Organoboronates are convenient and versatile reagents owing to their comparatively low toxicity, high functional group compatibility, and good stability.1 Moreover, these compounds can easily be oxidized to alcohols2 or used to construct new C–C bonds by Suzuki–Miyaura cross-couplings.3 Because of the broad applications of these boronates, many borylation methods have been developed.4–7 Amongst the routes reported for C–B bond formation the most common are Miyaura borylation,4 hydroboration,5 and the reaction of lithium or magnesium organometallic compounds with borate esters.1 In addition, recent developments in transition-metal-catalyzed C–H borylation reactions have also provided efficient access to boronates.6
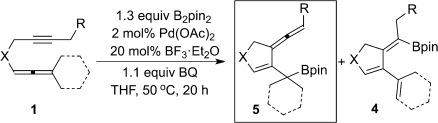
Furthermore, by combining the borylation with a C–C bond-forming cyclization, complex molecules suitable for various further functionalizations could be obtained in one step.8–11, 13b,d Such borylating carbocyclizations have been successfully developed by the group of Cárdenas.9–11 Starting from unsaturated compounds, such as enynes,9 enediynes,10 enallenes,11 and allenynes,11 homoallylic or allylic boronates were prepared under palladium(0) catalysis. For instance, the non-oxidative borylating carbocyclization of allenynes 1 in the presence of bis(pinacolato)diboron (B2pin2) yielded two isomers (2 and 3), where borylation occurred at the allene (Scheme 1 a).11
Scheme 1.
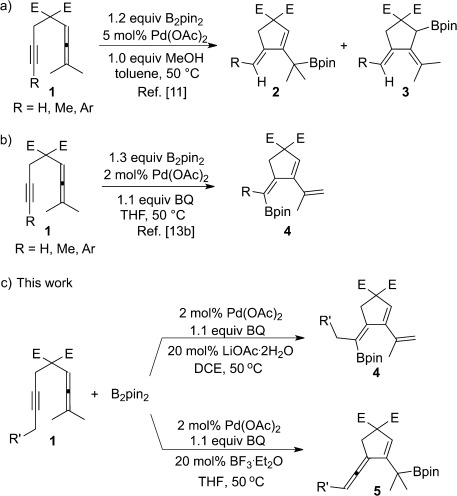
Palladium-catalyzed borylating carbocyclizations of allenynes: a) under non-oxidative conditions;11 b) under oxidative conditions;13b c) under selective oxidative conditions. E=CO2Me.
In ongoing investigations our research group has been studying oxidative PdII-catalyzed carbocyclizations of various unsaturated molecules.12–15 Recently we accomplished the carbocyclization/arylation of allenynes with arylboronic acids.13b Also, some preliminary results regarding carbocyclization/borylation were obtained with differently substituted 1,5-allenynes (1; R=H, Me, Ar), which only gave borylated triene products 4 (Scheme 1 b).13b However, under carbocyclization/arylation conditions alkyl-substituted allenynes afforded two different constitutional isomers (arylated trienes and arylated vinylallenes) in a ratio determined by the substitution on the starting allenyne.13b The aim of the present study was to develop a carbocyclization/borylation that can be directed towards either a borylated triene or a borylated vinyllallene by control of the reaction conditions (Scheme 1 c). We now report a highly selective oxidative carbocyclization/borylation of allenynes 1 with B2pin2 under PdII catalysis with p-benzoquinone (BQ) as the oxidant. The use of LiOAc⋅2 H2O in 1,2-dichloroethane (DCE) or BF3⋅Et2O in THF addressed the issue of selectivity, to give either borylated trienes 4 or borylated vinylallenes 5, respectively.
