Abstract
Many of the pathological effects of lipid peroxidation are mediated by aldehydes generated through fragmentation of lipid peroxides. Among these aldehydes, the γ-hydroxy- and γ-oxo-α,β-alkenals, e.g., 4-hydroxy-2-nonenal (HNE), and 4-oxo-2-nonenal (ONE), are especially prone to modify proteins and DNA through covalent adduction. In addition the “mirror image” γ-hydroxy- and γ-oxo- α,β-alkenal phospholipids, can serve as high affinity ligands for biological receptors triggering pathology. Therefore, the mechanisms by which these aldehydes are generated in vivo are under intense scrutiny. We now report observations supporting the intermediacy of a unique pseudo symmetrical diepoxycarbinyl radical that accounts for the coproduction of HNE, ONE and their “mirror image” analogues 9-hydroxy-12-oxo-dodec-10-enoic acid (HODA), 9-keto-12-oxo-dodec-10-enoic acid (KODA) upon fragmentation of 13-hydroperoxy-cis-9,10-epoxyoctadeca-11-enoic acid (13-HP-Epo-Acid).
Keywords: lipid peroxidation; linoleate-derived γ-hydroperoxy-α,β-unsaturated epoxide; HNE; ONE; HODA; KODA; pseudo symmetrical diepoxycarbinyl radical
Introduction
Lipid peroxidation is involved in many disease processes.[1–3] Aldehydes, generated through the fragmentation of lipid peroxides, mediate many of the pathological effects of lipid peroxidation.[4] The γ-hydroxy- and γ-oxo alkenals are especially prone to form biologically active covalent adducts with proteins and DNA.[5–10] Furthermore, oxidatively truncated phospholipids with γ-hydroxy (or oxo) α,β-unsaturated carbonyl functionality in the sn-2 acyl group are found on the surface of oxidatively damaged cell membranes or lipoprotein particles where they serve as high affinity ligands for biological receptors triggering pathology, e.g., through apoptosis[11, 12] or the innate immune system.[13]
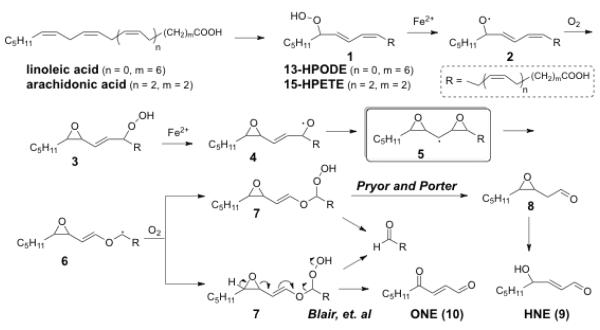
It now seems clear that a number of mechanisms are operable that lead to the formation of hydroxyl (oxo) alkenals from lipid peroxidation.[14–19] Both free radical-induced and enzyme-catalyzed, oxidation of polyunsaturated fatty acyls often involve initial abstraction of a doubly allylic hydrogen and generation of a hydroperoxydiene such as 13-hydroperoxy-9,11-octadecadienoate (13-HPODE, 1, n = 0, m = 7) from linoleic acid or 15-hydroperoxy-eicosa-5,7,10,13-tetraenoic acid (15-HPETE, 1, n = 2, m = 3) from arachidonic acid (Fig. 1). One mechanism proposed by Pryor and Porter[20] to account for the fragmentation of 13-HPODE or 15-HPETE to produce the γ-hydroxyα,β-unsaturated aldehyde 4-hydroxyl-2-nonenal (HNE, 9), involves fragmentation of a hydroperoxy epoxide intermediate 3 (Fig 1). Thus, reductive cleavage of hydroperoxide 1, e.g., by Fe(II), followed by cyclization of the resulting alkoxyl radical 2 delivers the allylic hydroperoxide 3. Reductive cleavage of 3 then gives alkoxyl radical 4, which can further cyclize to produce a diepoxycarbinyl radical 5. Homolysis of the oxirane C–C bond in 5 and capture of the carbinyl radical 6 by oxygen, is postulated to produce a vinyl ether 7 that is finally hydrolyzed to give 3,4-epoxy aldehyde 8. The epoxy aldehyde 8 was reported to rearrange to HNE (9) under very mild conditions.[21, 22] Because the reaction 13-hydroperoxylinoleic acid with 2'-deoxyguanosine produces adducts derived from 4-oxo-2-nonenal (ONE, 10), but not those expected from HNE (9), it was suggested that, rather than being generated through oxidation of HNE, ONE might be generated directly through fragmentation of 13-HPODE through a variation of Pryor and Porter's mechanism for the formation of HNE (9). Thus, the intermediate 7, instead of being hydrolyzed to deliver 3,4-epoxy aldehyde 8, eliminates water, a long chain fatty acid aldehyde and ONE (10).[7] This process would be driven by the generation of two C=O bonds and cleavage of the strained epoxy ring.
Figure 1.
