Abstract
Hypertonic shock of Saccharomyces cerevisiae activates the Hog1p MAP kinase cascade. In contrast, protein kinase C (Pkc1p) and the “cell integrity” MAP kinase cascade are critical for the response to hypotonic shock. We observed that hypertonic shock transiently relocated many, but not all, nuclear and nucleolar proteins to the cytoplasm. We hypothesized that the relocation of nuclear proteins was due to activation of the Hog1p kinase cascade, yet, surprisingly, Hog1p was not required for these effects. In contrast, Pkc1p kinase activity was required, although the Pkc1p MAP kinase cascade and several factors known to lie upstream and downstream of Pkc1p were not. Moreover, sudden induction of a hyperactive form of Pkc1p was sufficient to relocate nuclear proteins. Taken together, these observations show that the scope of involvement of Pkc1p in the organization of the nucleus considerably exceeds what has been characterized previously. The relocation of nuclear proteins is likely to account for the profound inhibition of RNA synthesis that was observed during hypertonic shock.
INTRODUCTION
The cellular effects of changes in tonicity have been investigated extensively (Robbins et al., 1970; Banuett, 1998; Gustin et al., 1998; Lang et al., 1998). In particular, analysis of the yeast Saccharomyces cerevisiae has elucidated major roles for two MAP kinase signaling pathways: the Pkc1p “cell integrity” pathway, which is stimulated by hypotonic shock (Watanabe et al., 1994; Davenport et al., 1995), and the Hog1p pathway, which is stimulated by hypertonic shock (Brewster et al., 1993; Posas and Saito, 1997). Each pathway is required for long-term survival in the corresponding medium. Hypertonic shock profoundly affects the actin cytoskeleton (Chowdhury et al., 1992; Mulholland et al., 1994; Delley and Hall, 1999).
The Pkc1p pathway can receive input from plasma membrane glycoproteins of the Wsc family and from the Rho GTPases and their regulatory factors, for example in the context of control of cell polarization and cell wall biosynthesis (Gustin et al., 1998). Interestingly, some signaling events that require Pkc1p appear not to require the corresponding downstream MAP kinase cascade, which is linked to activation of the transcription factors Rlm1p and Swi4p/Swi6p (Lee and Levin, 1992; Gustin et al., 1998; Delley and Hall, 1999; Li et al., 2000). In contrast, the Hog1p pathway is initiated by a two-component phosphorelay involving the cell surface transmembrane protein Sln1p and the cytosolic protein Ypd1p (Posas et al., 1996). Stimulation of this pathway causes phosphorylation and transient nuclear entry of the terminal kinase, Hog1p, as well as of the transcription factor Msn2p (Ferrigno et al., 1998; Görner et al., 1998; Reiser et al., 1999). The downstream substrate(s) of Hog1p kinase is not known.
We have observed recently that arrest of the secretory pathway in yeast inhibits nuclear import and causes many nucleolar and nucleoplasmic proteins to relocate to the cytoplasm. These events (the “arrest of secretion response”) are reversible and are prevented if Hog1p is overexpressed or if Pkc1p is deleted (Nanduri et al., 1999; our unpublished observations). These observations caused us to investigate the impact of changes in tonicity on the yeast nucleus.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
Yeast strains (Table 1) were grown to midlog phase at 23°C in YEPD or in synthetic media lacking individual components to select for the presence of plasmids of interest. The osmotically sensitive strain DL376 was grown in YEPD supplemented with 0.5 M sorbitol. Chromosomal integration of GFP-NSP1 (pSW956) and GFP-NIC96 (pSW950) into YPH500 was as described by Bucci and Wente (1998). Cells were transformed with pPS1739 (HOG1-GFP, URA3, CEN [Ferrigno et al., 1998]), pBM3495 (MIG 217–400-GFP-lacZ, URA3, 2 μ [De Vit et al., 1997]), pSW636 (NUP49-GFP, LEU2, CEN [Bucci and Wente, 1998]), or pGFP-TBP (GFP-TBP, HIS3, CEN [Patterson et al., 1998]) by standard procedures and maintained on appropriate selective plates. DL376 transformants carrying pDL468 (GAL-PKC1wt-HA, URA3, CEN [Gray et al., 1997]), pDL469 (GAL-PKC1pK853R-HA, URA3, CEN [Gray et al., 1997]), pBM743 (GAL-PKC1pR398A, URA3, CEN [Delley and Hall, 1999]), pHPS29 (PKC1C434S, C437S, C514S, C517S, TRP1, CEN [Jacoby et al., 1997]), or pHPS30 (pPKC1wt, TRP1, CEN [Jacoby et al., 1997]) were maintained on selective plates containing 0.5 M sorbitol.
