Abstract
Sphingomyelin- and cholesterol-enriched microdomains can be isolated as detergent-resistant membranes from total cell extracts (total-DRM). It is generally believed that this total-DRM represents microdomains of the plasma membrane. Here we describe the purification and detailed characterization of microdomains from Golgi membranes. These Golgi-derived detergent-insoluble complexes (GICs) have a low buoyant density and are highly enriched in lipids, containing 25% of total Golgi phospholipids including 67% of Golgi-derived sphingomyelin, and 43% of Golgi-derived cholesterol. In contrast to total-DRM, GICs contain only 10 major proteins, present in nearly stoichiometric amounts, including the α- and β-subunits of heterotrimeric G proteins, flotillin-1, caveolin, and subunits of the vacuolar ATPase. Morphological data show a brefeldin A-sensitive and temperature-sensitive localization to the Golgi complex. Strikingly, the stability of GICs does not depend on its membrane environment, because, after addition of brefeldin A to cells, GICs can be isolated from a fused Golgi-endoplasmic reticulum organelle. This indicates that GIC microdomains are not in a dynamic equilibrium with neighboring membrane proteins and lipids. After disruption of the microdomains by cholesterol extraction with cyclodextrin, a subcomplex of several GIC proteins including the B-subunit of the vacuolar ATPase, flotillin-1, caveolin, and p17 could still be isolated by immunoprecipitation. This indicates that several of the identified GIC proteins localize to the same microdomains and that the microdomain scaffold is not required for protein interactions between these GIC proteins but instead might modulate their affinity.
INTRODUCTION
G proteins, GPI-anchored proteins, palmitoylated proteins, and many other signaling molecules are found to be concentrated in microdomains of the plasma membrane (Simons and Ikonen, 1997; Anderson, 1998; Okamoto et al., 1998). The lipid scaffold for these microdomains is built mainly by sphingomyelin (SM) and cholesterol (Simons and Ikonen, 1997; Brown and London, 1998). In many cell types, caveolin appears to be the major protein in morphologically distinct microdomains named caveolae.
Low-density detergent-resistant membrane (DRM) complexes derived from total cell-lysates (total-DRMs) have characteristics similar to caveolar membranes with respect to the enrichment of lipids, signaling proteins, and caveolin (Brown and Rose, 1992; Sargiacomo et al., 1993; Chang et al., 1994). Recent observations suggest that total-DRMs are derived from at least two different types of microdomains at the plasma membrane (Simons and Ikonen, 1997). DRMs containing caveolin can be separated from DRMs containing GPI-anchored proteins (Schnitzer et al., 1995). In addition, from cells that lack caveolin, similar low-density glycosphingolipid- and GPI-anchored protein-enriched detergent-insoluble complexes can be isolated (Fra et al., 1994; Gorodinsky and Harris, 1995). Thus, DRMs are not restricted to caveolar structures. The equivalence of low-density detergent-insoluble complexes with microdomains within membranes has long been a matter of debate (Kurzchalia et al., 1995; Brown and London, 1998; Jacobson and Dietrich, 1999), and conflicting data are still reported. Several groups present evidence that microdomain-associated proteins have a predominant random distribution at the apical surface (Harder et al., 1998; Kenworthy and Edidin, 1998; Kenworthy et al., 2000). In contrast, recent observations of overexpression of the GPI-anchored folate receptor results in cholesterol-dependent clustering at the plasma membrane (Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998) provide evidence in favor of the existence of microdomains within the plasma membrane. Additional support in favor of the existence of microdomains comes from laser-trapping techniques (Pralle et al., 2000) and electron microscopy (Wilson et al., 2000). Indications for structural diversity of the microdomains by occupation of functionally different GPI-anchored proteins have also been reported (Madore et al., 1999).
Microdomains are not likely to be restricted to the plasma membrane. Indications for the existence of microdomains along the secretory pathway have been published (Sevlever et al., 1999; Bagnat et al., 2000; Heino et al., 2000). SM- and cholesterol-enriched microdomains were originally postulated to exist at the trans-Golgi network (TGN) and to function as sorting platform for apical and basolateral transport (Simons and van Meer, 1988; Simons and Ikonen, 1997). Although these microdomains have not been isolated so far, several microdomain constituents have been localized to the Golgi complex. There is compelling evidence that caveolin-1 and caveolin-2 are present at the Golgi complex (Luetterforst et al., 1999; Mora et al., 1999; Parolini et al., 1999; Machleidt et al., 2000). In addition to caveolin, heterotrimeric G proteins have been localized to the Golgi complex, and several lines of evidence support a role in intracellular protein transport (Bomsel and Mostov, 1992; Helms, 1995). More recently, heterotrimeric G proteins have been implicated in the regulation of Golgi structure (Jamora et al., 1997; Yamaguchi et al., 1997). We previously showed that a putative heterotrimeric G protein inhibits the fusion of COPI-coated vesicles at early stages of the Golgi complex and found that Golgi-localized G proteins reside in a detergent-insoluble complex (Helms et al., 1998). Here we describe the purification and characterization of these Golgi-derived detergent-insoluble complex (GICs).
MATERIALS AND METHODS
Reagents and Antibodies
Brefeldin A (Roche Diagnostics, Mannheim, Germany) was stored at 2.5 mM in ethyl alcohol at −20°C. Proteinase K and methyl-β-cyclodextrin (mean degree of substitution 10.5–14.7) was obtained from Sigma-Aldrich Chemie (Taufkirchen, Germany). Methyl-β-cyclodextrin was freshly prepared and used at 20 mM (final concentration) in aqueous solution. Saponin and Brij 96V were from Fluka Biochemica (Buchs, Switzerland). Eupergit C250L beads were kindly donated by Röhm (Darmstadt, Germany).
The monoclonal mouse antibody against ERGIC-53 (Schweizer et al., 1988) was kindly donated by Dr. Pepperkok (EMBL, Heidelberg, Germany). A polyclonal rabbit antibody against the C-terminal domain of caveolin (Dupree et al., 1993) was kindly donated by Dr. K. Simons (EMBL). A guinea pig antibody against the B-subunit of vacuolar (v)-ATPase was the kind gift of Dr. N. Nelson (Tel Aviv University, Israel). Caveolin-1–specific antibodies against the N-terminal domain of caveolin-1 (N-20) and calnexin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and BD Transduction Laboratories (San Diego, CA), respectively. Antibodies against Gαi3 (EC/2), Gαi1/2 (A/S7), and Gβc (SW/1) were obtained from NEN Life Science Products (Boston, MA). Other G protein antibodies specific for Gαic (AS266) Gαs (AS 348), Gα12 (AS 232), Gαq/11 (AS 369), and Gαoc (AS 6) were the kind gift of Dr. Bernd Nürnberg (Freie Universität Berlin, Germany). Polyclonal rabbit antibodies against p23 (Henriette) were generated against the overexpressed and purified luminal domain of this protein (K. Sohn, unpublished results). A β-COP-specific antibody was used as described by Duden et al. (1991). Rabbit anti-peptide antibodies against flotillin-1 were generated and affinity purified according to standard procedures by coupling of peptide 1768 (KELEARVRK, corresponding to AA 277–286 in flotillin-1) or 1769 (CEEIYKDRQKFSE, corresponding to AA 118–130) to keyhole limpet hemacyanin. A peptide antibody against p45 was generated according to similar procedures against the amino acid sequence ALIQEQEAQIK (antibody 1767), which was obtained by microsequencing (Table 2). For immunofluorescence, a mouse antibody against TGN-38 (ABR Affinity Bioreagents, Golden, CO) and against calreticulin (StressGen Biotechnologies, Victoria, Canada) was used. Fluorescein isothiocyanate-conjugated goat anti-mouse and tetramethylrhodamine B isothiocyanate-conjugated goat-anti-rabbit IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Table 2.
Protein identification of GIC proteins by microsequencing
| Band | Sequence | Protein |
|---|---|---|
| 1 | HFTEFVPLRTK | v-ATPase (subunit A) (van Hille et al., 1993) |
| LIKDDFLQQN | v-ATPase (subunit A) | |
| 2 | DLTGYITEGQIY | v-ATPase (subunit B) (Nelson et al., 1992) |
| 3 | ITLVSSGSGTMGAAK | Flotillin-1 (Bickel et al., 1997) |
| FSEQVFK | Flotillin-1 | |
| LEARVRK | Flotillin-1 | |
| 4 | K(T/Q)ALIQEQEAQIK | Unknown (p45) |
| 5 | YDEAASYIQS | Gαi2 (Sullivan et al., 1986) |
| DLFEEKR | Gαi1/Gαi2/Gαi3 (Kaziro et al., 1991) | |
| 6 | LLFEGA | v-ATPase (accessory subunit) (Wang et al., 1988) |
| NVADYYPEYK | v-ATPase (accessory subunit) | |
| 7 | NQIRDARK | Gβ2 (Fong et al., 1987) |
| 8 | GRLVQTQRLK | v-ATPase (subunit E) (Hirsch et al., 1988) |
| 9 | EIDLVNRDPK | Caveolin 1 and 3 (Kurzchalia et al., 1992; Tang et al., 1996) |
| 10 | LNQEAQ(Q)YS(E) | Unknown (p17) |
GIC proteins were isolated and separated by SDS-PAGE as described for Figure 2. The proteins identified by Coomassie blue were identified by microsequencing (Eckerskorn and Lottspeich, 1989).