Proposed mechanisms for the generation of HNE (9) and ONE (10) from arachidonate or linoleate hydroperoxides (1).
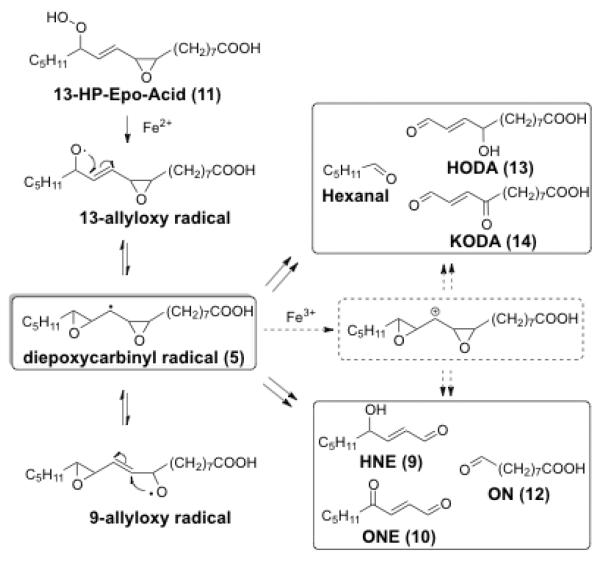
A noteworthy feature of the reaction pathways envisioned in Fig. 1 is the putative pseudo symmetrical diepoxycarbinyl radical intermediate 5. Owing to symmetry, if the formation of the diepoxycarbinyl radical is rapid and reversible, products arising from both a 9- and 13-allyloxy radical intermediates (Fig. 2) would be generated from the linoleate hydroperoxy epoxide 13-HP-Epo-Acid (11). If the putative 9- and 13-allyloxy radical intermediates equilibrate before fragmentation, analogous products – the saturated aldehydes hexanal and 9-oxononanoate (ON, 12), the γ-hydroxyenals 9-hydroxy-12-oxo-dodec-10-enoic acid (HODA, 13) and 4-hydroxynon-2-enal (HNE, 9), and the γ-ketoenals 9-keto-12-oxo-dodec-10-enoic acid (KODA, 14) and 4-oxo-2-nonenal (ONE, 10) – should be generated in similar yields.
Figure 2.
Proposed multiple fragmentations of 13-HP-Epo-Acid (11).
The equilibration of the 9- and 13-allyloxy radicals through the putative diepoxycarbinyl radical 5 required for the outcome predicted in Fig. 2 depends on cleavage of the oxirane C–O bond being favored over the oxirane C–C bond cleavage, e.g., 5 → 6 in Fig. 1. The interconversion of allyloxy radicals and epoxy carbinyl radicals[23–25] (the C–O bond formation and cleavage) is one of the fastest known radical reactions[26] (k1 = 2 × 109 s−1, k−1 = 3.2 × 1010 s−1, respectively[27, 28]). C–C bond cleavage of epoxy carbinyl radicals is also known but only if the substrate has radical-stabilizing groups, e.g., phenyl or vinyl.[29, 30] Ab initio molecular orbital calculations on a simple epoxy carbinyl radical model suggest that C–O bond cleavage is dominant in alkyl-substituted epoxy carbinyl radicals (Fig. 3).[31] Although the C–C bond cleavage product, the vinyloxycarbinyl radical, is predicted to be more stable than the C–O bond cleavage product, the allyoxy radical (− 16.5 kJmol−1 vs −5.0 kJmol−1), the barrier for C–C bond cleavage is significantly higher than the barrier for C–O bond cleavage (48.8 kJmol−1 vs 17.0 kJmol−1). Thus the C–O bond cleavage is favored kinetically. As in our model studies, lipid peroxidation in vivo occurs under mild conditions. This would favor the CO over the C–C bond cleavage proposed in Fig. 1.
Figure 3.
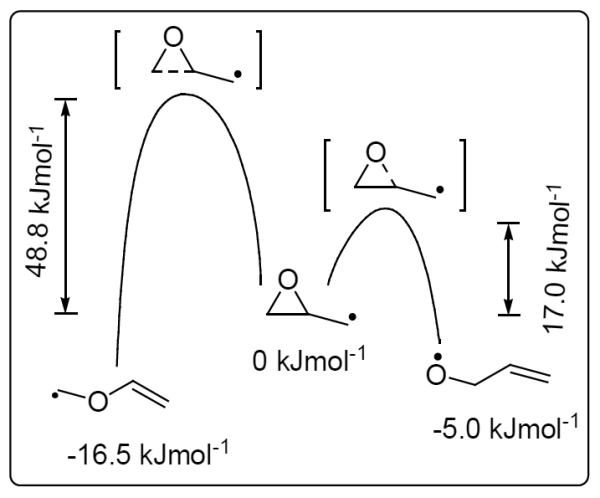
Epoxide C-O bond cleavage is kinetically favored over C-C bond cleavage for epoxycarbinyl radicals.
