Abstract
AMP-activated protein kinase (AMPK) is a highly conserved sensor of cellular energy status found in all eukaryotic cells. AMPK is activated by stimuli that increase the cellular AMP/ATP ratio. Essential to activation of AMPK is its phosphorylation at Thr-172 by an upstream kinase, AMPKK, whose identity in mammalian cells has remained elusive. Here we present biochemical and genetic evidence indicating that the LKB1 serine/threonine kinase, the gene inactivated in the Peutz-Jeghers familial cancer syndrome, is the dominant regulator of AMPK activation in several mammalian cell types. We show that LKB1 directly phosphorylates Thr-172 of AMPKα in vitro and activates its kinase activity. LKB1-deficient murine embryonic fibroblasts show nearly complete loss of Thr-172 phosphorylation and downstream AMPK signaling in response to a variety of stimuli that activate AMPK. Reintroduction of WT, but not kinase-dead, LKB1 into these cells restores AMPK activity. Furthermore, we show that LKB1 plays a biologically significant role in this pathway, because LKB1-deficient cells are hypersensitive to apoptosis induced by energy stress. On the basis of these results, we propose a model to explain the apparent paradox that LKB1 is a tumor suppressor, yet cells lacking LKB1 are resistant to cell transformation by conventional oncogenes and are sensitive to killing in response to agents that elevate AMP. The role of LKB1/AMPK in the survival of a subset of genetically defined tumor cells may provide opportunities for cancer therapeutics.
AMP-activated protein kinase (AMPK) is the primary regulator of the cellular response to lowered ATP levels in eukaryotic cells (1, 2). AMPK is activated by stimuli that include pathological stresses, such as oxidative damage, osmotic shock, hypoxia, and glucose deprivation, as well as physiological stimuli, such as exercise, muscle contraction, and hormones including leptin and adiponectin (1). Accordingly, AMPK phosphorylation of its downstream targets results in the up-regulation of ATP-producing catabolic pathways and the down-regulation of ATP-consuming processes. Recent studies have indicated that AMPK is a critical regulator of leptin-induced fatty acid metabolism and glucose uptake in skeletal muscle (3–5). Indeed, impaired energy metabolism is a primary defect in type 2 diabetes, and two major current diabetic therapeutics have been shown to act via stimulation of AMPK (3).
AMPK exists in cells as a heterotrimeric complex composed of a catalytic kinase subunit (α) and two regulatory subunits (β and γ). Because of the presence of cystathionine β synthase (CBS) domains, which can act as nucleoside-binding motifs in other proteins, as well as naturally occurring activating mutations, the γ subunit has been proposed to mediate direct binding of AMP (1). AMP binding has been proposed to induce a conformational change in the heterotrimeric AMPK that allows it to serve as a better substrate for an upstream activating kinase(s). Phosphorylation of a single invariant threonine residue in the activation loop of the catalytic subunit (Thr-172 in human AMPKα1) has been shown to be required to activate all known AMPK homologues (1). A number of laboratories have reported biochemical purification of a kinase activity, AMPK kinase (AMPKK), that is capable of phosphorylating Thr-172 (6–8). Calcium/calmodulin-dependent protein kinase kinase (CAMKK) has been demonstrated to serve as a surrogate AMPKK in vitro, although some of its biochemical properties suggest it may not be a bona fide AMPKK in vivo (9).
The LKB1 serine/threonine kinase is a divergent yet evolutionarily well conserved kinase that most closely resembles CAMKK in its catalytic domain. LKB1 inactivation is the genetic basis of Peutz-Jeghers syndrome, a familial colorectal polyp disorder in which patients are predisposed to early-onset cancers in other tissues (10). Recently, LKB1 has been shown to be an essential mediator of embryonic polarity in Caenorhabditis elegans and Drosophila (11, 12). STRAD, a recently identified obligate coactivator for LKB1, is the only known physiological substrate of LKB1 (13). Because of the homology of LKB1 to CAMKK, as well as the recently discovered AMPKKs in yeast (14, 15), we set out to determine whether LKB1 is a bona fide AMPKK in vivo and whether it regulates AMPK signaling under physiological circumstances. We present here genetic and biochemical evidence that LKB1 is the major AMPKK in several mammalian cell types in response to changes in AMP/ATP ratios. While an earlier version of this article was in review, articles appeared by Hawley et al. (16) and Woods et al. (17) that also support the idea that LKB1 is an AMPKK in vivo.
We further show here that LKB1 is a critical mediator of the effects of low energy on cell viability. We conclude that LKB1 is essential to protect cells from apoptosis in response to agents that elevate intracellular AMP and as such may act as a low-energy checkpoint in the cell. These results suggest a model to explain the paradox that loss of LKB1 in tumors can result in increased cell growth, yet LKB1-deficient cells are resistant to transformation and readily undergo apoptosis under conditions that elevate AMP.
Materials and Methods
Reagents. HT1080, LLC-PK1, and HeLa cells were all purchased from the American Type Culture Collection (ATCC). Murine embryonic fibroblasts (MEFs) were derived from embryos at postcoitum day 13.5 as previously described (18). LKB1-/- MEFs were produced by in vitro excision of the LKB1 lox allele as previously described (18). Phospho-Thr-172 AMPKα, total AMPKα, and phospho-acetyl CoA carboxylase (ACC) antibodies were from Cell Signaling Technology (Beverly, MA). SAMS peptide was from Upstate Biotechnology (Lake Placid, NY). Maltose-binding protein (MBP) AMPKα (1-312) bacterial fusion protein was prepared and purified as previously described (7). Heterotrimeric AMPK was expressed in and isolated from COS cells (7). LKB1 antibody (1G) was previously described (18). FLAG-tagged human LKB1 was generated by subcloning the human LKB1 cDNA into an N-terminal-tagged pCDNA3 vector. Human and mouse LKB1 retroviral constructs were generated by PCR and subcloning into pBABE-puro. Point mutations were generated by using QuikChange mutagenesis (Stratagene). STRAD was PCR-amplified from a human EST (Research Genetics, Huntsville, AL) and subcloned into pCDNA4/HisMax (Invitrogen). All constructs were fully sequenced to verify their integrity. Sorbitol, H2O2, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma, and 5-aminoimidizole-4-carboxamide riboside (AICAR) was obtained from Toronto Research Chemicals (Downsview, ON, Canada).
