Abstract
The Group VIA Phospholipase A2 (iPLA2β) is the first recognized cytosolic Ca2+-independent PLA2 and has been proposed to participate in arachidonic acid (20:4) incorporation into glycerophosphocholine lipids, cell proliferation, exocytosis, apoptosis, and other processes. To study iPLA2β functions, we disrupted its gene by homologous recombination to generate mice that do not express iPLA2β. Heterozygous iPLA2β+/− breeding pairs yield a Mendelian 1:2:1 ratio of iPLA2β+/+, iPLA2β+/−, and iPLA2β−/− pups and a 1:1 male:female gender distribution of iPLA2β−/− pups. Several tissues of wild-type mice express iPLA2β mRNA, immunoreactive protein, and activity, and testes express the highest levels. Testes or other tissues of iPLA2β−/− mice express no iPLA2β mRNA or protein, but iPLA2β−/− testes are not deficient in 20:4-containing glycerophosphocholine lipids, indicating that iPLA2β does not play an obligatory role in formation of such lipids in that tissue. Spermatozoa from iPLA2β−/− mice have reduced motility and impaired ability to fertilize mouse oocytes in vitro and in vivo, and inhibiting iPLA2β with a bromoenol lactone suicide substrate reduces motility of wild-type spermatozoa in a time- and concentration-dependent manner.Mating iPLA2β−/− male mice with iPLA2β+/+, iPLA2β+/−, or iPLA2β−/− female mice yields only about 7% of the number of pups produced by mating pairs with an iPLA2β+/+ or iPLA2β+/− male, but iPLA2β−/− female mice have nearly normal fertility. These findings indicate that iPLA2β plays an important functional role in spermatozoa, suggest a target for developing male contraceptive drugs, and complement reports that disruption of the Group IVA PLA2 (cPLA2α) gene impairs female reproductive ability.
Phospholipases A2 (PLA2s)1 catalyze hydrolysis of the sn-2 fatty acid substituent from glycerophospholipid substrates to yield a free fatty acid, e.g. arachidonic acid, and a 2-lysophospholipid that can initiate synthesis of lipid mediators (1, 2). Arachidonic acid (20:4), for example, is converted by various oxygenases to prostaglandins, leukotrienes, epoxy-trienes, and other mediators, and acetylation of 2-lysoplasmanylcholine yields the mediator platelet-activating factor (3). Both 20:4 and 2-lysophospholipids also have intrinsic mediator activities (4, 5).
Of mammalian PLA2s so far cloned, secretory PLA2s are low molecular weight enzymes that require millimolar Ca2+ concentrations for catalysis and affect eicosanoid generation, inflammation, and other processes (1). The platelet-activating factor-acetylhydrolase PLA2 family exhibits substrate specificity for platelet-activating factor and oxidized phospholipids. Of Group IV cytosolic PLA2 (cPLA2) family members (1), cPLA2α was the first identified and prefers substrates with sn-2 20:4 residues, catalyzes 20:4 release for subsequent metabolism, associates with its substrates in membranes upon rises in cytosolic [Ca2+] in stimulated cells, and is also regulated by phosphorylation (6).
The Group VI PLA2 (iPLA2) enzymes (1, 2, 7, 8) do not require Ca2+ for catalysis and are inhibited by a bromoenol lactone (BEL) suicide substrate that does not inhibit secretory PLA2 or cPLA2 at similar concentrations (9, 10). The Group VIA PLA2 (iPLA2β) resides mainly in the cytoplasm of resting cells, but the Group VIB PLA2 (iPLA2γ) contains a peroxisomal targeting sequence and is membrane-associated (11).
Many cells express multiple distinct PLA2s, and this might reflect redundancy or specific functions of an individual PLA2. Physiological roles for PLA2s can be studied with genetic gain-or loss-of-function manipulations. Overexpressing iPLA2β in insulinoma cells, for example, provides evidence for its participation in exocytosis, cell proliferation, and apoptosis (12–14), and cPLA2α gene disruption by homologous recombination has produced cPLA2α-null mice that reveal a role for cPLA2α in parturition, allergic responses, and post-ischemic brain injury (15, 16).
We have used homologous recombination to generate iPLA2β-null mice. Among various tissues, testes of wild-type mice express the highest iPLA2β levels, and male iPLA2β−/− mice produce spermatozoa with reduced motility and impaired ability to fertilize mouse oocytes in vitro and in vivo. Male iPLA2β−/− mice are also much less fertile than wild-type males, but female iPLA2β−/− mouse fertility is not markedly impaired. Our findings indicate that iPLA2β−/− plays an important functional role in spermatozoa.
EXPERIMENTAL PROCEDURES
Generating iPLA2−/− Knockout Mice
To prepare a knockout construct, we obtained a P1 clone with an iPLA2β gene fragment by screening a 129/SvJ mouse genomic DNA library with rat iPLA2β cDNA (8). The 7.8-kb EcoRV-BglII fragment containing exons 7–10 was sub-cloned into pBluescript SK-. A single XhoI site mapped to exon 9 near sequence encoding the 463GTSTG467 lipase motif. A pGK-neo-poly(A) cassette with a neomycin resistance gene (neo) was inserted at this site to disrupt iPLA2β coding sequence and provide a positive selection marker. A pGK-thymidine kinase gene was inserted into the BglII site of the genomic fragment as a negative selection marker. This yielded a vector with 4.1 and 3.7 kb of the 5′ and 3′ sequences, respectively, homologous to the native gene for recombination.
The targeting fragment was excised with EcoRV and BglII and introduced into 129/SvJ mouse embryonic stem cells by electroporation. Clones resistant to G418 and ganciclovir were isolated and screened for homologous recombination by Southern blotting of genomic DNA digested with EcoRV. Six embryonic stem clones contained 6.7-kb fragments characteristic of iPLA2β gene disruption and, as expected, 8.7-kb fragments from the wild-type allele. The clones were injected into C57BL/6 mouse blastocysts, which were implanted for gestation to yield chimeras that were mated with wild-type mice to yield heterozygotes. Mating iPLA2β+/− mice with each other gave iPLA2β−/−, iPLA2β−/+, and iPLA2β+/+ pups.
