Abstract
Tumor viruses can induce cell transformation by overcoming cellular defense mechanisms and promoting the ungoverned proliferation of infected cells. To this end, functionally related viral oncogenes have evolved in disparate viruses to override key proliferative and survival intracellular pathways, thus assuring efficient viral replication and contributing to tumor formation. Indeed, the study of viral oncogenes has been a powerful tool for disclosing fundamental insights into these basic cellular processes.
In this regard, the Kaposi’s sarcoma-associated Herpesvirus (KSHV or HHV8), the etiological agent of Kaposi’s sarcoma (KS), is an exemplary model of an oncogenic virus that includes within its genome several homologues of cellular genes implicated in the regulation of cell proliferation and apoptosis. However, emerging evidence now points to a single KSHV gene, ORF74, encoding for the viral G protein-coupled receptor (vGPCR), as essential for KS development. Expressed in only a fraction of cells within KS lesions, this viral receptor induces tumorigenesis through both autocrine and paracrine mechanisms. Indeed, work from several labs has demonstrated that vGPCR can promote cell proliferation, enhance cell survival, modulate cell migration, stimulate angiogenesis, and recruit inflammatory cells, both in expressing cells, as well as in neighboring (bystander) cells. Examination of this powerful viral oncogene may expose novel targets for the treatment of patients with KS and could ultimately provide a unique perspective into how GPCRs, and specifically chemokine receptors, contribute to angiogenesis and tumorigenesis.
Keywords: endothelial cell, Kaposi’s sarcoma, rapamycin, sirolimus, mTOR, Akt, PI3 kinase, NFκB, Rac1, Kaposi’s sarcoma associated herpesvirus, KSHV, Human herpesvirus-8, HHV-8, G protein-coupled receptor, vGPCR, paracrine neoplasia
Introduction
As we approach the Centennial anniversary of Peyton Rous’s landmark experiment demonstrating that extract solution from filtered avian tumors could be injected into unaffected chickens to produce sarcomas [Rous, 1911], we may benefit from looking back on this celebrated discovery – and the subsequent work it inspired – which introduced the idea that a “minute parasitic organism” could be the cause of cancer. In the 1930s, Richard Shope later demonstrated that a similar non-filterable agent from rabbit papillomas could produce both benign and malignant tumors when injected into rabbits [Shope RE, 1933]. John Bittner subsequently suggested that a similar vector may be passed from mothers to their offspring via breast milk [Bittner, 1936]. By the 1960s research on animal tumor viruses was flourishing; experiments demonstrating the transfer of tumors from affected to unaffected animals via injection of acellular extracts had been performed in hamsters, rats, cats, and non human primates [Javier and Butel, 2008].
However, despite the plethora of evidence that viruses were the etiologic agent for numerous animal tumors, experimental evidence demonstrating that viruses could also cause cancer in humans was less forthcoming. Our understanding of the molecular basis of human cancer was nonetheless transformed by further examination of the Rous sarcoma virus (RSV). In the 1970s, Bishop and Varmus demonstrated that the cellular homologue for “sarc” (later termed src), the presumed viral oncogene encoded by RSV, was found in numerous species, including humans [Stehelin et al., 1976]. They postulated that src was not an oncogene, but a normal cellular gene that played an important role in fundamental cellular processes (e.g. cell growth, proliferation, survival). They further suggested that this “proto-oncogene” was transferred to RSV during its evolution. Subsequent work by numerous laboratories has demonstrated that mutational activation (or inactivation) of proto-oncogenes (or tumor suppressors, respectively) is, indeed, the foundation of human cancer [Butel, 2000].
Ironically, in the last half century, only a handful of viruses have ultimately proved to be, at least in part, responsible for the development of cancer in humans (Table 1). Several of these pathogens encode viral oncogenes that, like v-src, have been pirated from their cellular host; and like c-src, their cellular cousins have been shown to play essential roles in basic cellular processes. Studies of these viral oncogenes have thus provided fundamental insight into both human cancer and the basic cellular processes that are perturbed in the development of human neoplastic disease.
Table 1.
Human tumor viruses, encoded oncogenes and their cellular targets.
| Tumor virus |
Year of discovery |
Proposed encoded oncogene |
Proposed cellular homologue |
Proposed cellular targets |
|---|---|---|---|---|
| HPV | 1907 | E6/E7 | - | p53, Dlg1, Scribble, PATJ, MAGI-1, MUPP1, Rb |
| EBV | 1965 | LMP1 | CD40 | TRAFs |
| HBV | 1967 | HBx | - | HBXIP, Ras-Raf-MAPK |
| HTLV-1 | 1980 | Tax | - | NFκB, p300/CBP, Dlg1, Scribble |
| HCV | 1989 | Core, NS3, NS5A | bcl-2 | p53, p21 |
| HHV-8 | 1994 | vGPCR | CCX2 | PI3K, mTOR |
The Kaposi’s Sarcoma-Associated Herpesvirus (KSHV)
Unique among the viruses associated with human cancer is the recently discovered Human Herpesvirus-8 (HHV-8) or Kaposi’s Sarcoma-Associated Herpesvirus (KSHV). A member of the gamma-herpesvirus family, KSHV was first isolated from human AIDS-KS lesions by Yuan Chang and Patrick Moore [Chang et al., 1994] and later found to be associated with all four forms of KS (classic, AIDS-associated, endemic (African) and iatrogenic) [Dourmishev et al., 2003; Moore and Chang, 2001]. KSHV was also identified as the etiological agent for two lymphoproliferative disorders: primary effusion lymphomas (PELs) and multicentric Castleman’s disease [Cesarman, 2002]; associations of KSHV with other diseases are less well established [Dourmishev et al., 2003].