Kinase Assays and Cellular Analysis. The kinase activity of AMPK was measured by using the SAMS peptide as previously described (7). LKB1 phosphorylation of AMPK was carried out in kinase buffer (50 mM Tris, pH 7.5/10 mM MgCl2/1 mM DTT/100 μm ATP) for 20 min at 30°C. For immunoprecipitations of active LKB1 kinase, FLAG-tagged LKB1 was cotransfected with an equimolar amount of STRAD expression plasmid into HT1080 cells and immunoprecipitated by using M2-agarose (Sigma) 24 h posttransfection in Nonidet P-40 lysis buffer (19). Before kinase assays, immunoprecipitated LKB1 was washed three times in lysis buffer, then twice in kinase buffer. Soluble MBP–AMPK was added at 5 μg per kinase reaction. The LKB1 peptide library screen was performed as previously described (20). Peptide libraries were fixed at indicated positions and degenerate for all amino acids except cysteine, threonine, and serine at all other positions indicated with an “x,” with at least four degenerate flanking positions on either side of all fixed sequences [e.g., the LxT library is composed of x-x-x-x-Leu-x-Thr-x-x-x peptides with a fixed lysine tail (K-K-K)]. Total cell extracts and immunoblotting were as previously described (21). Amphotropic and ecotropic retroviral infections and subsequent selections were as previously described (22). For the cell survival assays, cells were plated in triplicate for each condition on 48-well plates. MTT assays were performed according to the manufacturer's suggestions (Sigma).
Results
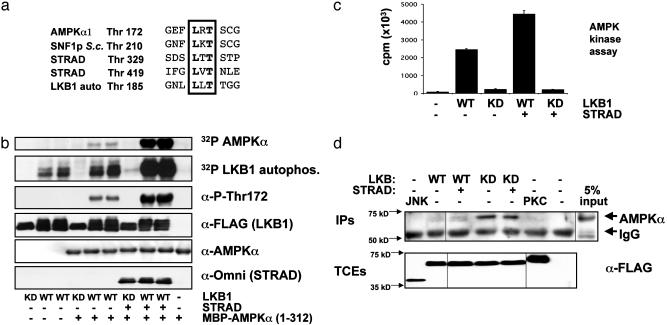
We set out to identify the optimal substrate motif for LKB1 in an attempt to identify other substrates. To examine the substrate specificity of LKB1, we coexpressed it in mammalian cells with its coactivator STRAD and then tested the ability of purified LKB1 immunoprecipitates to phosphorylate various degenerate peptide libraries (20). Interestingly, we found that LKB1 will only phosphorylate libraries with threonine as the phospho-acceptor site (Fig. 7, which is published as supporting information on the PNAS web site). Arginine in the -1 position was selected over random amino acids, and leucine in the -2 position was strongly selected, suggesting that a Leu-Arg-Thr motif would be a highly selected peptide substrate. Thr-172 of AMPKα has a leucine in the -2 position that is conserved in AMPK orthologues from other species, including the yeast SNF1 protein (Fig. 1a), and a well conserved arginine in the -1 position, suggesting it would make an excellent in vitro substrate for LKB1. In addition, the previously mapped LKB1 phosphorylation sites in STRAD conform to the LxT sequence.
Fig. 1.
LKB1 phosphorylates Thr-172 of AMPKα in vitro and activates its kinase activity. (a) Lineup of known LKB1 in vitro phosphorylation sites with sites of phosphorylation in human AMPKα and its yeast homologue SNF1 protein (SNF1p). S.c., Saccharomyces cerevisiae; auto, autophosphorylation. (b) HT1080 cells were transfected with WT or kinase-dead (KD; K78I) LKB1 with or without its coactivating protein, STRAD. As indicated, LKB1 immunoprecipitates were tested for their ability to transphosphorylate bacterial MBP–AMPKα in an in vitro kinase assay. Parallel in vitro kinase assays were performed by using [γ-32P]ATP followed by autoradiography or cold ATP followed by immunoblotting for phospho-Thr-172 AMPK (α-P-Thr172) and the indicated proteins. WT LKB1 immunoprecipitates were run in duplicate as shown. MBP–AMPK was also tested alone, as indicated. Results are typical of three separate experiments. (c) AMPKK assay. LKB1 phosphorylation of MBP–AMPK activates its kinase activity toward a peptide substrate (SAMS). LKB1 immunoprecipitates (as in a) were used to phosphorylate MBP–AMPK in vitro, and then MBP–AMPK was removed and tested for its ability to transphosphorylate the SAMS peptide in the presence of [γ-32P]ATP. Results were obtained from two separate experiments in triplicate. LKB1 alone was incapable of detectably phosphorylating the SAMS peptide, and equivalent levels of LKB1 and MBP–AMPK were used in each reaction (data not shown). Samples without LKB1, without SAMS peptide, or without MBP–AMPK all gave similar levels of background (data not shown). KD, kinase-dead. (d) Coimmunoprecipitation of endogenous AMPKα with LKB1. HT1080 cells were transfected with FLAG-tagged vectors encoding WT or kinase-dead (KD) LKB1 (with or without STRAD), or FLAG-JNK or FLAG-PKC ζ. FLAG immunoprecipitates (IPs) were immunoblotted with anti-AMPKα pan antisera (Upstate Biotechnology); 5% of the total input is shown at right. A significant amount of endogenous AMPK coimmunoprecipitates with kinase-dead LKB1. TCEs, total cell extracts.