Mice were genotyped with Southern blots of tail clipping genomic DNA digested with EcoRV using a 32P-labeled probe prepared by PCR amplification or restriction endonuclease digestion with EcoRV and BglII to yield an 0.95-kb fragment located downstream of the targeting sequence. This probe hybridizes with an 8.7-kb DNA fragment in iPLA2β+/+ mice, with a 6.7-kb fragment in iPLA2β−/− mice, and with both in iPLA2β+/− mice (see Fig. 1D). The iPLA2β gene-targeting sequence inserts a new EcoRV site in genomic DNA to yield a fragment that hybridizes with the probe that is shorter than that from wild-type genomic DNA.
FIG. 1. Scheme for disrupting the iPLA2β gene in mice, identifying disrupted and wild-type iPLA2β alleles, and determining genotypes of progeny of mating male and female iPLA2β+/− pairs.
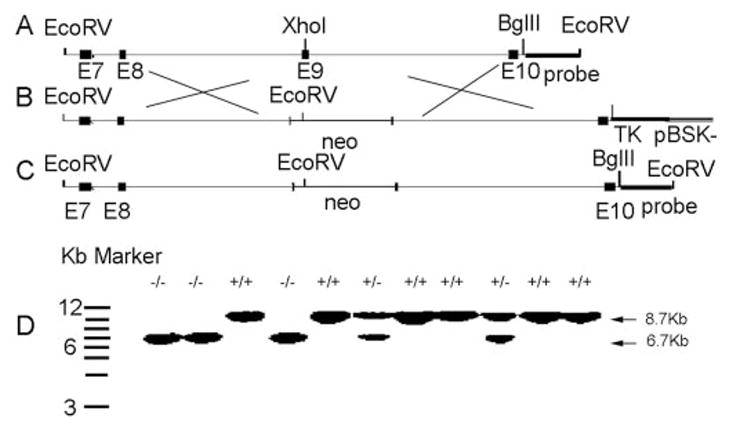
A–C, wild-type allele (A) and scheme for preparing the knockout construct and incorporating it into the iPLA2β gene (B and C). D, Southern blot identification of disrupted and wild-type iPLA2β alleles and genotypes of offspring from iPLA2β+/− mating pairs.
Northern and Western Blot Analyses
As described (8), tissue total RNA analyzed by electrophoresis was transferred to nylon membranes that were hybridized with iPLA2β cDNA probes labeled by random priming. The iPLA2β cDNA probe was amplified using reverse transcription-PCR (sense primer, 5′-TGTGACGTGGACAGCACTAGC; antisense primer, 5′-CCCCAGAGAAACGACTATGGA), which hybridizes to both short and long isoforms of iPLA2β (2). This region of cDNA represents the sequence that encodes amino acid residues 307–552 of the short isoform of iPLA2β. A final stringency wash was followed by autoradiography, and the filters were then stripped and hybridized with cDNA probes to rat glyceraldehyde-3-phosphate dehydrogenase to mark RNA load (8). Western blotting was performed as described with tissue homogenates and iPLA2β antibody that had been raised in rabbits (12).
Ca2+-independent Phospholipase A2 Activity Assay
Tissue Ca2+-independent PLA2 specific activity was determined as described (8) in cytosol by monitoring hydrolysis of 1-palmitoyl-2-[14C]linoleoyl-sn-glycero-3-phosphocholine in assay buffer (40 mM Tris, pH 7.5, 5 mM EGTA) to [14C]linoleate as measured by TLC and liquid scintillation spectrometry. Specific activity was calculated from released [14C]disintegrations/min and protein content.
Electrospray Ionization Mass Spectrometry of Lipids
Tissue lipids were extracted and infused into the ESI source of a triple stage quadrupole mass spectrometer in CHCl3/CH3OH containing LiOH, as described (17–19). Glycerophosphocholine (GPC) lipids and triacylglycerols (TAGs) were analyzed as [M+Li]+ ions. Seminolipid and glycerol-, inositol-, and ethanolamine-glycerophospholipids and phosphatidic acids were analyzed as [M-H]− ions. Tandem spectra were obtained by accelerating selected ions into a collision cell to induce dissociation, and product ions were analyzed in the third quadrupole. Internal standards, e.g. 14:0/14:0-GPC, were used for quantitation.
Analyses of Motility of Spermatozoa and Effect of the iPLA2β Inhibitor BEL
Male mice 8–10 weeks old were euthanized with pentobarbital in a protocol approved by our Animal Studies Committee. Caudae epididymides, and the vasa deferentia were removed and placed in prewarmed and pregassed human tubal fluid medium (Irvine Scientific, Irvine, CA). Morphology and viability of spermatozoa were assessed as described (20–22), did not differ between iPLA2β+/+ and iPLA2β−/− mice, and were unaffected by BEL. Sperm suspension was placed in an incubation chamber (37 °C), and motility was quantified using CEROS computer-assisted semen analyses (version 10; Hamilton Thorne Research, Beverly, MA), as discussed (23). Total and progressive motilities were analyzed in about 104 spermatozoa from each genotype. A swim-up motility assay was also used that involved centrifugation, incubation (1 h, 37 °C), and counting spermatozoa that migrated into supernatant, as described (22). Spermatozoa were treated in some cases with various concentrations of BEL (0–20 μM), and motility was analyzed after various periods (0–30 min).
In Vitro Fertilization Assay
Female mice about 30 days old were injected with pregnant mare serum gonadotropin (7.5 IU intraperitoneally) and 48 h later were injected with human chorionic gonadotropin (hCG; 7.5 IU intraperitoneally), as discussed (24). Oviducts were collected 13 h later, and the oocyte cumulus complexes were removed. One or two complexes were placed in a culture dish (100 mm, 50 μl minimum essential medium, 25 mM NaHCO3, 1% fatty acid-free bovine serum albumin; Sigma), and droplets were covered by embryo-tested mineral oil (Sigma). Spermatozoa collected from cauda epididymis were allowed to swim into minimum essential medium (10 min, 37 °C), aspirated, incubated (37 °C, 60 min, 5% CO2, 4 × 106/ml) to permit capacitation, diluted, and added to oocyte droplets to achieve a concentration of 105 or 106 spermatozoa/ml. Spermatozoa and oocytes were co-incubated (5–24 h, 37 °C, 5% CO2). Oocytes and zygotes were passed through a pipette to remove cumulus cells, fixed (1% paraformaldehyde), placed on a microscope slide with affixed coverslip, treated with acetic acid/ethanol (1/3 v/v, 2 min), stained (1% lacmoid/45% acetic acid), destained (45% acetic acid), and examined for oocyte fertilization reflected by a second polar body and two pronuclei with one near a sperm tail (24).