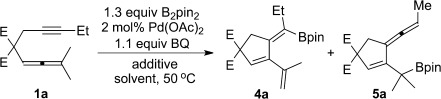
We first studied the reaction of ethyl-substituted allenyne 1 a with B2pin2 under the original carbocyclization/borylation conditions (Scheme 1 b).13b The use of a catalytic amount of palladium acetate (2 mol %) and stoichiometric amounts of BQ (1.1 equiv) in THF at 50 °C led to an isomeric mixture of borylated triene 4 a and borylated vinylallene 5 a in 28 % and 14 % yield, respectively (Table 1, entry 1). Analyzing the effect of different solvents showed that a higher selectivity for 4 a was obtained when DCE was used as the solvent (in Table 1, entry 4 vs. entries 1–3). Furthermore, upon the addition of catalytic amounts (20 mol %) of a basic salt, such as Na2CO3, NaOAc, or LiOAc⋅2 H2O, formation of triene 4 a was favored (Table 1, entries 5–7). Boronate 4 a was obtained in high selectivity in 73 % yield with LiOAc⋅2 H2O as the base additive and with DCE as the solvent (Table 1, entry 7; defined as Method A). An increase of the amount of LiOAc⋅2 H2O to 50 mol % gave no additional improvement in selectivity or yield (Table 1, entry 8).
Table 1.
Solvent and additive effect in the selective formation of triene 4 a or vinylallene 5 a.[a]
 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Additive (20 mol %) | Time [h] | Yield of4 a/5 a [%][b] | 4 a/5 a |
| 1 | THF | – | 15 | 28:14 | 2:1 |
| 2 | cyclohexane | – | 15 | 33:34 | 1:1 |
| 3 | CH2Cl2 | – | 15 | 39:35 | 1:1 |
| 4 | DCE | – | 15 | 61:13 | 5:1 |
| 5 | DCE | Na2CO3 | 15 | 70:7 | 10:1 |
| 6 | DCE | NaOAc | 15 | 67:6 | 11:1 |
| 7 | DCE | LiOAc⋅2H2O | 15 | 73:7 | 10:1 |
| 8[c] | DCE | LiOAc⋅2H2O | 15 | 71:10 | 7:1 |
| 9 | THF | HOAc | 20 | 19:16 | ca. 1:1 |
| 10 | THF | p-TSA | 20 | 0 | – |
| 11 | THF | BF3⋅Et2O | 20 | 3:78 | 1:26 |
| 12[d] | THF | – | 20 | 5:60 | 1:12 |
| 13 | THF | Et3B | 20 | 8:24 | 1:3 |
Unless otherwise noted, 1 a, B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), and indicated additive (20 mol %) were dissolved in the indicated solvent (5 mL mmol−1) and stirred at 50 °C in a sealed tube.
Yield was determined by 1H NMR spectroscopy using anisole as internal standard.
50 mol % of LiOAc⋅2H2O was added.
2 mol % of [Pd(CH3CN)4][(BF4)2] was used in place of Pd(OAc)2. E=CO2Me.
The finding that the addition of a basic salt substantially enhanced the selective formation of alkenyl boronate 4 encouraged us to study the effect of acidic reaction conditions. To our surprise, the addition of a Brønsted acid, such as HOAc, generated an approximately 1:1 mixture of 4 a and 5 a in moderate yields (Table 1, entry 9) and the use of p-toluenesulfonic acid (p-TSA) even did not afford any borylation products (Table 1, entry 10). However, the use of a Lewis acid, BF3⋅Et2O, resulted in a high selectivity for 5 and afforded products 4 a and 5 a in 3 % and 78 % yield, respectively (Table 1, entry 11; defined as Method B). Notably when the cationic palladium catalyst [Pd(CH3CN)4][(BF4)2] was used the same trend in selectivity was seen but a lower yield was obtained (Table 1, entry 12 vs. entry 11).16 The structurally similar Lewis acid BEt3 was also tried and moderate selectivity for 5 a over 4 a was seen with low yields of products (Table 1, entry 13).