Given these observations, we investigated whether LKB1 would phosphorylate Thr-172 of AMPK in vitro. As seen in Fig. 1b, WT, but not kinase-dead, LKB1 immunoprecipitated from mammalian cells efficiently phosphorylated a bacterially expressed MBP fusion product of the AMPKα catalytic subunit in vitro. Moreover, coexpression of LKB1 with STRAD led to a dramatic proportional increase in LKB1 autophosphorylation and transphosphorylation of MBP–AMPKα. Immunoblotting with phosphospecific AMPK Thr-172 antisera confirmed that LKB1 phosphorylated this site in vitro. As seen in Fig. 1b, the level of immunoblotting with anti-phospho-Thr-172 antibody was directly proportional to the amount of 32P incorporation into recombinant AMPK in a parallel radioactive in vitro kinase assay.
To examine whether in vitro phosphorylation of AMPK by LKB1 was sufficient to activate the bacterial MBP–AMPK fusion protein, we assayed the kinase activity of AMPKα by using a specific peptide substrate, SAMS (14). As previously reported (7), bacterial MBP–AMPK is inactive toward the peptide (Fig. 1c). In vitro phosphorylation of AMPK by WT LKB1 alone or STRAD-activated LKB1 induced the kinase activity of AMPK by an average of 27- and 50-fold, respectively. Kinase-dead LKB1 alone or coexpressed with STRAD was unable to activate AMPK.
Next we addressed whether agonists that activate the kinase activity of AMPK also serve to stimulate the kinase activity of LKB1. The MBP–AMPK fusion we used as a substrate is not stimulated by AMP in vitro (unlike the intact AMPK heterotrimer); therefore, any stimulated phosphorylation of this fusion protein by LKB1 only reflects the kinase activity of LKB1. We confirmed that AMP stimulates the kinase activity of heterotrimeric AMPK in vitro (Fig. 8b, which is published as supporting information on the PNAS web site). In contrast, LKB1 kinase activity was not directly stimulated in vitro by AMP (Fig. 8a). We then examined whether LKB1 kinase activity would be increased in cells treated with the AMPK agonists H2O2 or AICAR. Peroxide serves to activate AMPK by increasing intracellular AMP/ATP ratios. AICAR is a cell-permeable drug that is converted to AICAR monophosphate (ZMP) intracellularly and mimics the effect of AMP on AMPK signaling (1). The kinase activity of LKB1 immunoprecipitated from cells treated with peroxide or AICAR was not increased compared with that from untreated control cells, as measured both by autophosphorylation and transphosphorylation of the MBP–AMPK fusion (Fig. 8a). Both of these treatments led to activation of AMPK in vivo (see below). These results support a previously suggested model in which allosteric regulation of the AMPK heterotrimer by AMP binding allows it to better serve as substrate for the upstream kinase (2), as opposed to direct regulation of the activity of the AMPKK by AMP.
As a first means to address whether LKB1 is a bona fide AMPKK in vivo, we examined whether LKB1 and AMPK associated in cells. We transfected FLAG-tagged WT and kinase-dead LKB1 or control kinases into HT1080 cells and then immunoprecipitated with anti-FLAG antisera and immunoblotted for endogenous AMPKα. As seen in Fig. 1d, a significant amount of endogenous AMPKα specifically coimmunoprecipitated with kinase-dead LKB1, suggesting that it may act to trap substrates.
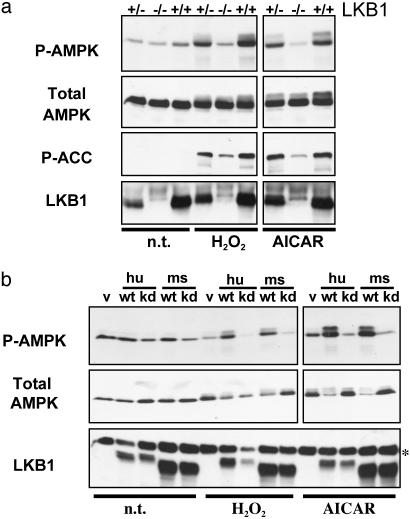
To rigorously test the requirement for LKB1 in AMPK activation in vivo, we derived LKB1-deficient MEFs from conditionally inactivated mouse embryos as previously described (18). Cells from littermate-matched embryos then were stimulated with peroxide or AICAR, and the response of AMPK was examined. As seen in Fig. 2a, LKB1-null MEFs, but not WT or heterozygous controls, showed a complete loss of stimulated Thr-172 phosphorylation in response to peroxide and AICAR. To determine whether AMPK activity was accordingly down-regulated in these cells, we examined the in vivo phosphorylation of one of its critical downstream substrates, ACC. AMPK inactivates ACC through phosphorylation of Ser-79, thereby stimulating fatty acid oxidation (1). Mirroring the level of phospho-AMPK, phosphorylation of ACC in response to both stimuli was nearly abolished in LKB1-null cells. There was still a small, but reproducible, amount of ACC phosphorylation in response to these stimuli in LKB1-null MEFs, suggesting the existence of other minor compensating AMPKKs in these cells or the existence of other ACC kinases also activated by these stimuli. However, the dramatic reduction in phospho-AMPK and phospho-ACC indicates that LKB1 is the dominant AMPKK activity in these cells in response to the stimuli tested. To demonstrate that LKB1 loss itself, and not a secondary defect arising in these cells, is responsible for impaired AMPK activation, we reintroduced WT and kinase-dead LKB1 alleles into an immortalized LKB1-null MEF line by retrovirus. Indeed, WT, but not kinase-dead, LKB1 expression reconstitutes AMPK activation (Fig. 2b) and downstream phosphorylation of its targets (data not shown). Interestingly, despite the absence of LKB1 in these cells, kinase-dead LKB1 reduced the basal and stimulated phosphorylation of AMPK and ACC to levels below the vector-infected control LKB1-null cells, suggesting that kinase-dead LKB1 might block endogenous AMPK from being available as a substrate for other compensatory AMPKKs.