In Vivo Fertilization Assay
Superovulation of 6–8-week-old female mice was induced by injecting pregnant mare serum gonadotropin (10 IU, intraperitoneally; Sigma) and, after 48 h, hCG (10 IU, intraperitoneal, Sigma), as described (21–24). Those mice were then mated with iPLA2β+/+ or iPLA2β−/− males overnight, and, after 48 h, the mice were euthanized with pentobarbital according to a protocol approved by our Animal Studies Committee. Dissected uterine horns were flushed with human tubal fluid medium containing 0.25% bovine serum albumin (Sigma) to retrieve one- and two-cell structures, which were then counted and cultured (human tubal fluid with 0.25% bovine serum albumin media microdroplets under mineral oil, 37 °C, 5% CO2) for 72 h. The blastocyst embryos were then counted.
Fertility Tests
Male mice were placed with females for 6 weeks and then removed, and the pups were counted, as described (21). Females mated with iPLA2β−/− males had a normal frequency of vaginal semen plugs, determined as described (25).
Statistical Analyses
Comparisons between two groups or among three or more groups were performed with Student’s t test or with analysis of variance using posthoc analysis (Statview 4.51, Abacus), respectively.
RESULTS
Generation of iPLA2β-null Mice
Fig. 1 illustrates our scheme to generate mice with a disrupted iPLA2β gene and to determine their genotypes. An iPLA2β gene-targeting construct was introduced into mouse embryonic stem cells, and those that incorporated it by homologous recombination, which disrupts the iPLA2β gene coding sequence (Fig. 1B), were introduced into mouse blastocysts that were then implanted into pseudo-pregnant female mice. Progeny included chimeras, which were mated with wild-type mice, and litters included iPLA2β+/− mice, reflecting iPLA2β− allele germ-line transmission. Mating pairs of iPLA2β+/− mice yielded iPLA2β+/+, iPLA2β+/−, and iPLA2β−/− pups in a nearly Mendelian 1:2: 1 distribution with a 1:1 male/female gender distribution (Table I).
Table I.
Distribution of genotypes and genders in offspring from mating male iPLA2β+/− heterozygous mice with female heterozygous iPLA2β+/− mice
| Genotype | Total no. of pups | Gender | No. of pups |
|---|---|---|---|
| iPLA2β+/+ | 31 | Male | 13 |
| Female | 18 | ||
| iPLA2β+/− | 72 | Male | 35 |
| Female | 37 | ||
| iPLA2β−/− | 35 | Male | 17 |
| Female | 18 |
Northern blots revealed that wild-type mouse testes iPLA2β mRNA content exceeds that of muscle, pancreas, kidney, liver, brain, heart, adipose, and epididymis (Fig. 2A). No iPLA2β mRNA was detected in testes or other tissues of iPLA2β−/− mice. Western blots also revealed high iPLA2β protein expression in wild-type testes, but no iPLA2β protein was detected in iPLA2β−/− testes or spermatozoa (Fig. 2B).
FIG. 2. Expression of iPLA2β mRNA in tissues and of iPLA2β immunoreactive protein in testes and spermatozoa of iPLA2β+/+ and iPLA2β−/− mice.
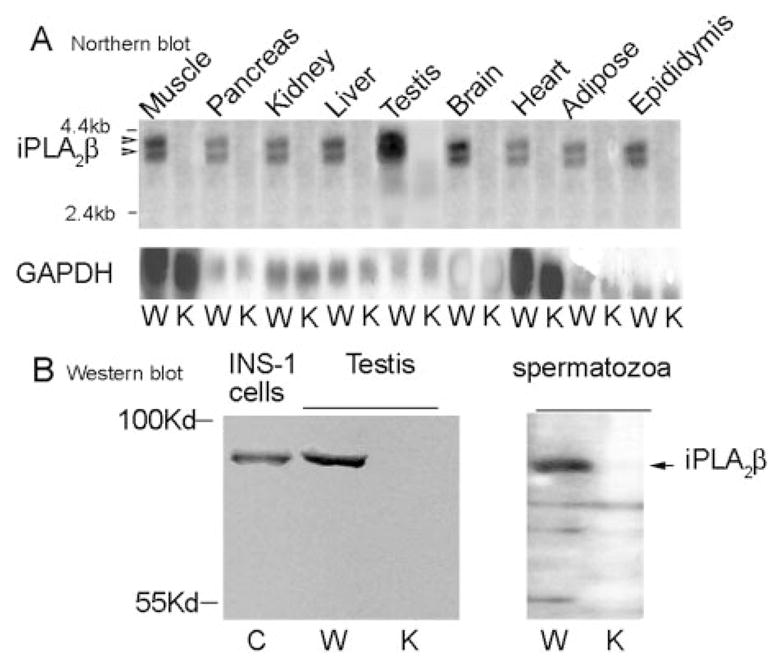
A, Northern blots of iPLA2β mRNA in tissues of wild-type (W) and iPLA2β−/− (K) mice. B, Western blots of iPLA2β immunoreactive protein in INS-1 insulinoma cells (C, control) and in testes (left panel) and spermatozoa (right panel) of wild-type (W) and iPLA2β−/− knockout (K) mice.
The highest level of Ca2+-independent PLA2 activity is also observed in the testes of wild-type mice (Fig. 3A), and, as characteristic of iPLA2β (10–12), it is stimulated by ATP and inhibited by a BEL suicide substrate (Fig. 3B). Testes and other tissues of iPLA2β−/− mice exhibit much less total Ca2+-independent PLA2 activity than wild-type mouse tissues (Fig. 3A), suggesting that iPLA2β is ordinarily the major tissue Ca2+−independent PLA2 and that there is little up-regulation of other Ca2+-independent PLA2s, such as cPLA2γ and iPLA2γ (1–2), to compensate for the loss of iPLA2β.