Similar to other members of the herpesvirus family, KSHV remains in latency in the majority of infected cells and the viral genome is maintained as a closed extrachromosomal episome within the nucleus. Gene expression in latently infected cells is limited to a few viral genes that maintain the latent state and may further function to help evade immune detection [Moore and Chang, 2001]. A small percentage of KSHV infected cells spontaneously exit latency to enter the lytic cycle, which is accompanied by the expression of viral replicative and structural genes, resulting in the lysis of the host cell and the release of a new progeny of infective virions [Dourmishev et al., 2003; Jenner and Boshoff, 2002].
The KSHV genome contains more than 80 open reading frames, including those genes required for viral replication and assembly [Moore and Chang, 2001]. Unlike most tumor viruses, KSHV encodes over a dozen homologues to mammalian proteins likely pirated by KSHV from its cellular host. Further investigation has demonstrated that several of these genes play a role in regulating host cell immune function, enhancing host cell survival, and promoting angiogenesis [Jenner and Boshoff, 2002]; consequently a role for these genes has been proposed in Kaposi’s sarcomagenesis. However, early studies have incited reasonable disagreement as to which KSHV gene(s) may be responsible for the initiation of cell transformation. Ironically, this was due not to the lack of candidate viral oncogenes, but rather to the remarkable redundancy of KSHV genes that bear transforming potential.
KSHV vGPCR as the viral gene responsible for KS development
Identification of the KSHV oncogene(s) is further confused by accumulating evidence that lytic genes may play an important role in Kaposi’s sarcomagenesis. Because lytic genes are expressed in cells ultimately destined to die (lyse), it was thought that they are unlikely to play a significant role in cell transformation. Nonetheless, emerging evidence from several laboratories supports a key role for a single lytic gene, the KSHV G-protein-coupled receptor (vGPCR), in the initiation of KS [Guo et al., 2003; Montaner et al., 2003; Yang et al., 2000]. vGPCR, a member of the CXC chemokine family of GPCRs, has a mutation within the highly conserved DRY sequence that renders the receptor constitutively-active. Numerous mitogenic and survival intracellular pathways are upregulated in vGPCR-expressing cells [Sodhi et al., 2004b] (Figure 2). vGPCR activation of these pathways further leads to the transcription and secretion of the growth factors, chemokines and cytokines that may promote the angiogenic phenotype characteristic of human KS [Sodhi et al., 2004b]. Indeed, early studies demonstrated that vGPCR harbors transforming potential in vitro; vGPCR was therefore among the leading candidates for the KSHV oncogene [Arvanitakis et al., 1997; Bais et al., 1998; Cesarman et al., 1996].
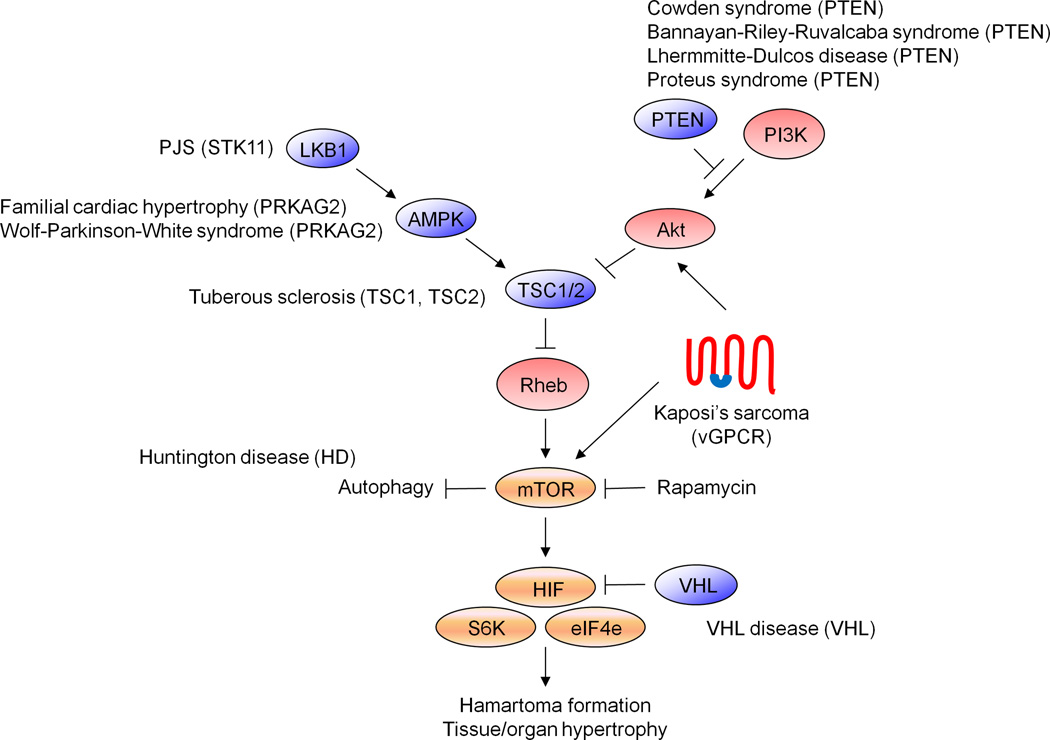
Figure 2. mTOR in human disease.
