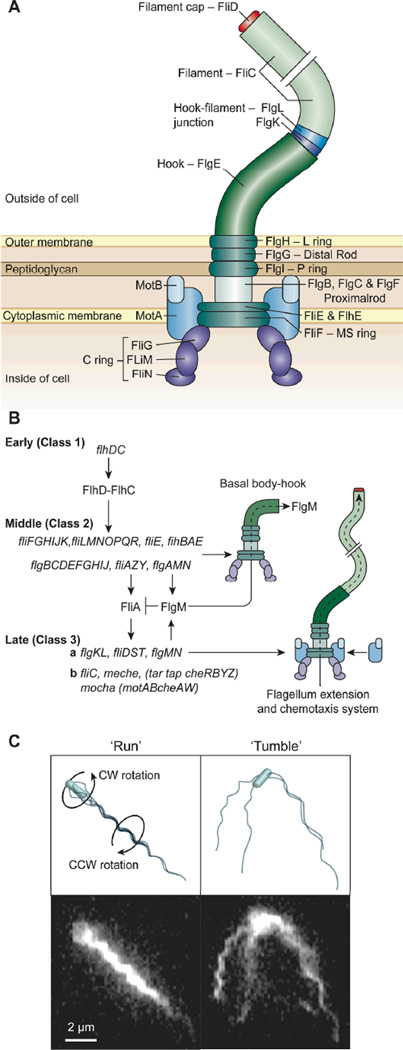
The biosynthesis, assembly, and function of the flagellum involves more than 50 genes that are divided across several operons.19 The expression of this flagellar regulon is divided into three hierarchical, temporally transcribed classes of genes: early (class 1), middle (class 2), and late genes (class 3). Global regulatory signals influence transcription of the early genes, flhD and flhC, which constitute the flhDC operon. FlhD and FlhC are transcriptional activators that direct transcription of the middle flagellar genes from their upstream class 2 promoters. The middle genes are responsible for expression of the basal body and hook of the flagella, as well as the class 3 promoter sigma factor, σ28 (FliA), and its corresponding anti-sigma factor, FlgM. Following completion of the basal body-hook structure, FlgM is secreted out of the nascent flagellum, allowing σ28 to activate transcription of the late (class 3) flagellar and chemotaxis genes responsible for synthesis of the flagellar filament and motility components (Figure 1b).19 The structure of the flagellum, the properties of this organelle, and its role in motility are described below.
Figure 1.
Structure, expression, assembly, and function of bacterial flagella. A) A cartoon depicting the components of flagella discussed in this review; some elements have intentionally been removed for clarity.34 B) A diagram of the hierarchy of the expression of flagellar genes in E. coli161 C) Fluorescence microscopy images of swimming E. coli cells and corresponding cartoons depicting a cell ‘running’ and ‘tumbling’.38 The cartoon on the top left depicts a cell with bundled flagella ‘running’; the image below shows a live cell in this configuration. When the cell ‘runs’ the bundle of flagella rotate CCW (as seen from behind the cell) and the cell body rotates CW. The cartoon on the top right depicts a cell ‘tumbling’ in which the flagella are splayed outward; the image immediately below shows a live cell in this configuration. Images are reprinted or modified with permission from A) Nature Publishing Group, Copyright 2008, B) American Association for the Advancement of Science, Copyright 2001, C) American Society for Microbiology, Copyright, 2000.
b. Structure and function of the flagellum
Bacteria have evolved efficient mechanisms of motility in bulk fluids and on surfaces using flagella. Swimming cells of E. coli and S. typhimurium are peritrichous and actuate 6–8 flagella to translate through fluids.20, 21 Each flagellum consists of three regions: 1) helical filament; 2) hook; and 3) basal body and motor components (Figure 1b).22 The helical filament is a polymer of the flagellin protein (FliC) which terminates in a filament cap (FliD).23 Eleven parallel rows of FliC form a left-handed helical filament, which may be up to 15-µm long with a diameter of 12–25 nm.24, 25 The filament is connected to the flagellar hook, which is a polymer consisting of the FlgE protein and the hook-filament junction proteins FlgK and FlgL.23 The basal body of the flagellum is embedded in the cell wall of Gram-negative bacteria and consists of four rings and a rod. The rings are located in distinct regions of the membrane and are illustrated in Figure 1a.23, 26
The rotary motor of the bacterial flagellum consists of a stator (MotA and MotB) and a rod (FlgB, FlgC, FlgF, and FlgG).25, 27 The components of the stator are stationary and embedded in the basal body; the rotor consists of FliG attached to the MS-ring (FliF). Together, the rotor and the stator (Mot proteins) generate torque.25, 28 A proton motive force, or in some circumstances, a gradient of sodium ions, causes the flagellar motor to rotate.29–31 The passage of protons through channels in MotA and MotB creates a conformational change in the stator, which subsequently turns the rotor.32, 33 The C-ring consists of FliG, FliM, and FliN and functions as a switch complex and rotor to control the direction of rotation of the motor that actuates the flagella. A recent review by Chevance and Hughes provides an excellent overview of the components of the bacterial flagellum.34
Rotation of the flagellar motor in the counterclockwise direction (CCW)—as viewed from the distal end of the filament—causes the left-handed helical flagellar filaments to form a bundle, which propels the cell forward in a phase of motility referred to as a ‘run’ (Figure 1c).35 The torque produced by rotating the bundle of flagella at an angular velocity of ~100 Hz is balanced by viscous drag from the clockwise (CW) rotation of the cell body around its long axis at a frequency of ~10 Hz. The viscous drag on flagella moving normal to its long axis produces thrust. When one or more motors rotate CW the flagella assume a right-handed waveform, the bundle of flagella unravels, and the cell ‘tumbles’ in place.12, 36–38 Motility in planktonic cells of E. coli consists of two components: 1) periods of running which typically lasts for 1 sec; and 2) randomly interspersed tumbles that last for ~0.1 sec.39 The interplay between smooth swimming and tumbling results in a random walk. The direction of motor rotation is biased by extracellular signals, including chemoattractants (e.g. sugars, amino acids, and dipeptides) and chemorepellents (e.g. phenol, Ni2+, and Co2+).23, 40–43 The chemotaxis system and the switching between directions of motor rotation play a role in swarming and are discussed in more detail in section 4c.
c. Viscosity
All motile microorganisms live in a regime in which viscosity dominates over inertial forces due to the intrinsic dimensions of cells and the velocity at which they translate through fluids.44, 45 This regime is characterized by a low Reynolds number (Re) which is a unit-less parameter that describes the ratio of inertial and viscous forces acting on a particle moving through a fluid. The Reynolds number is expressed as Re = lvρ/µ, where l is the length scale of the moving object (µm), v is the velocity (µm·sec−1), ρ is the density (g·cm−3), and µ is the viscosity (kg·m−1·sec−1). Swimming cells of E. coli (~2-µm long, 800-nm wide) translate through fluids with a velocity approaching 20–30 µm·sec−1 and a Re of ~10−5, which is similar to most other motile microorganisms.44 Bacteria are motile in fluids that have a narrow range of viscosity and are only sensitive to viscosity modifying agents that have a length scale that is smaller than the dimensions of cells (e.g. Percoll, Ficoll, or other branched polymers).
Several groups have investigated the motion of E. coli cells in aqueous solutions of polymers and have characterized the affect of the microviscosity of the fluid on cell motility.46 Greenberg and Canale-Parola observed that motility increased to a maximum of 30 µm·sec−1 at 8×10−3 Pa·s and then decreased exponentially with increasing viscosity, reaching 0 µm·sec−1 at 6×10−2 Pa·s.47 As we discuss in Section 3b, swarming is accompanied by the secretion of large amounts of polymers and other surface-active compounds, which modify the properties of fluids and surfaces and may increase viscosity beyond the normal range in which cells are motile. An important unanswered question is whether swarming cells, which are often longer and have more flagella than planktonic cells, are capable of motility in higher viscosity fluids. Can cells increase their torque by expressing and actuating a more flagella? An answer to this question will help us better understand the motility of cells on surfaces and in densely packed multicellular structures (e.g. biofilms).
3. Cell-surface and cell-cell interactions
Swarming begins with the introduction of one or more cells on a hydrogel surface. In the lab this is typically accomplished by inoculating the center of a gel with a small droplet of a liquid suspension of cells. The excess liquid is absorbed by the hydrogel and the cells are brought into contact with the surface. Strains of swarming bacteria that are in contact with hydrogel surfaces undergo a complex, and poorly understood developmental cycle of differentiation, motility, and dedifferentiation (Figure 2). When cells differentiate into the swarming phenotype, they generally elongate into multinucleate filaments and the density of flagella on their surface increases 3–50x per unit area of cell surface.10 This is not always the case, however, as some swarming genera, including species of Bacillus and Pseudomonas, do not elongate and may only double the number of flagella per unit area of cell surface after differentiation.48, 49 The addition of water to a swarming colony causes the cells to rapidly disperse into the fluid and appears to promote dedifferentiation.
Figure 2.
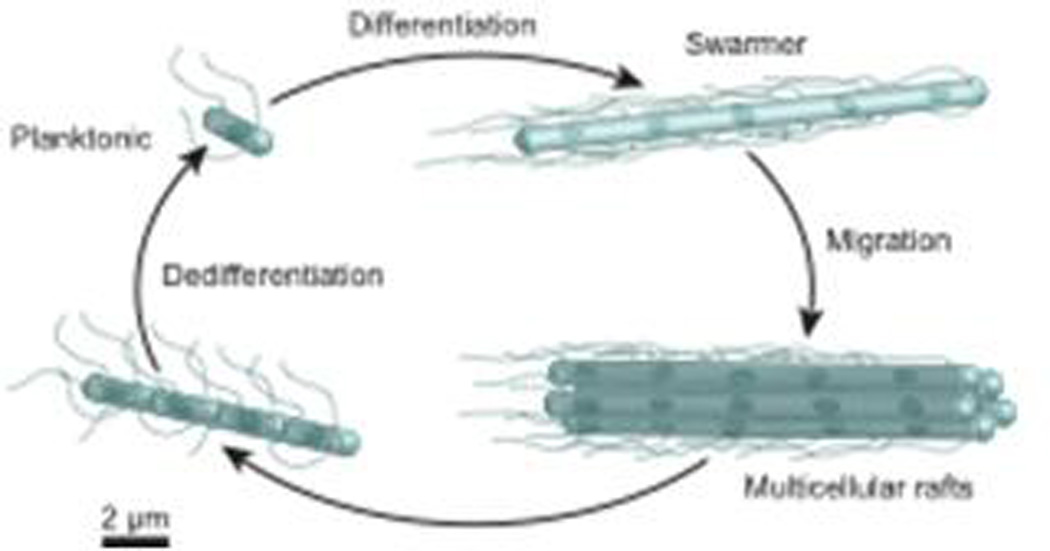
A cartoon depicting the general ‘life cycle’ of motile cells of bacteria as they swarm on surfaces. The length of the flagella (in relation to the length of cells) and the number of flagella per swarmer cell has been reduced for clarity. The blue spot depicts the bacterial chromosome. The scale bar is an approximate estimate of dimensions.