FIG. 3. Enzymatic activity of iPLA2β in tissues and effects of ATP and BEL on iPLA2β activity in testes of iPLA2β+/+ and iPLA2β−/− mice.
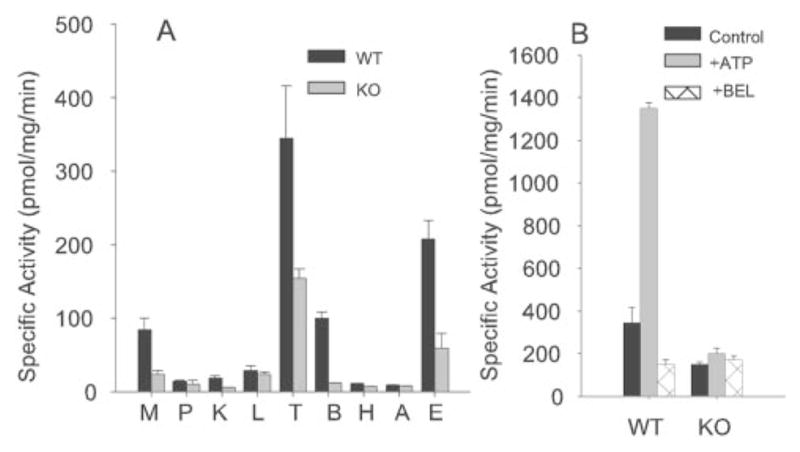
A, tissue iPLA2β activities in wild-type (WT, black bars) and iPLA2β−/− knockout (KO, shaded bars) mice determined as described under “Experimental Procedures” in muscle (M), pancreas (P), kidney (K), liver (L), testes (T), brain (B), heart (H), adipose (A), and epididymis (E). B, effect of ATP (1 mM) and the iPLA2β inhibitor BEL (10 μM) on testes Ca2+-independent PLA2 activity in wild-type (WT) and iPLA2β−/− knockout (KO) mice.
Effect of iPLA2β Gene Disruption on Tissue Content of Arachidonic Acid-containing Phosphatidylcholine and Other Lipids
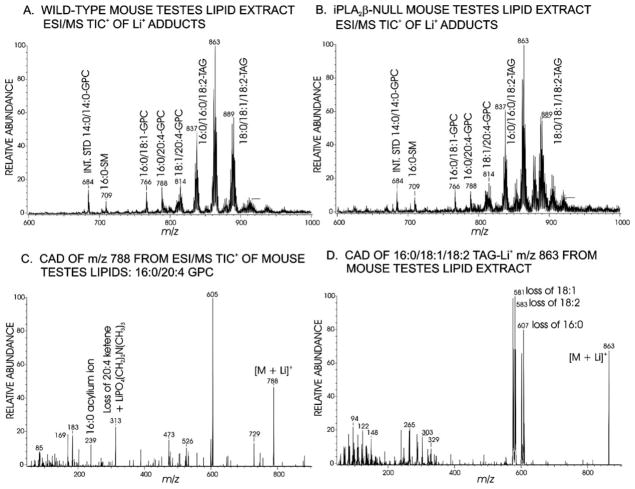
Studies of [3H8]arachidonic acid incorporation into P388D1 tumor cells have been taken to imply that the function of iPLA2β is to generate lysophosphatidylcholine acceptors for arachidonic acid (20:4) incorporation into GPC lipids (26), and it might thus be expected that iPLA2β−/− mouse tissues would be deficient in 20:4-containing GPC lipids. The lipids were extracted from wild-type and iPLA2β−/− mouse testes and analyzed by ESI/MS in the presence of LiOH. Fig. 4A is the ESI/MS positive total ion current spectrum for a lipid extract from wild-type mouse testes, and it contains ions with even m/z values for Li+ adducts (17) of internal standard (m/z 684) dimyristoyl-GPC (14:0/14:0-GPC) and of endogenous 16:0/18:1-GPC (m/z 766) and the 20:4-containing species 16:0/20:4-GPC (m/z 788) and 18:1/20:4-GPC (m/z 814).
FIG. 4. ESI/MS analyses of glycerolipids from testes of iPLA2β+/+ and iPLA2β−/− mice.
ESI/MS positive total ion current (TIC) tracing of a lipid extract from wild-type (A) or iPLA2β−/− knockout (B) mouse testes infused in LiOH solution. C, tandem mass spectrum from collisionally activated dissociation (CAD) of the ion of m/z 788 for 16:0/20:4-GPC-Li+. D, tandem mass spectrum from collisionally activated dissociation of the ion of m/z 863 for 16:0/18:1/18:2-TAG-Li+.
The spectrum also contains odd m/z value ions for Li+ adducts of sphingomyelins (e.g. 16:0-sphingomyelin at m/z 709) and of TAGs (18) with various fatty acid substituents (e.g. m/z 837, 863, and 889). Fig. 4C illustrates the tandem spectrum that identifies the species represented by the ion of m/z 788 as 16:0/20:4-GPC (20), and it contains ions for losses of trimethylamine (m/z 729), of phosphocholine (m/z 605), of Li+ phosphocholine (m/z 599), of palmitic acid (16:0, m/z 532), of Li+ 16:0 (m/z 526), of 20:4 (m/z 484), of Li+ 20:4 (m/z 478), of 16:0 plus trimethylamine (m/z 473), of the ketene of 20:4 plus Li+ phosphocholine (m/z 313), and 16:0 acylium ion (m/z 239). Fig. 4D illustrates tandem spectra that identify TAG Li+ adducts (18), such as that of the ion of m/z 863 for 16:0/18:1/18:2-TAGLi+ that contains ions reflecting losses of 16:0 (m/z 607), of Li+ 16:0 (m/z 601), of oleic acid (18:1, m/z 581), of Li+ 18:1 (m/z 575), of linoleic acid (18:2, m/z 583), and of Li+ 18:2 (m/z 577). Incomplete exchange of Li+ for Na+ on occasion resulted in satellite peaks in some TAG spectra (e.g. m/z 853, 879, and 905 in Fig. 4B) that did not occur in others (Fig. 4A).