Table 1.
S. cerevisiae strains used in this studya
| Name | Relevant genotype | Reference/source |
|---|---|---|
| YPH500 | wt | Sikorski and Hieter (1989) |
| YAT501 | YPH500 [pSW950] | This study |
| YAT502 | YPH500 [pSW956] | This study |
| JK9-3da | wt | Delley and Hall (1999) |
| AS135-1a | Δrho2 | Delley and Hall (1999) |
| AS138-1b | Δrom2 | Delley and Hall (1999) |
| NB75-1b | Δskn7 | Delley and Hall (1999) |
| PA39-1b | Δwsc1 | Delley and Hall (1999) |
| PA88-1a | Δwsc2 | Delley and Hall (1999) |
| PA62-2d | Δwsc3 | Delley and Hall (1999) |
| PE3-2a | Δmid2 | Delley and Hall (1999) |
| TB50a | wt | Delley and Hall (1999) |
| TS45-1a | Δmpk1 | Delley and Hall (1999) |
| IH1-5a | Δwsc4 | Delley and Hall (1999) |
| MH272-1da | wt | Delley and Hall (1999) |
| MB146-3d | Δfks1 | Delley and Hall (1999) |
| AS167-1d | Δbni1 | Delley and Hall (1999) |
| EG123 | wt | Lee and Levin (1992) |
| DL253 | Δbck1 | Lee and Levin (1992) |
| DL376 | Δpkc1 | Lee and Levin (1992) |
| YPH102 | wt | Sikorski and Hieter (1989) |
| JBY13 | Δhog1 | Schüller et al. (1994) |
| W303a | wt | |
| V1274 | Δpbs2 | S.M. O'Rourkeb |
| V1273 | Δsho1 | O'Rourkeb |
| V1275 | Δssk1 | O'Rourkeb |
| V1276 | Δste11 | O'Rourkeb |
| RH273-1A | Δgas1 | Nuoffer et al. (1991) |
| MYY260 | hsf1 | Smith and Yaffe (1991) |
Strains of the same genetic background are grouped together.
Strains derived from W303, provided by S.M. O'Rourke (O'Rourke and Herskowitz, 1998).
For hypertonic shock, the osmolarity was increased by adding 3 M sorbitol, 5 M NaCl, or 5 M KCl to reach a final concentration of 1 M sorbitol or 0.5 M NaCl or KCl. For heat shock, cultures were transferred to a shaking water bath at 37°C. For hypotonic shock, cultures in YEPD were diluted with 4 volumes of water.
Immunostaining
Cells were fixed and processed as described (Nanduri et al., 1999). A 1/10 volume of 37% formaldehyde was added directly to growing cultures for 10 min, followed by sedimentation and further fixation in 3.7% formaldehyde, 10% methanol, in 0.1 M potassium phosphate buffer (pH 6.5) for 10 min. Cells were spheroplasted with Zymolyase (ICN, Costa Mesa, CA; catalog number 320921), allowed to adhere to polylysine-coated slides, dehydrated with the use of −20°C methanol for 5 min, −20°C acetone for 30 s (Wente et al., 1992), and immunostained with the use of the antibodies described by Liu et al. (1996). GFP-tagged proteins were detected without fixation. DNA was stained with DAPI. Cells were examined with a Nikon Microphot-FX microscope with the use of a 100× objective. Images were collected with the use of a Diagnostic Instruments (Sterling Heights, MI) 1.1.0 SPOT camera. Final figures were produced by with the use of Adobe Photoshop.
Immunoblotting
Glass-bead extracts of cells prepared with 10 mM Tris-HCl, pH 7.5, containing 1 mM PMSF, 1 μg/ml leupeptin and aprotinin, and 1% SDS were electrophoresed on a 8% SDS-polyacrylamide gel and blotted onto nitrocellulose membranes. The blots were blocked with 2% nonfat dry milk, probed with polyclonal anti-Fpr3p (J. Thorner, University of California, Berkeley), polyclonal anti-phospho–specific p38 (Hog1p) (New England Biolabs, Beverly, MA, catalog numbers 9211 and 9212), and developed with the use of ECL chemiluminescence (Amersham/Pharmacia, Arlington, IL).
RNA Synthesis
Cells were exposed to hypertonic shock for 0–4 h. Aliquots were pulse-labeled with [3H-methyl]-methionine (NEN Life Sciences [Boston, MA]; NET-061x, 70–85 Ci/mmol) for 5 min over this period. Total RNA was then extracted with acid phenol at 65°C for 1 h. Aliquots were precipitated with trichloroacetic acid and counted.