Schematic showing the functional relationship between the mTOR signaling pathway and several genetic diseases, some of them characterized by the development of vascular tumors. In a similar manner, vGPCR, the KSHV gene hypothesized to be responsible for KS development, appears to trigger Kaposi’s sarcomagenesis by dysregulating this signaling pathway.
In 2003, a novel KS mouse model was engineered in which endothelial-specific retroviral transduction was used to express putative KSHV oncogenes in the vascular endothelium of mice, mimicking the infection of endothelial cells by KSHV in humans [Montaner et al., 2003]. Surprisingly, despite the redundancy in candidate KSHV oncogenes, vGPCR was the only gene that was able to produce vascular tumors using this model [Montaner et al., 2003]. Tumors induced by expression of vGPCR are strikingly similar to human KS lesions, suggestive of an important role for this gene in the initiation of KS. In addition to their histological, ultrastructural and immunohistochemical similarity to human KS lesions, vGPCR-induced tumors also have a unique predilection for the dermis, identical to human KS, further suggesting that dermal endothelial cells may be particularly vulnerable to vGPCR-induced sarcomagenesis. Indeed, two transgenic animals that express vGPCR under either a ubiquitous (SV40) or a T cell-specific (CD2) promoter also manifest dermal angioproliferative lesions that closely resemble those seen in KS [Guo et al., 2003; Yang et al., 2000]. Of note, in all vGPCR models – just as in human KS – expression of vGPCR is restricted to a small subset of cells within the lesions, supporting the hypothesis that vGPCR influences tumor growth by paracrine mechanisms promoted by the cytokines, chemokines and growth factors secreted by vGPCR-expressing cells.
How does KSHV vGPCR – a lytic gene – cause cancer?
As a lytic protein, vGPCR transcription is known to be activated by the KSHV replication and transcription activator (RTA; ORF50), the KSHV lytic master-switch protein [Moore and Chang, 2001]. Interestingly, vGPCR can also activate the ORF50 promoter, as part of a positive feedback mechanism resulting in sustained ORF50 expression, continued ORF50-dependent lytic transcription, and successful viral progeny formation [Bottero et al., 2009].
Although it is expressed in a fraction of cells within KS lesions, it is not unreasonable to suggest that vGPCR (and other KSHV lytic genes) may still contribute to KS pathogenesis. Indeed, interruption of lytic replication can impair KS tumor development at all stages of the natural history of KSHV infection, supportive of a critical role for lytic genes in KS tumor maintenance [Martin et al., 1999]. However, it remains unclear how a gene expressed in cells destined to die can play a role in cell immortalization.
Several theories have been proposed to explain this paradox. The simplest theory may be that vGPCR may initiate cell transformation when expressed transiently, early in the infectious process. The expression of this receptor may then create an environment in which subsequent tumor development can occur (perhaps in the presence of other KSHV survival genes), after which receptor expression may become unnecessary [Cesarman et al., 2000]. This “hit-and-run” mechanism has been previously documented for the major transforming protein of the human T cell leukemia virus-1 (HTLV-1), Tax, as well as for the human papilloma virus E5 gene product [DiMaio and Mattoon, 2001; Yoshida, 2001]. However, compelling data using novel KS animal models suggests that vGPCR is required for the maintenance of KS tumors. Despite the fact that vGPCR is only expressed in a minority of tumor cells, selective elimination of vGPCR-expressing cells in established allografts in nude mice results in tumor regression [Montaner et al., 2006]. Similarly, using an inducible transgenic system for the expression of vGPCR, it has been shown that continued vGPCR expression is required for tumor growth, and that cessation of the vGPCR stimulus leads to partial tumor regression [Jensen et al., 2005]. These observations argue against the simple “hit-and-run” hypothesis for vGPCR oncogenesis.
Alternatively, it has been suggested that transient expression in the few lytically-infected cells may be sufficient for vGPCR to participate in tumor initiation and maintenance. This theory presupposes that the primary function of vGPCR is to promote the secretion of angiogenic and inflammatory cytokines, rather than to directly transform expressing cells. While not sufficient to transform cells on their own, KSHV latent gene expression (in latently-infected cells) may instead prove to be oncogenic in the presence of the potent pro-angiogenic and pro-inflammatory mediators elaborated by vGPCR-expressing (lytically-infected) cells [Montaner et al., 2003]. A similar role for paracrine neoplasia has been suggested for the Reed-Sternberg cells in Hodgkin lymphoma [Schmitz et al., 2009]. In support of this hypothesis, a novel allograft model for KS using mixed cell populations of endothelial cells expressing either KSHV latent oncogenes (v-Cyclin and v-Flip) or vGPCR in a ratio that reproduces that observed in human KS lesions was used to demonstrate that KSHV latent genes are indeed sufficient to promote tumorigenesis in vivo in the presence of the paracrine growth factors secreted by vGPCR-expressing cells [Montaner et al., 2006]. However, this theory does not explain why infection with KSHV alone – although necessary – is not sufficient to cause KS. Ultimately, it remains unclear whether transient expression of vGPCR in lytically-infected cells (i.e., expression for only a few hours prior to cell lysis) is sufficient to produce the environment necessary for latent genes to promote tumor formation.