To test the viability of the diepoxycarbinyl radical as an intermediate in the interconversion of 9- and 13-allyloxy radicals and in the formation of mirror image γ-hydroxy- and γ-oxo-alkenals from 11, we set out to generate the 13-allyloxy radical from 13-HP-EPO-Acid (11) and monitor the formation of the predicted fragmentation products HODA (13), KODA (14), and hexanal and the mirror image products HNE (9), ONE (10), ON (12), shown in Fig. 2.
Materials and Methods
General Methods
Proton magnetic resonance (1H NMR) spectra were recorded on a Varian Inova AS400 spectrometer operating at 400 MHz. Proton chemical shifts are reported as parts per million (ppm) on the δ scale relative to CDCl3 (δ 7.24). 1H NMR spectral data are tabulated in terms of multiplicity of proton absorption (s, singlet; d, doublet; t, triplet; m, multiplet; br, broad), coupling constants (Hz), number of protons.
All solvents were distilled under a nitrogen atmosphere prior to use, and all materials were obtained from Aldrich unless specified. Chromatography was performed with ACS grade solvent. Rf values are quoted for plates of 0.25 mm thickness. The plates were visualized with iodine. Ferrous thiocyanate reagent was prepared and used to detect hydroperoxides.[32] Flash column chromatography was performed on 230–400 mesh silica gel supplied by E. Merk.
Liquid Chromatography/Mass Spectrometry
LC/ESI/MS/MS analysis of linoleate-derived hydroperoxy epoxide (13-HP-Epo-Acid, 11) fragmentation was performed on a Quattro Ultima (Micromass, Wythenshawe, UK) connected to a Waters 2690 solvent delivery system with an auto-injector. The source temperature was maintained at 100 °C, and the desolvation temperature was kept at 200 °C. The drying gas (N2) was maintained at ca. 450 L/h, and the core flow gas was kept at ca. 50 L/h. The multiplier was set at an absolute value of 500. Optimized parameters for aldehyde-DNPH derivatives, 13-HP-Epo-Acid (11) and the potential fragmentation products were established with authentic samples. MS scans at m/z 80–1000 were obtained for standard compounds (Table 1).
Table 1.
Optimized parameters for the mass spectrometer.
| acids | DNPH-derivatives | |
|---|---|---|
| ion mode | negative | negative |
| capillary (kV) | 3.00 | 3.00 |
| cone (V) | 60 | 60 |
| hex 1 (V) | 30.0 | 30.0 |
| aperture (V) | 0.0 | 0.0 |
| hex 2 (V) | 0.5 | 1.0 |
| LM 1 Resolution | 10.0 | 10.0 |
| HM 1 Resolution | 10.0 | 10.0 |
| ion Energy 1 | 1.0 | 2.0 |
| LM 2 Resolution | 15.0 | 15.0 |
| HM 2 Resolution | 15.0 | 15.0 |
| ion Energy 2 | 2.0 | 2.0 |
Argon was used as collision gas at a pressure of 5 psi for MS/MS analysis. For MS/MS analysis, the collision energy was optimized for each compound. For multiple reaction monitoring (MRM) experiments, the optimum collision energy (giving the strongest signal) was determined for each m/z parent-daughter ion pair (Table 2).
Table 2.
MRM transition ion pair and optimal collision energy for the analytes.
| Analytes | MRM transition ion pair (m/z) | Collision energy (eV) |
|---|---|---|
| Hexanal-DNPH | 279.2 > 163.0 | 15 |
| ON-DNPH | 351.2 > 152.0 | 25 |
| BA-DNPH | 285.2 > 181.0 | 25 |
| HNE-DNPH | 335.4 > 163.3 | 15 |
| ONE-DNPH | 513.4 > 182.0 | 40 |
| 13-HP-Epo-Acid | 327.6 > 155.4 | 20 |
| HODA | 227.0 > 84.0 | 25 |
| KODA | 225.0> 109.0 | 10 |
| HODA-methoxime | 256.3 > 197.0 | 20 |
| KODA-methoxime | 283.3 > 251.0 | 20 |
Online chromatographic separation was achieved using a 150×2.0 mm i.d. Prodigy ODS-5 μm column (Phenomenex, UK), with water and methanol binary solvent gradients. For DNPH-derivatives, the gradient started with 50% methanol and rose to 100% methanol in 10 min, then was held at 100% methanol for another 15 min. The gradient was then reversed to 50% methanol in 0.5 min, and held for 4.5 min before the next run. For the carboxylic acids, the gradient started with 5% methanol and rose to 100% methanol in 15 min, then was held at 100 % methanol for another 15 min. The gradient was then reversed to 5% methanol in 0.5 min, and held at 5% methanol for 4.5 min before the next run. NH4OAc was added (2 mM) in both the water and the methanol. The solvents were all delivered at 200 μL/ min.