Chinese hamster ovary (CHO) cells overexpressing the folate receptor was a gift from Dr. Teymuras Kurzchalia (Max-Delbrueck-Center for Molecular Medicine, Berlin, Germany). Folate-free HAM medium was obtained from PAN Biotech (Aidenbach, Germany). Antibodies against the folate receptor (Mov19) were kindly donated by Dr. Silvana Canevari (Istituto Nazionale Tumori, Milan, Italy).
CHO Golgi membranes were isolated as described previously (Balch et al., 1984; Brügger et al., 2000). For a careful analysis of contaminating membranes we refer to (Brügger et al., 2000). COPI-coated vesicles were isolated as described before (Malhotra et al., 1989; Serafini et al., 1991). Protein determination was according to the method of Lowry et al. (1951).
Methods
Isolation of Low-Density Detergent-insoluble Fractions from Golgi Membranes (GICs) and from Total Cell Lysates (total-DRM).
The isolation of low-density detergent-insoluble fractions from CHO or rabbit liver Golgi membranes is basically as described by Brown and Rose (1992) and modified (Sargiacomo et al., 1993; Gorodinsky and Harris, 1995). In short, 5 mg (protein) of CHO Golgi membranes (isolated as described above) were pelleted and resuspended in 2 ml of PEN buffer (25 mM PIPES, pH 6.5, 2 mM EDTA, 150 mM NaCl) containing 1% Triton X-100. The suspension was incubated for 30 min on ice, mixed with 2 ml of 80% (wt/vol) sucrose in PEN buffer and transferred to an SW41 rotor tube (Beckman, Fullerton, CA). The 40% sucrose fraction was overlaid with 1.3 ml of each 30, 25, 20, 15, 10, and 5% sucrose in PEN buffer. The samples were centrifuged for 22 h at 39,000 rpm and 4°C. After centrifugation, either the opalescent band at the 10–15% sucrose interface was collected, or the gradients were fractionated in 750-μl fractions starting from the top (fraction 1). Total-DRM was isolated from total cell lysates according to the same procedure by solubilization of a CHO cell homogenate or a CHO total cell suspension in 2 ml of PEN buffer plus 1% Triton X-100. Isolation of GICs in the presence of Brij 96 was done exactly as described above, except that 0.5% Brij 96 was used instead of Triton X-100.
Lipid Analysis.
Lipid analysis and quantitation was performed by nano-electrospray ionization tandem mass spectrometry (Brügger et al., 1997); cholesterol was determined as described by Sandhoff et al. (1999). Briefly, lipids were extracted according to the method of Bligh and Dyer (1959) in the presence of nonnatural phosphatidylcholine (14:0/14:0, 16:0/16:0, 20:0/20:0, and 22:0/22:0) and SM (14:0, 18:1 and 25:0) as standards for quantitation. For each quantitative measurement 100 (PC and SM) or 50 (cholesterol) consecutive scans of 4 s duration were averaged. Total phospholipid content was determined according to the method of Rouser et al. (1970).
Immunoprecipitation of GIC Subcomplexes.
Purified antibodies (3 mg, total IgG fraction) were coupled to 1 g of Eupergit C250L beads according to the method of Grassel et al. (1989). Isolated CHO Golgi membranes (500 μg) were resuspended in PEN plus 0.5% Brij 96 and incubated for 30 min on ice. The Golgi lysate was incubated with Eupergit-coupled antibodies (1 mg of antibody) overnight at 4°C in PEN plus 0.5% Brij 96. The beads were washed with PEN buffer, and the immunoprecipitated proteins were eluted from the beads with 100 mM glycine, pH 2.5. Proteins in the eluate were analyzed by SDS-PAGE and Western blotting.
Cholesterol Extraction of Membranes with the Use of Methyl-β-Cyclodextrin.
Intact CHO cells overexpressing the folate receptor were incubated for 30 min at 37°C in serum-free medium in the presence of 20 mM methyl-β-cyclodextrin. Cells were washed twice with phosphate-buffered saline and twice with PEN buffer and collected by use of a rubber policeman. The mixture was adjusted to 1% Triton X-100 and incubated for 30 min at 0°C. After incubation, the samples were adjusted to 40% sucrose, overlaid, and centrifuged as described above for the isolation of GICs. After centrifugation, the top fractions (low density, detergent-insoluble proteins) and bottom fractions (high density, soluble proteins) were pooled. CHO Golgi membranes and CHO total membranes (isolated as described above) were incubated in buffer AB (25 mM Hepes/KOH, pH 7.2, 2.5 nM Mg[OAC]2) with 20 mM methyl-β-cyclodextrin for 30 min at 37°C, washed once in buffer AB by centrifugation, and resuspended in PEN plus 1% Triton X-100. Further manipulation was as described for CHO cells.
Immunofluorescence.
NRK cells were cultured to ∼70% confluency and prepared for indirect immunofluorescence according to standard procedures. Cells were fixed in methanol (1 min at −20°C) and embedded in Fluoromount G (Biozol, Eching, Germany). Images were taken with the use of a Zeiss (Oberkochen, Germany) inverted fluorescence microscope (Axiovert 10), equipped with a photometrics cooled CCD camera or with the use of a Zeiss inverted fluorescence microscope (Axiovert 35), equipped with a Zeiss AxioCam. The specificity of immunofluorescence for flotillin-1 and p45 was demonstrated by loss of immunofluorescence signal after preincubation of the antibodies with their respective peptide antigen (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). Flotillin-1 and p45 localize to the Golgi complex both in the absence and presence of cycloheximide (5 h at 50 μM before fixation of the cells). PC12 cells were cultured as described by Tooze and Huttner (1990) and were kindly provided by Rut Jellinek (University of Heidelberg, Germany). HeLa 229 cells were cultured under standard conditions in DMEM plus 10% fetal calf serum.
RESULTS
Isolation of a Low-Density Detergent-insoluble Complex from Golgi Membranes
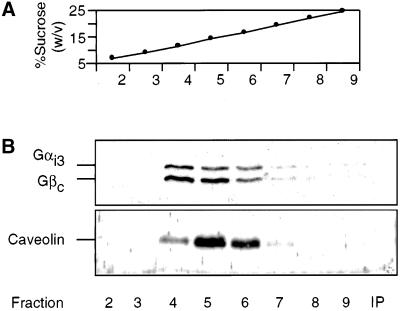
We previously found that trimeric G proteins from isolated Golgi membranes were partially insoluble in Triton X-100 and 3-[(cholamidopropyl)dimethylammonio]-1-propanesulfonic acid but soluble in octylglucoside and cholate (Helms et al., 1998). This behavior is reminiscent of the solubility of caveolar structures in various detergents. On solubilization in Triton X-100, caveolar structures form a low-density detergent-insoluble fraction resulting in flotation on an isopycnic sucrose gradient (Brown and Rose, 1992; Simons and Ikonen, 1997). We therefore tested whether the Triton X-100-insoluble fraction that contains Gαi3 behaved similarly on a sucrose gradient. Golgi membranes, isolated from CHO cells, were treated with 1% Triton X-100 and fractionated by isopycnic sucrose density centrifugation. Subsequently, the gradient was analyzed for the presence of trimeric G proteins. The Gαi3-subunit of G proteins, which is localized to the cis side of the Golgi stack, was taken as a representative of the Gα class of proteins (Stow et al., 1991; Wilson et al., 1994; Denker et al., 1996). As shown in Figure 1B, GICs, containing both Gαi3- and Gβ-subunits, flotate to fraction 4–6, corresponding to a density of ∼13–19% (wt/vol) sucrose (Figure 1A). Caveolin-1, a marker for caveolae-derived complexes, is also present in these fractions (Figure 1B). Golgi marker proteins such as p23 (Sohn et al., 1996) and marker proteins from contaminating membranes such as calnexin from the endoplasmic reticulum (ER) are absent from the GIC preparation (Figure 2B).
Figure 1.
Isolation of a low-density detergent-insoluble fraction from Golgi membranes. CHO Golgi membranes were resuspended in PEN buffer containing 1% Triton X-100, and the low-density detergent-insoluble fractions were isolated by isopycnic sucrose density centrifugation as described in MATERIALS AND METHODS. Fractions (750 μl) were collected from top to bottom, and the sucrose concentration was determined by refractive index (A). Fraction 2–9 of the gradient (100 μl) and the high-density insoluble fraction (IP) were analyzed by SDS-PAGE and subsequent Western blotting with the use of various antibodies as indicated (B).