A third theory to explain the potent sarcomagenic potential of this viral lytic gene is that dysregulated expression of vGPCR in non-lytic cells may trigger KS development [Sodhi et al., 2004a]. According to this hypothesis, during the normal viral life cycle, vGPCR is indeed a lytic gene, and its potent transforming potential is kept in check through multiple mechanisms: 1) restricted expression (vGPCR is transcribed within the 3’ end of a bicistronic mRNA, in cells ultimately destined to die); 2) the inhibition of its signaling by cellular host proteins (SDF1α and IP10 have been shown to down regulate vGPCR constitutive signaling); and 3) the inhibition of vGPCR signaling by KSHV-encoded proteins (viral monocyte inflammatory protein-II, or vMIP2, has also been shown to down regulate vGPCR signaling). Consequently, proliferative signals initiated by vGPCR may serve only to prolong lytic cell survival while secreted angiogenic and inflammatory growth factors may function to recruit bystander cells for infection; vGPCR may thereby function to help ensure efficient viral replication. However, under special circumstances (e.g., immunosuppression, HIV co-infection, inflammation, aborted lytic cycle progression), dysregulation of the normal viral program may result in nonlytic expression and enhanced signaling of vGPCR, which, together with other unknown factors, may ultimately manifest as KS [Sodhi et al., 2004a]. Indeed, HIV Tat has been shown to increase expression of KSHV lytic genes, including vGPCR, whose expression is significantly enhanced in aggressive AIDS-KS as compared with the more benign classical KS lesions [Yen-Moore et al., 2000]. Moreover, several inflammatory cytokines (interleukin-8, GROα) released by HIV infected cells can increase vGPCR signaling [Couty and Gershengorn, 2004]. Of note, two vGPCR-encoding transcripts have been identified in KSHV-induced lesions: the lytic 2.7 kb bicistronic K14/vGPCR transcript and a smaller 1.4 kb monocistronic vGPCR transcript [Nador et al., 2001]. It is tempting to speculate that the latter may prove to be a nonlytic transcript of dysregulated vGPCR-expressing cells.
Ultimately, the role of vGPCR in KS initiation and maintenance suggest that this viral gene may hold the answer to understanding KS and may represent an appropriate molecular target for the treatment of patients with KS lesions. Just as studies of other viral oncogenes (e.g. viral src) has provided fundamental insights into basic cellular processes, examining the role of vGPCR in KS has provided a unique perspective into how GPCRs, and specifically CXCR chemokine receptors, contribute to endothelial cell proliferation and dysregulated angiogenesis.
vGPCR and Kaposi’s sarcomagenesis
Just as KSHV encodes a redundancy of candidate oncogenes, vGPCR appears to contribute to KS pathogenesis through a plethora of mechanisms. Among its many proposed functions, vGPCR has been shown to promote cell proliferation, enhance cell survival, modulate cell migration, stimulate angiogenesis and recruit inflammatory cells; all of which occurs in both expressing endothelial cells as well as in neighboring “bystander” endothelial cells [Sodhi et al., 2004b]. Thus, although the KSHV oncogene responsible for initiating KS may have been narrowed to one principal suspect, the mechanism(s) whereby vGPCR promotes Kaposi’s sarcomagenesis remain under active investigation. A common theme among the disparate properties of vGPCR is the deregulation of normal endothelial cell signaling, which together manifest as dysregulated angiogenesis. Examination of vGPCR oncogenesis has therefore provided insights into normal and abnormal endothelial cell function.
Rac1 plays a key role in vGPCR paracrine neoplasia
Although established late-stage human KS tumors are comprised by a majority of cells infected with KSHV, only approximately 10% of spindle and endothelial cells in early KS lesions are KSHV positive [Dupin et al., 1999]. Similarly, when human primary endothelial cells are infected with KSHV in vitro, KSHV is present in only 1–6% of cells in each infected culture [Flore et al., 1998]; nonetheless, this is sufficient to promote an immortalized phenotype in almost 100% of cultured cells. Thus, transformation by KSHV appears to be mediated – in part – through paracrine mechanisms.
In this regard, it has been shown that vGPCR oncogenesis is also mediated through paracrine mechanisms. vGPCR stimulates a network of intracellular signaling cascades which upregulate the activity of numerous transcription factors, including hypoxia-inducible factor-1α (HIF-1α), nuclear factor-kappaB (NF-κB), activator protein-1 (AP-1), cAMP response element binding protein (CREB) and nuclear factor of activated T cells (NFAT), resulting in the expression of angiogenic growth factors and pro-inflammatory chemokines and cytokines such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, tumor necrosis factor α (TNFα), and MIP-1 as well as VEGF receptor-2/ KDR and several adhesion molecules (VCAM-1, ICAM-1 and E-selectin) [Bais et al., 1998; Couty et al., 2001; Montaner et al., 2001; Montaner et al., 2004; Pati et al., 2001; Pati et al., 2003; Schwarz and Murphy, 2001; Sodhi et al., 2000]. This, in turn, promotes angiogenesis and inflammatory cell recruitment, hallmarks of KS.
Since small guanosine triphosphate (GTP)-binding proteins represent critical links between GPCRs and nuclear transcription factors, the involvement of members of the Rho family of small guanosine triphosphatases (GTPases) in vGPCR-induced transcriptional regulation has been investigated [Montaner et al., 2004]. These experiments revealed that vGPCR potently activates the small G protein, Rac1. Preventing the activation of Rac1 by vGPCR blocks the stimulation of the transcriptional activity of NF-κB, AP-1, and NFAT, resulting in the inhibition of cytokine secretion in vitro and vGPCR sarcomagenesis in vivo [Montaner et al., 2004]. Moreover, expression of activated Rac1 is sufficient to promote endothelial cell transformation in mice [Ma et al., 2009; Montaner et al., 2004]. More recently, an animal model with endothelial cell-specific deletion of Rac1 has demonstrated an essential role for this GTPase in endothelial cell function and vascular development, corroborating a role for Rac1-mediated pathways in aberrant neovascularization [Tan et al., 2008]. These findings suggest that further examination of these pathways may provide fundamental insights into the role of Rac1 in pathological angiogenesis. Indeed, ongoing work has begun to uncover the roles of the Rho GTPases, including Rac1, in the regulation of normal and pathologic endothelial cell function [Bryan and D'Amore, 2007; Mammoto et al., 2008].