13-Hydroperoxy-cis-9,10-epoxyoctadeca-11-enoic acid (13-HP-Epo-Acid, 11)
Freshly prepared dioxirane[33] (3 mL, 6–8 mM) was added dropwise to a solution of 13-HPODE (15, 50 mg, 0.16 mmol) in chloroform (5 mL). The resulting mixture was stirred for 15 min. The solvents were then removed by rotary evaporation. The desired compound was cleanly obtained in quantitative yield. 1H NMR (400 MHz, CDCl3) δ 5.84 (dd, J = 15.7, 7.5 Hz, 1H), 5.5–5.7 (m, 1H), 4.2–4.4 (m, 1H), 3.42 (dd, J = 7.1, 4.6 Hz, 1H), 3.0–3.2 (m, 1H), 2.32 (t, J = 7.3 Hz, 2 H), 1.1–1.8 (20H), 0.85 (t, J = 6.6 Hz, 3H). ESI-MS/MS analysis yields the following diagnostic peaks: m/z 327.5 [M-H]−, m/z 209.4, m/z 171.4, m/z 155.5, m/z 137.5 (supporting information, Fig. S6).
Dinitrophenylhydrazine (DNPH) derivatization
DNPH (25 mg) was dissolved in CH3CN (10 mL) and acidified with formic acid (0.2 mL). The DNPH solution was stable for 1 week when stored at 4 °C. Derivatization of aldehydes (hexanal, ON (12), HNE (9) and ONE(10)) was performed by mixing various amounts of standard aldehydes or reaction mixtures with 100 μL of the derivatizing agent and incubating at room temperature for 1h. Then solvents were removed under a stream of nitrogen and kept at − 80 °C before analysis by LC/MS/MS.
Quantification of hexanal, ON (12), HNE (9) and ONE (10) as the DNPH derivatives
13-HP-Epo-Acid (11, 10 μg, 0.03 μmol) dissolved in methanol (100 μL) was placed in small glass vials. Then various amounts of Fe2+ (FeSO4·7H2O dissolved in water, 1mM solution) were added. The mixtures were incubated at 37 °C for 1 h. Then benzaldehyde in methanol solution (100 μg/mL, 5 μL) was added as internal standard. Then DNPH derivatizing agent (100 μL) was added and the mixture was kept at room temperature for another 1 h. The solvent was removed under a stream of nitrogen and the residue was kept at −80 °C until LC/MS/MS analysis.
Quantification of HODA (13) and KODA (14)
13-HP-Epo-Acid (11, 10 μg, 0.03 μmol) dissolved in methanol (100 μL) was placed in small glass vials. Then various amounts of Fe2+ (FeSO4·7H2O dissolved in water, 1mM solution) were added. The mixture was incubated at 37 °C for 1 h. The solvent was removed under a stream of nitrogen and the residue was kept at −80 °C until LC/MS/MS analysis.
HODA methoxime and KODA methoxime
To authentic HODA (13, 50 μg), or KODA (14, 50 μg) in a small vial was added pyridine (200 μL) containing 2 wt% of methoxylamine hydrochloride. The mixtures were kept under argon at room temperature over night. Pyridine was then removed under a stream of argon. The residues were dissolved in 0.5 mL of water (pH = 3). The solutions were extracted with ethyl ether (1 mL and then 0.5 mL). Solvents were removed from the extract and the residues were then analyzed with ESI-MS/MS, producing the following fragments, HODA methoxime: m/z 256.3 [M–H]−; m/z 197, KODA methoxime: m/z 283.3 [M–H]−; m/z 251 (supporting information, Fig. S8, S10).
Identification of HODA and KODA methoximes in the reaction product mixture of 13-HP-Epo-Acid (11) after derivatization
13-HP-Epo-Acid (11, 50 μg, 0.15 μmol) was dissolved in methanol (100 μL) in a small glass vial. Then 2 equivalents of Fe2+ (FeSO4·7H2O dissolved in water, 1mM solution) were added. The mixture was incubated at 37 °C for 1 h. The solvent was removed under a stream of nitrogen. Then pyridine (200 μL) containing 2 wt% of methoxylamine hydrochloride was added. The mixture was kept under argon at room temperature over night. Pyridine was then removed under a stream of argon. The residue was then dissolved in 0.5 mL of water (pH = 3). The solution was extracted with ethyl ether (1 mL and then 0.5 mL). Solvent was then removed from the combined extracts and the residue was sealed under argon and kept at −80 °C until LCMS/MS analysis.