The ESI/MS spectrum for Li+ adducts of iPLA2β−/− mouse testes lipids (Fig. 4B) is nearly identical to that for iPLA2β+/+ mice (Fig. 4A), and abundances of ions for 20:4-containing GPC lipids (m/z 788 and m/z 814) relative to the internal standard (m/z 684) are virtually identical in panels A and B of Fig. 4, indicating that iPLA2β−/− mouse testes are not deficient in 20:4-containing GPC lipids. Negative ion ESI/MS analyses (19) of testes lipids reveal 20:4-containing glycerophosphoethanolamine and glycerophosphoinositol lipids, e.g. 18:0/20:4-glycerophosphoethanolamine and 18:0/20:4-glycerophosphoinositol, and other lipid species. Negative ion ESI/MS spectra for iPLA2β−/− and iPLA2β+/+ testes lipids are also nearly identical (not shown). Lack of iPLA2β thus has little effect on testes phospholipid composition or 20:4-content, and iPLA2β−/− and iPLA2β+/+ testes also do not differ in gross or microscopic anatomy or weight.
Motility of Spermatozoa from Wild-type and iPLA2β−/− Mice
Although there is a modest reduction in number of spermatozoa produced by iPLA2β−/− mice (1.5 ± 0.4 × 107/mouse) compared with wild-type mice (3.4 ± 0.7 × 107/mouse), there is a marked reduction in motility of iPLA2β−/− mouse spermatozoa. Fig. 5A summarizes swim-up motility analyses and illustrates that motility of spermatozoa from iPLA2β−/− mice is less than 5% of that of spermatozoa from iPLA2β+/+ or iPLA2β+/− mice. Fig. 5B illustrates computer-assisted spermatozoa analyses of motility and also demonstrates reduced motility of spermatozoa from iPLA2β−/− compared with iPLA2β+/+ or iPLA2β+/− mice.
FIG. 5. Motility of spermatozoa from iPLA2β+/+, iPLA2β+/−, and iPLA2β−/− mice.
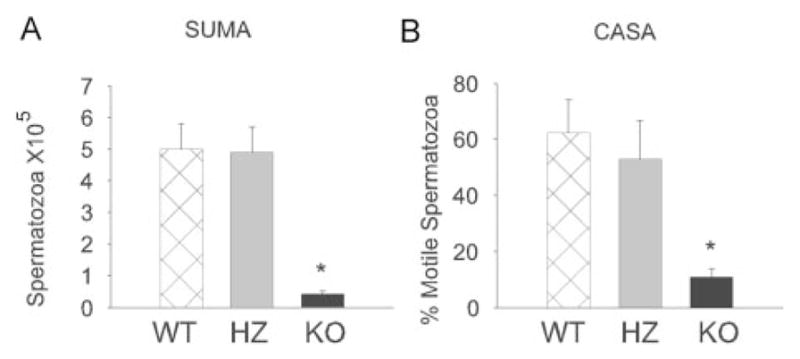
Motility of spermatozoa from wild-type (WT), heterozygous (HZ), and iPLA2β−/− knockout (KO) mice examined by swim-up motility assay (A) or computer-assisted semen analyses (B) studies described under “Experimental Procedures.” The error bars reflect S.E. (n = 4; *, p < 0.001).
Effect of Inhibiting iPLA2β Activity with a BEL Suicide Substrate on Motility of Spermatozoa
To examine further the role of iPLA2β in motility, suspensions of spermatozoa were treated with varied concentrations of the iPLA2β inhibitor BEL (9–10) for various intervals, and motility of spermatozoa was determined. BEL reduced motility of spermatozoa in a concentration- and time-dependent manner at [BEL] as low as 5 μM (Fig. 6A) and at times as early as 5 min (Fig. 6B).
FIG. 6. Effect of the iPLA2β inhibitor BEL on motility of wild-type mouse spermatozoa.
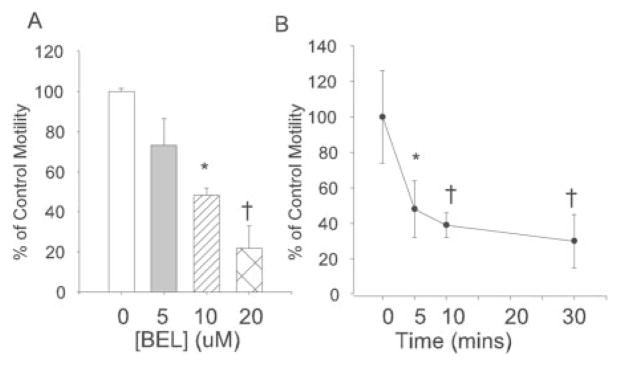
Motility of spermatozoa was examined with varied concentrations of BEL (A) at 60 min or with 20 μM BEL for various periods (B). The error bars reflect S.E. (n = 7; *, p < 0.05; †, p < 0.01).
In Vitro Fertilization of Mouse Oocytes
The reduced motility of iPLA2β−/− spermatozoa suggested that they might be impaired functionally. Fig. 7A illustrates that wild-type spermatozoa exhibit a concentration-dependent ability to fertilize oocytes from wild-type female mice in vitro, but spermatozoa from iPLA2β−/− mice achieved fertilization only about 5% as often as wild-type spermatozoa at the highest concentration tested, indicating that iPLA2β−/− spermatozoa are functionally impaired.
FIG. 7. Fertilization of mouse oocytes in vitro and in vivo by spermatozoa from iPLA2β+/+ and iPLA2β−/− mice.