Fig. 2.
LKB1-deficient MEFs are defective in AMPK activation. (a) Littermate MEFs of the indicated LKB1 genotypes were left untreated (n.t.) or were treated with 0.1 mM H2O2 for 20 min or 2 mM AICAR for 2 h. Total cell extracts were immunoblotted for phospho-Thr-172 AMPK (P-AMPK) or phospho-Ser-79 ACC (P-ACC), as well as for total AMPK and LKB1. (b) An immortalized LKB1-deficient MEF cell line was reconstituted with human (hu) or mouse (ms) WT (wt) or kinase-dead (kd) LKB1-expressing retroviruses. v, Vector control cells; hu wt, human FLAG-tagged WT LKB1-expressing cells; hu kd, human FLAG-tagged kinase-dead LKB1-expressing cells; ms wt, untagged mouse WT LKB1-expressing cells; ms kd, untagged mouse kinase-dead LKB1-expressing cells. Cells were treated as in a. The asterisk indicates a background band that serves as a loading control.
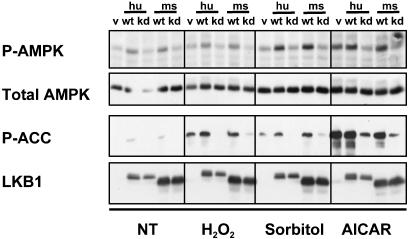
Given the requirement for LKB1 in AMPK activation in MEFs, we examined the ability of LKB1 to modulate AMPK activation in other cell types. As above, we used retroviruses to introduce WT or kinase-dead human and mouse LKB1 into a number of cell types. As seen in Fig. 3, in HT1080 human fibrosarcoma cells, kinase-dead LKB1 specifically inhibited AICAR, peroxide, and osmotic shock-induced Thr-172 phosphorylation to levels below those seen in the vector-infected cells. Additionally, expression of WT LKB1 increased the basal and stimulated level of Thr-172 phosphorylation (Fig. 3). As in the MEFs, phosphorylation of Ser-79 of ACC is also increased basally and in response to all stimuli by WT LKB1 overexpression, further indicating that AMPK activity is regulated by LKB1 in vivo. Similarly, expression of kinase-dead LKB1 nearly abolishes the AMPK-induced phosphorylation of ACC in response to all three stimuli in HT1080 cells. Similar results were found in LLC-PK1 and IEC18 epithelial cells, as well as in HeLa cells, which are deficient in LKB1 protein because of promoter methylation (24).
Fig. 3.
LKB1 regulates activation of AMPK in response to the AMP analogue AICAR as well as to oxidative or osmotic stress in HT1080 cells. HT1080 cells stably expressing WT or kinase-dead (kd) LKB1 as in Fig. 2 were treated with 0.1 mM H2O2 for 20 min, 0.6 M sorbitol for 30 min, or 2 mM AICAR for 2 h. Total cell extracts were analyzed as in Fig. 2. v, Vector control cells; hu, human; ms, mouse; P-AMPK, phospho-Thr-172 AMPK; P-ACC, phospho-Ser-79 ACC.
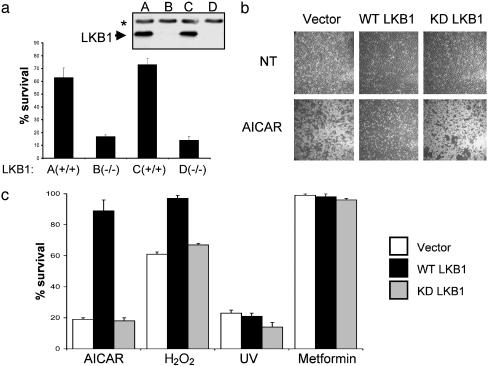
To determine whether LKB1 could mediate biological effects in response to low energy, we investigated whether LKB1 might modulate cell death under circumstances in which AMPK would be activated. AMPK activation has been shown to lead to an inhibition of apoptosis in a number of cell types. Treatment of quiescent cells with AICAR protects them from glucocorticoid-induced apoptosis, and AICAR also protects astrocytes and endothelial cells from cell death in response to different stimuli (25–28). Furthermore, reduction of AMPK levels was recently shown to reduce cellular viability after glucose deprivation in a number of human tumor cell lines (28). We therefore compared the response of LKB1-deficient cells with that of LKB1-expressing cells as above in their cellular response to apoptotic stimuli, including stimuli known to activate AMPK. To that end, we used WT and LKB1-deficient MEFs as well as HeLa cells that were reconstituted with WT or kinase-dead LKB1 (Fig. 9, which is published as supporting information on the PNAS web site). Two independent lines of LKB1-deficient MEFs, but not their WT littermate controls, underwent rapid apoptosis (<12 h) when treated with AICAR (Fig. 4a). AICAR treatment did induce some cell death in the WT MEFs, but this death was delayed until 36 h posttreatment (data not shown). Similar to MEFs, vector-infected or kinase-dead LKB1-expressing HeLa cells died rapidly after AICAR treatment, unlike their counter-parts reconstituted with WT LKB1 (Fig. 4 b and c). In contrast, UV treatment killed all cell lines regardless of LKB1 status, to a similar extent (Fig. 4c) and with similar kinetics (data not shown).