Results and Discussion
Synthesis of the linoleate-derived hydroperoxy epoxide 13-HP-Epo-Acid (11)
Regioselective epoxidation of the cis double bond in 13-HPODE (15) with dimethyl dioxirane delivers 13-HP-Epo-Acid (11) in quantitative yield.[33] Besides the inductive effect of the allylic hydroperoxy group that presumably makes the adjacent trans C=C bond more electron deficient than the remote cis C=C bond, the latter is also sterically more accessible toward epoxidation. The potential fragmentation products ON (12), HNE (9), ONE (10), HODA (13) and KODA (14) were also synthesized as previously described.[34–36]
Formation and LC/MS/MS analysis of dinitrophenylhydrazine (DNPH)-derivatives of hexanal, HNE (9) and ONE (10), ON (12)
The fragmentation products generated upon treatment of 13-HP-Epo-Acid (11) with Fe+2 were analyzed by LC/MS/MS. Since low molecular weight aldehydes (hexanal, HNE (9) and ONE (10), ON (12)) were not readily detected by LC/MS/MS, derivatization was recommended. Pentafluorobenzyl hydroxylamine [37], cyclohexanedione[38] and dinitrophenylhydrazine (DNPH) are widely used as derivatization reagents for aldehydes, and the derivatization products can be easily analyzed by GC/MS or LC/MS. However, some of these derivatizations require severe reaction conditions, e.g., high incubation temperature and very acidic conditions. The challenge was to find a mild derivatization method which can quickly derivatize the aldehydes in the reaction mixture while not promoting fragmentation of unreacted starting 13-HP-Epo-Acid (11) during the derivatization. DNPH was used as derivatization reagent, and instead of using strong acid sulfuric acid, a catalytic amount of formic acid was added to facilitate hydrazone formation [39] Aldehydes were incubated with DNPH at room temperature for 1 h. After incubation, the reaction mixture was directly injected onto an LC/MS/MS. No extraction or further purification was required.
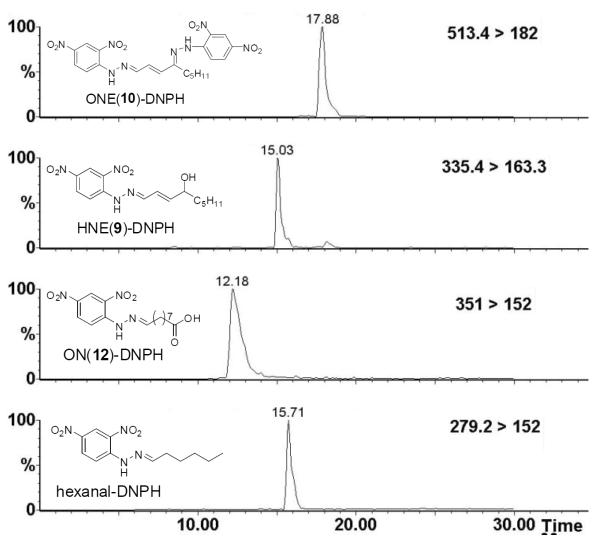
Authentic hexanal, HNE (9), ONE (10) and ON (12) were incubated with DNPH, and the derivatized products (Fig. S1, in supporting information) were analyzed by ESIMS/MS in the negative ion mode. Parent ions [M-H]− and characteristic fragments for each aldehyde derivative were detected. Hexanal-DNPH has a parent ion [M-H]− at m/z 279.2 and a dominant fragment at m/z 152.1 which agree with a previous report.[39] ONDNPH has a parent ion [M-H]− at m/z 351.2 and major fragments at m/z 152.1, 163.0. HNE-DNPH has a parent ion [M-H]− at m/z 335.4 and fragments at m/z 152.2, 163.3, 167.2 and 182.2 while ONE-DNPH has a parent ion [M-H]− at m/z 513 and a dominant fragment at m/z 182 (Fig. S2–5). Based on the above ESI-MS/MS study of these aldehyde derivatives, an LC/MS/MS method was developed to quantify hexanal, HNE (9), ONE (10) and ON (12) derivatives based on monitoring their unique transitions between the m/z of their parent ions and their characteristic daughter ions as well as their characteristic LC retention times (Fig. 4).
Figure 4.
HPLC chromatogram of ONE (10), HNE (9), ON (12), and hexanal-DNPH derivatives. All chromatograms were monitored by LC/MS/MS in the negative ion mode with MRM of appropriate mass transitions as noted.
Fragmentation of 13-HP-Epo-Acid (11) generates hexanal, HNE (9), ONE (10), ON (12), HODA (13) and KODA (14)
Hydroperoxy epoxide 13-HP-Epo-Acid (11) was dissolved in a mixed solvent of methanol and water in open glass vials. Various amounts of Fe2+ in water were added, and the resulting mixture was incubated at 37 °C for 1 h. Then an internal standard benzaldehyde and DNPH were added. The yields of hexanal, HNE (9), ONE (10) and ON (12) from 13-HP-Epo-Acid (11) were determined by LC/MS/MS analysis. As predicted, both hexanal and ON (12) were produced from 13-HP-Epo-Acid (11) upon addition of Fe2+ (Fig. 5). The yields of hexanal and ON (12) increased to ~15% and ~20% respectively when three equivalents of Fe2+ were added. The slightly higher yield of ON (12) compared with hexanal may be the consequence of some loss of the latter owing its high volatility. HNE (9) and ONE (10) were also generated during the fragmentation of 13-HP-Epo-Acid (11) in the presence of various amounts of Fe2+ (Fig. 5). The yield of ONE (10, ~7%) was much higher than HNE (9, ~1%), when three equivalents of Fe2+ were added. These results are consistent with a previous study where ONE (10), and not HNE (9), was found to be the dominant product from fragmentation of 13-HPODE (15). [7]
Figure 5.