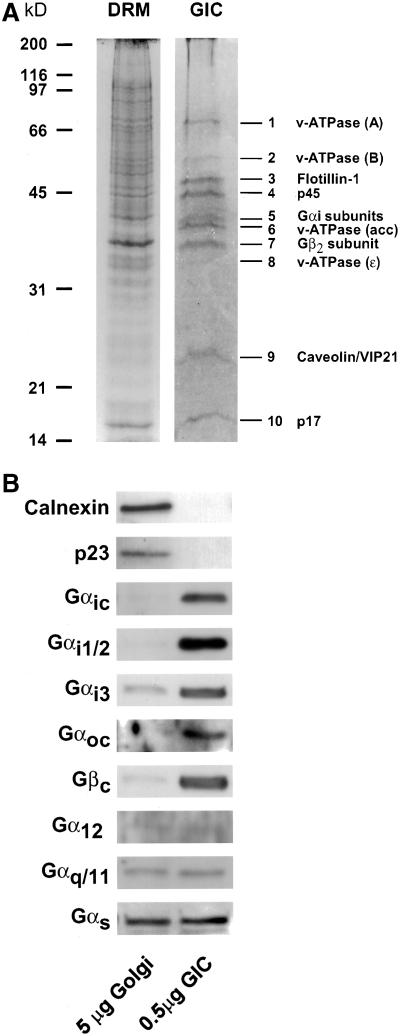
Figure 2.
Protein composition of GIC. (A) GICs and total-DRM were isolated from CHO Golgi membranes and total cell lysates, respectively, as described in MATERIALS AND METHODS. Protein (150 μg) was loaded on a 12% SDS-PAGE gel and stained with Coomassie blue. (B) Enrichment of G protein subunits in GIC. Proteins from isolated Golgi membranes and GICs were separated by SDS-PAGE, and the enrichment of various G protein subunits and of marker proteins for the Golgi complex (p23) and the ER (calnexin) was determined by subsequent Western blotting with the use of (subunit-)specific antibodies described in MATERIALS AND METHODS.
Lipid Composition of GICs
The low density of caveolae-derived detergent-insoluble complexes is attributed to an enrichment of lipids (especially SM and cholesterol) in these complexes. The protein to phospholipid ratio of isolated GICs was determined and calculated to be 0.13 ± 0.03 (Table 1). For comparison, this ratio for isolated CHO Golgi membranes is on the average 1.6 ± 0.2. This explains the low density characteristics for GICs, running at a buoyant density of 1.04–1.05 g/cm3.
Table 1.
Quantitation of lipid content of various CHO compartments
| PC (% of PL) | SM (% of PL) | CHOL (mol/mol) | Protein/Lipid (wt/wt) | |
|---|---|---|---|---|
| CHOGIC | 46 | 38 | 0.96 | 0.13 |
| CHO total-DRM | 35 | 28 | 0.71 | 0.17 |
| CHO Golgi | 37 | 14 | 0.57a | 1.6 |
| CHO cells | 49bc | 8bc | 0.35a | 8.2d |
| CHO PM | 39c | 18c | 0.59e | Not determined |
PC, phosphatidylcholine; PM, plasma membrane. Lipids were measured and quantified as described in MATERIALS AND METHODS. Various CHO compartments were isolated as described in MATERIALS AND METHODS. As indicated, some data were published recently by Brügger et al. (1997)
and by Sandhoff et al. (1999).
The lipid content of CHO-derived plasma membranes was determined by Cezanne et al. (1992)
and Warnock et al. (1993).
From CHO postnuclear supernatant. PL, total phospholipid; mol/mol, mol cholesterol/mol total phospholipid. The data are expressed as mean values (±10%) from two different determinations.
The average yield of Golgi membranes from total cell homogenate and of GICs from isolated Golgi membranes is 1‰ and 2.1%, respectively, based on total protein. Based on these data and their respective protein to lipid ratios, it can be calculated that 25% of total phospholipids in the Golgi complex are present in the detergent-insoluble fraction.
The lipid composition of GICs was compared with that of Golgi membranes and total-DRM by nano-electrospray ionization tandem mass spectrometry. Similar to analyses of total-DRMs (Brown and Rose, 1992; Fiedler et al., 1993), we find that a total-DRM fraction isolated from CHO cell homogenate is enriched in SM and cholesterol (Table 1). The protein to lipid ratio of a total-DRM preparation does not significantly differ from the GIC preparation. Comparison of the lipid content of GICs with total-DRM, however, clearly shows that GICs are more enriched in SM and cholesterol. This is remarkable considering that the Golgi membranes (the source of GICs) have less SM (as percentage of total phospholipids) than plasma membranes, the predominant source of total-DRM (see DISCUSSION and Table 1). Based on the yield of the various isolated fractions (see above) and on the protein to (phospho)lipid ratio in these fractions, it can be calculated that 32% of PC, 67% of SM, and 43% of cholesterol of the Golgi complex can be recovered in isolated Golgi-derived microdomains. These calculations might be subject to some variation because of contaminating organelles in the Golgi preparation, but because membranes with a low and high SM content are present in approximately equal amounts (Brügger et al., 2000), these data are not likely to change significantly.
Protein Composition of GICs
The protein constituents in the GIC preparation were analyzed by SDS-PAGE and compared with that of total-DRM (Figure 2A). The protein composition of GICs is clearly distinct from that of total-DRM and shows a relatively simple set of 10 proteins, which were identified by microsequencing (Table 2). As expected, both the α- and β-subunits of heterotrimeric G proteins are present in GICs (Figure 2A, bands 5 and 7, respectively). We find a peptide specific for Gαi2 and a peptide shared by all Gαi-subunits. To determine the presence and enrichment of other G protein isoforms, Western blot analysis was used to compare the enrichment of various G protein subunits in GICs and Golgi membranes. As shown in Figure 2B, most G protein subunits can be detected and are enriched in the GIC preparation. Gαs, Gαq/11, and Gα12 are enriched ∼10-fold in GICs, compared with their donor membranes. In contrast, the entire Gαi-subclass of G proteins and Gβ-subunits are much more enriched, ∼50–100 fold. The Gαo-subclass is barely detectable in the Golgi fraction but also seems to be highly enriched in GICs. The Western blot staining obtained with the Gαoc antibody is specific for the Gαo2-isoform, because in CHO cells only this isoform is expressed (Exner et al., 1999). The high enrichment of Gαi- (and possibly Gαo-) subclasses is comparable to that of flotillin-1 (see below).
In addition to G proteins, four subunits of the v-ATPase are present in GICs (Figure 2A, bands 1, 2, 6, and 8; Table 2). The v-ATPase is a proton pump that causes acidification of the lumen of several intracellular organelles including the Golgi complex (Finbow and Harrison, 1997; Forgac, 1997). The pump is a hetero-oligomeric complex that consists of a membrane (V0) domain and a peripheral (V1) domain. All the subunits found in GICs belong to the V1 domain.
A major component of GICs, at 47 kDa, was identified as flotillin-1 (Figure 2A, band 3; Table 2). Flotillin-1 has been found in a Triton-insoluble membrane fraction from mouse brain (Bickel et al., 1997). Flotillin-1 and flotillin-2 (also known as reggie-2 and reggie-1, respectively) have also been cloned from goldfish and rat, and the synthesis of these proteins is up-regulated during axon regeneration by retinal ganglion cells after optic nerve injury (Schulte et al., 1997; Lang et al., 1998).
Caveolin-1 is also a major component of GICs (Figure 2A, band 9; Table 2). An antibody against the C-terminal domain of caveolin-1 specifically recognizes the cis-Golgi associated form (Luetterforst et al., 1999). Three different family members of caveolin have been identified so far. Interestingly, coexpression of caveolin-1 is necessary to transport caveolin-2 from the Golgi to the plasma membrane (Mora et al., 1999; Parolini et al., 1999). The sequence derived from band 9 in Figure 2A (Table 2) exists in caveolin 1 and 3, but because the latter is not expressed in CHO cells, this sequence corresponds to caveolin 1. By Western blotting, we could also determine the presence of caveolin-2 in GICs with the use of anti-caveolin-2–specific antibodies (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results).
The sequences derived from bands 4 and 10 (p45 and p17, respectively, Table 2) do not match any protein known to date.