Individual, isolated swarming cells are not motile on surfaces. As the cells replicate and the population increases there is a threshold in cell density beyond which the cells begin moving collectively. Following the inoculation of swarm agar, the timeframe for the development of swarming motility from a single isolated cell is not universally known, although it has been suggested that the initiation of swarming motility depends on the water content of the substrate.50 The lag phase that occurs before the onset of swarming motility in P. mirabilis involves cell growth, differentiation into swarmer cells, and the dynamic self-assembly of swarmer cells into multicellular packs that migrate collectively across surfaces.51 The lag phase for P. mirabilis swarming is inversely proportional to the concentration of the cells on the substrate.51, 52 Models of P. mirabilis swarming that are based on experimental data suggest a threshold value of ~10−3 cells·µm−2 is required to initiate differentiation into the swarmer phenotype.51–53 It is currently unclear why a quorum is required before the cells begin translating on substrates.
Populations of swarmer cells migrate cooperatively and rapidly across surfaces in search of new resources (Figure 3). In swarming strains of E. coli, cells at the edge of a swarm colony are differentiated, arranged in a monolayer, and are relatively immobile. These cells appear to be pinned at the surface/liquid interface due to surface tension but this idea has not been conclusively confirmed. Cells located directly behind the monolayer (i.e. toward the center of the colony) are differentiated and are among the most rapidly moving cells in the colony. These cells typically form a multilayer that is several layers thick and they collide with swarmers at the edge, exchanging places with them, which pushes the population outward. Closer to the center of the colony, the cells become less motile and are stacked in a three-dimensional structure that is many layers thick. These cells are present in both the differentiated and dedifferentiated states.12, 13
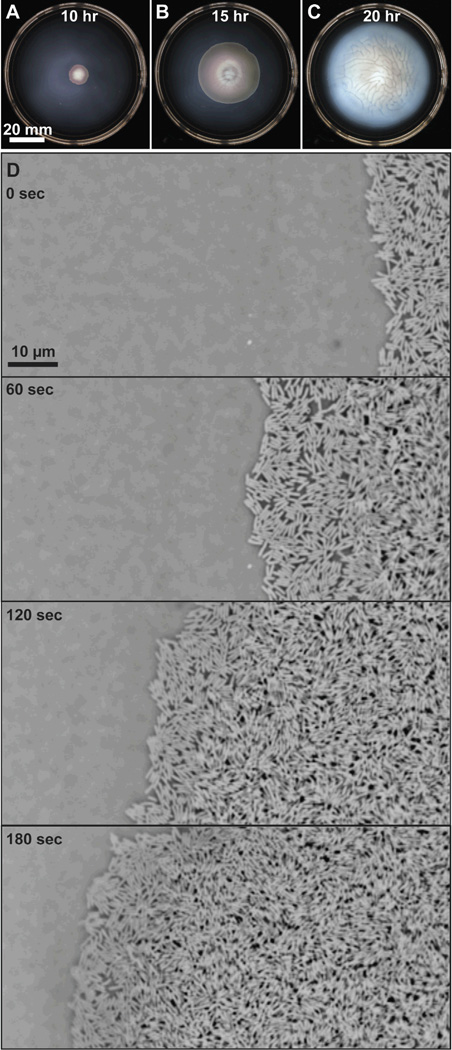
Figure 3.
A-C) Time lapse images depicting the macroscopic migration of a swarming colony of wild-type E. coli strain RP437 on the surface of 0.45% Eiken agar (w/v) infused with nutrient broth (1.0% peptone, 0.5% NaCl, 0.3% beef extract, and 0.5% glucose). The agar solidified overnight at 25 °C before the center of the gel was inoculated with 2 µl of a saturated overnight culture of bacteria. The plate was incubated at 30 °C. The expansion of the swarm colony over time is shown at 10, 15, and 20 hours after inoculation. D) A sequence of microscopy images that demonstrate the time-dependent spreading of swarmer cells across an agar surface. The panels show the progression of the edge of the swarm colony over a three-minute period. The media and inoculation procedure was identical to the conditions used for images A-C. The images were acquired using phase contrast microscopy and a CCD camera; the images were inverted to improve the contrast between the cells and background. The cells are moving from the right-hand side of the image to the left.
A tracking algorithm was used recently to study the velocity fields and pattern formation of swarming cells of Serratia marcescens.54 The study found that the average cell velocity and maximum correlation length was largest at a region 500 µm−1 mm away from the edge of a swarming colony and that the motion decreased suddenly at a region 2−4 mm away from the edge. These data are in agreement with earlier qualitative observations made by other groups.54
a. Cell-surface interactions
Surface motility appears to require ‘wet’ substrates.10, 12 In fact, the presence of water in the underlying substrate appears to be one of the most critical determinants of swarming. A number of lines of evidence suggest that swarming cells move through a two-dimensional layer of fluid that the cells extract from the substrate;51, 55 the exception to this rule are strains of Pseudomonas syringae, which can swarm on leaves and other plant tissues by moving through their own secreted lubricant.56 The most widely used materials for studying swarming have been agar, in part, because the polymer is biologically compatible, inexpensive, readily available, simple to prepare, and is not decomposed by bacteria. Agar gels contain a large fraction of bound water with very little ‘free’ water on the surface. The concentration of agar that supports the swarming phenotype varies across genera. Many genera, including species of Escherichia,57Salmonella,57Bacillus,11, 58 and Serratia59, 60 swarm rapidly across 0.45−0.8% agar (w/v) infused with nutrient-rich media but do not move on higher concentrations of agar. Species of Vibrio15 and Proteus17, 51, 61 swarm on the surface of higher concentrations of agar (1.5−2%, w/v) and have been reported to move on several abiotic surfaces.12, 62, 63
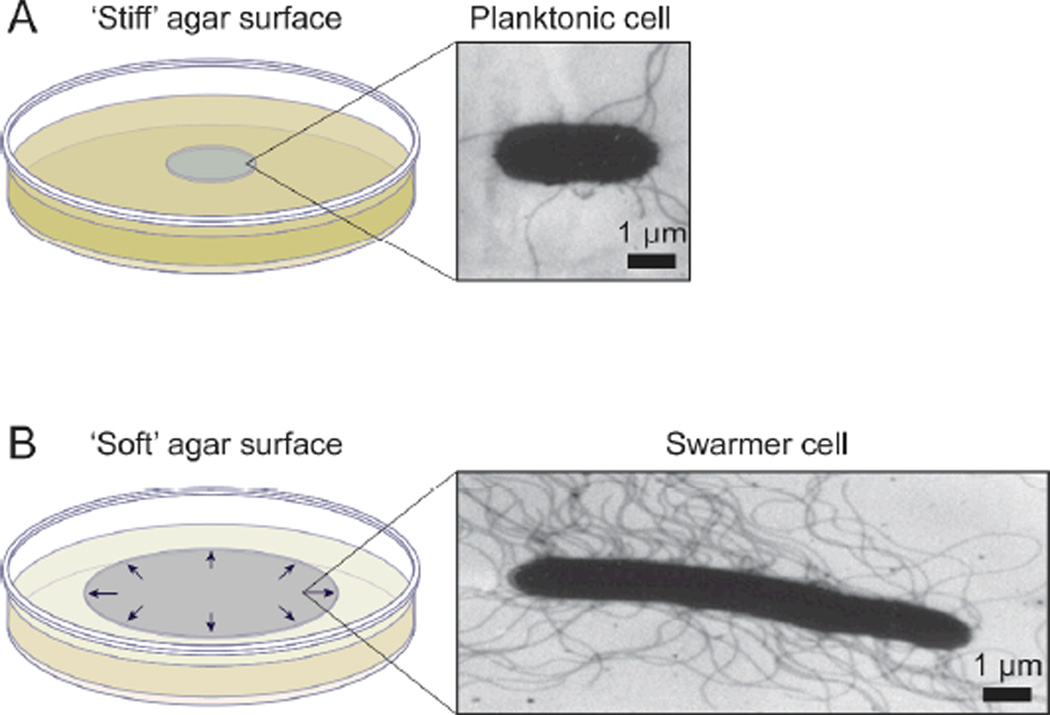
When cells are grown on the surface of agar gels (>2%, w/v) that typically do not support swarming motility, the cells grow into nonmotile colonies and retain their planktonic morphology and phenotype (Figure 4). Conversely, cells that are introduced on the surface of nutrient-limited hard agar grow slowly and form a colony that resembles a fractal, which can be predicted by the diffusion-limited aggregation (DLA) model.64, 65 The term ‘colony’ in this review is context dependent and can be used to describe both non-motile populations of planktonic cells and migrating populations of swarming cells; the distinction is illustrated in Figure 4.
Figure 4.
A) A schematic diagram depicting a colony of bacteria grown on the surface of a ‘stiff’ agar gel (e.g. 1.5%, w/v) and a characteristic planktonic cell isolated from the colony. The TEM image shows a planktonic cell of S. liquefaciens MG1.83 B) A swarming colony of bacteria grown on the surface of a ‘soft’ agar gel (e.g. 0.45%, w/v) and a TEM image of a swarmer cell of S. liquefaciens MG1 isolated from the outer edge of a swarm colony.83 The arrows depict the radial outward expansion of the colony on the surface. TEM images in A and B are reprinted with permission from the American Society for Microbiology, Copyright 1999.