RESULTS
Reversible Relocation of Nuclear Proteins
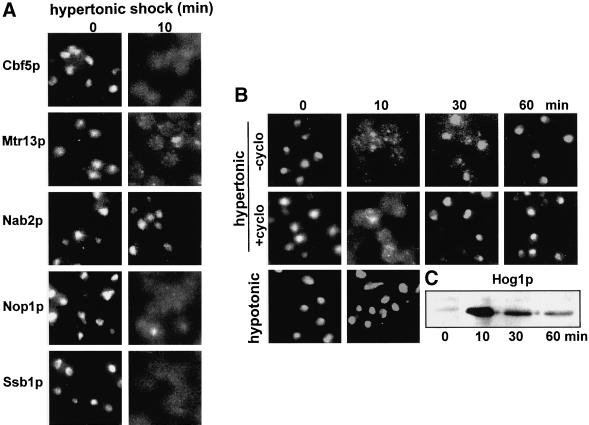
When wild-type cells are exposed to 0.5 M NaCl, many nuclear proteins rapidly relocate to the cytoplasm. Extensive relocation was seen within 2 min and relocation was complete by 10 min. Figure 1A illustrates the relocation of the nucleolar proteins Cbf5p, Nop1p, and Ssb1p (Jordan and Shaw, 1995; Liu et al., 1996). The nucleolar proteins Fpr3p (Figure 1B) and Nsr1p (our unpublished results) also relocated. In addition, several nucleoplasmic proteins relocated, e.g., the shuttling nucleoplasmic hnRNP-like protein Npl3p/Mtr13p (Flach et al. 1994; Singleton et al., 1995) (Figure 1A) and the transcription factor fusion Mig1p-GFP-β-galactosidase (De Vit et al., 1997) (our unpublished results). In contrast, Figure 1A shows that the hnRNP-like protein Nab2p (Anderson et al., 1993) did not relocate. This was also the case for a chromatin-associated protein, a GFP fusion of TBP (Patterson et al., 1998) (our unpublished results). To learn whether hypertonic shock per se is responsible for the relocation of Fpr3p and Nop1p, we have also used 0.5 M KCl and 1 M sorbitol. Both had effects comparable to those of 0.5 M NaCl (our unpublished results), but strong hypotonic shock did not cause relocation (Figure 1B). To simplify the further description of these phenomena, although we have monitored several proteins that relocate, we focus on the nucleolar prolyl isomerase Fpr3p.
Figure 1.
Relocation of nuclear proteins during osmotic shock. (A) Several nuclear proteins were localized in wild-type cells (strain YPH500) before and after 10 min of hypertonic shock (0.5 M NaCl). (B) Time course of transient relocation of the nucleolar protein Fpr3p in YPH500 cells during hypertonic shock, lack of effect of 100 μg/ml cycloheximide added 15 min before the osmotic shock, and lack of effect of hypotonic shock (see MATERIALS AND METHODS) on Fpr3p localization. (C) YPH500 cells were subjected to hypertonic shock as in B, and samples containing equal amounts of protein were analyzed to detect activation of Hog1p by Western blotting.
On continued incubation in hypertonic medium, Fpr3p reentered the nucleus within 1 h (Figure 1B). This cycle was not affected by adding 100 μg/ml cycloheximide 15 min before the shock (Figure 1B). Judging from these observations and from Western blotting (our unpublished results), the copies of Fpr3p that leave the nucleus are those that return. Interestingly, the kinetics of transient Hog1p activation and nuclear entry were comparable to the kinetics of Fpr3p exit and return to the nucleus (Figure 1C) (Ferrigno et al., 1998).
The Pathway of Signaling
To define the pathway by which hypertonicity signals to the nucleus, we have examined the effects of 1 M sorbitol and 0.5 M NaCl on the localization of nucleolar proteins in strains that are deleted for individual candidate genes of interest.
Hog1p Pathway.
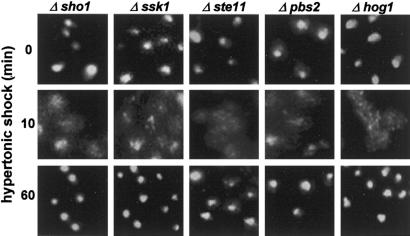
Because the Hog1p pathway is stimulated by hypertonic shock, we hypothesized that it would be required for the relocation or return of proteins that relocate. Nevertheless, Δsho1, Δssk1, Δste11, Δpbs2, and Δhog1 strains showed reversible relocation of Fpr3p (Figure 2), and of Nop1p and Npl3p/Mtr13p (our unpublished results), that was equivalent to that in wild-type cells. The impact of hypertonicity on the nucleus thus involves intermediates other than the Hog1p MAP kinase cascade.