Subcomplexes in the Isolated GIC Preparation
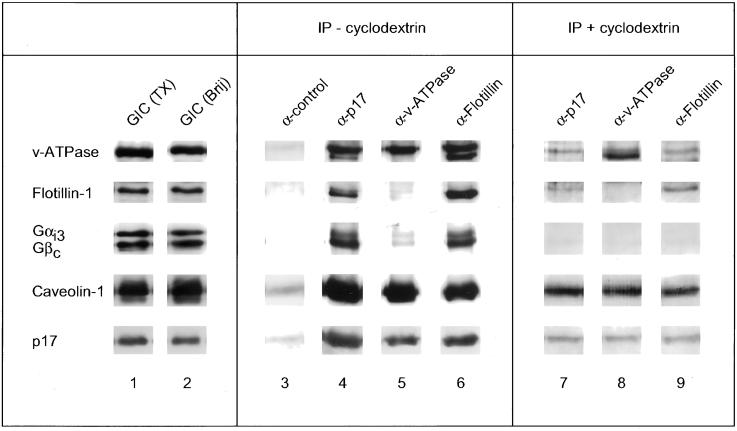
Isolation of lipid-enriched microdomains by use of detergent results in comigration of functionally distinct microdomains to the low-density fractions during density centrifugation (Schnitzer et al., 1995; Simons and Ikonen, 1997). In addition, Triton X-100 may cause the artifactual mixing of protein constituents from distinct microdomains into one complex (Madore et al., 1999). Therefore, we also purified the GIC complex from Golgi membranes by use of Brij 96, which does not cause artificial mixing of distinct microdomains (Madore et al., 1999). As shown in Figure 3 (left), the GIC complex isolated in the presence of Brij 96 contains all the GIC proteins isolated in the presence of Triton X-100 and in approximately the same relative abundance. To determine the presence of subcomplexes in the GIC preparation, antibodies against three different GIC proteins were used to immunoprecipitate their respective antigen in the presence of Brij 96 and the coimmunoprecipitation of various GIC proteins was determined. As shown in Figure 3 (middle), immunoprecipitation of p17 or flotillin-1 caused the coimmunoprecipitation of all other GIC proteins analyzed. In contrast, immunoprecipitation with an antibody against the B-subunit of the v-ATPase caused the coimmunoprecipitation of caveolin-1 and p17 but only very little of the other GIC proteins. This indicates that there are at least two different subcomplexes in the GIC preparation, one consisting of all the GIC proteins and another consisting of the v-ATPase with p17 and caveolin-1, although we cannot formally exclude the possibility that the antibody against the B-subunit of the v-ATPase interferes with the formation of the complex.
Figure 3.
Protein subcomplexes in the GIC preparation. Left, GICs were isolated in the presence of Triton-X100 (lane 1) or Brij 96 (lane 2) as described in MATERIALS AND METHODS. Middel, 500 μg of Golgi membranes were solubilized in 0.5% Brij 96, and GIC subcomplexes were immunoprecipitated from the lysate with the use of a control antibody (an antibody against p17 that did not immunoprecipiate; lane 3), an antibody against p17 (lane 4), an antibody against the B-subunit of the v-ATPase (lane 5), and an antibody against flotillin-1 (lane 6). Right, after incubation of 500 μg of Golgi membranes with 20 mM methyl-β-cyclodextrin for 1 h at 37°C, the membranes were reisolated by centrifugation and resuspended in PEN buffer plus 0.5% Brij. The detergent-soluble fraction was obtained after a 30-min incubation on ice by centrifugation for 1 h at 100,000 × g, and GIC subcomplexes were immunoprecipitated from the detergent-soluble fraction. The reduced signal of immunoprecipitated GIC proteins from the detergent-soluble fraction after cyclodextrin treatment is most likely due to the stringent centrifugation conditions, under which only 20% of the Golgi proteins are recovered in the detergent-soluble phase. The coupling of the antibodies to Eupergit beads and subsequent immunoprecipitation procedure are described in MATERIALS AND METHODS. The samples were analyzed by SDS-PAGE (13% acrylamide) and Western blotting with the use of antibodies against the indicated GIC proteins.
To determine whether the subcomplexes are defined by the lipid scaffold or by protein-protein interactions, the lipid scaffold was disrupted by methyl-β-cyclodextrin treatment of the membranes (see also Figure 6). Subsequently, the membranes were solubilized with Brij 96 and the incubation was centrifuged for 1 h at 100,000 × g and the supernatant, i.e., the detergent-soluble phase, was used for immunoprecipitation. Under these conditions, the same subcomplexes could be immunoprecipitated, except that they did not contain the G protein subunits anymore (Figure 3, right). This indicates that the microdomain scaffold is not required for the formation of protein complexes between several GIC proteins, including the B-subunit of the v-ATPase, flotillin-1, caveolin-1, and p17, but might instead modulate their relative affinities. It remains to be established whether the G protein subunits that are not present in the immunoprecipitated complex after disruption of the lipid scaffold are present in different microdomains or whether their interaction with the core complex is modulated by the microdomain scaffold.
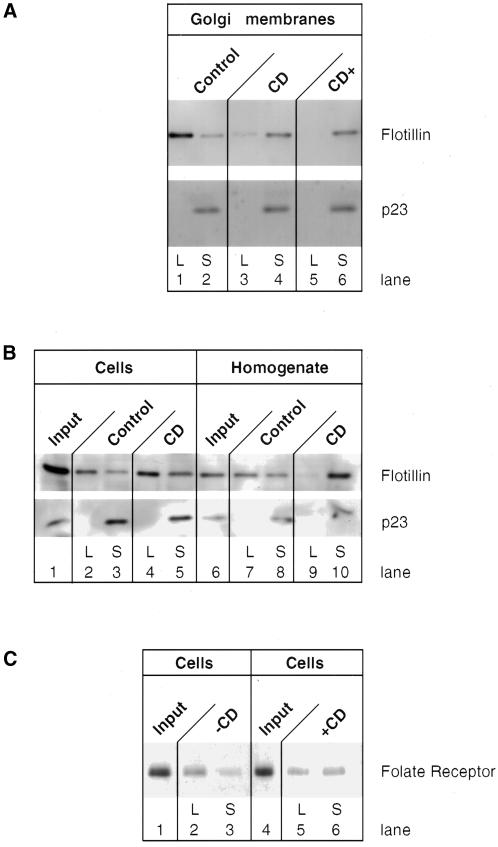
Figure 6.
Cholesterol extraction affects the integrity of GIC. Isolated CHO Golgi membranes (1.4 mg; A), CHO total membrane fraction (2.5 mg; B), or intact CHO cells that overexpress the folate receptor (B and C) were incubated for 30 min at 37°C in the absence or presence of 20 mM methyl-β-cyclodextrin (CD). The detergent-soluble (S) and detergent-insoluble (L for low density) were isolated from these incubations as described in MATERIALS AND METHODS. The fractions were analyzed for the presence of proteins as indicated in the panels by SDS-PAGE and subsequent Western blotting. In case cholesterol was added back to the incubation (a, CD+), 450 μg of cholesterol (solubilized in ethyl alcohol (10 mg/ml, freshly prepared) was added after solubilization of the Golgi membrane fraction in PEN plus 1% Triton X-100 (final ethyl alcohol concentration of 2.5%) and incubated for another 30 min at 0°C before loading on the gradient.
Topology and Enrichment of Flotillin-1
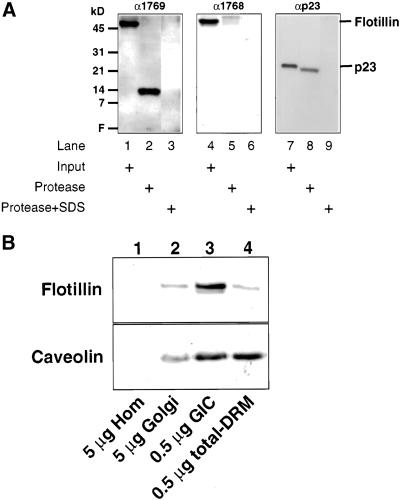
Flotillin-1 was recently cloned, and based on its sequence it was suggested (Bickel et al., 1997) that two hydrophobic domains (flotillin-1 residues 10–36 and 134–151) might function as potential transmembrane-spanning domains, although the first one would be very atypical (Bretscher and Munro, 1993). A transmembrane domain could allow flotillin-1 to function as a structural protein in SM/cholesterol-enriched microdomains. We generated peptide antibodies against epitopes on each side of the potential transmembrane-spanning domain of flotillin-1 (residues 134–151) as tools to determine the topology of flotillin-1 in the Golgi membrane. As shown in Figure 4A (middle), epitope 1768 is completely digested by proteinase K, indicating that this epitope is cytosolically oriented. Under the same conditions, only a short cytosolic tail (∼1 kDa) is cleaved from p23, a transmembrane protein of the Golgi complex (Sohn et al., 1996), showing that the Golgi membranes are sealed (Figure 4A, right). However, when probed with an antibody against epitope 1769, a protease-resistant peptide of ∼14 kDa is observed, indicating that this epitope has a luminal orientation (Figure 4A, left). The apparent size of the protease-resistant peptide is in good agreement with the localization of an N-terminal part of amino acid residues 1–151 to the lumen of the Golgi complex that would include a predicted transmembrane segment (amino acid residues 134–151).