It is well known that on concentrations of agar <0.4% (w/v) the pore size of the gel is larger than the radius of most bacterial cells and they penetrate into the polymer network and swim. This phenomenon is related to a possible source of confusion surrounding the terminology ‘swarm’. Adler and other pioneers of bacterial chemotaxis first used this phrase to refer to the motility of cells within low concentration agar plates (typically 0.25% agar, w/v).40, 66 Thus, the phrase ‘swarming’ has unfortunately also been used interchangeably with ‘swimming’, and has confused this nomenclature.67 In this review, we refer to swarming exclusively in the context of surface migration.
A narrow concentration range of agar (0.45−0.5%, w/v) supports swarming of E. coli K-12 strains and may be related to the lack of an intact O-antigen on the lipopolysaccharide (LPS) of these cells. The O-antigen is a component of the outer membrane that may play a role in the wettability of cells on hydrogel surfaces through its influence on the surface energy of cells.68–71 Harshey and colleagues used Eiken agar to recover swarming motility in mutants of S. marcescens that are defective in surfactant production, and mutants in LPS biosynthesis in S. typhimurium typhimurium.12, 70, 72 The chemical composition of this material differs from other sources of agar on which these cells were not motile.57 Unfortunately, the heterogeneity of agar makes it very difficult to pinpoint how this material complements these strains.
A fundamental unanswered question in this area is what is the limitation of surfaces that support swarming? The systematic investigation of the chemical and physical properties of materials required for swarming motility will be an important step forward for this field. The development of chemically characterized hydrogels as substitutes for agar will make it possible to precisely vary the conditions of substrates and observe how these parameters affect swarming. A variety of techniques are available for modifying hydrogels that may be particularly useful in this area of microbiology.73–75 We are not arguing for the global replacement of agar. Instead, the introduction of more homogenous polymeric materials may pinpoint physical and chemical ‘triggers’ for the emergence of this behavior.
b. Surface-active molecules
Several groups have suggested that swarming cells translate across surfaces using a mechanism that resembles swimming motility in a two-dimensional layer of fluid that is extracted from the underlying gel by syneresis (e.g. the contraction of a gel accompanied by the exudation of liquid).51, 55, 76 This layer of fluid may be formed during or after the secretion of extracellular biomolecules by swarming cells, which modify the surface tension of water and makes it possible for cells to become immersed in a thin film of fluid.4, 11, 12 There are primarily four classes of compounds that modify the surface tension of liquids and are secreted by bacteria: polysaccharides, lipopolysaccharides, lipoproteins, and glycolipids. In the following sub-sections we review their role in swarming.
Polysaccharides
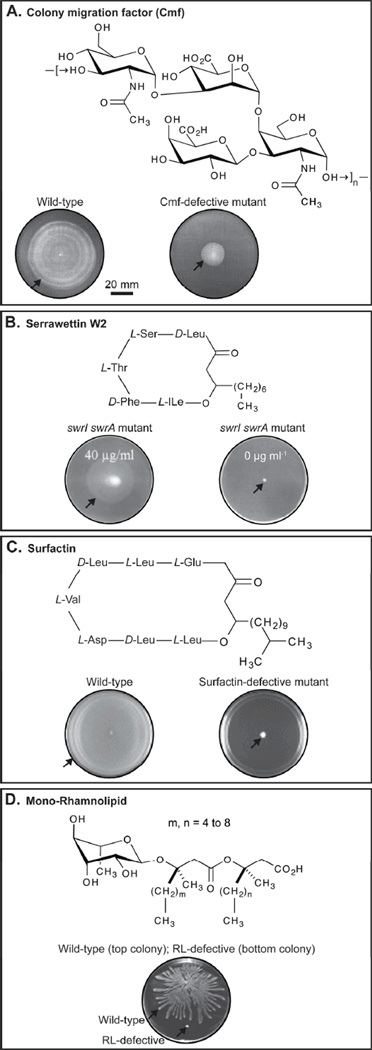
Swarmer cells secrete a matrix that consists of polysaccharides, peptides, proteins, and other compounds.12, 70, 77, 78 Polysaccharides are the predominant component of the mixture produced by P. mirabilis during swarming migration.79, 80 Gygi et al. isolated a mutant of P. mirabilis that does not migrate across surfaces.78 This mutation mapped to an open-reading frame responsible for the assembly of the ‘colony migration factor’, an acidic capsular polysaccharide that may be responsible for extracting fluid from the underlying agar (Figure 5a).51, 81 Boles et al. demonstrated that the increase in production of capsular polysaccharides in V. parahaemolyticus decreases swarming motility and promotes the adhesion of cells to surfaces, suggesting that these polymers may have different roles in surface colonization in different organisms.82
Figure 5.
Examples of surface-active molecules involved in swarming migration. A) The structure of colony migration factor (CMF) from P. mirabilis. The two images show the swarming migration of wild-type P. mirabilis (left) and a mutant defective in production of CMF on agar gels.114 B) The structure of serrawettin W2. The images show the rescue of swarming motility on agar gels of a S. liquefaciens MG1 mutant (swrI/swrA) that does not synthesize serrawettin W2.85 The concentration of serrawettin W2 added to each plate is indicated. C) The structure of surfactin. The swarm plate on the left displays the swarming migration of wild-type B. subtilis strain 3610. The swarming defective B. subtilis mutant (right) contains a mutation in the srfAA gene, which encodes a translation product important for surfactin synthesis.92 D) The structure of mono-rhamnolipid. The image shows the swarming migration of wild-type P. aeruginosa (top structure) and an rhlA mutant (bottom structure), which is unable to synthesize rhamnolipids, on agar gels.162 The black arrows on each agar plate depict the edge of the swarming colony. Images are reprinted with permission from (A) Blackwell Science Ltd, Copyright 1995, (B) American Society for Microbiology, Copyright 1998, (C) Blackwell Publishing Ltd, Copyright 2004, and (D) American Society for Microbiology, Copyright 2000.
Lipopolysaccharides
Toguchi et al. screened S. typhimurium mutants for deficiencies in swarming motility and isolated several mutants that were defective in LPS biosynthesis.70 The authors suggest that LPS plays a role in surface motility by modulating the wettability of the cell surface. S. typhimurium mutants of waaG, a gene involved in the biosynthesis of the LPS core structure, produced extensive amounts of extracellular matrix, which led to the early onset of swarming.70 Chen et al. demonstrated recently that swarming cells of S. typhimurium use an osmotic agent—which may be LPS—to extract fluid from agar.76
Lipopeptides
The differentiation of Serratia liquefaciens MG1 (S. liquefaciens) into swarmers and their collective spreading is controlled by the flhDC flagellar master regulator and a quorum-sensing biosynthetic pathway, respectively.59, 83–85 Cells use the quorum-sensing signal molecules, C4-HSL (N-butanoyl-L-homoserine lactone) and C6-HSL (N-hexanoyl-L-homoserine lactone) to sense the density of cells in the nascent swarming population.83, 86 The S. liquefaciens quorum-sensing system uses SwrI, an N-acyl-L-homoserine lactone (AHL) synthase, and SwrR, a transcriptional repressor and a receptor for the signal generated by the AHL synthase; collectively, these components activate transcription of swrA, which encodes a protein complex responsible for production of serrawettin W2.83, 87–90 Serrawettin W2 is a cyclic lipodepsipentapeptide that reduces the surface tension of water and is essential for Serratia swarming migration; the addition of serrawettin W2 rescued the surface motility of swrI and swrI/swrA mutants (Figure 5b).83, 85, 88
B. subtilis secretes a surface-active agent, surfactin, during the colonization of agar surfaces.91–93 Kearns and Losick discovered that surfactin was essential for swarming migration in B. subtilis.48 Laboratory strains of B. subtilis that do not swarm contain a frameshift mutation in the sfp gene required for surfactin synthesis.94 In addition to the sfp gene, the srf operon—consisting of srfAA, srfAB, and srfAC—encodes the enzyme complex, surfactin synthetase, which is essential for swarming (Figure 5c).92, 95,48
Glycolipids
Rhamnolipids are a class of amphiphilic glycolipids secreted primarily by the Gram-negative opportunistic human pathogen, P. aeruginosa.96 These compounds reduce the surface tension of liquids and are implicated in pathogenesis. Swarming colonies of P. aeruginosa have a characteristic pattern of tendrils that radiate away from the center of the swarm plate that is partially due to the secretion of rhamnolipids.97,98P. aeruginosa produces two types of rhamnolipids: L-rhamnosyl-3-hydroxydecanoyl-3-hydroxydecanoate (mono-RL) and L-rhamnosyl-L-rhamnosyl-3-hydroxydecanoyl-3-hydroxydecanoate (di-RL).96 These two compounds and the rhamnolipid precursor, 3-(3-hydroxyalkanoyloxy)alkanoic acid (HAA) are secreted by P. aeruginosa and facilitate swarming motility (Figure 5d).97–99 Tremblay et al. demonstrated recently that the characteristic swarming pattern of P. aeruginosa arises from the properties of di-RLs, mono-RLs, and HAAs.97 Their data suggests that di-RLs are chemoattractants, HAAs are chemorepellents, and mono-RLs are wetting agents.97 None of these molecules appears to affect swimming motility.
c. Cell/cell interactions
In contrast to the growing body of literature on the role of physical interactions between mammalian cells that regulate physiology and behavior 100, 101, our understanding of cell/cell interactions in bacteria is still unclear. Cell-cell interactions are particularly relevant to swarming bacteria in which a large population of cells is packed into a confined volume. Physical interactions between bacterial cells may be responsible for many of the observed phenotypes associated with swarming, including: 1) alignment of adjacent bacterial cells and their coordinated movement in ‘rafts’; 2) formation of dynamic, circular vortices; and 3) coordination of the motility of colonies across surfaces. Below we summarize data that suggests that contact between cells may be important in swarming motility, behavior, and emergence.