Figure 2.
Lack of involvement of the Hog1p map kinase cascade in the relocation of Fpr3p. Fpr3p was localized 0, 10, and 60 min after hypertonic shock. The deletion strains are Δsho1 (V1273), Δssk1 (V1275), Δste11 (V1276), Δpbs2 (V1274), and Δhog1 (JBY13).
Pkc1p Pathway.
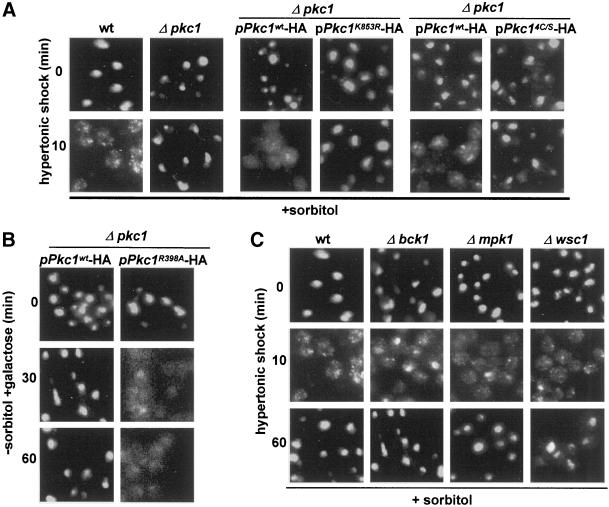
The Pkc1p pathway is not known to be stimulated by hypertonic shock in yeast, but it is stimulated by hypotonic shock. Nevertheless, we observed that a Δpkc1 strain resisted relocation of nuclear proteins during hypertonic shock for 10–60 min (Figure 3A, four panels at the left). To inquire whether the kinase activity of Pkc1p is needed to relocate Fpr3p, we have transformed Δpkc1 with plasmids that drive expression of wild-type Pkc1p or an active site mutant (K853R) from a galactose-inducible promoter. As shown in Figure 3A (middle four panels), hypertonic shock relocated Fpr3p only in the former strain. Figure 3A (four panels at the right) also shows that relocation did not occur in cells that express a mutant form of Pkc1p in which the putative diacylglycerol-binding site has been destroyed (Pkc1p4C/S) (Jacoby et al., 1997). In all of the above experiments, wild-type and Δpkc1 strains from the same background (EG123, DL376) were grown with osmotic support, because it is required for survival in the absence of functional Pkc1p.
Figure 3.
Involvement of Pkc1p, but not of functionally related proteins, in relocation. (A) Localization of Fpr3p in wild-type cells (EG123); Δpkc1 (DL376); DL376 carrying pDL468 for expression of wild-type Pkc1p-HA; DL376 carrying pDL469 for expression of the active site mutant Pkc1K853R-HA; DL376 carrying either pHPS30, encoding wild-type Pkc1p, or pHPS29, encoding Pkc1p4C/S, which is purported not to bind DAG. All cells were shocked for 0 or 10 min. The strains in which Pkc1p expression is driven by a galactose-regulated promoter (middle panels) were grown in raffinose/sorbitol medium that was supplemented with 2% galactose for 4 h before the hypertonic shock. (B) Localization of Fpr3p in Δpkc1 (DL376) cells carrying pDL468 for expression of Pkc1p-HA or a corresponding plasmid, pBM743, for expression of the autophosphorylation site (hyperactive) mutant Pkc1pR398A-HA. Because both plasmids drive Pkc1p expression from a galactose-regulated promoter, both strains were grown in raffinose medium, received 2% galactose at time zero, and were processed for localization of Fpr3p after 0, 30, or 60 min. (C) Localization of Fpr3p in wild-type cells (EG123), Δbck1 (DL253), Δmpk1 (TS45–1a), and Δwsc1 (PA39–1b) after hypertonic shock for 0, 10, or 60 min.
Evidence that Pkc1p is sufficient to relocate nuclear proteins in the absence of complexities attributable to the presence of osmotic support came from studies of cells that express a pseudosubstrate (“hyperactive”) mutant of Pkc1p. These experiments made use of Δpkc1 (pGAL-PKC1R398A) cells, which like Δpkc1 (pGAL-PKC1wt) cells can grow without osmotic support, presumably because of a low level of PKC1 transcription even in glucose medium (Watanabe et al., 1994). On addition of 2% galactose to induce Pkc1pR398A, Fpr3p relocated to the cytoplasm between 30 and 60 min, whereas no relocation was seen with cells carrying the wild-type plasmid (Figure 3B). This relocation is likely to account for the reported inviability of this strain in galactose medium (Watanabe et al., 1994).