Figure 4.
Topology and enrichment of flotillin-1 in Golgi membranes. (A) Topology. Isolated intact (lanes 1, 2, 4, 5, 7, and 8) or solubilized (lanes 3, 6, and 9) CHO Golgi membranes (40 μg) were incubated for 30 min at 37°C in the absence (lanes 1, 4, and 7) or presence of 500 μg/ml proteinase K (lanes 2, 3, 5, 6, 8, and 9). After incubations, the reactions were stopped by addition of phenylmethylsulfonyl fluoride and analyzed by SDS-PAGE and subsequent Western blotting with the use of antibodies against distinct epitopes of flotillin-1 (α1768 and α1769, generated against flotillin-1 peptides 277–286 and 118–130, respectively) and an antibody against the luminal domain of p23, a Golgi-localized protein (Sohn et al., 1996). Golgi membranes were solubilized in 1% SDS (which completely solubilizes flotillin-1; Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results) for digestion of luminal domains. (B) Enrichment. Five micrograms of homogenate (lane 1), 5 μg of Golgi membranes (lane 2), 0.5 μg of GIC (lane 3), and 0.5 μg of a caveolar fraction (lane 4) were analyzed by SDS-PAGE and Western blotting for the presence of flotillin-1 (top) and caveolin (bottom). Homogenate and Golgi membranes were prepared from CHO cells as described (Balch et al., 1984; Brügger et al., 2000). CHO-GIC was prepared from isolated CHO Golgi membranes as described in MATERIALS AND METHODS. A caveolar fraction was prepared from CHO cells as described for GIC but instead with the use of CHO homogenate as a source.
To determine the intracellular localization of GICs, we focussed on flotillin-1. As expected for a Golgi-localized protein, flotillin-1 is enriched in Golgi membranes and highly enriched in GICs, compared with total cell homogenate (Figure 4B, lanes 1–3). When a total-DRM (representing a caveolar fraction) was isolated from a total cell homogenate and compared with GICs for the presence of flotillin-1, it became evident that flotillin-1 is much more enriched in GICs than in the total-DRM fraction (Figure 4B, lane 3 vs. 4), although both GICs and total-DRM contain similar amounts of caveolin-1 (Figure 4B, bottom) and caveolin-2 (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). Because a total-DRM fraction also includes the GIC fraction, the weak signal of flotillin-1 in total-DRM might be due to the presence of GICs in this preparation, explaining the original suggestion for the caveolar localization of flotillin-1 (Bickel et al., 1997).
Golgi Localization of GIC Proteins
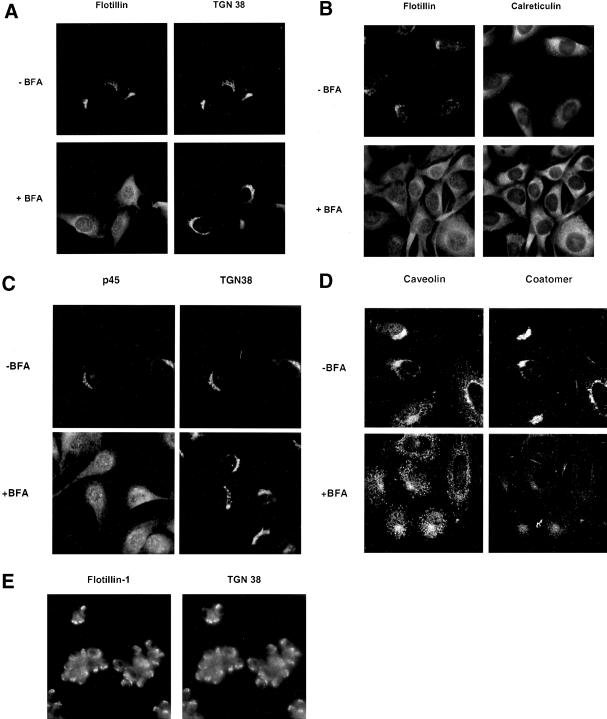
The Golgi localization of flotillin-1 and another GIC protein, p45, was confirmed by immunofluorescence in NRK cells (Figure 5) and CHO cells (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). Both flotillin-1 and p45 colocalized with TGN38, a marker for the Golgi apparatus (Figure 5, A and C). Because microdomains have been postulated for the TGN but not for earlier Golgi compartments, it came as a surprise that the Golgi localization of both GIC proteins disappeared upon treatment of cells with brefeldin A (Figure 5, A and C), known to preferentially disrupt Golgi cisternae (Klausner et al., 1992). In agreement with this, the localization of TGN38 was hardly affected (Figure 5, A and C), whereas under the same conditions, mannosidase II, a marker for early Golgi compartments, behaved like flotillin-1 and p45 (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). Based on these images, it cannot be excluded that some fluorescent signal is brefeldin A insensitive, i.e., localizes to the TGN. After brefeldin A treatment, Golgi membranes are known to fuse with the ER (Lippincott-Schwartz et al., 1989), resulting in uptake of the Golgi complex into the ER. As shown in Figure 5B, in the presence of BFA, flotillin-1 colocalizes with calreticulin, a marker for the ER. By use of an antibody that specifically recognizes a Golgi-localized form of caveolin-1 (Luetterforst et al., 1999), we could also confirm that this pool of caveolin-1 is sensitive to brefeldin A (Figure 5D). In neuronal cells, flotillin-1 (reggie-2) has been localized to the cell surface (Lang et al., 1998). We therefore determined the localization of flotillin-1 in PC12 cells. Although these cells are much smaller than CHO and NRK cells, flotillin-1 clearly colocalizes with TGN38 to the Golgi complex. A relatively high background signal is observed in these cells, which might represent plasma membrane staining, but because the TGN38 antibody produces a similar background signal under the fixation procedures used, this likely reflects nonspecific staining.
Figure 5.
Immunolocalization of GIC proteins. NRK cells (A, B, and C), CHO cells (D), or PC12 cells (E) in monolayer culture were incubated in the absence (−brefeldin A [BFA]) or presence (+BFA) of 5 μM brefeldin A (10 min). After fixation, the localization of flotillin-1 (A, B, and E, left), p45 (C, left), and caveolin-1 (D, left) was determined by use of primary antibodies against the respective proteins. Colocalization was performed with antibodies against TGN38 (A, C, and E, right) or coatomer (D, right) as Golgi markers or calreticulin (B, right) as an ER marker. Immunofluorescence on NRK cells and PC12 cells was performed as described in MATERIALS AND METHODS. CHO cells were fixed with 3% paraformaldehyde (20 min at room temperature) and permeabilized in 0.1% saponin. Further treatment was as described for NRK cells. CHO cells (D) were analyzed with the use of a TCS-NT confocal microscope (Leica, Deerfield, IL).
The GIC Scaffold Is Disrupted by Methyl-β-Cyclodextrin
We used methyl-β-cyclodextrin, which is known to remove cholesterol from membranes, thus interfering with the scaffold of SM/cholesterol-based microdomains and rendering detergent-insoluble complexes detergent soluble (Scheiffele et al., 1997). As depicted in Figure 6A, treatment of isolated Golgi membranes with cyclodextrin strongly increases the detergent solubility of flotillin-1 (compare lanes 1 and 3 and 2 and 4). The amount of input membranes was controlled by Western blotting with the use of an antibody against p23, a Golgi-resident and detergent-soluble type I transmembrane protein. Detergent resistance of the GIC structure was due to the presence of cholesterol in microdomains (before detergent extraction) and not simply due to a lack of cholesterol in the detergent phase, because addition of a twofold excess of cholesterol (over endogenous cholesterol present without cyclodextrin treatment) to the detergent extract of cyclodextrin-treated samples did not rescue detergent insolubility (Figure 6A, lanes 5 and 6). Under these conditions, cholesterol did not precipitate from the aqueous phase, which is due to the presence of detergent (as determined with [3H]cholesterol, Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). Methyl-β-cyclodextrin was then used to remove cholesterol from the plasma membrane of intact cells. In contrast to isolated Golgi membranes, cyclodextrin did not affect the detergent insolubility of flotillin-1 (Figure 6B). As a positive control, alteration of the detergent solubility after treatment of intact cells with cyclodextrin is shown for the folate receptor, a typical plasma membrane protein containing a GPI anchor and localized to microdomains (Figure 6C). In addition, flotillin-1 became detergent soluble again after homogenization of cells and subsequent treatment with methyl-β-cyclodextrin (Figure 6B).