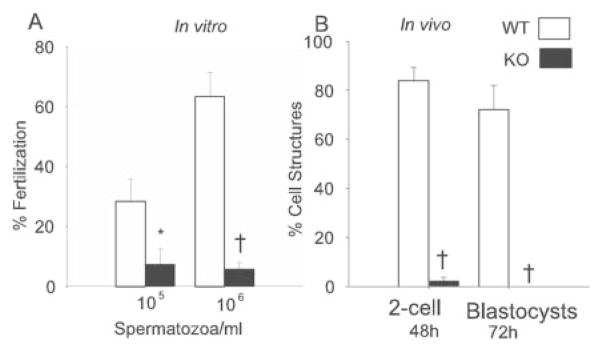
A, fertilization of mouse oocytes in vitro by varied concentrations of spermatozoa from wild-type iPLA2β+/+ (WT, shaded bars) mice and of iPLA2β−/− knockout (KO, black bars) mice determined as under “Experimental Procedures.” B, fertilization of mouse oocytes in vivo by control male mice of normal reproductive competence (shaded bars) or iPLA2β−/− knockout male (black bars) mice determined as under “Experimental Procedures” (*, p < 0.05; †, p < 0.001).
In Vivo Fertilization of Mouse Oocytes
Fertilization experiments in vivo were performed by inducing wild-type female mice to ovulate by treatment with injected pregnant mare serum gonadotropin and hCG. They were then mated with control male mice of normal reproductive competence or with iPLA2β−/− male mice. Products from oocytes were recovered from the uterus, and it was determined whether fertilization had occurred and whether there had been subsequent development of blastocysts. Fig. 7B illustrates that when female mice were mated with control, reproductively competent male mice, 84 two-cell structures were observed by 48 h after hCG injection, and 86% of them later developed into blastocysts. In contrast, when female mice were mated with iPLA2β−/− male mice, only 2 two-cell structures were observed by 48 h after hCG injection, and neither developed into a blastocyst (Fig. 7B). Both in vitro (Fig. 7A) and in vivo (Fig. 7B) fertilization experiments thus demonstrate functional impairment of spermatozoa from iPLA2β−/− mice.
Fertility of iPLA2β−/− Male Mice
The reduced motility of iPLA2β−/− mouse spermatozoa and their impairment in fertilizing oocytes raised the question of whether these mice would exhibit reduced fertility. Male iPLA2β+/+, iPLA2β+/−, and iPLA2β−/− mice were thus mated with female iPLA2β+/+, iPLA2β+/−, and iPLA2β−/− mice. Ten breeding pairs were examined for each possible genotypic pairing. Each pair was allowed to mate for 6 weeks, and the number of pups each pair produced during this period was determined. Table II illustrates that with wild-type iPLA2β+/+ male partners, female iPLA2β+/+ mice bore 59 pups and female iPLA2β−/− mice produced 70 pups, indicating that female iPLA2β−/− mice exhibit no severe reproductive defect.
Table II.
Number of offspring from mating pairs of male and female mice of different genotypes
| Male | Female | Pups in 6 weeks with 10 mating pairs |
|---|---|---|
| +/+ | +/+ | 59 |
| +/− | 85 | |
| −/− | 70 | |
| Total | 214 | |
| +/− | +/+ | 89 |
| +/− | 98 | |
| −/− | 48 | |
| Total | 235 | |
| −/− | +/+ | 15 |
| +/− | 1 | |
| −/− | 0 | |
| Total | 16 |
Similar numbers of pups were produced by pairs of either wild-type iPLA2β+/+ males or heterozygous iPLA2β+/− males with female mice of all three genotypes. A total of 214 pups were sired by iPLA2β+/+ males, and 235 pups were sired by iPLA2β+/− males (Table II), indicating that iPLA2β+/− males are not reproductively impaired. In contrast, iPLA2β−/− male mice sired only 16 pups or about 7% of the number sired by iPLA2β+/+ or iPLA2β+/− males, reflecting severe reproductive impairment in iPLA2β−/− males despite a normal frequency of vaginal semen plugs in females with which they were mated.
Male iPLA2β−/− males were not completely infertile and sired more pups with wild-type iPLA2β+/+ female partners than with iPLA2β+/− or iPLA2β−/− female partners (Table II). No mechanistic explanation for that finding is obvious because iPLA2β+/− females produced more pups (total of 183) than did iPLA2β+/+ females (total of 148 pups) when mated with iPLA2β+/+ or iPLA2β+/− males. The severe impairment of fertility of male iPLA2β−/− mice is reflected by the fact that they sired only a single pup with iPLA2β+/− female partners under these conditions. The larger number of pups resulting from matings of iPLA2β+/+ females with iPLA2β−/− males might reflect chance variation, although an occult mechanistic basis cannot be excluded.
DISCUSSION
Male iPLA2β−/− knockout mice have greatly reduced fertility that would impose a selection bias against that genotype, although female iPLA2β−/− mouse reproductive ability is not dramatically impaired. The low iPLA2β−/− male fertility is associated with markedly reduced motility of spermatozoa. Inhibition of iPLA2β with BEL also reduces motility of wild-type spermatozoa in a concentration- and time-dependent manner that resembles the effect of eliminating iPLA2β by gene disruption. The iPLA2β-null mouse thus joins a group of recently reported mouse models involving gene disruption that produce selective impairment of male (but not female) fertility associated with reduced motility of spermatozoa. Other such models include disruption of the genes for soluble adenylyl cyclase (20), for the voltage-gated cation channels Catsper1 and CatSper2 (27–29), and for plasma membrane Ca2+-ATPase 4 (25).
Signals that regulate motility of spermatozoa include changes in cAMP and intracellular [Ca2+] (27–33), and both parameters are affected by products of PLA2 action, which include a free fatty acid and a 2-lysophospholipid. Mice deficient in soluble adenylyl cyclase activity are infertile because of a severe sperm motility defect (20), and both abnormalities also occur in mice null for the catalytic subunit of cAMP-dependent protein kinase A (33). Products of iPLA2β action affect downstream effects of cAMP, and iPLA2β overexpression in insulinoma cells amplifies secretion induced by glucose and agents that elevate [cAMP] (12). The iPLA2β reaction products 2-lysophosphatidylcholine (LPC) and 2-lysoplasmenylcholine activate cAMP-dependent protein kinase A and enhance phosphorylation of the cAMP response element-binding protein in cardiac myocytes (34), and LPC generated by iPLA2β also regulates cAMP- and cAMP-dependent protein kinase A-dependent events in macrophages and endothelial cells (35, 36).