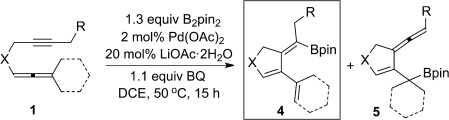
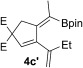
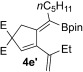
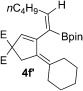
With the optimized conditions for the selective formation of borylated triene 4 a established, we applied them to differently substituted allenynes (Table 2). The allenynes bearing a methyl group on the alkyne moiety (1 b and 1 c) afforded the borylated trienes as the sole products (Table 2, entries 2 and 3). For substrates with a longer alkyl group (1 d and 1 f) on the alkyne moiety, the competing allene formation took place to a notable extent (Table 2, entries 4 and 6), but the corresponding triene products 4 d and 4 f/4 f′ could be isolated in good to moderate yields. In those cases where the substrates are unsymmetrically substituted at the allene moiety (1 c and 1 e) a comparatively high selectivity for the triene products was observed (Table 2, entries 3 and 5). However, a mixture of borylated triene products was obtained, with a preference for formation of the products with the more substituted double bond. Products 4 c and 4 e were obtained as a mixture of Z/E isomers in a ratio of 3.3:1 and 3.5:1, respectively. The allenyne 1 f with a cyclohexylidene group on the allene cyclized to give a mixture of isomers 4 f and 4 f′, where the formation of 4 f′ could be explained by a Pd-catalyzed isomerization of 4 f (Table 2, entry 6; for the detailed mechanism, please see the Supporting Information). Moreover, the reaction of allenyne 1 g, having two benzyl ether groups on the linker part X, also gave borylated triene product 4 g selectively in 57 % yield (Table 2, entry 7), thus proving that the malonate group of linker X is not necessary for a successful transformation.
Table 2.
Selective carbocyclization of allenynes 1 yielding borylated trienes 4[a]
 | ||||
|---|---|---|---|---|
| Entry | Allenyne | Product | 4/5[b] | Yield of4[%],[c] ratio[b] |
| 1 |  |
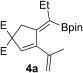 |
10:1 | 73 |
| 2 | 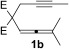 |
 |
99:1 | 92 |
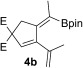
| 3 | 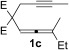 |
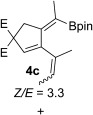 |
99:1 | 55 4 c/4 c′=2.4:1 |
 | ||||
| 4 | 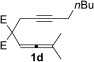 |
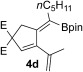 |
9:1 >11:1 | 81 92[d] |
| 5 | 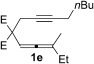 |
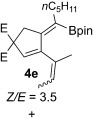 |
18:1 | 65 4 e/4 e′=2.3:1 |
 | ||||
| 6 | 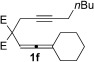 |
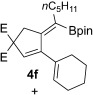 |
5:1 | 48 4 f/4 f′=1.4:1 |
 |
||||
| 7 | 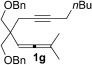 |
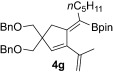 |
>20:1 | 57 |
Unless otherwise noted, 1 (0.1–0.2 mmol), B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), and LiOAc⋅2 H2O (20 mol %) were dissolved in DCE (5 mL mmol−1) and stirred at 50 °C for 15 h.
The ratio was determined by 1H NMR analysis of the reaction mixture.
Yield of the isolated product.
1 mmol of 1 d was used. E=CO2Me.
The optimized reaction conditions for the selective formation of borylated vinylallene 5 (20 mol % of BF3⋅Et2O, Method B) were applied to various allenynes (Table 3). Allenynes 1 a–1 f were transformed into vinylallenic boronates 5 a–5 f; for most cases the yield was between 70 % and 80 % and the formation of the corresponding triene isomers 4 a–4 f was efficiently suppressed. Even the methyl-substituted substrate 1 b, which intrinsically favors formation of triene 4 b,13b displayed opposite selectivity under these reaction conditions, that is, favoring vinylallene formation (Table 3, entry 2). The reaction of allenyne 1 g under the standard conditions of Table 3 was sluggish and did not give the desired product 5 g, probably because of the incompatibility between the benzyl ether group and BF3⋅Et2O. However, by switching the palladium catalyst to [Pd(CH3CN)4][(BF4)2] and in the absence of BF3⋅Et2O, product 5 g was obtained in 37 % yield (entry 7).
Table 3.