Dynamics of GIC Microdomains along the Early Secretory Pathway
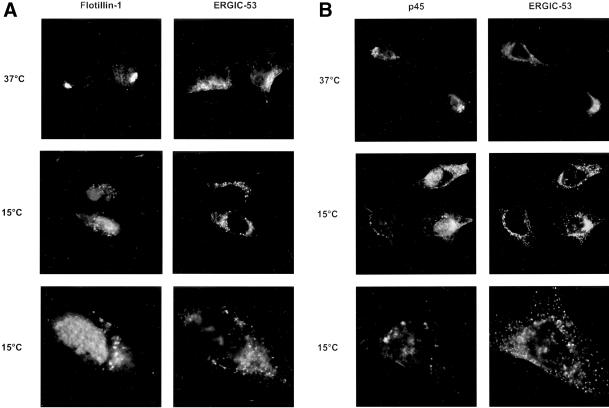
To determine whether GICs in the Golgi complex are cycling between the Golgi and the ER, we used specific conditions to inhibit cycling between these compartments and studied the localization of flotillin-1 and p45 under these conditions. Initial experiments revealed a change in the intracellular localization of these GIC proteins from a typical perinuclear staining to a more punctuate distribution, possibly representing the intermediate compartment (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). Therefore, these experiments were repeated in HeLa cells, which have a morphologically well defined intermediate compartment. Antibodies against ERGIC-53, a marker for the intermediate compartment (Hauri et al., 2000), was used for colocalization with GIC proteins. As shown in Figure 7, both flotillin-1 and p45 redistribute upon incubation of the cells at 15°C from a typical perinuclear structure into large vesicle-like structures. Comparison of the localization of these structures with ERGIC-53 revealed, however, that these structures do not significantly colocalize with the intermediate compartment (Figure 7). Similar results were obtained for p17 (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished observations). These data indicate that GIC components show a dynamic distribution through the early secretory pathway, which is inhibited by incubation at 15°C, but the site of inhibition, i.e., the identity of these vesicle-like globular structures, remains to be established.
Figure 7.
Flotillin-1 and p45 redistribute at 15°C. HeLa cells were incubated for 2 h at 37°C (top) or 15°C (middle and bottom). The localization of flotillin-1 (A, left ), p45 (B, left), and ERGIC-53 (A and B, right) was determined by immunofluorescence spectroscopy as described in MATERIALS AND METHODS and analyzed with the use of a Zeiss Axiovison fluorescent microscope. Images were taken with the use of a 63− objective (top and middle) or 100× objective (bottom).
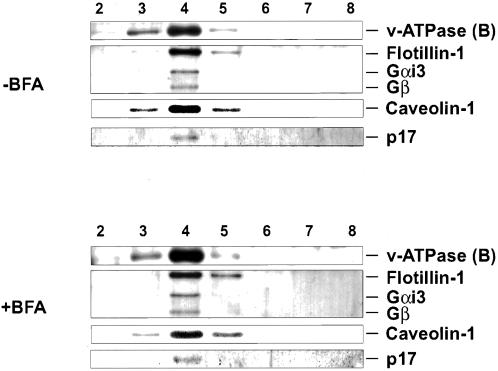
The GIC Scaffold Is Not in a Dynamic Equilibrium with Other Membrane Lipids
Because the ER contains strikingly lower levels of SM and cholesterol than the Golgi (van Meer, 1998), we determined whether the characteristics of detergent insolubility of GICs would change in an environment as different as the ER. To this end, cells were treated with brefeldin A, and low-density detergent-insoluble complexes were isolated from these cells by isopycnic sucrose density centrifugation. As shown in Figure 8, all GIC proteins examined (subunit B of the v-ATPase, Gαi3, Gβc, flotillin-1, caveolin-1, and p17) remain in a low-density detergent-insoluble fraction, indicating that once these microdomains have formed, they are stable enough to survive in a membrane with an SM and cholesterol content as low as the ER. Even prolonged incubations of the cells with brefeldin A (up to 1 h) did not affect the detergent insolubility of the GIC proteins. Both in the absence (see also Figure 2B) and presence of brefeldin A, p23 (a Golgi marker) and calnexin (an ER marker) could not be detected in the detergent-insoluble fraction (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results).
Figure 8.
GIC structure is insensitive to brefeldin A. CHO cells were grown to subconfluency. Cells were then incubated in the absence (top) or presence (bottom) of 5 μM brefeldin A for 15 min at 37°C. The absence of Golgi localization of flotillin-1 and p45 was confirmed by concomitant immunofluorescence (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). After incubation, the cells were directly solubilized in PEN plus 1% Triton X-100 and incubated for 30 min at 0°C. The lysate was analyzed for the presence or absence of low-density detergent insoluble complexes by isopycnic sucrose density centrifugation as described for the preparation of GICs (MATERIALS AND METHODS). The gradients were fractionated and fractions 1–9 were analyzed by SDS-PAGE and Western blotting for the presence of v-ATPase (subunit B), flotillin-1, Gαi3, Gβc, caveolin-1, and p17 with the use of the corresponding antibodies.
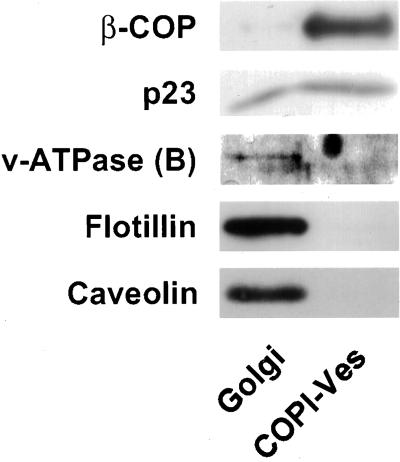
Segregation of GIC Proteins from COPI-coated Vesicles
By analogy to microdomains in the TGN (Simons and Ikonen, 1997), GICs may provide a sorting platform for lipids and proteins at earlier Golgi compartments. We previously showed that heterotrimeric G proteins are excluded from COPI-coated vesicles and speculated that strong interactions with other proteins might be responsible for this segregation (Helms et al., 1998). If the segregation of G proteins is due to their presence in GICs, then the prediction is that other GIC constituents are also segregated from COPI-coated vesicles. Figure 9 shows a comparison of equivalent amounts of donor Golgi and COPI-coated vesicles, based on their phospholipid content. As expected, β-COP, a subunit of the coatomer complex, is enriched in the vesicle fraction (Figure 9). In addition, p23, a Golgi-localized protein, is enriched both in Golgi membranes and in COPI-coated vesicles (Sohn et al., 1996). Antibodies against various GIC proteins were then used to identify the presence of these proteins in the vesicles. Figure 9 shows that like Gα- and Gβ-subunits (Helms et al., 1998), flotillin-1, subunit B of the v-ATPase, and caveolin-1 are also absent from COPI-coated vesicles, in agreement with a possible role of GICs in sorting events.
Figure 9.
Exclusion of GIC proteins from COPI-coated vesicles. Equivalent amounts of isolated Golgi membranes and COPI-coated vesicles (10.8 μg of total phospholipid) were analyzed by SDS-PAGE and Western blotting for the presence of subunit B of the v-ATPase, flotillin-1, and caveolin-1 as indicated in the figure by use of their respective antibody. As positive controls for COPI-coated vesicles, the blots were probed with antibodies against β-COP and p23. Golgi membranes and COPI-coated vesicles were isolated as described in MATERIALS AND METHODS.
DISCUSSION
General Characteristics of Golgi-localized Microdomains
Here we have described the isolation and detailed characterization of GICs, Golgi-derived low-density detergent-insoluble complexes enriched with a unique subset of proteins and lipids compared with other DRMs. We have identified 10 major protein components of GICs (subunits of heterotrimeric G proteins and v-ATPase, flotillin-1, caveolin, and two unknown proteins). Remarkably, although only ∼2% of Golgi proteins are present in GICs, they contain 25% of Golgi phospholipids, leaving the possibility that 25% of the Golgi membrane is relatively empty, i.e., has a relatively low protein abundance, although it cannot be excluded that because of the detergent extraction method used to isolate GICs, several proteins physiologically associated with this microdomain have been extracted from the scaffold. We have identified a core complex of GIC proteins consisting of the B-subunit of the v-ATPase, flotillin-1, caveolin-1, and p17. The interaction between these proteins is not dependent on the lipid scaffold but relies on protein-protein interactions. It remains to be established whether the other GIC proteins that are not present in the immunoprecipitated complex are present in different microdomains or whether their interaction with the core complex is modulated by the microdomain scaffold. Because p45 and flotillin-1 show a similar dynamic Golgi localization (a brefeldin A-sensitive Golgi localization and an accumulation in large globular structures upon 15°C incubation), this protein might also be part of the same core complex. Caveolin-1 is also part of the core complex, but the brefeldin A-induced redistribution of caveolin-1 results in a more punctuate redistribution compared with the brefeldin A-induced redistribution of flotillin and p45 to the ER. This indicates that the Golgi-localized caveolin-1 is associated with various Golgi-localized microdomains, some of which may behave differently from microdomains that contain the GIC core complex. Because the antibodies used to detect caveolin-1 at the Golgi complex by immunofluorescence visualize only a subpopulation of the caveolin-1 (Luetterforst et al., 1999), it cannot be excluded that this antibody preferentially recognizes the pool of caveolin-1 that relocates to vesicular structures upon brefeldin A treatment and that the caveolin-1 associated with the GIC core complex (causing a relocation to the ER upon brefeldin A treatment) is underrepresented in the immunofluorescence data.