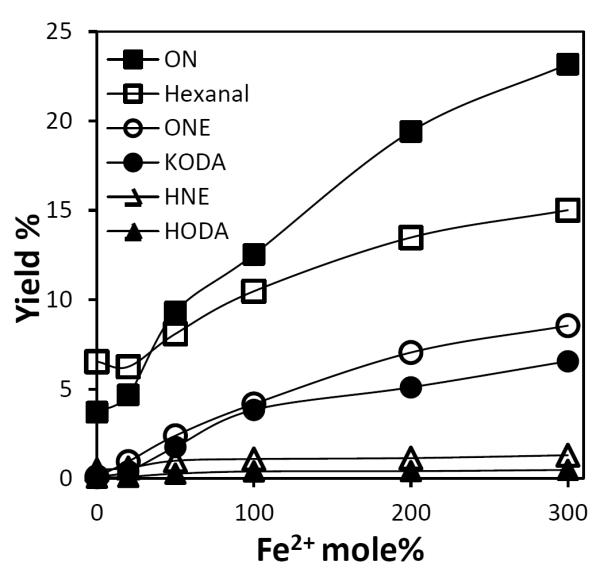
Generation of hexanal, ON (12), ONE (10), KODA (14), HNE (9) and HODA (13) from 13-HP-Epo-Acid (11) in the presence various amounts of Fe2+.
Authentic HODA (13) and KODA (14) were synthesized as described previously [34–36]. An ESI-MS/MS study of HODA (13) and KODA (14) production in the negative ion mode gave parent ions [M-H]− at m/z 227, 225 and dominant fragments at m/z 84, 109, respectively. LC/MS/MS methods were established to monitor these products by MRM.13-HP-Epo-Acid (11) was dissolved in a mixed solvent of methanol and water in open glass vials. Then different amounts of Fe2+ in water were added, and the resulting mixture was incubated at 37 °C for 1 h. The yields of HODA (13) and KODA (14) from 13-HP-Epo-Acid (11) were obtained by LC/MS/MS analysis (Fig. 5). HODA (13) and KODA (14) were both generated during the fragmentation, and similar to the formation of HNE (9) and ONE (10), the yield of KODA (14) was higher than HODA (13), ~7% and 0.5% respectively, when excess of Fe2+ was added.
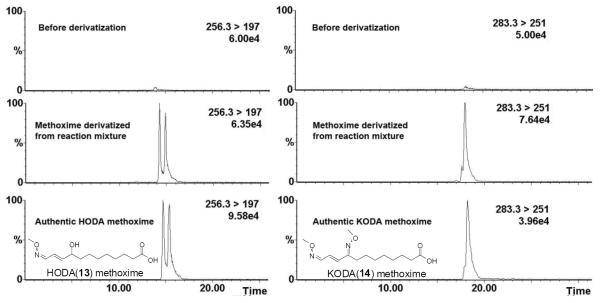
The formation of HODA (13) and KODA (14) were further verified through methoxyamine derivatization. Authentic samples of HODA (13), KODA (14) and the fragmentation reaction product mixture from 13-HP-Epo-Acid (11) were derivatized with methoxyamine hydrochloride in pyridine. The HODA methoxime derivative ([M-H]−, m/z 256) gives a dominant daughter ion at m/z 197, while the KODA methoxime derivative ([M-H]−, m/z 283) gives a dominant daughter ion at m/z 251. For HPLC analysis, the HODA and KODA methoxime derivatives were detected by ESI-MS/MS using MRM of the 256 → 197 and 283 → 251 mass transitions. After derivatization, both HODA and KODA methoxime derivatives were detected in the reaction product mixture from 13-HP-Epo-Acid (11, Fig. 6).
Figure 6.
HPLC chromatogram of the HODA methoxime derivative (left) and KODA methoxime derivative (right). All chromatograms were monitored by LC/MS in the negative mode with the MRM of appropriate mass transitions as noted. The HODA and KODA methoxime derivatives were detected among the 13-HP-Epo-Acid (11) fragmentation reaction products.