Elaboration of vGPCR paracrine secretions is mediated by NF-κB
The identity and relative contribution of vGPCR pro-angiogenic and pro-inflammatory mediators has also been under intensive investigation. Regulation of host gene expression by vGPCR has been examined using DNA microarray technology, in both B cells and endothelial cells [Polson et al., 2002]. Several genes affecting endothelial/vascular growth and remodeling are induced by vGPCR, including interleukin-6, GROα, plasminogen, thrombomodulin, the urokinase-type plasminogen activator receptor, and to a modest extent vascular endothelial growth factor C. The angiogenic program triggered upon inducible expression of vGPCR in mice similarly reveals upregulation of a wide battery of angiogenic factors including PIGF, iNOS, PDGF-B, TNFα, angiopoietin-2, MMP-9 and MMP-13 metalloproteases as well as angiogenic factor receptors including VEGF receptors (VEGFR1 and VEGFR2), angiopoietin receptors (Tie1 and Tie2) and the PDGF receptor β (PDGFβ) [Jensen et al., 2005].
Interestingly, using full-genome microarray analysis, it has been shown that vGPCR stimulates a NF-κB-related gene expression signature in endothelial cells, both directly (endothelial cells expressing vGPCR) or indirectly (endothelial cells exposed to vGPCR-induced secretions) and activation of NF-κB appears to be required for vGPCR-induced transformation [Martin et al., 2008]. Surprisingly, these two processes (direct and indirect activation of NF-κB) seem to be highly interrelated, as vGPCR promotes the NF-κB-dependent expression of cytokines and chemokines that can then activate NF-κB in endothelial cells not expressing this KSHV oncogene. The expression of cytokines by endothelial cells exposed to vGPCR-induced secretions may contribute to propagate the vGPCR-initiated NF-κB paracrine/autocrine signaling network to the surrounding and even distant endothelial cells, thereby promoting their unrestricted growth. Indeed, the link between inflammation and angiogenesis has long been appreciated and emerging evidence suggests that NF-κB may play a fundamental role linking the immune response to pathological angiogenesis [Ono, 2008].
Direct and paracrine activation of Akt/TSC/mTOR in Kaposi’s sarcomagenesis
vGPCR belongs to the rhodopsin/β-adrenergic subfamily of G protein-coupled receptors and shows closest homology to the human C-X-C chemokine receptor type 1 (CXCR1) and 2 (CXCR2) [Couty and Gershengorn, 2004]. Unlike its cellular homologues, the KSHV-encoded receptor does not require ligand binding to transduce signals inside the cell; rather, expression of vGPCR leads to constitutive activation of intracellular molecules [Sodhi et al., 2004b] (Figure 2). Despite its constitutive activity, vGPCR binds a variety of ligands that can further modulate its signaling [Couty and Gershengorn, 2004; Rosenkilde and Schwartz, 2000]. Indeed, vGPCR is capable of interacting with a much broader array of chemokines than do most mammalian receptors. Gro-α binding activates vGPCR above its constitutive signaling level, behaving as a full agonist of the receptor. As mentioned above, IP-10, SDF-1 and the KSHV-encoded CC chemokine v-MIP2 are inverse agonists, inhibiting vGPCR activity. Other chemokines, including IL-8, neutrophil activating peptide-2 (NAP-2, CXCL7) and epithelial cell-derived neutrophil-activating 78 (ENA78, CXCL5), are neutral antagonists for vGPCR, since they do not affect constitutive signaling but would compete for binding with receptor agonists or inverse agonists [Couty and Gershengorn, 2004; Rosenkilde and Schwartz, 2000].
Among the intracellular cascades activated by vGPCR, the Akt signaling pathway appears to play a central role in Kaposi’s sarcomagenesis [Montaner et al., 2001; Sodhi et al., 2004c]. This serine/threonine protein kinase is a major signal transducer of the phosphoinositide 3-kinase (PI3K) pathway and plays a pivotal role in numerous cellular phenotypes associated with cancer including cell proliferation, survival, angiogenesis and tissue invasion [Chin and Toker, 2009]. Initial in vitro experiments in primary cultured human endothelial cells showed that expression of vGPCR is able to induce PI3K-dependent Akt activation through the release of βγ subunits from both pertussis toxin-sensitive and -insensitive G proteins [Montaner et al., 2001]. Akt activation by vGPCR promotes the protection of endothelial cells from apoptosis, suggestive of its key role in promoting the survival of KSHV-infected cells. In vivo studies have corroborated the critical role of Akt in vGPCR sarcomagenesis [Sodhi et al., 2004c]. Immunohistochemical analysis of biopsies of murine (KS-like) vGPCR tumors as well as cutaneous and visceral human KS lesions revealed high levels of phosphorylated Akt in tumor cells, demonstrating that activation of Akt is a molecular hallmark of KS, further supporting a role for this pathway in human Kaposi’s sarcomagenesis [Sodhi et al., 2004c].