Because Pkc1p was required for relocation of nuclear proteins, we hypothesized that the downstream kinases including Bck1p and Mpk1p/Slt2p would also be required. However, as shown in Figure 3C, a wild-type strain and a Δbck1 strain from the same genetic background both showed relocation. A Δmpk1 strain from a different background also showed relocation (Figure 3C). In agreement with others, we also observed that Mpk1p/Slt2p was not phosphorylated during hypertonic stimulation for 10 min (Davenport et al., 1995) (our unpublished results). Thus, although Pkc1p is required, the kinase cascade downstream of Pkc1p is not required for perturbation of the nucleus and is not activated.
In addition, a plasma membrane glycoprotein that can signal to Pkc1p under other circumstances, Wsc1p (Gray et al., 1997; Verna et al., 1997; Delley and Hall, 1999), is not needed to signal to Pkc1p during hypertonic shock, judging from relocation of Fpr3p in Δwsc1 cells (Figure 3C). Equivalent experiments show that the similar proteins, Wsc2p, Wsc3p, Wsc4p, and Mid2p, and the major GPI-anchored protein, Gas1p, also are not needed for signaling (our unpublished results). Deletion of additional proteins that interact with Pkc1p (Bni1p, Fks1p, Rho2p, Rom2p, Skn7p) also does not affect relocation (our unpublished results).
These experiments indicated whether specific factors are needed for relocation of nuclear proteins during hypertonic shock. Because the incubations were extended to 60 min, they also indicated which components are needed for return of the relocated proteins to the nucleus. We observed that none of the mutations studied strongly interrupted the return phase for Fpr3p. However, deletion of potential upstream regulators of Pkc1p (Bni1p, Fks1p, Rho2p, Skn7p) resulted in only incomplete return of proteins to the nucleus at the 1-h time point (our unpublished results).
Protection of the Nucleus Against the Effects of Hypertonic Shock
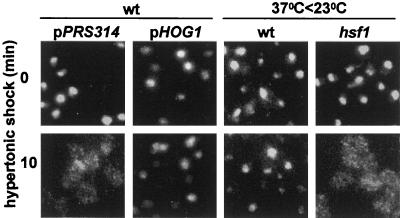
Because Pkc1p and Hog1p often have opposing effects, we inquired whether overexpression of Hog1p might protect the nucleus from the effects of osmotic shock. Indeed, as shown in Figure 4 (left four panels), cells that carry a high-copy plasmid for expression of Hog1p did not relocate nuclear proteins during hypertonic shock.
Figure 4.
Conditions that protect against relocation of nuclear proteins. Fpr3p was localized 0 and 10 min after hypertonic shock in YPH500 cells carrying either the control plasmid pRS314 or a high-copy plasmid (pPS1739) for expression of Hog1p. In parallel experiments, YPH500 or hsf1–1 (MYY260) cells were incubated for 1 h at 37°C before being returned to 23°C and subjected to hypertonic shock for 0 or 10 min.
When cells are transferred to 37°C, the induction of heat-shock proteins can protect them against subsequent stress (Liu et al., 1996). We therefore inquired whether 1 h of exposure to 37°C would allow cells to withstand subsequent hypertonic shock at room temperature. As shown in Figure 4 (third pair of images), this pretreatment greatly reduced the relocation of Fpr3p in wild-type cells, suggesting that some heat-shock proteins do contribute to protection. To test this hypothesis further, we used an equivalent protocol to study a heat-shock transcription factor mutant, hsf1–1. When this strain was incubated at 37°C for 1 h before being subjected to osmotic shock at 23°C, Fpr3p relocated as in wild-type cells that had not been preincubated at 37°C (Figure 4, right panels). Thus, some consequences of heat shock that depend on heat-shock transcription factor are responsible for the protection.
Functional Consequences of Shock
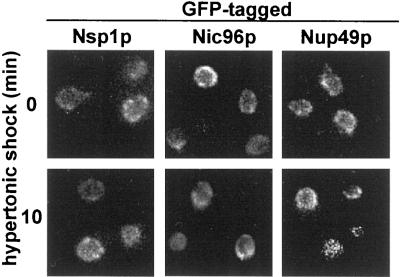
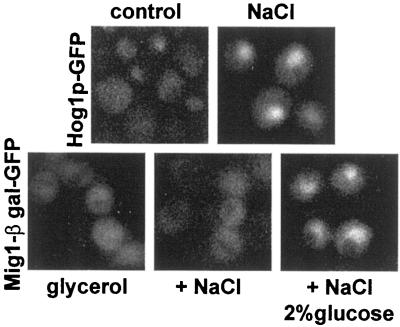
In protocols equivalent to those that documented relocalization of other nuclear proteins, the localization of at least three nucleoporins, Nsp1p, Nic96p, and Nup49p, remained unchanged (Figure 5). Moreover, nuclear import continued for both Hog1p-GFP and a Mig1p-GFP fusion (Figure 6). Both are known to be imported by importin β family members (De Vit et al., 1997; Ferrigno et al., 1998; Reiser et al., 1999).