Interactions between translating swarming cells
Physical contact between swarming cells is important for surface migration as individual swarming cells isolated from a colony do not move unless the agar is supplemented with a surfactant or has a layer of liquid on the surface.12 Several early observations of Proteus demonstrated that swarmer cells align parallel to their long axis and migrate across surfaces as multicellular rafts (Figure 6a).4, 10, 102 This behavior may be due to the interactions between flagella of adjacent swarming cells during their migration across surfaces in high density populations of cells.13 In a study of the extracellular matrix surrounding swarming colonies of P. mirabilis, Stahl and Williams discovered the presence of flagellar bundles between adjacent swarmer cells using transmission electron microscopy (TEM).79 Jones and coworkers confirmed this observation in cells of P. mirabilis swarming across catheters using scanning electron microscopy (SEM) and TEM (Figure 6e,f).103 Furthermore, the reversal of the flagellar motors is important for swarming motility and suggests a potential mechanism for flagella to unbundle and rebundle with organelles on adjacent cells. It is unclear how the structure and dynamics of flagellar bundles on swarmer cells function during coordinated motility. The confirmation of the bundling of flagella between live, unfixed swarmer cells, will be an important step forward in understanding whether flagella/flagella interactions between cells plays a role in the coordinated motility of swarming cells.
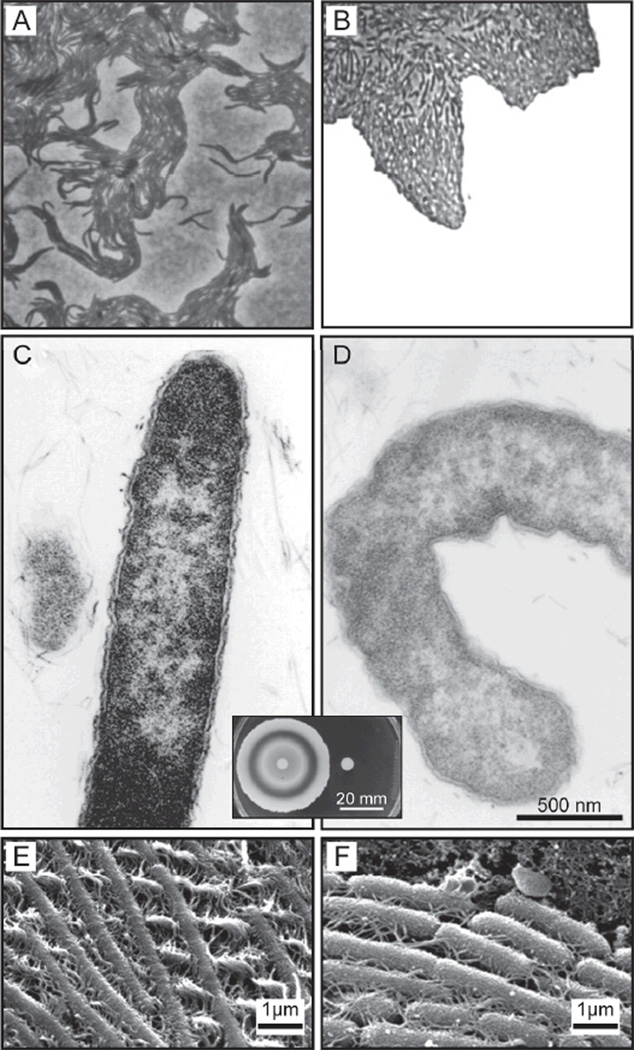
Figure 6.
Examples of cell-cell contact in swarming motility. A) An image of the edge of a swarming colony of P. mirabilis (300x). The swarmer cells are arranged into tightly packed rafts of cells.10 B) S. liquefaciens MG1 cells at the leading edge of a swarming colony migrate together.83 No scale was provided in the original publication for images A) and B). C-D) TEM images of a wild-type P. mirabilis swarmer cell (C) and a mutant of the ccmA gene in P. mirabilis (D).104 The image (inset) shows the swarming motility of colonies of wild-type P. mirabilis (left) and the ccmA mutant (right) on an agar gel. E-F) SEM images of vapor-fixed swarmer cells of wild-type P. mirabilis (E) and a mutant with reduced swarming motility (~85% reduction in surface migration) (F).103 Images are reprinted with permission from (A) American Society for Microbiology, Copyright 1972, (B) American Society for Microbiology, Copyright 1999, (C) and (D) American Society for Microbiology, Copyright 1999, and (E) and (F) American Society for Microbiology, Copyright 2004.
Another possible mechanism for cell/cell interactions is based on the packing of complementary shapes. Cells that do not align properly along their long axis are defective in swarming motility. Hay et al. described a ccmA mutant of P. mirabilis that produces irregularly curved cells, which prevents physical interaction between swarmers, disrupts the formation of multicellular rafts, and inhibits swarming migration (Figure 6c, d).104 These studies suggest physical interactions play an important role in the migration of multicellular rafts and the emergence of coordinated behavior during swarming.
Hydrodynamic interactions between motile bacterial cells
Complementary to physical interactions, hydrodynamic interactions between cells may be important for the collective dynamics of motile bacteria. Hernandez-Ortiz et al. simulated suspensions of self-propelled particles at low Re.105 When the particles were at a high density, the hydrodynamic interactions between the particles produced large-scale vortex motions in the fluid that emerged from the collective motion of the particles.105,106 Mendelson et al. studied populations of B. subtilis cells on agar plates that had a thin layer of liquid on the surface. The authors observed motile, undifferentiated cells that exhibited dynamic, correlated movement (‘whirls and jets’) that were similar to the characteristic circular motion observed in populations of swarmer cells.107 In another study, the formation of swirling patterns in a dense population of bacterial cells within a quasi-two-dimensional environment (i.e. a freely suspended horizontal soap film) increased the diffusion of passive beads within the film over short time scales.108 This observation may aid efforts to model the dispersion of nutrients in an actively swarming population. These examples of collective motion require sufficiently dense populations of cells and can be explained by hydrodynamic interactions without the requirement for chemical signaling.109–112 Importantly, these observations and models of collective swimming explain the patterns of whirls and vortices that are formed during bacterial swarming and may shed further insight into the mechanisms of transient cell-cell contact observed in swarming multicellular rafts.
4. The physiology and behavior of bacteria on surfaces
In contrast to swimming motility in which cells typically move independently at low density in bulk fluids using mechanisms that are well understood, swarming is characterized by the coordinated migration of high-density groups of cells over surfaces via mechanisms that are poorly understood. The earliest stages in swarming involve several phenomena: 1) cells sensing surfaces and extracellular signals; 2) cell sensors that relay extracellular information to the transcriptional machinery in cells; and 3) the regulation of metabolic and behavioral pathways that leads to adaptation and differentiation into swarmer cells. In this section we discuss several mechanisms for extracellular sensing that plays a role in differentiation and the metabolic changes that accompany swarming in several species.
a. Differentiation into swarmer cells
Proteus mirabilis, the archetypal swarmer cell, differentiates into swarmer cells when cultured on hydrogel surfaces (1.5−3% agar, w/v).13, 17, 113, 114 Macroscopically, P. mirabilis colonies alternate between periodic phases of migration (swarming) and phases of growth without movement (consolidation). The cyclic repetition of these stages results in regularly spaced concentric terraces that are geometrically similar to a bulls-eye pattern.14 The migration and consolidation pattern of swarming strains of P. mirabilis as well as that of other swarming genera have been modeled with reaction-diffusion theory using partial differential equations and/or kinetic equations that incorporate a parameter for the age-dependence of swarmer cell behavior.115–118
Swarmer cells of P. mirabilis are 10–80 µm long with approximately 10 to 50 times more flagella than planktonic cells; the formation of swarmers is accompanied by the inhibition of cell septation, but not DNA synthesis, as swarmer cells are multinucleate and have the same ratio of DNA/cell length as planktonic cells (Table 1).17, 119,120 Harshey has suggested that the large number of flagella on swarmer cells reduces friction between the cell body and the surface and facilitates swarming.12 The flagella that play a role in surface motility by most swarming strains of Gram-negative and Gram-positive bacteria are the same organelles used for individual swimming motility.4, 11, 13 Some genera of bacteria, including species of Vibrio and Aeromonas, however, encode a separate flagellar system for motility on surfaces and in viscous fluids.121–124 These bacteria use a sheathed polar flagellum for translating through bulk fluids and express unsheathed lateral flagella for motility on surfaces. The synthesis and assembly of lateral flagella in Vibrio parahaemolyticus (V. parahaemolyticus) is activated when cells are grown in viscous fluids (e.g. 18% Ficoll 400 w/v, 8×10−2 Pa·s), on agar surfaces (<2% agar, w/v) or in the presence of a polyclonal antibody that binds specifically to the polar flagellum and causes agglutination.121, 122, 125 The induction of the lateral flagella genes in V. parahaemolyticus is also triggered by iron-limited conditions.126
Pioneering research by McCarter et al. demonstrated that the polar flagellum in V. parahaemolyticus acts as a mechanosensor; the inhibition of rotation of the polar flagellum induces the expression and assembly of lateral flagella and leads to differentiation into the swarming phenotype.113, 122, 127 Alavi et al. showed that this concept extends to P. mirabilis in which planktonic cells differentiate into swarmers when the cells are on surfaces and the rotation of the flagellum is inhibited by the substrate.113 Swarmer cells of P. mirabilis dedifferentiate into planktonic cells soon after they are transferred to a liquid, presumably as a result of loss of the surface stimulus.4, 51, 113, 128 Additionally, the inhibition of flagella rotation as a result of surface contact (0.7−0.8% agar, w/v) or growth in a solution of Ficoll 400 (5−10%, w/v, 2–5×10−3 Pa·s) stimulates increased flagellin production and the swarming phenotype in Serratia marcescens (strain 274) (S. marcescens).60 The flagellum in many strains of bacteria appears to be a mechanical sensor of the extracellular environment, which relays signals that affect the transcription of genes and leads to differentiation.113
Not surprisingly, mutations of flagellar genes produce abnormal swarmer cell phenotypes, presumably as a result of a perturbation of flagella-based mechanisms of sensing surfaces.114, 120 Mutants of the flaA gene in P. mirabilis, which encodes the subunit of the flagellar filament, produce cells that are not motile.129 The FlaA mutants do not differentiate into swarmers when grown using conditions that stimulate swarmer cell development in wild-type Proteus.13, 14, 129 Belas and colleagues identified other flagellar genes in P. mirabilis that result in abnormal swarmer cell development.120 These genes include fliL, a component of the flagellar basal body, fliG, part of the flagellar switch, and flgH, which forms the basal-body L-ring.120 Gygi et al. demonstrated that a P. mirabilis mutant of the flhA gene, encoding a flagellum export protein, does not produce flagella and is unable to differentiate into a swarmer cell.114, 130 Furthermore, a transposon inserted into the flgN gene in P. mirabilis, which encodes a protein involved in flagella filament assembly, resulted in cells which were motile in liquid, but not on surfaces, potentially due to a reduction in the number of flagella on swarmer cells.131 In V. parahaemolyticus, mutants that are unable to form a polar flagellum generate a constitutive swarmer phenotype.122, 132 Collectively, these studies demonstrate that the biosynthesis, assembly, and function of flagella in some bacteria are essential for sensing environmental stimuli, including surfaces that produce the swarming phenotype.