Emerging evidence suggest that mTOR may be the key Akt effector in KS development [Sodhi et al., 2006]. Akt has been recognized as an essential link between the PI3K pathway and the mammalian target of rapamycin (mTOR) through the inactivation of the tuberous sclerosis complex (TSC) [Manning and Cantley, 2003; Richardson et al., 2004]. The TSC complex, formed by hamartin (TSC1) and tuberin (TSC2), functions as a GTPase-activating protein (GAP) for Rheb, a Ras-related small GTP binding protein that promotes the activation of mTOR [Garami et al., 2003; Inoki et al., 2003; Tee et al., 2003]. Phosphorylation of the tumor suppressor TSC2 by Akt results in its inactivation, thereby promoting the accumulation of (active) Rheb-GTP and the induction of mTOR activity. mTOR then triggers the phosphorylation of key regulators of the cellular translation machinery, including ribosomal p70 S6 kinase (p70 S6K) and eukaryote initiation factor 4E binding protein 1 (4EBP1) [Aoki et al., 2001; Gingras et al., 1998]. Of interest, mutations in different signaling proteins of the TSC/mTOR pathway are associated with the onset of other human diseases characterized by the development of vascular tumors (i.e. tuberous sclerosis, Cowden disease) [Inoki et al., 2005] (Figure 3). In a similar manner, vGPCR may trigger Kaposi’s sarcomagenesis by dysregulating this signaling pathway.
Indeed, expression of vGPCR in endothelial cells induces tuberin (TSC2) phosphorylation and inactivation, thereby promoting the phosphorylation of mTOR and its downstream molecules, including p70 S6K and 4EBP1 (in both expressing cells as well as neighboring bystander cells through a paracrine mechanism) [Sodhi et al., 2006]. Pharmacological inhibition of either PI3K or mTOR in vGPCR-expressing cells dramatically reduced the proliferation of these cells. Overexpression of Rheb strongly protected vGPCR-expressing cells from the ability of the PI3K-inhibitor, LY294002, (but not rapamycin) to inhibit cell proliferation, implicating mTOR as an essential Akt effector in the promotion of endothelial cell growth by vGPCR. Indeed, pharmacologic inhibition of mTOR with rapamycin prevented the growth of vGPCR tumors in vivo, suggesting that the TSC/mTOR signaling pathway is required for vGPCR sarcomagenesis [Sodhi et al., 2006].
Immunohistochemical analysis of both vGPCR-induced murine tumors and human KS lesions demonstrated elevated levels of phosphorylated (active) mTOR downstream effectors [Sodhi et al., 2006]. Of note, it has been shown that vGPCR conditioned media also induces Akt and mTOR in treated endothelial cells. Indeed, most cells in vGPCR murine tumors as well as in human KS lesions express elevated levels of activated (phosphorylated) Akt and the downstream mTOR target, S6 ribosomal protein, suggestive of a paracrine activation of these pathways [Sodhi et al., 2006; Sodhi et al., 2004c]. Experiments using allografts derived from the injection of nude mice with endothelial cells co-expressing vGPCR along with a rapamycin-insensitive mTOR mutant demonstrated equal sensitivity to treatment with rapamycin as did endothelial cells expressing vGPCR alone [Sodhi et al., 2006]. These findings along with other unpublished results from our group suggest that direct activation of TSC/mTOR in vGPCR-expressing cells is not sufficient to explain the potent oncogenic potential of vGPCR; rather, the paracrine activation of TSC/mTOR by vGPCR angiogenic factors may be essential for vGPCR tumorigenesis. Since it is known that rapamycin and its analogs have potent antiangiogenic properties related to the inhibition of HIF-1α and the suppression of VEGF signal transduction, it is tempting to hypothesize that vGPCR paracrine activation of TSC/mTOR could be acting in KS as a mediator between inflammation and VEGF-mediated angiogenesis [Lee and Hung, 2007].
Supportive of the key role of the TSC/mTOR pathway in vGPCR sarcomagenesis, mice haploinsufficient for TSC2 are predisposed to vascular sarcomas remarkably similar to KS. These observations suggest that the sarcomagenic potential of KSHV may be a direct consequence of the profound sensitivity of endothelial cells to vGPCR dysregulation of the TSC2/mTOR pathway [Kwiatkowski et al., 2002; Onda et al., 1999; Sodhi et al., 2006]. This hypothesis is further supported by recent evidence demonstrating that inhibition of mTOR with sirolimus results in the resolution of classic KS and KS lesions in renal transplant patients [Campistol, 2009; Guenova et al., 2008; Merimsky et al., 2008; Montaner, 2007] Taken together, these findings implicate vGPCR activation of the mTOR pathway as a critical event in the development of KS [Sodhi et al., 2006]. Emerging evidence points to a key role for the mTOR pathway in pathological angiogenesis [Guertin and Sabatini, 2007] and further suggests that this pathway may play an essential role in regulating endothelial cell growth and survival.
Concluding remarks
Just as the v-src oncogene has been a powerful tool for helping us understand the role of c-src in cell growth and proliferation, vGPCR may similarly provide a greater appreciation for the role of chemokine receptors in angiogenesis and tumorigenesis. Studies of this unique viral oncogene may further provide fundamental insights into normal and pathological endothelial cell growth and survival, thereby exposing novel molecular targets for the treatment of pathological angiogenesis.