Conclusions
The generation of both saturated aldehydes hexanal and ON (12) as major products in similar yields, the generation of both “mirror image” γ-hydroxyalkenals HNE (9) and HODA (13) as minor products, and the generation of both “mirror image” γ-hydroxyalkenals ONE (10) and KODA (14) in similar yields provides compelling evidence supporting the postulated formation of a pseudo symmetrical diepoxycarbinyl radical intermediate 5 during the fragmentation of 13-HP-Epo-Acid (11), and suggest that this intermediate is in rapid equilibrium with both the 9- and 13-allyloxy radicals. Thus, fragmentation of the diepoxycarbinyl radical 5, e.g., by the Pryor-Porter mechanism of Fig. 1, delivers both γ-hydroxy-α,β-unsaturated aldehydes HNE (9) and HODA (13) as minor products. An alternative fragmentation of 5, e.g., by the Blair mechanism of Fig. 1, delivers both γ-keto-α,β-unsaturated aldehydes ONE (10) and KODA (14) as major products in similar yields. An immediate practical consequence of the present study is that, owing to a common diepoxycarbinyl radical intermediate, not only free radical-induced oxidation of linoleate, that non regioselectively generates both 9- and 13-hydroperoxy octadecadienoates (HODEs), but also enzyme-induced oxidation of linoleate, that regioselectively delivers either 9- or 13-HODE, can initiate the oxidative fragmentation that produces both “mirror image” γ-hydroxy-α,β-unsaturated aldehydes 9 plus 13 and both γ-keto-α,β-unsaturated aldehydes 10 plus 14.
Supplementary Material
Highlights.
We investigated the generation of the γ-hydroxy- and γ-oxo-α,β-alkenals from the fragmentation of a linoleate-derived γ-hydroperoxy-α,β-unsaturated epoxide. > Through chemical synthesis and LC/MS/MS analysis combined with various derivatization methods, we found that not only 4-hydroxy- and 4-oxo-2-nonenals but also their “mirror image” analogues 9-hydroxy- and 9-keto-12-oxo-dodec-10-enoic acid are generated in similar yields. > These observations support the intermediacy of a unique pseudo symmetrical diepoxycarbinyl radical.
Acknowledgements
The National Institutes of Health supported this research through grants from the National Eye Institute (EY016813), the National Institute of General Medical Sciences (GM021249) and the National Heart, Lung and Blood Institute (HL053315) to RGS.
Abbreviations
- DNPH
dinitrophenylhydrazine
- ESI
electrospray ionization
- HNE
4-hydroxyl-2-nonenal
- HODA
9-hydroxy-12-oxo-10(E)-dodecenoic acid
- 13-HP-Epo-Acid
13-hydroperoxy-cis-9,10-epoxyoctadeca-11-enoic acid
- 13-HPODE
13-hydroperoxy-9,11-octadecadienoic acid
- KODA
9-keto-12-oxo-10-dodecenoic acid
- LC/MS
liquid chromatography/mass spectrometry
- MRM
multiple reaction monitoring
- ON
9-oxononanoic acid
- ONE
4-oxo-2-nonenal
References
- [1].Clausen J. Demential syndromes and the lipid metabolism. Acta Neurol Scand. 1984;70:345–355. doi: 10.1111/j.1600-0404.1984.tb00835.x. [DOI] [PubMed] [Google Scholar]
- [2].Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- [3].Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- [4].Spiteller G. Linoleic acid peroxidation--the dominant lipid peroxidation process in low density lipoprotein--and its relationship to chronic diseases. Chem Phys Lipids. 1998;95:105–162. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- [5].Esterbauer H. Aldehydic products of lipid peroxidation. Academic Press; London: 1982. [Google Scholar]
- [6].Lin D, Lee HG, Liu Q, Perry G, Smith MA, Sayre LM. 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem Res Toxicol. 2005;18:1219–1231. doi: 10.1021/tx050080q. [DOI] [PubMed] [Google Scholar]
- [7].Rindgen D, Nakajima M, Wehrli S, Xu K, Blair IA. Covalent modifications to 2'-deoxyguanosine by 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem Res Toxicol. 1999;12:1195–1204. doi: 10.1021/tx990034o. [DOI] [PubMed] [Google Scholar]
- [8].Jian W, Lee SH, Mesaros C, Oe T, Elipe MV, Blair IA. A novel 4-oxo-2(E)-nonenal-derived endogenous thiadiazabicyclo glutathione adduct formed during cellular oxidative stress. Chem Res Toxicol. 2007;20:1008–1018. doi: 10.1021/tx700001t. [DOI] [PubMed] [Google Scholar]
- [9].Lee SH, Silva Elipe MV, Arora JS, Blair IA. Dioxododecenoic acid: a lipid hydroperoxide-derived bifunctional electrophile responsible for etheno DNA adduct formation. Chem Res Toxicol. 2005;18:566–578. doi: 10.1021/tx049716o. [DOI] [PubMed] [Google Scholar]
- [10].Lee SH, Rindgen D, Bible RH, Jr., Hajdu E, Blair IA. Characterization of 2'-deoxyadenosine adducts derived from 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem Res Toxicol. 2000;13:565–574. doi: 10.1021/tx000057z. [DOI] [PubMed] [Google Scholar]