Selective carbocyclization of allenynes 1 yielding borylated vinylallenes 5[a]
 | ||||
|---|---|---|---|---|
| Entry | Allenyne | Product | 5/4[b] | Yield of 5 [%][c] |
| 1 |  |
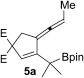 |
>20:1 | 77 |
| 2 |  |
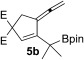 |
>20:1 | 73 |
| 3 |  |
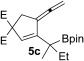 |
>20:1 | 56 |
| 4 |  |
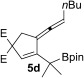 |
>20:1 | 79 87[d] |
| 5 |  |
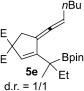 |
20:1 | 77 |
| 6 |  |
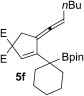 |
>20:1 | 70 |
| 7[e] |  |
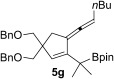 |
>20:1 | 37 |
Unless otherwise noted, 1 (0.1–0.2 mmol), B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), and BF3⋅Et2O (20 mol %) were dissolved in THF (5 mL mmol−1) and stirred at 50 °C for 20 h.
Ratio determined by 1H NMR analysis of the crude reaction mixture.
Yield of the isolated product.
1 mmol of 1 d was used.
2 mol % of [Pd(CH3CN)4][(BF4)2] was used in place of Pd(OAc)2 and BF3⋅Et2O. E=CO2Me.
To gain further insights into the mechanism of the oxidative carbocyclization/borylation, kinetic deuterium isotope effects were studied (Scheme 2). An intermolecular competition experiment using 1 d and its hexadeuterated derivative [D6]-1 d under the conditions for selective triene formation for 1 h provided a large intermolecular KIE value of 6.717 (Scheme 2 a). This result indicates that the allylic C–H bond cleavage involved has to occur prior to any irreversible step of the reaction, for example, the carbocyclization step.18 On the other hand, when a 1:1 mixture of 1 d and [D2]-1 d was subjected to the conditions for selective vinylallene formation for 1 h the ratio between 5 d and [D1]-5 d was 2.4, from which the KIE was determined to 2.719 (Scheme 2 b).17 The intrinsic KIE from intramolecular competition for vinylallene formation was determined to 5.317 by the use of [D1]-1 d as the allenyne substrate (Scheme 2 c). The results in Scheme 2 b and 2 c indicate that the propargylic C–H bond cleavage does not fully determine the selectivity between 5 d and [D1]-5 d in the competitive experiment (Scheme 2 b).18
Scheme 2.
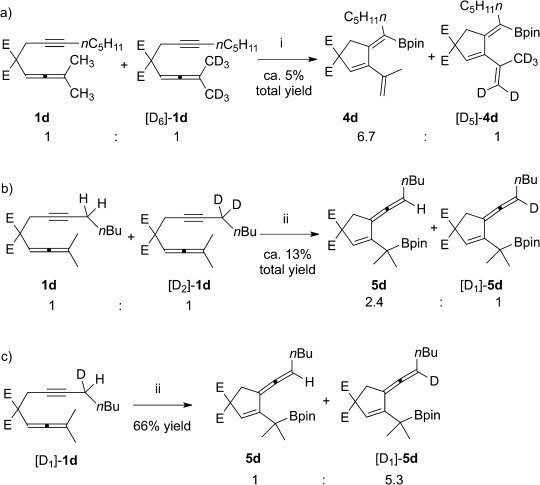
Kinetic isotope effect study. Reaction conditions: i) B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), LiOAc⋅2H2O (20 mol %), DCE, 50 °C, 1 h. ii) B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), BF3⋅Et2O (20 mol %), THF, 50 °C, 1 h for b) and 20 h for c). E=CO2Me.
Three control experiments with allenyne 1 d and the corresponding deuterium-labeled allenynes [D2]-1 d and [D6]-1 d were conducted under palladium catalysis in the absence of any additional basic or acidic additive and using DCE as the solvent (Scheme 3). Under these conditions the reaction of 1 d gave a mixture of 4 d and 5 d in a ratio of 3.8:1 (Scheme 3 a). When substrate [D2]-1 d was employed (Scheme 3 b) under the same reaction conditions, the ratio increased to 12.3:1.20 Allenyne [D6]-1 d showed the opposite selectivity, with [D5]-4 d and [D6]-5 d being formed in a ratio of 1:5.120 (Scheme 3 c).
Scheme 3.