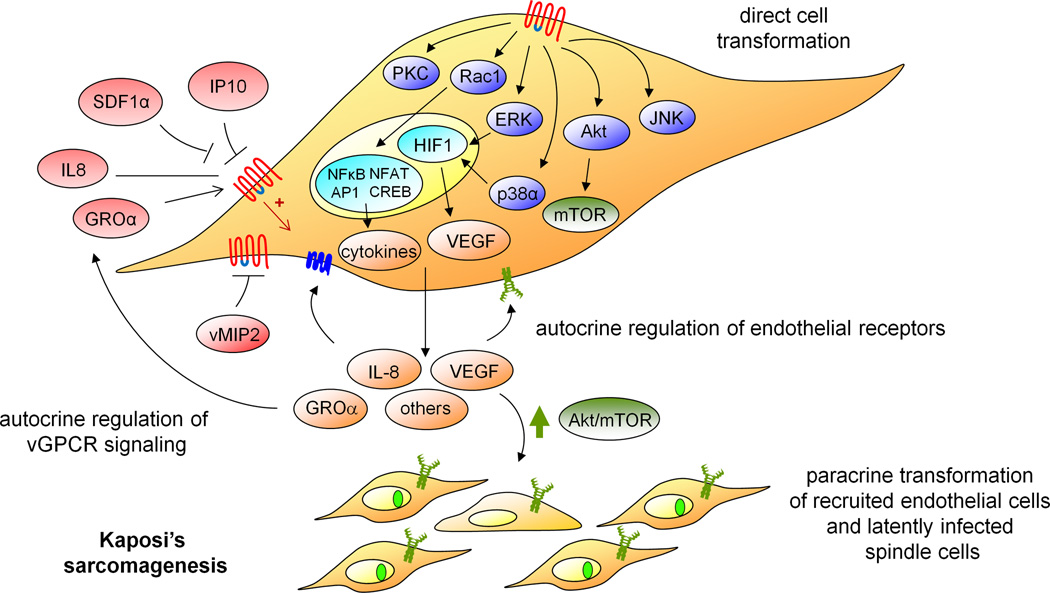
Figure 1. vGPCR signaling pathways in Kaposi’s sarcomagenesis.
The expression of Kaposi’s-sarcoma associated herpesvirus (KSHV) G-protein-coupled receptor (vGPCR) in KSHV-infected endothelial cells appears to be essential for triggering Kaposi’s sarcomagenesis. Signaling pathways that are upregulated by vGPCR in endothelial cells induce endothelial-cell transformation through both direct and indirect (paracrine) mechanisms. vGPCR directly activates different intracellular pathways, including PKC, Akt/mTOR as well as MAPK cascades (such as JNK, ERK and p38). vGPCR, through the small GTPase Rac1, stimulates the activity of key cellular transcription factors (including AP-1, NF-κB, and NFAT) and the secretion of multiple inflammatory/angiogenic molecules. Phosphorylation of HIF1 by ERK and p38 leads to HIF activation and VEGF secretion. vGPCR secreted angiogenic factors may then bind to and activate endogenous cellular receptors in neighboring cells, leading to the indirect (paracrine) activation of signaling pathways, such as Akt/mTOR. Several of these secreted chemokines and cytokines, as well as the KSHV-encoded vMIP2, could also function as ligands for vGPCR, modulating its signalling through an autocrine mechanism.
Acknowledgments
This work was supported by grant R01CA119911 (National Cancer Institute, NIH). BCJ is a recipient of a predoctoral fellowship from the CNPq-Brazil. We thank Dr. Akrit Sodhi for his insightful comments.
References
- Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc Natl Acad Sci U S A. 2001;98:136–141. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein- coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA, Gerhengorn MC. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Bittner JJ. Some Possible Effects Of Nursing On The Mammary Gland Tumor Incidence In Mice. Science. 1936;84:162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- Bottero V, Sharma-Walia N, Kerur N, Paul AG, Sadagopan S, Cannon M, Chandran B. Kaposi sarcoma-associated herpes virus (KSHV) G protein-coupled receptor (vGPCR) activates the ORF50 lytic switch promoter: a potential positive feedback loop for sustained ORF50 gene expression. Virology. 2009;392:34–51. doi: 10.1016/j.virol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan BA, D'Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21:405–426. doi: 10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- Campistol JM. Minimizing the risk of posttransplant malignancy. Transplantation. 2009;87:S19–S22. doi: 10.1097/TP.0b013e3181a07a57. [DOI] [PubMed] [Google Scholar]
- Cesarman E. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 2002;159:27–37. doi: 10.1007/978-3-642-56352-2_4. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Mesri EA, Gershengorn MC. Viral G protein-coupled receptor and Kaposi's sarcoma: a model of paracrine neoplasia? J Exp Med. 2000;191:417–422. doi: 10.1084/jem.191.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, Moore PS, Chang Y, Knowles DM. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal. 2009;21:470–476. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couty JP, Geras-Raaka E, Weksler BB, Gershengorn MC. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J Biol Chem. 2001;276:33805–33811. doi: 10.1074/jbc.M104631200. [DOI] [PubMed] [Google Scholar]
- Couty JP, Gershengorn MC. Insights into the viral G protein-coupled receptor encoded by human herpesvirus type 8 (HHV-8) Biol Cell. 2004;96:349–354. doi: 10.1016/j.biolcel.2004.03.011. [DOI] [PubMed] [Google Scholar]
- DiMaio D, Mattoon D. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene. 2001;20:7866–7873. doi: 10.1038/sj.onc.1204915. [DOI] [PubMed] [Google Scholar]
- Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol Biol Rev. 2003;67:175–212. doi: 10.1128/MMBR.67.2.175-212.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flore O, Rafii S, Ely S, O'Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi's sarcoma- associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenova E, Metzler G, Hoetzenecker W, Berneburg M, Rocken M. Classic Mediterranean Kaposi's sarcoma regression with sirolimus treatment. Arch Dermatol. 2008;144:692–693. doi: 10.1001/archderm.144.5.692. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Guo HG, Sadowska M, Reid W, Tschachler E, Hayward G, Reitz M. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol. 2003;77:2631–2639. doi: 10.1128/JVI.77.4.2631-2639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier RT, Butel JS. The history of tumor virology. Cancer Res. 2008;68:7693–7706. doi: 10.1158/0008-5472.CAN-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner RG, Boshoff C. The molecular pathology of Kaposi's sarcoma-associated herpesvirus. Biochim Biophys Acta. 2002;1602:1–22. doi: 10.1016/s0304-419x(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Jensen KK, Manfra DJ, Grisotto MG, Martin AP, Vassileva G, Kelley K, Schwartz TW, Lira SA. The human herpes virus 8-encoded chemokine receptor is required for angioproliferation in a murine model of Kaposi's sarcoma. J Immunol. 2005;174:3686–3694. doi: 10.4049/jimmunol.174.6.3686. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- Lee DF, Hung MC. All roads lead to mTOR: integrating inflammation and tumor angiogenesis. Cell Cycle. 2007;6:3011–3014. doi: 10.4161/cc.6.24.5085. [DOI] [PubMed] [Google Scholar]
- Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermont PJ. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci U S A. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Curr Opin Hematol. 2008;15:228–234. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- Martin D, Galisteo R, Ji Y, Montaner S, Gutkind JS. An NF-kappaB gene expression signature contributes to Kaposi's sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene. 2008;27:1844–1852. doi: 10.1038/sj.onc.1210817. [DOI] [PubMed] [Google Scholar]
- Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340:1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- Merimsky O, Jiveliouk I, Sagi-Eisenberg R. Targeting mTOR in HIV-Negative Classic Kaposi's Sarcoma. Sarcoma. 2008;2008:825093. doi: 10.1155/2008/825093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S. Akt/TSC/mTOR activation by the KSHV G protein-coupled receptor: emerging insights into the molecular oncogenesis and treatment of Kaposi's sarcoma. Cell Cycle. 2007;6:438–443. doi: 10.4161/cc.6.4.3843. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–2648. [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Ramsdell AK, Martin D, Hu J, Sawai ET, Gutkind JS. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi's sarcoma. Cancer Res. 2006;66:168–174. doi: 10.1158/0008-5472.CAN-05-1026. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Servitja JM, Ramsdell AK, Barac A, Sawai ET, Gutkind JS. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903–2911. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nador RG, Milligan LL, Flore O, Wang X, Arvanitakis L, Knowles DM, Cesarman E. Expression of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor monocistronic and bicistronic transcripts in primary effusion lymphomas. Virology. 2001;287:62–70. doi: 10.1006/viro.2001.1016. [DOI] [PubMed] [Google Scholar]
- Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S, Cavrois M, Guo HG, Foulke JS, Jr, Kim J, Feldman RA, Reitz M. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J Virol. 2001;75:8660–8673. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati S, Foulke JS, Jr, Barabitskaya O, Kim J, Nair BC, Hone D, Smart J, Feldman RA, Reitz M. Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat. J Virol. 2003;77:5759–5773. doi: 10.1128/JVI.77.10.5759-5773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson AG, Wang D, DeRisi J, Ganem D. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 2002;62:4525–4530. [PubMed] [Google Scholar]
- Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147–159. doi: 10.1016/j.semcdb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. Potency of ligands correlates with affinity measured against agonist and inverse agonists but not against neutral ligand in constitutively active chemokine receptor. Mol Pharmacol. 2000;57:602–609. doi: 10.1124/mol.57.3.602. [DOI] [PubMed] [Google Scholar]
- Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13:397–399. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R, Stanelle J, Hansmann ML, Kuppers R. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol. 2009;4:151–174. doi: 10.1146/annurev.pathol.4.110807.092209. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Murphy PM. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167:505–513. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- Shope RE HE. Infectious papillomatosis of rabbits; with a note on the histopathology. J Exp Med. 1933;58:607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, Sawai ET, Molinolo A, Gutkind JS, Montaner S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10:133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Gutkind JS. Does dysregulated expression of a deregulated viral GPCR trigger Kaposi's sarcomagenesis? Faseb J. 2004a;18:422–427. doi: 10.1096/fj.03-1035hyp. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Gutkind JS. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat Rev Mol Cell Biol. 2004b;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. Akt plays a central role in sarcomagenesis induced by Kaposi's sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004c;101:4821–4826. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873–4880. [PubMed] [Google Scholar]
- Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260:170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. Faseb J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, Lira SA. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med. 2000;191:445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen-Moore A, Hudnall SD, Rady PL, Wagner RF, Jr, Moore TO, Memar O, Hughes TK, Tyring SK. Differential expression of the HHV-8 vGCR cellular homolog gene in AIDS-associated and classic Kaposi's sarcoma: potential role of HIV-1 Tat. Virology. 2000;267:247–251. doi: 10.1006/viro.1999.0125. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]