FliL is a protein that is transcribed from the middle class 2 flagellar operon, fliLMNOPQR of S. typhimurium and E. coli that had an unknown function until recently.19 Knockouts of this protein in S. typhimurium133 and E. coli134 produce cells that are not motile on surfaces.135 Flagellar filaments in cells of fliL mutant strains grown on swarm agar (0.6% agar, w/v) are severed at the rod. The flagella are not broken off if the movement of the cells on the swarm agar is disrupted by mutations to the Mot proteins. Attmannspacher and coworkers suggest that a significant amount of torque is transmitted to the flagellar motor when cells are on the surface of swarm agar; this situation requires the stabilization of the flagella by FliL.135 A fliL mutant in P. mirabilis produced cells that had no flagella and were non-motile. Interestingly, these cells elongated into swarmers during growth in liquid and became hyper-elongated on agar.128 These data suggest that FliL may play a role in transmitting an external, surface-sensing stimuli into a change in gene expression within the cell.128
It is clear that flagella play a role in sensing surfaces in species of Proteus, Serratia, and Vibrio but it is unclear if this mechanism for relaying information on the extracellular environment of cells to the transcriptional machinery is widespread among Eubacteria. Remarkably few other mechanisms that bacteria use to sense physical boundaries and surfaces are known. Sensing mechanisms based on receptor/ligand binding between cells and surfaces are commonly used by eukaryotes but were overlooked in bacteria until recently. Mignot et al. found that cells of Myxococcus xanthus form transient adhesion complexes between the cell surface and the substrate. These complexes play a role in surface motility and are reminiscent of mammalian focal adhesions.136 We anticipate that the introduction of biocompatible polymers and gels with controlled physical properties and surface chemistry will play an important role in the study of sensors that bacterial cells use to detect changes in their physical environment. These materials will make it possible to study the role of a variety of stimuli, including: 1) stiffness (e.g. Young’s modulus); 2) porosity; 3) surface gradients; and 4) ligand/receptor interactions.
b. Metabolism and respiration
Inoue et al. screened the Keio collection, a set of single-gene knockouts of all the nonessential genes in E. coli K-12,5 and identified 294 knockouts that were no longer able to swarm.137 Seventy of these genes were involved in general metabolism and included genes affiliated with the tricarboxylic acid cycle, ATP synthase, and the electron-transport chain, suggesting that swarming motility may require an increase in energy production.
Cells of E. coli and S. typhimurium require nutrient-rich media supplemented with a carbon source, such as glucose, for development into swarmers.57, 138 Using proteomics, Kim and Surette found that enzymes associated with anabolism were upregulated in swarmer cells of S. typhimurium and enzymes involved in the catabolism of peptides as well as nucleotide salvage were down-regulated relative to planktonic cells. The permeability of the outer membrane was reduced in swarmer cells, which suggests that these cells may be relying on de novo biosynthesis pathways to obtain amino acids and nucleotides rather than importing them.138 Wang and coworkers used DNA-arrays to profile mRNA transcripts of swarming cells of S. typhimurium and observed that genes involved in iron metabolism and lipopolysaccharide (LPS) biosynthesis were upregulated.139 These observations of general cellular metabolism suggest that swarming cells are physiologically and biochemically different from planktonic, swimming cells for reasons that are not yet clear.
c. Chemotaxis
The chemotaxis system consists of sensory receptors linked to a cytoplasmic phosphorylation cascade that alters the rotational bias of the flagella motors and produces net cell motility toward attractants and away from repellents. This area has been reviewed several times,21, 43, 140, 141 including an excellent, recent review by Hazelbauer et al.142 The chemotaxis pathway is important for differentiation into the swarming phenotype and colony migration.143–145 The addition of glutamine to minimal media that does not support swarming motility, triggers the differentiation of cells of P. mirabilis into swarmers and leads to the colonization of surfaces.143 Allison et al. demonstrated that glutamine is specific for chemotaxis by swarmers and does not elicit a response from swimming cells.143 The chemotaxis machinery, but not necessarily the act of chemosensing itself, is important for the development of swarming in E. coli, S. typhimurium, and S. marcescens.57, 144, 145 Wang et al. observed that chemotaxis mutants of S. typhimurium have fewer and shorter flagella and generate bacterial lawns that have less fluid than lawns of wild-type cells.146 Based on these observations and the downregulation of late (class 3) flagellar genes in chemotaxis mutants, Harshey and colleagues suggest that the flagellum is able to sense environmental conditions (external wetness) that are favorable for filament assembly.146 Subsequent studies by Mariconda et al. demonstrated that chemotaxis mutants unable to switch the direction of flagellar motor rotation are defective in their ability to efficiently migrate across the surface.147 It is currently unknown why these mutants produce swarm colonies that are drier than wild type colonies. The connection between chemotaxis and swarming is not yet fully understood.
d. Quorum sensing and signal transduction
The population density of cells and signaling molecules influences the development and migration of swarmer cells. Signaling molecules produced by bacteria are referred to as autoinducers (AIs). Threshold concentrations of AIs produce changes in gene expression through the modulation of transcriptional regulators in a process referred to as ‘quorum sensing’. Multiple quorum-sensing systems exist in bacteria, including the N-acyl-homoserine lactone (AHL) quorum sensing machinery of Gram-negative bacteria and the more recently discovered AI-2 and AI-3 systems for interspecies communication.148, 149 Several species of swarming bacteria use AHLs to trigger the production of surfactants that play an important role in surface motility.86, 97 We highlight examples of the role of signaling molecules in swarming, such as the induction of surfactant expression, in this section and section 3b. For an extensive review of the relationship between swarming and quorum sensing, see Daniels et al.150
Motlity and swarming in many species are regulated by two-component phosphorelay signaling systems that may not be modulated by quorum sensing autoinducers. The Rcs phosphorelay system of the Enterobacteriaceae family is one such system, which regulates a variety of processes, including: expression of genes involved in colanic acid synthesis, cell division, cell wall integrity, and virulence.151 Importantly, this phosphorelay system is also implicated in regulation of swarming motility, primarily through modulation of the flhDC operon.152, 153 Two-component signaling systems are used by microorganisms to sense and respond to environmental changes. The basic constituents of these systems are two signal transducers, specifically a sensor histidine kinase and a response regulator. Of the signals activating the Rcs system, including: osmotic shock, overproduction of a membrane chaperone protein, and growth at low-temperature (20°C) in the presence of glucose and high zinc concentrations; the activation of RcsC during growth on a solid surface is intriguing because of its potential role in affecting the gene expression of swarmer cells.152, 154
Fatty acids can also inhibit swarming in P. mirabilis through RsbA, which encodes a homologue of the E. coli RcsD protein.155 This pathway was originally implicated in swarming motility in P. mirabilis based on the observation that a rsbA mutant swarmed precociously.156 This phenotype was also observed in rcsC and rcsD mutants in E. coli as well as a rcsB mutant in S. typhimurium.153, 157 In agreement with these mutant phenotypes, the RcsCDB phosphorelay system is a negative regulator of the flhDC operon in E. coli. Repression is achieved via binding of the response regulator, RscB, and a cofactor, RcsA, to a RcsAB box in the promoter region of the flhDC operon.158 As described previously, repression of the flhDC operon will negatively affect swarming development and migration. Interestingly, the RcsC/RcsD/RcsB phosphorelay system is also involved in the transcriptional activation of the Wzz gene, which encodes a protein involved in determining the chain length of the O-antigen in S. typhimurium.159 As described earlier, the O-antigen is important for swarming migration in Salmonella.70 The RcsCDB system was also originally noted for its activation of genes involved in the biosynthesis of colanic acid, which is an inhibitor of swarming motility.153, 160 In conclusion, these observations suggest that the Rcs phosphorelay system plays an important role in swarming.
5. Outlook
Bacterial swarming may be one of the most tractable models for studying dynamic self-assembly in a biological system. After a century of experiments, however, there are still many fundamental unanswered questions about the system, including: 1) the stimuli, sensors, and biochemistry involved in the differentiation of cells into swarmers on surfaces; 2) chemical and physical interactions between cells and surfaces; 3) the mechanism of cell motility on surfaces; 4) the microscopic and macroscopic organization of swarming colonies and the dynamics of cells in these structures; and 5) the importance of cell/cell and cell/fluid interactions in the coordination of growth and motility. Microbiological tools may not provide enough leverage to dissect this system at a level of detail that is required to understand how collective behavior emerges and how this phenotype plays a role in multicellular homeostasis, survival, evolution, and pathogenesis.