Heterotrimeric G Proteins in GICs
Gα- and Gβ-subunits represent major constituents of GICs. As shown in Figure 2B, the various Gα-subunits show different enrichment factors. Because Gαi and possibly Gαo protein subunits show enrichment factors similar to other GIC proteins (Figure 4B for flotillin-1; Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results), these Gα-subunits are likely to be GIC-resident proteins. The relatively low enrichment of the other Gα-subunits indicates that they may be derived from another DRM. Isolated Golgi membranes are known to be contaminated with plasma membrane, and the corresponding DRM may contaminate our GIC fraction. Alternatively or in addition, they could be derived from a TGN-derived microdomain, for which indirect evidence exists but that to date has not been purified (see below). The differential enrichment of Gα-subunits in GICs are in good agreement with the distribution of Gα-subunits on Golgi membranes (Stow et al., 1991; Wilson et al., 1994; Denker et al., 1996).
Flotillin-1 in GICs
Flotillin-1 is a major GIC component that was originally described as a caveolar protein, based on its presence in DRMs (Bickel et al., 1997; Galbiati et al., 1998). Lang et al. (1998) observed a plasma membrane localization in neuronal cells with the use of antibodies against reggie-2 (flotillin-1). This antibody gives rise to a clustered cell surface staining (indicative of microdomains) that is unchanged after treatment of cells with filipin, a cholesterol-extracting agent known to affect caveolin clustering at the plasma membrane (Lang et al., 1998). We find, however, that flotillin-1 has a cholesterol-dependent microdomain localization. The reasons for these discrepancies are not clear and require further investigation. The observed Golgi localization with an antibody against peptide 1769 is not epitope specific, because an antibody against peptide 1768 also gives rise to a perinuclear staining in the immunofluorescence (Gkantiragas, Brügger, Stüven, Kaloyanova, Li, Löhr, Lottspeich, Wieland, and Helms, unpublished results). In addition, we show by several other independent criteria that flotillin-1 behaves as a Golgi-resident protein. 1) Flotillin-1 is enriched in an isolated Golgi fraction and shows a much higher enrichment in GICs compared with a total-DRM preparation (Figure 4B). In fact, flotillin-1 is a major component of GICs (Figure 2A). 2) Treatment of intact cells with cholesterol-extracting agents such as cyclodextrin did not affect the detergent insolubility of flotillin-1, whereas treatment of isolated Golgi membranes caused solubilization of flotillin-1 in Triton X-100. 3) The length of the transmembrane-spanning domain of flotillin-1 (18 amino acids) correlates with Golgi-resident proteins but not with plasma membrane proteins (Munro, 1998). In summary, we have strong evidence to show that flotillin-1 is localized to the Golgi complex in NRK, CHO, and HeLa cells, but at this point we cannot rule out the possibility that in specialized cells such as neuronal cells, flotillin-1 (and possibly other GIC proteins as well) might localize to the cell surface. Interestingly, in undifferentiated PC12 cells, we could localize flotillin-1 to the Golgi complex (Figure 5E). In differentiated PC12 cells (7 d in the presence of nerve growth factor), flottilin-1 could be localized to the cell surface (Lang et al., 1998). This indicates that flotillin-1 shows a cell-type–dependent subcellular localization. In support of this, it was recently shown that in another specialized cell type (macrophages), flotillin-1 was found to be present on phagosomes (Garin et al., 2001).
Flotillins contain a so-called SPFH domain and for many of these stomatin-related proteins, an atypical membrane topology has been suggested, including a hairpin loop in the membrane (Tavernarakis et al., 1999). Although our results of the protease digest experiments (Figure 4A) are very difficult to explain if this were the case, it remains to be established whether the atypical hydrophobic N-terminal region of flotillin-1 is anchored in the membrane.
v-ATPase Subunits in GICs
The vacuolar H+-ATPase is a multisubunit complex, consisting of a peripheral and a membrane domain (V1 and V0 domain, respectively; Finbow and Harrison, 1997; Forgac, 1997). There is a report in the literature of the presence of the B-subunit (V1 domain) in caveolin-containing DRMs (Mineo and Anderson, 1996). By microsequencing, we have identified four subunits (A-, B-, E-, and accessory subunit) as major components of GICs (Figure 2A; Table 2). Surprisingly, all of these subunits belong to the V1 domain. Very little is known about the regulation of v-ATPase activity. In this context, the presence of V1 domain proteins, but not V0 domain proteins in GICs, could provide a new and unexpected mechanism of spatial regulation of luminal pH and would support the hypothesis that association/dissociation of V1- and V0-subunits could regulate the activity of the proton pump (Finbow and Harrison, 1997; Forgac, 1997). Recently, the proteolipid in the V0 domain of the v-ATPase has been implicated in vacuolar membrane fusion (Peters et al., 2001). It remains to be established whether this mechanism is generally applicable, but it could provide an indication for the function of the GIC complex.
Caveolin in GICs
Although caveolin-1 polymerizes already in the ER upon de novo synthesis, it only becomes detergent insoluble during passage through the Golgi (Monier et al., 1995). In vivo, caveolin-1 is localized both to the plasma membrane and to the TGN (Dupree et al., 1993; Smart et al., 1994). Because GICs are derived from an isolated Golgi fraction, which includes TGN, our GIC preparation should contain a caveolin-containing precursor for caveolae, equivalent to the apical sorting platforms (Simons and Ikonen, 1997). Thus, it is likely that caveolin in our preparation is derived from microdomains of early Golgi compartments (GICs) as well as from apical sorting platforms at the TGN. In immunofluorescence, the C-terminal antibody against caveolin-1 might recognize predominantly the early Golgi form, because most of the immunofluorescence Golgi signal is sensitive to treatment with brefeldin A.
The Lipid Scaffold and Intracellular Transport of GICs
Lipid analysis revealed an enrichment of SM and cholesterol in GICs, similar to that observed in other types of DRM. The fact that the detergent insolubility of GIC proteins is sensitive to cyclodextrin indicates that lipids are an essential part of the scaffold of GIC microdomains. As shown in Table 1, SM and PC make up 84% of total phospholipids in GICs and 63% of total phospholipids in total-DRM. Many other lipids have been reported to be present in total-DRM, including phosphoinositides, GM1, ceramide, and diacylglycerol (Anderson, 1998).
The stability of the GIC scaffold, once formed, is underscored by the fact that conditions under which GIC proteins are redistributed into the ER, they remain intact, although the concentration of SM and cholesterol in the ER is very low (van Meer, 1993), and microdomains are not likely to exist in the ER of mammalian cells (Brown and London, 1998, but see Sevlever et al. 1999). This indicates that, once microdomains have formed, they are thermodynamically quite stable.
It therefore seems likely that (at least) the core complex travels through the early secretory pathway within one type of microdomain. Because the GIC proteins (Figure 9) and lipids (Brügger et al., 2000) are efficiently segregated from COPI-coated vesicles, the mechanism by which the GIC microdomains are transported remains to be identified. In agreement with a COPI-independent mechanism, we find that GIC proteins do not show a typical inhibition of transport at 15°C, which would result in an accumulation in the ER or intermediate compartment. COPI-independent transport through the early secretory pathway has been described (Girod et al., 1999; White et al., 1999) and it will be interesting to determine whether the vesicular or globular structures in which flotillin-1 and p45 accumulate upon incubation at 15°C are related to this alternative pathway.
Perspectives
GICs are novel microdomains and distinct from other low-density detergent-insoluble complexes, based on its localization and unique protein composition. What could be a function of GICs? One possibility is that, like the sorting platforms at the TGN, GICs are involved in the sorting of proteins and lipids at early stages of the secretory pathway. All GIC proteins analyzed so far are segregated from COPI-coated vesicles and it is likely that the remainder are segregated as well. Interestingly, SM and cholesterol are also segregated from COPI-coated vesicles (Brügger et al., 2000).
GICs contain similar amounts of G protein α- and β-subunits, suggesting that this microdomain can act in signal transduction at the Golgi complex. G protein subunits and v-ATPase subunits make six of the 10 proteins we have characterized in GICs. A future challenge will be to study a possible connection of the G proteins with the v-ATPase subunits, e.g., a possible regulation of association of V1- to V0-subunits. Golgi-localized G proteins have been implicated in membrane fusion (Helms et al., 1998) and in maintenance of the Golgi structure (Jamora et al., 1997; Yamaguchi et al., 1997). The identification of GIC (proteins) may provide a new starting point to study alternative G protein-mediated signal transduction cascades at the Golgi complex.