- [11].Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- [12].Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- [13].Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science (New York, N.Y. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- [15].Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9-and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- [16].Schneider C, Porter NA, Brash AR. Autoxidative transformation of chiral omega6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem Res Toxicol. 2004;17:937–941. doi: 10.1021/tx049913n. [DOI] [PubMed] [Google Scholar]
- [17].Schneider C, Boeglin WE, Yin H, Porter NA, Brash AR. Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem Res Toxicol. 2008;21:895–903. doi: 10.1021/tx700357u. [DOI] [PubMed] [Google Scholar]
- [18].Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yin H, Xu L, Porter NA. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chemical reviews. 2011 doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- [20].Pryor WA, Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Bio Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- [21].Yadagiri P, Lumin S, Mosset P, Capdevila J, Falck JR. Enantiospecific total synthesis of 8- and 12-hydroxyeicosatetraenoic acid. Tetrahedron Lett. 1986;27:6039–6040. [Google Scholar]
- [22].Gardner HW, Bartelt RJ, Weisleder D. A facile synthesis of 4-hydroxy-2(E)-nonenal. Lipids. 1992;27:686–689. [Google Scholar]
- [23].Sabatino EC, Gritter RJ. The free-radical chemistry of cyclic ethers. V. β-hydrogen atom abstraction from epoxides and a thioepoxide. J Org Chem. 1963;28:3437. [Google Scholar]
- [24].Gansauer A, Lauterbach T, Narayan S. Strained heterocycles in radical chemistry. Angewandte Chemie (International ed. 2003;42:5556–5573. doi: 10.1002/anie.200300583. [DOI] [PubMed] [Google Scholar]
- [25].Suginome H, Wang JB. Intramolecular β,γ-addition of allylic alkoxy radicals. A new general synthesis of a-iodoepoxides by photolysis of allylic alcohol hypoiodites in the presence of mercury (II) oxide, iodine and pyridine in benzne. J Chem Soc, Chem Commun. 1990:1629. [Google Scholar]
- [26].Newcomb M. In: Radicals in Organic Synthesis. Renaud P, Sibi MP, editors. Wiley-VCH; Weinheim: 2001. p. 317. [Google Scholar]
- [27].Ziegler FE, Petersen AK. Alkoxy radicals are formed reversibly from oxiranylcarbinyl radicals: a kinetic study. J Org Chem. 1995;60:2666. [Google Scholar]
- [28].Krishnamurthy V, Rawal VH. Kinetics of the oxiranylcarbinyl radical rearrangement. J Org Chem. 1997;62:1572. [Google Scholar]
- [29].Stogryn EL, Gianni MH. Oxirane C-C bond homolysis. Tetrahedron Lett. 1970;11:3025. [Google Scholar]
- [30].Cook M, Hares O, Johns A, Murphy JA, Patterson CW. Novel synthesis of vinyl ethers induced by carbon-halogen bond homolysis. J Chem Soc, Chem Commun. 1986:1419. [Google Scholar]
- [31].Smith DM, Nicolaides A, Golding BT, Radom L. Ring opening of the cyclopropylcarbinyl radical and its N- and O- substituted analogues: a theoretical examination of very fast unimolecular reactions. J Am Chem Soc. 1998;120:10223–10233. [Google Scholar]
- [32].Kelly RJ. Chemical Health &Safety. 1996;4:33–36. [Google Scholar]
- [33].Sun M. Synthetic and mechanistic studies of lipid peroxidation in vitro and in vivo. Case Western Reserve University; Cleveland: 2003. p. 262. [Google Scholar]
- [34].Deng Y, Salomon RG. Total synthesis of γ-hydroxy-α,β-unsaturated aldehydic esters of cholesterol and 2-lysophosphatidylcholine. J Org Chem. 1998;63:7789–7794. [Google Scholar]
- [35].Jian W, Lee SH, Arora JS, Silva Elipe MV, Blair IA. Unexpected formation of etheno-2'-deoxyguanosine adducts from 5(S)-hydroperoxyeicosatetraenoic acid: evidence for a bis-hydroperoxide intermediate. Chem Res Toxicol. 2005;18:599–610. doi: 10.1021/tx049693d. [DOI] [PubMed] [Google Scholar]
- [36].Sun M, Deng Y, Batyreva E, Sha W, Salomon RG. Novel bioactive phospholipids: practical total syntheses of products from the oxidation of arachidonic and linoleic esters of 2-lysophosphatidylcholine. J Org Chem. 2002;67:3575–3584. doi: 10.1021/jo0105383. [DOI] [PubMed] [Google Scholar]
- [37].Kawai Y, Takeda S, Terao J. Lipidomic analysis for lipid peroxidation-derived aldehydes using gas chromatography-mass spectrometry. Chem Res Toxicol. 2007;20:99–107. doi: 10.1021/tx060199e. [DOI] [PubMed] [Google Scholar]
- [38].O'Brien-Coker IC, Mallet GPAI. Aldehyde analysis by high performance liquid chromatography/tandem mass spectrometry. Rapid Commun in Mass Spectrom. 2001;15:920–928. doi: 10.1002/rcm.324. [DOI] [PubMed] [Google Scholar]
- [39].Andreoli R, Manini P, Corradi M, Mutti A, Niessen WM. Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:637–645. doi: 10.1002/rcm.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.