Figure 5.
Integrity of the nuclear pore complex during hypertonic shock. Cells expressing Nsp1p-GFP (strain YAT502), Nic96p-GFP (strain YAT501), or Nup49p-GFP (strain YPH500 carrying plasmid pSW636) were exposed to hypertonic shock (0.5 M NaCl) for 0 or 10 min at 23°C. Note: the magnification of the cells in these images is twice that in the other figures.
Figure 6.
Nuclear import of GFP-tagged proteins. Top, YPH500 cells carrying pPS1739 (HOG1-GFP) were grown overnight in glucose medium to localize Hog1p-GFP to the cytoplasm (control). The cells were then exposed to hypertonic shock for 10 min (NaCl). Bottom, YPH500 cells carrying pBM3495 (encoding Mig1p-GFP-β-galactosidase) were grown in glucose medium and then diluted into medium containing 5% glycerol for 60 min to cause Mig1p-GFP-β-galactosidase to concentrate in the cytoplasm (glycerol). Cells were then exposed to hypertonic shock while being supplemented (or not) with 2% glucose to initiate nuclear import. After 10 min, aliquots were washed with 2% glucose in water and examined.
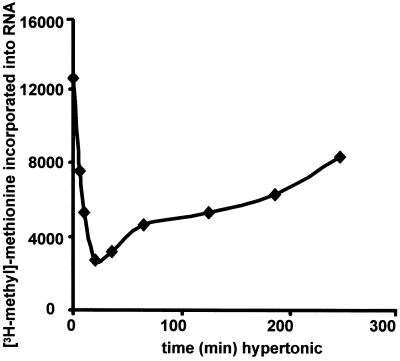
To evaluate nucleolar function during hypertonic shock, we monitored RNA synthesis after exposing cells to 0.5 M NaCl. Incorporation of label into RNA was radically inhibited for >1 h and then gradually returned to normal after several hours (Figure 7). These kinetics of normalization are notably slower than those of protein reimport. This suggests that some yet-unidentified protein returns more slowly, that some component has been degraded and must be resynthesized, or that, on return, the nuclear and nucleolar proteins must “reorganize” before rRNA synthesis resumes.
Figure 7.
RNA synthesis during hypertonic shock. YPH500 cells were exposed to hypertonic shock for 0–4 h. Aliquots were pulse-labeled with [3H-methyl]-methionine, and incorporation into RNA was quantitated.
DISCUSSION
Dramatic transient alterations of nuclear organization occur during hypertonic shock. These changes must accompany standard manipulations of yeast that are widely used, e.g., spheroplasting in the presence of sorbitol. They also raise a set of fundamental questions regarding signaling pathways and the factors that govern the stable organization of the nucleus, which clearly entails the dynamic flux of many macromolecules.
Hypertonic shock quickly causes water efflux across the plasma membrane and corresponding fluxes across intracellular membranes. The resulting reduction of turgor pressure must alter the surface tension of the plasma membrane and therefore affect the relationship of plasma membrane proteins to the cell wall. Hypertonic shock is followed within minutes by a decrease in the permeability of the yeast plasma membrane and by an increase of cytoplasmic solutes (e.g., glycerol), which reduces the initial osmotic insult (Banuett, 1998; Gustin et al., 1998). Hypertonic shock characteristically activates the Hog1p MAP kinase cascade via the plasma membrane proteins Sln1p and Sho1p. This cascade is implicated in many events, including the driving of transcription (e.g., of genes responsible for synthesis of glycerol) and the reorganization of Golgi functions (Reynolds et al., 1998). It is opposed by RAS-cAMP-PKA signaling (Gustin et al., 1998). Strikingly, both Hog1p and one of the factors implicated in STRE-mediated stress responses, Msn2p, quickly concentrate in the nucleus during osmotic shock (Ferrigno et al., 1998; Görner et al., 1998; Reiser et al., 1999).