Fig. 4.
LKB1 protects cells from apoptosis induced by agents that elevate intracellular AMP. (a) Two independent littermate-matched WT and LKB1-deficient primary MEF cell lines (plated in triplicate) were treated with 2 mM AICAR for 8 h. Cell viability was quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and expressed as a percentage of untreated controls. Results represent three independent experiments. (Inset) Immunoblot demonstrating LKB1-deletion. The arrowhead indicates LKB1; the asterisk denotes the background band. (b) Phase-contrast images of HeLa cells stably expressing vector, WT LKB1, or kinase-dead (KD) LKB1 5 h after treatment with 2.5 mM AICAR. Results represent four independent experiments. (c) LKB1 expression protects HeLa cells from AICAR and peroxide, but not UV-induced cell death. Cell viability is expressed as a percentage of untreated controls and quantified by MTT assays run in triplicate on indicated HeLa stable cell lines treated with 2.5 mM AICAR, 100 μM H2O2, 50 J/cm2 UV, or 10 mM metformin for 12 h. HeLa cells were stably infected with vector, WT LKB1-, or kinase-dead (KD) LKB1-expressing retroviruses as indicated.
To address whether LKB1-deficient cells might also be sensitive to cell death induced by other AMPK agonists, we treated MEFs and HeLa cells with peroxide or the mitochondrial uncoupler oligomycin. Similar to treatment with AICAR, treatment with these agents selectively induced apoptosis in LKB1-deficient MEFs (data not shown) and HeLa cells (Fig. 4c), although not as efficiently as AICAR. Finally, we were interested in whether other AMPK agonists that do not alter AMP/ATP ratios would also selectively induce apoptosis in the LKB1-deficient cells. Metformin is a widely used treatment for type 2 diabetes that has been demonstrated to activate AMPK without altering intracellular AMP or ATP. We found that, in MEFs, metformin activated AMPK and that this response was ablated in the LKB1-deficient cells, as seen for peroxide and AICAR treatment (data not shown). Strikingly, metformin treatment did not result in any cell death in the LKB1-deficient HeLa cells (Fig. 4c). This result suggests that elevation of AMP is the critical event that drives apoptosis in the LKB1-deficient cells.
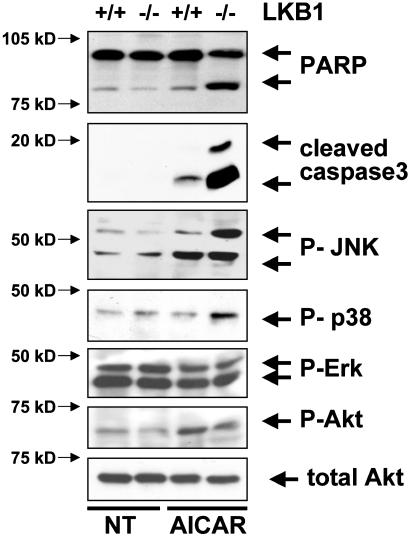
To begin to examine the mechanism by which death occurs in the LKB1-deficient cells, we examined the activation of various signaling pathways in WT or LKB1-deficient MEFs after AICAR treatment. Caspase-3 was selectively activated in LKB1-deficient cells after AICAR treatment (Fig. 5), as visualized by immunoblotting for the presence of activation-specific caspase-3 and cleavage of the caspase-3 substrate poly(ADP-ribose) polymerase (PARP). In addition, c-Jun NH2-terminal kinase (JNK) and p38, but not Erk or Akt, were selectively activated in the LKB1-null cells. Akt signaling was not suppressed in response to AICAR. In fact, a slight but consistent increase in phospho-Akt was observed in both genotypes after AICAR treatment, suggesting that down-regulation of this major survival signaling pathway is not involved in the cell death observed.
Fig. 5.
Caspase-3, JNK, and p38 signaling are hyperactivated in LKB1-null MEFs in response to AICAR. Immunoblot analysis on LKB1 WT or -null MEFs without treatment (NT) or 5 h after treatment with 2 mM AICAR. Cleaved (activated) caspase-3, poly(ADP-ribose) polymerase (PARP), phospho-JNK (P-JNK), phospho-p38 (P-p38), phospho-AKT (P-AKT; Ser-473), and total Akt were detected by immunoblotting (Cell Signaling Technology).
Discussion
Taken together, the results presented here suggest that LKB1 is a bona fide AMPKK and is the major AMPKK present in a number of cell types. Moreover, we show that LKB1 protects cells from apoptosis induced by elevated AMP levels. These findings provide genetic and biochemical evidence that LKB1 is a critical regulator of AMPK in vivo. As such, LKB1 may play an unexpected role in multiple organ systems that mediate the diverse effects of AMPK on mammalian physiology. Importantly, AMPK has been shown to be a critical mediator of glucose uptake in skeletal muscle in mice (5), and the kinase activity of AMPK is stimulated by two major diabetes therapeutics (29, 30). Therefore, identification of LKB1 as a major activator of AMPK in vivo may introduce a set of potential avenues to explore in the effort to boost AMPK activity for the treatment of diabetes. It will be critical to define the specific tissues in which LKB1 serves as the principal AMPKK and to determine which AMPK-activating stimuli use LKB1 as opposed to other AMPKKs. The presence of three functionally redundant AMPKKs in yeast (14, 15), along with the residual AMPK phosphorylation seen in LKB1-deficient cells, suggests that there will be additional mammalian AMPKKs.