ACKNOWLEDGMENTS
We thank Liyun Zhao (Biochemie Zentrum Heidelberg, Heidelberg, Germany) for determination of the total membrane lipid content of COPI-coated vesicles, Narayan Agrawal (Biochemie Zentrum Heidelberg, Heidelberg, Germany), and Rainer Saffrich (EMBL) for assistance with immunofluorescence techniques, Rainer Pepperkok, (EMBL, Germany), Kai Simons (EMBL, Germany), Nathan Nelson (Tel Aviv University, Israel), Bernd Nürnberg (Freie Universität Berlin, Germany), and Silvana Canevari (Istituto Nazionale Tumori, Italy) for antibodies against ERGIC-53, the C-terminal domain of caveolin, the B-subunit of v-ATPase, the various subunits of heterotrimeric G proteins, and the folate receptor respectively. CHO cells overexpressing the folate receptor were a gift from Dr. Teymuras Kurzchalia (Max-Delbrueck-Center for Molecular Medicine). This work was supported by a grant (SPP 312 to J.B.H. and F.T.W.) from the German Research Council, from the German-Israeli Foundation of Scientific Research and Development (to J.B.H. and F.T.W.), and from the European Commission Research Training Networks (grant HPRN-CT-2000-00077 to J.B.H.)
This paper is dedicated to Jan Helms, deceased on October 30, 1999, who initiated J.B.H.'s interest in science and who taught him the basic principles of chemistry with great fatherly love and dedication.
Abbreviations used:
- CHO
Chinese hamster ovary
- DRM
detergent-resistant membranes
- ER
endoplasmic reticulum
- GIC
Golgi-derived detergent-insoluble complex
- SM
sphingomyelin
- TGN
trans-Golgi network
- v
vacuolar
REFERENCES
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Bagnat M, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. Lipid extraction. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992;3:1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brügger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brügger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, Helms JB, Lehmann W, Nickel W, Wieland FT. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol. 2000;151:507–518. doi: 10.1083/jcb.151.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WJ, Ying YS, Rothberg KG, Hooper NM, Turner AJ, Gambliel HA, De Gunzburg J, Mumby SM, Gilman AG, Anderson RG. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994;126:127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Allan V, Kreis T. Involvement of beta-COP in membrane traffic through the Golgi complex. Trends Cell Biol. 1991;1:14–19. doi: 10.1016/0962-8924(91)90064-g. [DOI] [PubMed] [Google Scholar]
- Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerskorn C, Lottspeich F. Internal amino acid sequence analysis of proteins separated by gel electrophoresis after tryptic digestion in polyacrylamide matrix. Chromatographia. 1989;28:92–94. [Google Scholar]
- Exner T, Jensen ON, Mann M, Kleuss C, Nurnberg B. Posttranslational modification of Galphao1 generates Galphao3, an abundant G protein in brain. Proc Natl Acad Sci USA. 1999;96:1327–1332. doi: 10.1073/pnas.96.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Kobayashi T, Kurzchalia TV, Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993;32:6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Finbow ME, Harrison MA. The vacuolar H+-ATPase: a universal proton pump of eukaryotes. Biochem J. 1997;324:697–712. doi: 10.1042/bj3240697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPases. Annu Rev Cell Dev Biol. 1997;13:779–808. doi: 10.1146/annurev.cellbio.13.1.779. [DOI] [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Goltz JS, Steele Z, Sen J, Jurcsak J, Stein D, Stevens L, Lisanti MP. Identification, sequence and developmental expression of invertebrate flotillins from Drosophila melanogaster. Gene. 1998;210:229–237. doi: 10.1016/s0378-1119(98)00064-x. [DOI] [PubMed] [Google Scholar]
- Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassel S, Roling A, Hasilik A. Immunoprecipitation of labeled antigens with Eupergit C1Z. Anal Biochem. 1989;180:72–78. doi: 10.1016/0003-2697(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri HP, Kappeler F, Andersson H, Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci USA. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB. Role of heterotrimeric GTP binding proteins in vesicular protein transport: indications for both classical and alternative G protein cycles. FEBS Lett. 1995;369:84–88. doi: 10.1016/0014-5793(95)00620-o. [DOI] [PubMed] [Google Scholar]
- Helms JB, HelmsBrons D, Brugger B, Gkantiragas I, Eberle H, Nickel W, Nurnberg B, Gerdes HH, Wieland FT. A putative heterotrimeric G protein inhibits the fusion of COPI-coated vesicles: segregation of heterotrimeric G proteins from COPI-coated vesicles. J Biol Chem. 1998;273:15203–15208. doi: 10.1074/jbc.273.24.15203. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- Jamora C, Takizawa PA, Zaarour RF, Denesvre C, Faulkner DJ, Malhotra V. Regulation of Golgi structure through heterotrimeric G proteins. Cell. 1997;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 angstrom using imaging fluorescence resonance energy transfer. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott SJ. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia TV, Hartmann E, Dupree P. Guilt by insolubility: does a protein's detergent insolubility reflect a caveolar location? Trends Cell Biol. 1995;5:187–189. doi: 10.1016/s0962-8924(00)88990-4. [DOI] [PubMed] [Google Scholar]
- Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, Wiechers MF, Plattner H, Stuermer CA. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol. 1998;37:502–523. doi: 10.1002/(sici)1097-4695(199812)37:4<502::aid-neu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luetterforst R, Stang E, Zorzi N, Carozzi A, Way M, Parton RG. Molecular characterization of caveolin association with the Golgi complex: identification of a cis-Golgi targeting domain in the caveolin molecule. J Cell Biol. 1999;145:1443–1459. doi: 10.1083/jcb.145.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machleidt T, Li WP, Liu P, Anderson RG. Multiple domains in caveolin-1 control its intracellular traffic. J Cell Biol. 2000;148:17–28. doi: 10.1083/jcb.148.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore N, Smith KL, Graham CH, Jen A, Brady K, Hall S, Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Mineo C, Anderson RG. A vacuolar-type proton ATPase mediates acidification of plasmalemmal vesicles during potocytosis. Exp Cell Res. 1996;224:237–242. doi: 10.1006/excr.1996.0133. [DOI] [PubMed] [Google Scholar]
- Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E. Caveolin-2 localizes to the Golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem. 1999;274:25708–25717. doi: 10.1074/jbc.274.36.25708. [DOI] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the Golgi complex. J Biol Chem. 1999;274:25718–25725. doi: 10.1074/jbc.274.36.25718. [DOI] [PubMed] [Google Scholar]
- Peters C, Bayer M, Bühler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–588. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JKH. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1007. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sandhoff R, Brügger B, Jeckel D, Lehmann WD, Wieland FT. Determination of cholesterol at the low picomole level by nano-electrospray ionization tandem mass spectrometry. J Lipid Res. 1999;40:126–132. [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Roth MG, Simons K. Interaction of influenza virus hemagglutinin with sphingolipid cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schulte T, Paschke KA, Laessing U, Lottspeich F, Stuermer CA. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–587. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JA, Bachi T, Ginsel L, Hauri HP. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Stenbeck G, Brecht A, Lottspeich F, Orci L, Rothman JE, Wieland FT. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991;349:215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- Sevlever D, Pickett S, Mann KJ, Sambamurti K, Medof ME, Rosenberry TL. Glycosylphosphatidylinositol-anchor intermediates associate with Triton-insoluble membranes in subcellular compartments that include the endoplasmic reticulum. Biochem J. 1999;343(pt 3):627–635. [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Conrad PA, Anderson RG. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi derived COPI coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow JL, de Almeida JB, Narula N, Holtzman EJ, Ercolani L, Ausiello DA. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J Cell Biol. 1991;114:1113–1124. doi: 10.1083/jcb.114.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernarakis N, Driscoll M, Kyrpides NC. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci. 1999;24:425–427. doi: 10.1016/s0968-0004(99)01467-x. [DOI] [PubMed] [Google Scholar]
- Tooze SA, Huttner WB. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990;60:837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993;5:661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- van Meer G. Lipids of the Golgi membrane. Trends Cell Biol. 1998;8:29–33. doi: 10.1016/s0962-8924(97)01196-3. [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Gill S, Reinach S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Komuro M, Farquhar MG. Cellular variations in heterotrimeric G protein localization and expression in rat pituitary. Endocrinology. 1994;134:233–244. doi: 10.1210/endo.134.1.8275939. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Oliver JM. Observing FcepsilonRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Yamamoto A, Furuno A, Hatsuzawa K, Tani K, Himeno M, Tagaya M. Possible involvement of heterotrimeric G proteins in the organization of the Golgi apparatus. J Biol Chem. 1997;272:25260–25266. doi: 10.1074/jbc.272.40.25260. [DOI] [PubMed] [Google Scholar]