Physical scientists and engineers are in a unique position to have an impact on this area of microbiology through the application of materials and techniques from chemistry and materials science and engineering. Below we outline and summarize three areas where the intersection of microbiology and materials science and engineering may find synergy in answering fundamental questions about bacterial swarming and the origins of emergent behavior.
a. Surface sensing
Some cells use their polar flagellum to sense their microenvironment and detect boundaries between fluids and surfaces. We described this mechanism for V. parahaemolyticus and P. mirabilis in Section 4a and pointed out that it may not be a sensor that is universally conserved in Eubacteria. What other mechanisms do bacterial cells use to sense surfaces? Are there examples of mechanisms that are conserved across many different genera? To understand these questions at the molecular level, we need to determine the stimuli that cells sense, the sensors that couple information on the extracellular microenvironment to the transcription of genes, and the biochemical pathways that play a role in coordinating the differentiation of cells into swarmers.
One approach to the study of this problem is to build new platforms for studying swarming. The application of polymer substrates that have defined chemical and physical properties that can be systematically varied will make it possible to explore the stimuli that trigger differentiation. The fusion of different classes of soft, biocompatible polymers and genetic and genomic techniques will lead to the study of the signaling pathways and the resulting transcriptional changes that occur during differentiation at a new level of detail.
b. Surface motility
The mechanisms that are involved in the movement of swarming cells across surfaces are still not understood in detail. Several observations have led to the hypothesis that swarmers translate through a two-dimensional lay of fluid that is extracted from the hydrogel. The study of the temporal emergence of swarming from a single cell on a surface may provide insight into this hypothesis. The application of techniques for imaging and measuring the thickness of films of fluids will provide direct evidence of this mechanism. The ability to change the surface energy of the polymer will make it possible to manipulate the thickness of the layer of fluid on the substrate and determine how this parameter affects swarming motility and collective movement.
Some species of bacteria swarm on materials that provide little or no source of endogenous water (e.g. silicone catheters). These strains of bacteria differentiate into swarmers that produce and secrete significant amounts of extracellular polymers, which may provide the layer of fluid that the cells move through. Although it has not been determined experimentally, it seems reasonable that these fluids have a viscosity that approaches or exceeds the limited range that supports the swimming of planktonic cells (Section 2c). How do swarmers move through fluids that have a viscosity that inhibits the motility of planktonic cells? Swarmers have a higher density of flagella than planktonic cells. Does an increase in the number of flagella increase the torque produced by the cell?
c. The dynamics of flagella
The structure and dynamics of a single bundle of flagella in planktonic cells of E. coli has been the model system for our understanding of the mechanisms of bacterial cell motility. As we have pointed out in this review, swarmers typically have a higher density of flagella than planktonic cells. Surprisingly little is known about the spatial and temporal structure of the bundles of flagella on swarming cells. In contrast to studies of motility of planktonic bacterial cells in bulk fluids in which cell/cell interactions are uncommon, swarming colonies are characterized by frequent interactions between cells. What happens to the structure and dynamics of the flagella when two swarming cells are brought in close proximity (ie. close enough for their flagella to interact)? Do the flagella on adjacent swarming cells interact and become intertwined? The high density of cells in swarming colonies and the large number of flagella per cell makes it seem reasonable that the flagella may form intercellular bundles. This hypothesis is indirectly supported by the observation that the motors on swarming cells undergo frequent reversals in direction, which causes intracellular bundles of flagella on cells to break apart and reform. Could changes in the direction of the motors provide a mechanism for the formation of intercellular bundles of flagella between adjacent swarmers? These observations lead to the untested hypothesis that the apparent coordination of motility in swarming populations may be a consequence of the physical tethering of swarmers to their neighbors.
Swarming is a phenotype that plays a role in bacterial pathogenesis and biofilm formation and may be a common method for motile strains of bacteria adsorbed or in contact with surfaces to regain motility. Table 2 summarizes genera of swarming bacteria and includes a brief summary of their ‘native’ habitat, although not all of the species within each genus are capable of surface motility. The table provides a general idea of how widely this behavior is distributed in Eubacteria. Interestingly, many of the genera included in the table live in habitats that are not dominated by bulk fluids and may more accurately be thought of in environments that are at interfaces between surfaces and liquids. This observation brings into question whether cells evolved flagella for swimming in bulk fluids or for moving on surface. Perhaps the movement of bacterial cells on a surface is the norm and not the exception.
Table 2.
A comparison of the genera of bacteria that have been observed to swarm and their natural habitat.
| Swarming Genera of Bacteria | Natural habitat |
|---|---|
| Aeromonas | Fresh water; GI tract |
| Azospirillum | Soil; rhizosphere |
| Bacillus | Soil; air (spores) |
| Burkholderia | Soil; fresh water |
| Clostridium | Soil; marine sediment; GI tract |
| Escherichia | GI tract |
| Myxococcus | Soil |
| Proteus | Soil; GI/urinary tract; water; plant surfaces |
| Pseudomonas | Water; soil; plant surfaces |
| Rhizobium | Soil; root |
| Rhodospirillum | Water |
| Salmonella | GI tract; feces |
| Serratia | Soil; water; plant surfaces |
| Vibrio | Salt water |
| Yersinia | Rodent; flea; GI tract |
Swarming presents a unique opportunity for studying dynamic self-assembly in a biological system that is relevant to biomedicine and ecology. The infusion of new techniques based on soft matter will have an important impact on understanding bacterial surface motility and the origins of emergent behavior, and will almost certainly inspire new directions in materials science and the study of microbiological systems.
Supplementary Material
References
- 1.Whitesides GM, Grzybowski B. 2002;vol. 295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin M, Shapiro JA. Bacteria as multicellular organisms. New York: Oxford University Press; 1997. [Google Scholar]
- 3.Shapiro JA. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Fraser GM, Hughes C. Curr Opin Microbiol. 1999;2:630–635. doi: 10.1016/s1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 5.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butland G, Babu M, Diaz-Mejia JJ, Bohdana F, Phanse S, Gold B, Yang W, Li J, Gagarinova AG, Pogoutse O, Mori H, Wanner BL, Lo H, Wasniewski J, Christopolous C, Ali M, Venn P, Safavi-Naini A, Sourour N, Caron S, Choi JY, Laigle L, Nazarians-Armavil A, Deshpande A, Joe S, Datsenko KA, Yamamoto N, Andrews BJ, Boone C, Ding H, Sheikh B, Moreno-Hagelseib G, Greenblatt JF, Emili A. Nat Methods. 2008. [DOI] [PubMed] [Google Scholar]
- 7.Typas A, Nichols RJ, Siegele DA, Shales M, Collins SR, Lim B, Braberg H, Yamamoto N, Takeuchi R, Wanner BL, Mori H, Weissman JS, Krogan NJ, Gross CA. Nat Methods. 2008 doi: 10.1038/nmeth.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weibel DB, DiLuzio WR, Whitesides GM. Nat Rev Microbiol. 2007;5:209–218. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- 9.Weibel DB, Lee A, Mayer M, Brady SF, Bruzewicz D, Yang J, Diluzio WR, Clardy J, Whitesides GM. Langmuir. 2005;21:6436–6442. doi: 10.1021/la047173c. [DOI] [PubMed] [Google Scholar]
- 10.Henrichsen J. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harshey RM. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 12.Harshey RM. Annu Rev Microbiol. 2003;57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- 13.Allison C, Hughes C. Sci Prog. 1991;75:403–422. [PubMed] [Google Scholar]
- 14.Belas R, Shapiro M, JA D, editors. Bacteria as multicellular organisms. edition edn. Oxford, England: Oxford University Press; 1996. [Google Scholar]
- 15.McCarter L. J Mol Microbiol Biotechnol. 1999;1:51–57. [PubMed] [Google Scholar]
- 16.Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J, Michiels J. Trends in Microbiology. 2008;16:496–506. doi: 10.1016/j.tim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Williams FD, Schwarzhoff RH. Annu Rev Microbiol. 1978;32:101–122. doi: 10.1146/annurev.mi.32.100178.000533. [DOI] [PubMed] [Google Scholar]
- 18.Bray D. Cell Movements: From Molecules to Motility. Garland Publishing; 2001. [Google Scholar]
- 19.Chilcott GS, Hughes KT. Microbiol Mol Biol Rev. 2000;64:694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman M, Simon M. Nature. 1974;249:73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- 21.Manson MD, Armitage JP, Hoch JA, Macnab RM. J Bacteriol. 1998;180:1009–1022. doi: 10.1128/jb.180.5.1009-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macnab RM, Neidhardt FC, Curtiss R, Ingraham JL, Lin ECC, editors; Neidhardt FC, Curtis R III, Ingraham J, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Vol. 1. 1996. pp. 123–145. [Google Scholar]