AMPK is the first identified substrate of the LKB1 tumor suppressor that may mediate its downstream biological effects. Interestingly, we have found that multiple disparate types of LKB1-deficient cells are sensitized to death by the AMP analogue AICAR. These data suggest that LKB1/AMPK signaling plays a role in protection from apoptosis, specifically in response to agents that increase the cellular AMP/ATP ratio. We hypothesize that active AMPK signaling offers a protective effect by allowing the cell time to attempt to reverse the aberrantly high ratio of AMP/ATP. If unable to reverse this ratio, the cell eventually will undergo cell death. However, in the absence of active AMPK signaling, the onset of this death is rapid, as is observed in the LKB1-deficient MEFs and HeLa cells. These results offer the provocative suggestion of a potential therapeutic window in which LKB1-deficient tumor cells might be acutely sensitive to AMP analogues or sensitized to cell death by other stimuli when treated in combination with agents that increase the AMP/ATP ratio. Interestingly, the diabetic treatment rosiglitazone is known to stimulate AMPK signaling through alterations in the intracellular AMP/ATP ratio (30), suggesting that it may be useful in the treatment of LKB1-deficient tumors as well.
The observation that altered AMP/ATP ratios result in cell death in the absence of AMPK signaling indicates that other cellular proteins that are regulated by AMP may contribute to the cell death observed. One such enzyme is PFK-1, which catalyzes the rate-limiting conversion of fructose 6-phosphate to fructose 1,6 bisphosphate in glycolysis. Perhaps by uncoupling steps of glycolysis from the ability to reverse the high AMP/ATP ratio, loss of AMPK may bypass the pentose phosphate shunt, altering the total reduction power of the cell by lowering NADPH levels, and predisposing cells to apoptosis by oxidative stress (31). In addition, AMPK signaling has been suggested to regulate mitochondrial biogenesis through stimulation of the peroxisome-activated receptor γ coactivator-1 (PGC-1), which could mediate some of the protective effects observed (32). Decreased intracellular glucose due to defective Glut1 recruitment in the absence of AMPK signaling is another possible contributor to the death observed (33). Further studies will be needed to delineate the critical mediators of the protective effect of AMPK on cell death. It is worth noting that several connections between glucose metabolism and apoptosis have been examined recently (34–36).
The results presented here lead to the paradox that LKB1 acts as a tumor suppressor in vivo, yet cells lacking LKB1 are sensitized to killing by agents that elevate AMP and also cannot be transformed by Ras (18). A possible explanation for the role of LKB1 in tumor suppression is suggested by emerging evidence that AMPK activation negatively regulates the mTOR pathway. AICAR treatment of cells was recently shown to inhibit the mTOR pathway (37), and we have found that this effect of AICAR does not occur in LKB1-deficient MEFs (R.J.S., unpublished data). These results are consistent with AMPK down-regulating major ATP-consuming processes, such as protein synthesis. The biochemical basis for the effects of AMPK in down-regulating mTOR signaling was recently reported to be through direct phosphorylation of the TSC2 tumor suppressor by AMPK (38). TSC2 is inactivated in tuberous sclerosis, another cancer syndrome characterized by a predisposition to hamartomatous polyps. Taken together, these results offer a potential common biochemical explanation for the clinical similarities between Peutz-Jeghers syndrome and other hamartomatous syndromes such as Cowden's disease and tuberous sclerosis. Loss of the tumor suppressors mutated in all three of these disorders (PTEN, TSC1, TSC2, and LKB1) results in aberrant up-regulation of mTOR signaling under conditions of cellular stress (e.g., growth factor withdrawal, nutrient deprivation, low energy, etc.) wherein mTOR signaling is normally inhibited. The resulting unregulated protein synthesis and cell growth may result in the formation of the benign polyps that characterize these three disorders. Interestingly, the same study (38) demonstrated that TSC2 is essential for protection from apoptosis induced by glucose deprivation, analogous to the role of LKB1 in low-energy apoptosis. Future studies should aim at determining whether loss of TSC2 phosphorylation by AMPK and subsequently deregulated mTOR signaling is the critical downstream effector of LKB1 in apoptosis as well as tumorigenesis.
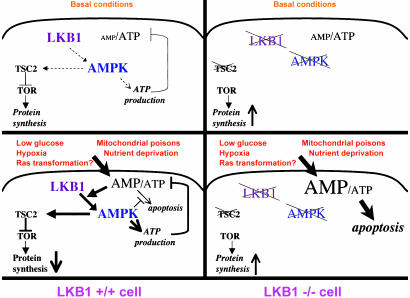
Altogether, these data suggest a model for LKB1 as a low-energy-checkpoint tumor suppressor (Fig. 6). In WT cells, LKB1 serves as a sensor to induce AMPK signaling, which keeps ATP-consuming processes, including macromolecular synthesis and cell division, from occurring under conditions of low cellular energy. LKB1-dependent phosphorylation of AMPK triggers the cell to reverse its lowered energy state by inhibiting anabolic pathways and stimulating catabolic processes. In the absence of the LKB1/AMPK sensor, such as is found in Peutz-Jeghers syndrome patients, growth and proliferation pathways (e.g., mTOR) may be aberrantly elevated under basal conditions, thereby increasing the tumorigenic potential of certain cell types. When these cells are challenged with agonists that further increase the AMP/ATP ratio, our results predict that LKB1-deficient cells will selectively undergo cell death. These results may in fact explain the perplexing observation that LKB1-deficient MEFs are resistant to oncogenic transformation (unlike most tumor suppressor-lacking MEFs, which are sensitized to transformation). Cellular transformation may create a higher energy demand on the cell, triggering activation of AMPK to fulfill the cell's energy needs. In the absence of AMPK signaling to stimulate ATP production, transformation may be impossible, which perhaps simply drives LKB1-deficient cells into apoptosis.