In contrast, hypotonic shock and heat shock activate the Pkc1p cell integrity MAP kinase cascade, which is implicated in cell wall synthesis, bud morphogenesis, and polarized cell growth. This pathway is also stimulated by mating pheromone and G1 cyclin-Cdc28p. Upstream factors that activate Pkc1p, at least during heat shock, are plasma membrane glycoproteins of the Wsc family and the Rho GTPases (Levin and Bartlett-Heubusch, 1992; Igual et al., 1996; Kamada et al., 1996; Zarzov et al., 1996; Gustin et al., 1998).
Given this background, it is surprising that Pkc1p, not Hog1p, is required for the hypertonic stress-induced relocation of nuclear proteins. Since the kinase activity of Pkc1p is required and since activation of Pkc1p itself can cause relocation, it becomes important to identify the upstream and downstream factors that function in conjunction with Pkc1p. Judging from our observations, the Wsc glycoproteins are not required, but stimulation by diacylglycerol may be involved. In addition, kinases that are part of the Pkc1p MAP kinase cascade (Bck1p, Mpk1p) are not required. We therefore conclude that Pkc1p can stimulate an “alternative signaling pathway.” Consistent with this conclusion, other investigators have shown that deletion of Pkc1p has more severe phenotypic consequences than does deletion of individual downstream kinases (Lee and Levin, 1992). Moreover, Pkc1p, but not the downstream kinases, is required both for heat shock-mediated depolarization of the actin cytoskeleton and for attenuation of ribosome biogenesis during treatment of cells with tunicamycin (Delley and Hall, 1999; Li et al., 2000). We observed that HA-tagged Pkc1p is normally distributed throughout the cytoplasm and the nucleus, but that, like many nuclear proteins, it is excluded from the nucleus during hypertonic shock (our unpublished results). Its immediate targets along the alternative pathway may therefore be outside the nucleus. The normally broad distribution of wild-type Pkc1p may nevertheless be a dynamic average, judging from the evidence on the dynamics of Pkc localization in animal cells (Mochly-Rosen and Kauvar, 2000).
Our observations have also indicated a further complexity in the nuclear response to hypertonic shock: excess Hog1p antagonizes those effects of hypertonicity that are mediated by Pkc1p. It is not clear at what level this cross-talk occurs; however, Hog1p activity may normally play a stabilizing role with regard to the integrity of the nucleus.
Relocation of nuclear proteins has also been reported to occur in other situations. For example, slower Pkc1p-dependent relocation occurs when transport along the secretory pathway is inhibited (Nanduri et al., 1999) (our unpublished observations). Pkc1p itself therefore may target key factors that are needed for stable retention of nuclear proteins. Among other conditions that inhibit ribosome biosynthesis are amino acid starvation and treatment with rapamycin (Warner, 1989; Moehle and Hinnebusch, 1991; Neuman-Silberberg et al., 1995; Powers and Walter, 1999); however, these treatments did not cause relocation of Fpr3p over a 2-h period (our unpublished results).
ACKNOWLEDGMENTS
Plasmids, antibodies and yeast strains were from R. Ballester, J. Broach, J. Carbon, E. Craig, P.-A. Delley, J. Gray, M. Gustin, J. Heinisch, P. Hieter, D. Levin, S. Lindquist, S. O'Rourke, H. Reizman, J. Thorner, J. Woolford, and M. Yaffe. This work was supported by the Tobacco Research Council.