- 23.Berg HC. Annu Rev Biochem. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 24.Yonekura K, Maki-Yonekura S, Namba K. Nature. 2003;424:643–650. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- 25.Macnab RM. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 26.Jarrell KF, McBride MJ. Nat Rev Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 27.Berg HC. Nature. 1975;254:389–392. doi: 10.1038/254389a0. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Lloyd SA, Blair DF. Proc Natl Acad Sci U S A. 1998;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen SH, Adler J, Gargus JJ, Hogg RW. Proc Natl Acad Sci U S A. 1974;71:1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima S, Blair DF. Int Rev Cytol. 2004;233:93–134. doi: 10.1016/S0074-7696(04)33003-2. [DOI] [PubMed] [Google Scholar]
- 31.McCarter LL. J Mol Microbiol Biotechnol. 2004;7:18–29. doi: 10.1159/000077866. [DOI] [PubMed] [Google Scholar]
- 32.Blair DF, Berg HC. Cell. 1990;60:439–449. doi: 10.1016/0092-8674(90)90595-6. [DOI] [PubMed] [Google Scholar]
- 33.Kojima S, Blair DF. Biochemistry. 2001;40:13041–13050. doi: 10.1021/bi011263o. [DOI] [PubMed] [Google Scholar]
- 34.Chevance FF, Hughes KT. Nat Rev Microbiol. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg HC. E. Coli in Motion. Springer; 2003. [Google Scholar]
- 36.Larsen SH, Reader RW, Kort EN, Tso WW, Adler J. Nature. 1974;249:74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- 37.Macnab RM, Ornston MK. J Mol Biol. 1977;112:1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- 38.Turner L, Ryu WS, Berg HC. J Bacteriol. 2000;182:2793–2801. doi: 10.1128/jb.182.10.2793-2801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg HC, Brown DA. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 40.Adler J. Science. 1966;153:708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- 41.Blair DF. Annu Rev Microbiol. 1995;49:489–522. doi: 10.1146/annurev.mi.49.100195.002421. [DOI] [PubMed] [Google Scholar]
- 42.Adler J. J Bacteriol. 1966;92:121–129. doi: 10.1128/jb.92.1.121-129.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stock JB, Surette MG. In: Cellular and Molecular Biology. Neidhardt FC, Curtiss RI, Ingraham JL, Lin ECC, Low KB, Magasanik B, Riley M, Schaechter M, Umbarger HE, editors. 1996. pp. 1103–1129. [Google Scholar]
- 44.Berg HC. Random Walks in Biology. Princeton University Press; 1993. [Google Scholar]
- 45.Purcell EM. Am. J. Phys. 1977;45:3–11. [Google Scholar]
- 46.Berg HC, Turner L. Nature. 1979;278:349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg EP, Canale-Parola E. J Bacteriol. 1977;132:356–358. doi: 10.1128/jb.132.1.356-358.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kearns DB, Losick R. Molecular Microbiology. 2003;49:581–590. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- 49.Rashid MH, Kornberg A. Proc Natl Acad Sci U S A. 2000;97:4885–4890. doi: 10.1073/pnas.060030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bees MA, Andresen P, Mosekilde E, Givskov M. Bull Math Biol. 2002;64:565–587. doi: 10.1006/bulm.2002.0287. [DOI] [PubMed] [Google Scholar]
- 51.Rauprich O, Matsushita M, Weijer CJ, Siegert F, Esipov SE, Shapiro JA. J Bacteriol. 1996;178:6525–6538. doi: 10.1128/jb.178.22.6525-6538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itoi-i H, Wakita J, Matsuyama T, Matsushita M. Journal of the Physical Society of Japan. 1999;68:1436–1443. [Google Scholar]
- 53.Czirók A, Matsushita M, Vicsek T. Physical Review E. 2001;63:31915. doi: 10.1103/PhysRevE.63.031915. [DOI] [PubMed] [Google Scholar]
- 54.Steager EB, Kim CB, Kim MJ. Physics of Fluids. 2008;20:73601–73601. [Google Scholar]
- 55.Bees MA, Andresen P, Mosekilde E, Givskov M. J Math Biol. 2000;40:27–63. doi: 10.1007/s002850050004. [DOI] [PubMed] [Google Scholar]
- 56.Quinones B, Dulla G, Lindow SE. Mol Plant Microbe Interact. 2005;18:682–693. doi: 10.1094/MPMI-18-0682. [DOI] [PubMed] [Google Scholar]
- 57.Harshey RM, Matsuyama T. Proc Natl Acad Sci U S A. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Senesi S, Celandroni F, Salvetti S, Beecher DJ, Wong AC, Ghelardi E. Microbiology. 2002;148:1785–1794. doi: 10.1099/00221287-148-6-1785. [DOI] [PubMed] [Google Scholar]
- 59.Eberl L, Christiansen G, Molin S, Givskov M. J Bacteriol. 1996;178:554–559. doi: 10.1128/jb.178.2.554-559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alberti L, Harshey RM. J Bacteriol. 1990;172:4322–4328. doi: 10.1128/jb.172.8.4322-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeffries CD, Rogers HE. J Bacteriol. 1968;95:732–733. doi: 10.1128/jb.95.2.732-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stickler D, Hughes G. Eur J Clin Microbiol Infect Dis. 1999;18:206–208. doi: 10.1007/s100960050260. [DOI] [PubMed] [Google Scholar]
- 63.Watterson JD, Cadieux PA, Stickler D, Reid G, Denstedt JD. J Endourol. 2003;17:523–527. doi: 10.1089/089277903769013711. [DOI] [PubMed] [Google Scholar]
- 64.Matsuyama T, Matsushita M. Critical Reviews in Microbiology. 1993;19:117–135. doi: 10.3109/10408419309113526. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro J. BioEssays. 1995;17:597–607. doi: 10.1002/bies.950170706. [DOI] [PubMed] [Google Scholar]
- 66.Wolfe AJ, Berg HC. Proc Natl Acad Sci U S A. 1989;86:6973–6977. doi: 10.1073/pnas.86.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Pichel F. J Bacteriol. 1989;171:3560–3563. doi: 10.1128/jb.171.6.3560-3563.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu D, Reeves PR. Microbiology. 1994;140((Pt 1)):49–57. doi: 10.1099/13500872-140-1-49. [DOI] [PubMed] [Google Scholar]
- 69.Stevenson G, Neal B, Liu D, Hobbs M, Packer NH, Batley M, Redmond JW, Lindquist L, Reeves P. J Bacteriol. 1994;176:4144–4156. doi: 10.1128/jb.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toguchi A, Siano M, Burkart M, Harshey RM. J Bacteriol. 2000;182:6308–6321. doi: 10.1128/jb.182.22.6308-6321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raetz CRH. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, DC: ASM Press; 1996. pp. 1035–1063. [Google Scholar]
- 72.Matsuyama T, Bhasin A, Harshey RM. J Bacteriol. 1995;177:987–991. doi: 10.1128/jb.177.4.987-991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell CJ, Klajn R, Fialkowski M, Grzybowski BA. Phys. Lett. 2003;83:4444. [Google Scholar]
- 74.Grzybowski BA, Campbell CJ. Materials Today. 2007;10:38–46. [Google Scholar]
- 75.Martin BD, Brandow SL, Dressick WJ, Schull TL. Langmuir. 2000;16:9944–9946. [Google Scholar]
- 76.Chen BG, Turner L, Berg HC. J Bacteriol. 2007;189:8750–8753. doi: 10.1128/JB.01109-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mireles JR, 2nd, Toguchi A, Harshey RM. J Bacteriol. 2001;183:5848–5854. doi: 10.1128/JB.183.20.5848-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gygi D, Rahman MM, Lai HC, Carlson R, Guard-Petter J, Hughes C. Mol Microbiol. 1995;17:1167–1175. doi: 10.1111/j.1365-2958.1995.mmi_17061167.x. [DOI] [PubMed] [Google Scholar]
- 79.Stahl SJ, Stewart KR, Williams FD. J Bacteriol. 1983;154:930–937. doi: 10.1128/jb.154.2.930-937.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuscoe FJ. Med Lab Technol. 1973;30:373–382. [PubMed] [Google Scholar]
- 81.Rahman MM, Guard-Petter J, Asokan K, Hughes C, Carlson RW. J Biol Chem. 1999;274:22993–22998. doi: 10.1074/jbc.274.33.22993. [DOI] [PubMed] [Google Scholar]
- 82.Boles BR, McCarter LL. J Bacteriol. 2002;184:5946–5954. doi: 10.1128/JB.184.21.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eberl L, Molin S, Givskov M. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Givskov M, Ostling J, Eberl L, Lindum PW, Christensen AB, Christiansen G, Molin S, Kjelleberg S. J Bacteriol. 1998;180:742–745. doi: 10.1128/jb.180.3.742-745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindum PW, Anthoni U, Christophersen C, Eberl L, Molin S, Givskov M. J Bacteriol. 1998;180:6384–6388. doi: 10.1128/jb.180.23.6384-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eberl L, Winson MK, Sternberg C, Stewart GS, Christiansen G, Chhabra SR, Bycroft B, Williams P, Molin S, Givskov M. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 87.Van Houdt R, Givskov M, Michiels CW. FEMS Microbiol Rev. 2007;31:407–424. doi: 10.1111/j.1574-6976.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- 88.Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I. J Bacteriol. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stachelhaus T, Marahiel MA. FEMS Microbiol Lett. 1995;125:3–14. doi: 10.1111/j.1574-6968.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 90.Turgay K, Krause M, Marahiel MA. Mol Microbiol. 1992;6:529–546. doi: 10.1111/j.1365-2958.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 91.Connelly MB, Young GM, Sloma A. Journal of Bacteriology. 2004;186:4159–4167. doi: 10.1128/JB.186.13.4159-4167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kearns DB, Chu F, Rudner R, Losick R. Mol Microbiol. 2004;52:357–369. doi: 10.1111/j.1365-2958.2004.03996.x. [DOI] [PubMed] [Google Scholar]
- 93.Mendelson NH, Salhi B. Journal of Bacteriology. 1996;178:1980. doi: 10.1128/jb.178.7.1980-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakano MM, Corbell N, Besson J, Zuber P. Mol Gen Genet. 1992;232:313–321. doi: 10.1007/BF00280011. [DOI] [PubMed] [Google Scholar]