Fig. 6.
Model for LKB1 as a sensor of low energy and negative regulator of tumorigenesis and apoptosis. Under basal conditions, LKB1 serves as a sensor of low energy, keeping ATP-consuming processes including protein synthesis in check via AMPK phosphorylation of TSC2. In response to stresses such as low glucose, hypoxia, nutrient deprivation, or mitochondrial poisons, LKB1 phosphorylates AMPK, which shuts off ATP-consuming processes and up-regulates ATP production to offset the elevated AMP/ATP ratio. This activity prevents the cells from going into apoptosis in response to elevated AMP. In LKB1-deficient cells, under some basal conditions, there may be increases in TOR signaling due to the lack of TSC2 phosphorylation by AMPK, resulting in increased growth or tumorigenic potential. In response to further increases in intracellular AMP, these cells have no mechanism to offset the elevated AMP and go straight into apoptosis.
LKB1/AMPK signaling also may play a role in other cellular responses to environmental stress. LKB1-deficient MEFs are resistant to passage-induced senescence (18). Recently, AMPK activity was found to increase in cells undergoing senescence (39), and artificial hyperactivation of AMPK promoted senescence in primary human fibroblasts, suggesting that perhaps a loss of AMPK signaling promotes the immortalization of LKB1-deficient MEFs. Finally, defining the potential role of AMPK in tumorigenesis or as a potential regulator of cellular transformation or senescence will provide many further insights into the fundamental ties among energy metabolism, apoptosis, and aberrant cell growth and suggest chemotherapeutic intervention tailored to genetic defects that affect this pathway.
Supplementary Material
Acknowledgments
We thank S. Soltoff and C. Carpenter for reagents and advice, and K. Cichowski, B. Turk, and B. Manning for comments on the manuscript. R.J.S. is supported by a National Institutes of Health postdoctoral fellowship. N.B. is supported by a fellowship from the Lustgarten Foundation for Pancreatic Cancer Research. R.A.D. is an American Cancer Society Professor and recipient of the Steven and Michele Kirsch Foundation Investigation Award. This work was supported by National Institutes of Health Grants R01 GM56203, P01 CA89021, and DK35712 (to L.C.C., R.A.D., and L.A.W.).
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 1, 2001.
Abbreviations: AMPK, AMP-activated protein kinase; AMPKK, AMPK kinase; MEF, murine embryonic fibroblast; ACC, acetyl CoA carboxylase; MBP, maltose-binding protein; AICAR, 5-aminoimidizole-4-carboxamide riboside.
See accompanying Biography on page 3327.
References
- 1.Hardie, D. G., Scott, J. W., Pan, D. A. & Huson, E. R. (2003) FEBS Lett. 546, 1113-1120. [DOI] [PubMed] [Google Scholar]
- 2.Kemp, B. E., Stapleton, D., Campbell, D. J., Chen, Z. P., Murthy, S., Walter, M., Gupta, A., Adams, J. J., Katsis, F., van Denderen, B., et al. (2003) Biochem. Soc. Trans. 31, 162-168. [DOI] [PubMed] [Google Scholar]
- 3.Rutter, G. A., DaSilvaXavier, G. & Leclerc, I. (2003) Biochem. J. 375, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339-343. [DOI] [PubMed] [Google Scholar]
- 5.Mu, J., Brozinick, J. T., Jr., Valladares, O., Bucan, M. & Birnbaum, M. J. (2001) Mol. Cell 7, 1085-1094. [DOI] [PubMed] [Google Scholar]
- 6.Hawley, S. A., Davison, M., Woods, A., Davies, S. P., Beri, R. K., Carling, D. & Hardie, D. G. (1996) J. Biol. Chem. 271, 27879-27887. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton, S. R., O'Donnell, J. B., Jr., Hammet, A., Stapleton, D., Habinowski, S. A., Means, A. R., Kemp, B. E. & Witters, L. A. (2002) Biochem. Biophys. Res. Commun. 293, 892-898. [DOI] [PubMed] [Google Scholar]
- 8.Woods, A., Vertommen, D., Neumann, D., Turk, R., Bayliss, J., Schlattner, U., Wallimann, T., Carling, D. & Rider, M. H. (2003) J. Biol. Chem. 278, 28434-28442. [DOI] [PubMed] [Google Scholar]
- 9.Hawley, S. A., Selbert, M. A., Goldstein, E. G., Edelman, A. M., Carling, D. & Hardie, D. G. (1995) J. Biol. Chem. 270, 27186-27191. [DOI] [PubMed] [Google Scholar]
- 10.Boudeau, J., Sapkota, G. & Alessi, D. R. (2003) FEBS Lett. 546, 159-165. [DOI] [PubMed] [Google Scholar]
- 11.Martin, S. G. & St. Johnston, D. (2003) Nature 421, 379-384. [DOI] [PubMed] [Google Scholar]
- 12.Watts, J. L., Morton, D. G., Bestman, J. & Kemphues, K. J. (2000) Development (Cambridge, U.K.) 127, 1467-1475. [DOI] [PubMed] [Google Scholar]