The flagellar motion underlying sperm motility is cyclic and is associated with [Ca2+] oscillations at the base of the flagellum that occur at the frequency of the flagellar beat (37). Hyperactivated motility involves Ca2+ release from intracellular sequestration sites, such as the redundant nuclear envelope that surrounds the axoneme at its origin in the flagellar base (30). The fact that motility of spermatozoa is impaired by knockout of channels that mediate Ca2+ entry (27–29) or a plasma membrane pump that extrudes Ca2+ (25) is consistent with a requirement for Ca2+ oscillations for flagellar motion.
Insulin secretion by β-cells is oscillatory and associated with [Ca2+] oscillations produced by a cyclic process that involves glucose-induced Ca2+ release from endoplasmic reticulum (ER) and resultant activation of a nonselective plasma membrane cation channels (38). Activation of these store-operated channels (SOC) depolarizes the plasma membrane and causes voltage-operated Ca2+ channels to mediate Ca2+ influx. This results in refilling of ER Ca2+ stores and inactivation of the depolarizing SOC (38). This cycle repeats itself in an oscillatory manner in the continued presence of stimulus.
Recently, iPLA2β has been found to participate in regulating SOC (39). Ca2+ store depletion-induced activation of depolarizing SOC and resultant activation of voltage-operated Ca2+ channels in smooth muscle cells involves production of Ca2+ influx factor by ER. Ca2+ influx factor then interacts with calmodulin so as to release it from and relieve its tonic inhibition of iPLA2β, which then catalyzes phospholipid hydrolysis. LPC produced by iPLA2β then activates SOC (39). Pharmacologic and biochemical evidence supports operation of this pathway in vascular smooth muscle cells and β-cells (38–41). The operation of a similar pathway in spermatozoa could rationalize the requirement for Ca2+ store release (30), iPLA2β (this report), Ca2+ entry channels (27–29), and a Ca2+ extrusion pump (25) in the oscillatory flagellar motion that underlies the motility of spermatozoa.
The free fatty acid product of iPLA2β action could also participate in regulating [Ca2+] in spermatozoa subcellular compartments. Arachidonic acid (20:4) facilitates Ca2+ entry from the extracellular space and Ca2+ release from ER (42), and 20:4-containing plasmenylethanolamine species are abundant in ER and are excellent iPLA2β substrates (43). Moreover, Ca2+ influx factor is an arachidonate oxygenation product (44), and its production could involve iPLA2β action.
The fact that spermatozoa from iPLA2β−/− mice are defective in in vitro fertilization in which a high concentration of spermatozoa are placed in close proximity to oocytes suggests that these spermatozoa might be defective in properties in addition to motility. Ca2+ signaling in the tail of spermatozoa is involved in regulating flagellar motion, and [Ca2+] in the head of spermatozoa is involved in the acrosomal reaction induced in spermatozoa by oocyte zona pellucida (45), a reaction in which iPLA2β could participate. A PLA2 is activated during induction of the acrosomal reaction by zona pellucida and releases arachidonic acid and LPC from spermatozoa membrane phospholipids (46). LPC also induces the acrosome reaction in spermatozoa (30, 32), and one species of LPC (2-lysoplasmanylcholine) is the precursor of the platelet-activating factor, which is produced by spermatozoa and is an autocrine inducer of capacitation (47).
Our finding that homozygous iPLA2β gene disruption impairs male reproductive ability by causing production of spermatozoa with reduced motility complements reports that cPLA2α gene disruption impairs female reproductive ability by preventing parturition (15–16). PLA2 activities are thus involved in multiple steps of the reproductive process, and the reduced motility and fertilization competence of spermatozoa from male iPLA2β−/− mice coupled with the reduction in motility of spermatozoa induced by inhibiting iPLA2β with BEL suggest that iPLA2β is a potential target for developing male contraceptive agents.
Acknowledgments
We thank Dr. Marie La Regina for assistance in examining histological slides of testes. We also thank Sheng Zhang, Alan Bohrer, and Wu Jin for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants R37-DK34388, PO1-HL57278, P41-RR00954, P60-DK20579, and P30-DK56341 (to J. T.), Grant RO1-HD38311 (to D. J. M.), Grant R01-DK063076 (to Z. M.), and Grant HD40390 (to K. M.) and by an award from the American Diabetes Association (to S. R.).