- 95.Cosmina P, Rodriguez F, de Ferra F, Grandi G, Perego M, Venema G, van Sinderen D. Mol Microbiol. 1993;8:821–831. doi: 10.1111/j.1365-2958.1993.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 96.Soberón-Chávez G, Lépine F, Déziel E. Applied Microbiology and Biotechnology. 2005;68:718–725. doi: 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- 97.Tremblay J, Richardson AP, Lepine F, Deziel E. Environ Microbiol. 2007;9:2622–2630. doi: 10.1111/j.1462-2920.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 98.Caiazza NC, Shanks RM, O'Toole GA. J Bacteriol. 2005;187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deziel E, Lepine F, Milot S, Villemur R. Soc General Microbiol. (Edition edn.) 2003;vol. 149:2005–2013. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- 100.Liebner S, Cavallaro U, Dejana E. Arterioscler Thromb Vasc Biol. 2006;26:1431–1438. doi: 10.1161/01.ATV.0000218510.04541.5e. [DOI] [PubMed] [Google Scholar]
- 101.Matsuo K, Irie N. Arch Biochem Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 102.Morrison RB, Scott A. Nature. 1966;211:255–257. doi: 10.1038/211255a0. [DOI] [PubMed] [Google Scholar]
- 103.Jones BV, Young R, Mahenthiralingam E, Stickler DJ. Infect Immun. 2004;72:3941–3950. doi: 10.1128/IAI.72.7.3941-3950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hay NA, Tipper DJ, Gygi D, Hughes C. J Bacteriol. 1999;181:2008–2016. doi: 10.1128/jb.181.7.2008-2016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hernandez-Ortiz JP, Stoltz CG, Graham MD. Physical Review Letters. 2005;95:204501. doi: 10.1103/PhysRevLett.95.204501. [DOI] [PubMed] [Google Scholar]
- 106.Underhill PT, Hernandez-Ortiz JP, Graham MD. Phys Rev Lett. 2008;100:248101. doi: 10.1103/PhysRevLett.100.248101. [DOI] [PubMed] [Google Scholar]
- 107.Mendelson NH, Bourque A, Wilkening K, Anderson KR, Watkins JC. J Bacteriol. 1999;181:600–609. doi: 10.1128/jb.181.2.600-609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu XL, Libchaber A. Physical Review Letters. 2000;84:3017–3020. doi: 10.1103/PhysRevLett.84.3017. [DOI] [PubMed] [Google Scholar]
- 109.Dombrowski C, Cisneros L, Chatkaew S, Goldstein RE, Kessler JO. Physical Review Letters. 2004;93:98103. doi: 10.1103/PhysRevLett.93.098103. [DOI] [PubMed] [Google Scholar]
- 110.Narayan V, Ramaswamy S, Menon N. Science. 2007;317:105. doi: 10.1126/science.1140414. [DOI] [PubMed] [Google Scholar]
- 111.Riedel IH, Kruse K, Howard J. American Association for the Advancement of Science. (Editon edn.) 2005;vol. 309:300–303. [Google Scholar]
- 112.Sokolov A, Aranson IS, Kessler JO, Goldstein RE. Physical Review Letters. 2007;98:158102. doi: 10.1103/PhysRevLett.98.158102. [DOI] [PubMed] [Google Scholar]
- 113.Alavi M, Belas R. Methods Enzymol. 2001;336:29–40. doi: 10.1016/s0076-6879(01)36575-8. [DOI] [PubMed] [Google Scholar]
- 114.Gygi D, Bailey MJ, Allison C, Hughes C. Mol Microbiol. 1995;15:761–769. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 115.Ayati BP. Journal of Mathematical Biology. 2006;52:93–114. doi: 10.1007/s00285-005-0345-3. [DOI] [PubMed] [Google Scholar]
- 116.Esipov SE, Shapiro JA. Journal of Mathematical Biology. 1998;36:249–268. [Google Scholar]
- 117.Mimura M, Sakaguchi H, Matsushita M. Physica A: Statistical Mechanics and its Applications. 2000;282:283–303. [Google Scholar]
- 118.Zorzano MP, Hochberg D, Cuevas MT, Gomez-Gomez JM. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:031908. doi: 10.1103/PhysRevE.71.031908. [DOI] [PubMed] [Google Scholar]
- 119.Hoeniger JF. Can J Microbiol. 1966;12:113–123. doi: 10.1139/m66-017. [DOI] [PubMed] [Google Scholar]
- 120.Belas R, Goldman M, Ashliman K. J Bacteriol. 1995;177:823–828. doi: 10.1128/jb.177.3.823-828.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belas R, Simon M, Silverman M. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCarter L, Hilmen M, Silverman M. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 123.Merino S, Shaw JG, Tomas JM. FEMS Microbiol Lett. 2006;263:127–135. doi: 10.1111/j.1574-6968.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 124.Shimada T, Sakazaki R, Suzuki K. Jpn J Med Sci Biol. 1985;38:141–145. doi: 10.7883/yoken1952.38.141. [DOI] [PubMed] [Google Scholar]
- 125.Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. Mol Microbiol. 1996;20:693–699. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 126.McCarter L, Silverman M. J Bacteriol. 1989;171:731–736. doi: 10.1128/jb.171.2.731-736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCarter L, Silverman M. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 128.Belas R, Suvanasuthi R. J Bacteriol. 2005;187:6789–6803. doi: 10.1128/JB.187.19.6789-6803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Belas R. J Bacteriol. 1994;176:7169–7181. doi: 10.1128/jb.176.23.7169-7181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Furness RB, Fraser GM, Hay NA, Hughes C. J Bacteriol. 1997;179:5585–5588. doi: 10.1128/jb.179.17.5585-5588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gygi D, Fraser G, Dufour A, Hughes C. Mol Microbiol. 1997;25:597–604. doi: 10.1046/j.1365-2958.1997.5021862.x. [DOI] [PubMed] [Google Scholar]
- 132.Sar N, McCarter L, Simon M, Silverman M. J Bacteriol. 1990;172:334–341. doi: 10.1128/jb.172.1.334-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schoenhals GJ, Macnab RM. Microbiology. 1999;145((Pt 7)):1769–1775. doi: 10.1099/13500872-145-7-1769. [DOI] [PubMed] [Google Scholar]
- 134.Raha M, Sockett H, Macnab RM. J Bacteriol. 1994;176:2308–2311. doi: 10.1128/jb.176.8.2308-2311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Attmannspacher U, Scharf BE, Harshey RM. Mol Microbiol. 2008;68:328–341. doi: 10.1111/j.1365-2958.2008.06170.x. [DOI] [PubMed] [Google Scholar]
- 136.Mignot T, Shaevitz JW, Hartzell PL, Zusman DR. Science. 2007;315:853. doi: 10.1126/science.1137223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Inoue T, Shingaki R, Hirose S, Waki K, Mori H, Fukui K. J Bacteriol. 2007;189:950–957. doi: 10.1128/JB.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim W, Surette MG. Mol Microbiol. 2004;54:702–714. doi: 10.1111/j.1365-2958.2004.04295.x. [DOI] [PubMed] [Google Scholar]
- 139.Wang Q, Frye JG, McClelland M, Harshey RM. Mol Microbiol. 2004;52:169–187. doi: 10.1111/j.1365-2958.2003.03977.x. [DOI] [PubMed] [Google Scholar]
- 140.Bourret RB, Stock AM. J Biol Chem. 2002;277:9625–9628. doi: 10.1074/jbc.R100066200. [DOI] [PubMed] [Google Scholar]
- 141.Falke JJ, Hazelbauer GL. Trends Biochem Sci. 2001;26:257–265. doi: 10.1016/s0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hazelbauer GL, Falke JJ, Parkinson JS. Trends Biochem Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allison C, Lai HC, Gygi D, Hughes C. Mol Microbiol. 1993;8:53–60. doi: 10.1111/j.1365-2958.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 144.Burkart M, Toguchi A, Harshey RM. Proc Natl Acad Sci U S A. 1998;95:2568–2573. doi: 10.1073/pnas.95.5.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Rear J, Alberti L, Harshey RM. J Bacteriol. 1992;174:6125–6137. doi: 10.1128/jb.174.19.6125-6137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Q, Suzuki A, Mariconda S, Porwollik S, Harshey RM. EMBO J. 2005;24:2034–2042. doi: 10.1038/sj.emboj.7600668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mariconda S, Wang Q, Harshey RM. Mol Microbiol. 2006;60:1590–1602. doi: 10.1111/j.1365-2958.2006.05208.x. [DOI] [PubMed] [Google Scholar]
- 148.Sperandio V, Torres AG, Giron JA, Kaper JB. Journal of Bacteriology. 2001;183:5187. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Proc Natl Acad Sci U S A. 2003;100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Daniels R, Vanderleyden J, Michiels J. FEMS Microbiol Rev. 2004;28:261–289. doi: 10.1016/j.femsre.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 151.Majdalani N, Gottesman S. Annual Review of Microbiology. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 152.Huang YH, Ferrières L, Clarke DJ. Research in Microbiology. 2006;157:206–212. doi: 10.1016/j.resmic.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 153.Wang Q, Zhao Y, McClelland M, Harshey RM. The Journal of Bacteriology. 2007 doi: 10.1128/JB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ferrieres L, Clarke DJ. Molecular Microbiology. 2003;50:1665–1682. doi: 10.1046/j.1365-2958.2003.03815.x. [DOI] [PubMed] [Google Scholar]
- 155.Liaw SJ, Lai HC, Wang WB. Infection, Immunity. 2004;72:6836. doi: 10.1128/IAI.72.12.6836-6845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Belas R, Schneider R, Melch M. J Bacteriol. 1998;180:6126–6139. doi: 10.1128/jb.180.23.6126-6139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takeda S, Fujisawa Y, Matsubara M, Aiba H, Mizuno T. Mol Microbiol. 2001;40:440–450. doi: 10.1046/j.1365-2958.2001.02393.x. [DOI] [PubMed] [Google Scholar]
- 158.Francez-Charlot A, Laugel B, Van Gemert A, Dubarry N, Wiorowski F, Castanie-Cornet MP, Gutierrez C, Cam K. Mol Microbiol. 2003;49:823–832. doi: 10.1046/j.1365-2958.2003.03601.x. [DOI] [PubMed] [Google Scholar]
- 159.Delgado MA, Mouslim C, Groisman EA. Molecular Microbiology. 2006;60:39–50. doi: 10.1111/j.1365-2958.2006.05069.x. [DOI] [PubMed] [Google Scholar]
- 160.Gottesman S, Trisler P, Torres-Cabassa A. Journal of Bacteriology. 1985;162:1111. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, Leibler S, Surette MG, Alon U. Science. 2001;292:2080. doi: 10.1126/science.1058758. [DOI] [PubMed] [Google Scholar]
- 162.Kohler T, Curty LK, Barja F, van Delden C, Pechere JC. J Bacteriol. 2000;182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Senesi S, Ghelardi E, Celandroni F, Salvetti S, Parisio E, Galizzi A. Journal of Bacteriology. 2004;186:1158–1164. doi: 10.1128/JB.186.4.1158-1164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Magnuson R, Solomon J, Grossman AD. Cell. 1994;77:207–216. doi: 10.1016/0092-8674(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 165.Belas R, Erskine D, Flaherty D. Journal of Bacteriology. 1991;173:6279. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sturgill G, Rather PN. Molecular Microbiology. 2004;51:437–446. doi: 10.1046/j.1365-2958.2003.03835.x. [DOI] [PubMed] [Google Scholar]
- 167.Stafford GP, Hughes C. Microbiology. 2007;153:541–547. doi: 10.1099/mic.0.2006/002576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.