- 13.Baas, A. F., Boudeau, J., Sapkota, G. P., Smit, L., Medema, R., Morrice, N. A., Alessi, D. R. & Clevers, H. C. (2003) EMBO J. 22, 3062-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong, S. P., Leiper, F. C., Woods, A., Carling, D. & Carlson, M. (2003) Proc. Natl. Acad. Sci. USA 100, 8839-8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland, C. M., Hawley, S. A., McCartney, R. R., Leech, A., Stark, M. J., Schmidt, M. C. & Hardie, D. G. (2003) Curr. Biol. 13, 1299-1305. [DOI] [PubMed] [Google Scholar]
- 16.Hawley, S. A., Boudeau, J., Reid, J. L., Mustard, K. J., Udd, L., Makela, T. P., Alessi, D. R. & Hardie, D. G. (2003) J. Biol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods, A., Johnstone, S. R., Dickerson, K., Leiper, F. C., Fryer, L. G., Neumann, D., Schlattner, U., Wallimann, T., Carlson, M. & Carling, D. (2003) Curr. Biol. 13, 2004-2008. [DOI] [PubMed] [Google Scholar]
- 18.Bardeesy, N., Sinha, M., Hezel, A. F., Signoretti, S., Hathaway, N. A., Sharpless, N. E., Loda, M., Carrasco, D. R. & DePinho, R. A. (2002) Nature 419, 162-167. [DOI] [PubMed] [Google Scholar]
- 19.Shanahan, F., Seghezzi, W., Parry, D., Mahony, D. & Lees, E. (1999) Mol. Cell. Biol. 19, 1460-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Songyang, Z. & Cantley, L. C. (1998) Methods Mol. Biol. 87, 87-98. [DOI] [PubMed] [Google Scholar]
- 21.Shaw, R. J., Paez, J. G., Curto, M., Yaktine, A., Pruitt, W. M., Saotome, I., O'Bryan, J. P., Gupta, V., Ratner, N., Der, C. J., et al. (2001) Dev. Cell 1, 63-72. [DOI] [PubMed] [Google Scholar]
- 22.Zhao, J. J., Gjoerup, O. V., Subramanian, R. R., Cheng, Y., Chen, W., Roberts, T. M. & Hahn, W. C. (2003) Cancer Cell 3, 483-495. [DOI] [PubMed] [Google Scholar]
- 23.Davies, S. P., Carling, D. & Hardie, D. G. (1989) Eur. J. Biochem. 186, 123-128. [DOI] [PubMed] [Google Scholar]
- 24.Tiainen, M., Ylikorkala, A. & Makela, T. P. (1999) Proc. Natl. Acad. Sci. USA 96, 9248-9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanelli, C., Stanic, I., Bonavita, F., Flamigni, F., Pignatti, C., Guarnieri, C. & Caldarera, C. M. (1998) Biochem. Biophys. Res. Commun. 243, 821-826. [DOI] [PubMed] [Google Scholar]
- 26.Ido, Y., Carling, D. & Ruderman, N. (2002) Diabetes 51, 159-167. [DOI] [PubMed] [Google Scholar]
- 27.Culmsee, C., Monnig, J., Kemp, B. E. & Mattson, M. P. (2001) J. Mol. Neurosci. 17, 45-58. [DOI] [PubMed] [Google Scholar]
- 28.Kato, K., Ogura, T., Kishimoto, A., Minegishi, Y., Nakajima, N., Miyazaki, M. & Esumi, H. (2001) Oncogene 21, 6082-6090. [DOI] [PubMed] [Google Scholar]
- 29.Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., et al. (2001) J. Clin. Invest. 108, 1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fryer, L. G., Parbu-Patel, A. & Carling, D. (2002) J. Biol. Chem. 277, 25226-25232. [DOI] [PubMed] [Google Scholar]
- 31.Tian, W. N., Braunstein, L. D., Apse, K., Pang, J., Rose, M., Tian, X. & Stanton, R. C. (1999) Am. J. Physiol. 276, C1121-C1131. [DOI] [PubMed] [Google Scholar]
- 32.Zong, H., Ren, J. M., Young, L. H., Pypaert, M., Mu, J., Birnbaum, M. J. & Shulman, G. I. (2002) Proc. Natl. Acad. Sci. USA 99, 15983-15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fryer, L. G., Foufelle, F., Barnes, K., Baldwin, S. A., Woods, A. & Carling, D. (2002) Biochem. J. 363, 167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danial, N. N., Gramm, C. F., Scorrano, L., Zhang, C. Y., Krauss, S., Ranger, A. M., Datta, S. R., Greenberg, M. E., Licklider, L. J., Lowell, B. B., et al. (2003) Nature 424, 952-956. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden, M. G., Plas, D. R., Rathmell, J. C., Fox, C. J., Harris, M. H. & Thompson, C. B. (2001) Mol. Cell. Biol. 21, 5899-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathmell, J. C., Fox, C. J., Plas, D. R., Hammerman, P. S., Cinalli, R. M. & Thompson, C. B. (2003) Mol. Cell. Biol. 23, 7315-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura, N., Tokunaga, C., Dalal, S., Richardson, C., Yoshino, K., Hara, K., Kemp, B. E., Witters, L. A., Mimura, O. & Yonezawa, K. (2003) Genes Cells 9, 65-79. [DOI] [PubMed] [Google Scholar]
- 38.Inoki, K., Zhu, T. & Guan, K. L. (2003) Cell 115, 577-590. [DOI] [PubMed] [Google Scholar]
- 39.Wang, W., Yang, X., Lopez de Silanes, I., Carling, D. & Gorospe, M. (2003) J. Biol. Chem. 278, 27016-27023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.