REFERENCES
- Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Signaling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol Mol Biol Rev. 1998;62:249–274. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Bucci M, Wente SR. A novel fluorescence-based genetic strategy identifies mutants of Saccharomyces cerevisiae defective for nuclear pore complex assembly. Mol Biol Cell. 1998;9:2439–2461. doi: 10.1091/mbc.9.9.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Smith KW, Gustin MC. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE, Gustin MC. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J Biol Chem. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Delley P-A, Hall MN. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vit MJ, Waddle JA, Johnston M. Regulation of nuclear transport of the Mig1 glucose repressor. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno P, Posas F, Koepp D, Saito H, Silver PA. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J. 1998;17:5606–5614. doi: 10.1093/emboj/17.19.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach J, Bossie M, Vogel J, Corbett A, Jinks T, Willins DA, Silver PA. A yeast RNA-binding protein shuttles between the nucleus and the cytoplasm. Mol Cell Biol. 1994;14:8399–8407. doi: 10.1128/mcb.14.12.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schüller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JV, Ogas JP, Kamada Y, Stone M, Levin DE, Herskowitz I. A role for the Pkc1-MAP kinase pathway of S. cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 1997;16:4924–4937. doi: 10.1093/emboj/16.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin MC, Alertyn J, Alexander M, Davenport K. MAP kinase pathways in the yeast S. cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igual JC, Johnson AL, Johnston LH. Coordinated regulation of gene expression by the cell cycle transcription factor SWI4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 1996;15:5001–5013. [PMC free article] [PubMed] [Google Scholar]
- Jacoby JJ, Schmitz HP, Heinisch JJ. Mutants affected in the putative diacylglycerol binding site of yeast protein kinase C. FEBS Lett. 1997;417:219–222. doi: 10.1016/s0014-5793(97)01287-8. [DOI] [PubMed] [Google Scholar]
- Jordan E, Shaw P. The nucleolus. Annu Rev Cell Biol. 1995;11:93–122. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Qadota H, Python C, Anraku Y, Ohya Y, Levin DE. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:248–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lee KS, Levin DE. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a S. cerevisiae protein kinase C homolog. Mol Cell Biol. 1992;12:172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle specific osmotic stability defect. J Cell Biol. 1992;161:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Moir RD, Sethy-Coraci IK, Warner JR, Willis IM. Repression of ribosome and tRNA synthesis in secretion defective cells is signaled by a novel branch of the cell integrity pathway. Mol Cell Biol. 2000;20:3843–3851. doi: 10.1128/mcb.20.11.3843-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang S, Tartakoff AM. Heat shock disassembles the nucleolus and inhibits nuclear protein import and poly(A)+RNA export. EMBO J. 1996;15:6750–6757. [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Kauvar LM. Pharmacological regulation of network kinetics by protein kinase C localization. Semin Immunol. 2000;12:44–61. doi: 10.1006/smim.2000.0207. [DOI] [PubMed] [Google Scholar]
- Moehle CM, Hinnebusch AG. Association of RAP1 binding sites with stringent control of ribosomal protein gene transcription in S. cerevisiae. Mol Cell Biol. 1991;11:2723–2735. doi: 10.1128/mcb.11.5.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Mitra S, Andrei C, Liu Y, Yu Y, Hitomi M, Tartakoff AM. An unexpected link between the secretory pathway and the organization of the nucleus. J Biol Chem. 1999;274:33785–33789. doi: 10.1074/jbc.274.47.33785. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Bhattacharya S, Broach JR. Nutrient availability and the RAS/cyclic AMP pathway both induce expression of ribosomal protein genes in S. cerevisiae but by different mechanisms. Mol Cell Biol. 1995;15:3187–3196. doi: 10.1128/mcb.15.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C, Jeno P, Conzelmann A, Riezman H. Determinant for glycophospholipid anchoring of the S. cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991;11:27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke SM, Herskowitz I. The Hog1p MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in S. cerevisiae. Genes Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, Schroeder SC, Bai Y, Weil A, Piston DW. Quantitative imaging of TATA-binding protein in living yeast cells. Yeast. 1998;14:813–825. doi: 10.1002/(SICI)1097-0061(19980630)14:9<813::AID-YEA280>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P. Regulation of ribosome biogenesis by rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V, Ruis H, Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1147–1161. doi: 10.1091/mbc.10.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds TB, Hopkins BD, Lyons MR, Graham TR. The high osmolarity glycerol response (HOG) MAP kinase pathway controls localization of a yeast Golgi glycosyltransferase. J Cell Biol. 1998;143:935–946. doi: 10.1083/jcb.143.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E, Pederson T, Klein P. Comparison of mitotic phenomena and effects induced by hypertonic solutions in HeLa cells. J Cell Biol. 1970;44:400–416. doi: 10.1083/jcb.44.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994;13:4382–4389. doi: 10.1002/j.1460-2075.1994.tb06758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in S. cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton DR, Chen S, Hitomi M, Kumagai C, Tartakoff AM. A yeast protein that bidirectionally affects nucleocytoplasmic transport. J Cell Sci. 1995;108:265–272. doi: 10.1242/jcs.108.1.265. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Yaffe MP. A mutation in the yeast heat shock factor causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol Cell Biol. 1991;11:2647–2655. doi: 10.1128/mcb.11.5.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna J, Lodder A, Lee K, Vagts A, Ballester R. A family of genes required for maintenance of cell wall integrity and for the stress response in S. cerevisiae. Proc Natl Acad Sci USA. 1997;94:13804–13809. doi: 10.1073/pnas.94.25.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989;53:256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Chen CY, Levin DE. Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J Biol Chem. 1994;269:16829–16836. [PubMed] [Google Scholar]
- Wente S, Rout MP, Blobel G. A new family of yeast nuclear pore complex proteins. J Cell Biol. 1992;119:705–723. doi: 10.1083/jcb.119.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov P, Mazzoni C, Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]