The abbreviations used are: PLA2, phospholipase A2; cPLA2, cytosolic PLA2; iPLA2, Group VI PLA2; BEL, bromoenol lactone; ESI, electrospray ionization; MS, mass spectrometry; GPC, glycerophosphocholine; TAG, triacylglycerol; hCG, human chorionic gonadotropin; LPC, 2-lysophosphatidylcholine; ER, endoplasmic reticulum; SOC, store-operated channel
References
- 1.Six D, Dennis E. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 2.Ma Z, Turk J. Prog Nucleic Acids Res Mol Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 3.Murphy RC, Fitzpatrick FA. Methods Enzymol. 1990;187:1–628. [Google Scholar]
- 4.Brash AR. J Clin Investig. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radu CG, Yang LV, Riedinger M, Au M, Witten ON. Proc Natl Acad Sci U S A. 2004;101:245–250. doi: 10.1073/pnas.2536801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gijon M, Spencer D, Kaiser A, Leslie C. J Cell Biol. 1999;145:1219–1232. doi: 10.1083/jcb.145.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. J Biol Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Ramanadham S, Kempe K, Chi XS, Ladenson J, Turk J. J Biol Chem. 1997;272:11118–11127. [PubMed] [Google Scholar]
- 9.Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW. J Biol Chem. 1991;266:7227–7232. [PubMed] [Google Scholar]
- 10.Ma Z, Ramanadham S, Hu Z, Turk J. Biochim Biophys Acta. 1998;1391:384–400. doi: 10.1016/s0005-2760(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 11.Mancuso D, Jenkins CM, Gross RW. J Biol Chem. 2000;275:9937–9945. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- 12.Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. J Biol Chem. 2001;276:13198–13208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- 13.Ma Z, Bohrer A, Wohltmann M, Ramanadham S, Hsu FF, Turk J. Lipids. 2001;36:689–700. doi: 10.1007/s11745-001-0774-9. [DOI] [PubMed] [Google Scholar]
- 14.Ramanadham S, Hsu FF, Zhang S, Jin C, Bohrer A, Song H, Bao S, Ma Z, Turk J. Biochemistry. 2004;43:918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, Sapirstein A. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 16.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 17.Hsu FF, Bohrer A, Turk J. J Am Soc Mass Spectrom. 1998;9:516–526. doi: 10.1016/S1044-0305(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 18.Hsu FF, Turk J. J Am Soc Mass Spectrom. 1999;10:587–600. doi: 10.1016/S1044-0305(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramanadham S, Hsu FF, Bohrer A, Nowatzke W, Ma Z, Turk J. Biochemistry. 1998;37:4533–4567. doi: 10.1021/bi9722507. [DOI] [PubMed] [Google Scholar]
- 20.Esposito G, Jaiswal B, Xie F, Krajnc-Franken M, Robben T, Strik A, Kuil C, Philipsen R, van Duin M, Conti M, Gossen J. Proc Natl Acad Sci U S A. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagaman J, Moyer J, Bachman E, Sigony S, Magyar P, Welch J, Smithies O, Krege J, O’Brien D. Proc Natl Acad Sci U S A. 1998;95:2252–2257. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz D, Goldfinger N, Kam Z, Rotter V. Cell Growth & Differ. 1999;10:665–675. [PubMed] [Google Scholar]
- 23.Moley KH, Vaughn WK, DeCherney AH, Diamond MP. J Reprod Fert. 1991;93:325–332. doi: 10.1530/jrf.0.0930325. [DOI] [PubMed] [Google Scholar]
- 24.Miller D, Gong X, Decker G, Schur B. J Cell Biol. 1993;123:1431–1440. doi: 10.1083/jcb.123.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Armesilla AL, Emerson M, Oceandy D, Knobeloch KP, Neyses L. J Biol Chem. 2004;279:28220–28226. doi: 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- 26.Balsinde J, Bianco I, Ackermann E, Conde-Frieboes K, Dennis E. Proc Natl Acad Sci U S A. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson A, Westenbroek R, Quill T, Ren D, Clapham D, Hille B, Garbers DL, Babcock D. Proc Natl Acad Sci U S A. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quill T, Ren D, Clapham D, Garbers DL. Proc Natl Acad Sci U S A. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quill T, Sugden SA, Rossi K, Doolittle L, Hammer R, Garbers DL. Proc Natl Acad Sci U S A. 2003;100:14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho HC, Suarez SS. Biol Reprod. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 31.Ho HC, Suarez SS. Biol Reprod. 2003;68:1590–1596. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- 32.Marquez B, Suarez SS. Biol Reprod. 2004;70:1626–1633. doi: 10.1095/biolreprod.103.026476. [DOI] [PubMed] [Google Scholar]
- 33.Skalhegg BS, Huang Y, Su T, Iddzerda RL, McKnight GS, Burton KA. Mol Endocrinol. 2002;16:630–639. doi: 10.1210/mend.16.3.0793. [DOI] [PubMed] [Google Scholar]
- 34.Williams S, Ford D. Am J Physiol. 2001;281:H168–H176. doi: 10.1152/ajpheart.2001.281.1.H168. [DOI] [PubMed] [Google Scholar]
- 35.Maggi LB, Jr, Moran JM, Scarim AL, Ford DA, Yoon JW, McHowat J, Buller RM, Corbett JA. J Biol Chem. 2002;277:38449–38455. doi: 10.1074/jbc.M206247200. [DOI] [PubMed] [Google Scholar]
- 36.Martinson B, Albert C, Corbett J, Wysolmerski R, Ford DA. J Lipid Res. 2003;44:1686–1691. doi: 10.1194/jlr.M300018-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Suarez SS, Varosi M, Dai X. Proc Natl Acad Sci U S A. 1993;90:4660–4664. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roe MW, Worley JF, Qian F, Tamarina N, Mittal AA, Dralyuk F, Blair NT, Mertz RJ, Philipson LH, Dukes ID. J Biol Chem. 1998;273:10402–10410. doi: 10.1074/jbc.273.17.10402. [DOI] [PubMed] [Google Scholar]
- 39.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova E, Bolotina VM. Nature Cell Biol. 2004;6:113–121. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 40.Wolf MJ, Wang J, Turk J, Gross RW. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]
- 41.Nowatzke W, Ramanadham S, Hsu FF, Ma Z, Bohrer A, Turk J. Endocrinology. 1998;139:4073–4085. doi: 10.1210/endo.139.10.6225. [DOI] [PubMed] [Google Scholar]
- 42.Ramanadham S, Gross RW, Turk J. Biochem Biophys Res Commun. 1992;184:647–653. doi: 10.1016/0006-291x(92)90638-2. [DOI] [PubMed] [Google Scholar]
- 43.Ramanadham S, Bohrer A, Gross RW, Turk J. Biochemistry. 1993;32:13499–13509. doi: 10.1021/bi00212a015. [DOI] [PubMed] [Google Scholar]
- 44.Rzigalinski BA, Willoughby KA, Hoffman SW, Falck JR, Ellis EF. J Biol Chem. 1999;274:175–182. doi: 10.1074/jbc.274.1.175. [DOI] [PubMed] [Google Scholar]
- 45.Talbot P, Schur B, Myles D. Biol Reprod. 2003;68:1–9. doi: 10.1095/biolreprod.102.007856. [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y, Chen W, Shi Q, Mao L, Yu Q, Fang X, Roldan E. Biol Reprod. 2003;68:904–913. doi: 10.1095/biolreprod.102.005777. [DOI] [PubMed] [Google Scholar]
- 47.Wu C, Stojanov T, Cham O, Ishi S, Shimuzu T, Li A, O’Neill C. J Biol Chem. 2001;276:26962–26968. doi: 10.1074/jbc.M103107200. [DOI] [PubMed] [Google Scholar]