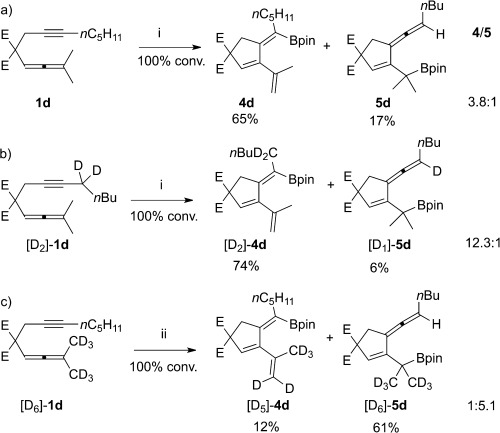
Effect of isotope substitution on product distribution. Reaction conditions: i) B2pin2 (1.3 equiv), Pd(OAc)2 (2 mol %), BQ (1.1 equiv), DCE, 50 °C, 15 h. ii) B2pin2 (1.3 equiv), Pd(OAc)2 (5 mol %), BQ (1.1 equiv), DCE, 50 °C, 15 h. E=CO2Me.
The results in Scheme 2 and Scheme 3 indicate that competing allylic and propargylic C–H bond cleavage occurs in 1, and this determines the ratio of boronates 4 and 5 (Scheme 4). The allene attack on PdII complex A through allylic C–H bond cleavage12, 13a–d would give B and subsequent alkyne insertion would generate intermediate C. Transmetalation of C with B2pin2 and reductive elimination would form product 4. The competing alkyne attack through propargylic C–H bond cleavage in A would produce allenylpalladium intermediate D. Intramolecular vinylpalladation of the allene moiety would generate (π-allyl)palladium intermediate E. Transmetalation with B2pin2 and subsequent reductive elimination would give 5. The mechanism in Scheme 4 is supported by the kinetic isotope effects and the experiments with deuterium-labeled compounds (Scheme 2 and Scheme 3). The lower kinetic isotope effect observed for the competitive experiment in Scheme 2 b compared to the intramolecular experiment in Scheme 2 c may reflect that 1 and A are not in full equilibrium under the conditions for formation of 5. In the path for formation of 5 it is likely that BF3⋅Et2O creates a cationic palladium species, which interacts better with the acetylene compared to the allene in A.21
Scheme 4.
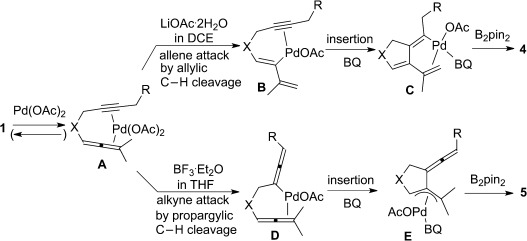
Proposed mechanism for palladium-catalyzed oxidative selective carbocyclization/borylation of allenyne 1.
In summary, we have developed an unprecedented selective PdII-catalyzed carbocyclization/borylation of allenynes under oxidative conditions. By controlling the reaction conditions the reaction can be directed to either the triene 4 or the vinylallene 5. On the basis of the results of deuterium-labeling experiments, we propose that the reactions of allenynes proceed through competing allylic and propargylic C–H bond cleavage pathways to give borylated trienes and borylated vinylallenes, respectively.
Experimental Section
Typical experimental procedure for palladium-catalyzed oxidative borylating carbocyclization of allenyne 1 to boronate 4: 1 a (26.0 mg, 0.10 mmol) and 0.5 mL of DCE were added to a mixture of B2pin2 (33.1 mg, 0.13 mmol), BQ (12.2 mg, 0.11 mmol), Pd(OAc)2 (0.5 mg, 0.002 mmol), and LiOAc⋅2H2O (1.8 mg, 0.02 mmol) at RT. The reaction was stirred at 50 °C for 15 h. After the reaction was complete, as monitored by TLC, evaporation and column chromatography on silica gel (pentane/ethyl acetate=10:1) afforded 4 a (27.9 mg, 73 %) as a liquid; 1H NMR (500 MHz, CDCl3): δ=5.93 (s, 1 H), 5.02–5.00 (m, 2 H), 3.72 (s, 6 H), 3.20 (s, 2 H), 2.19 (q, J=7.5 Hz, 2 H), 1.95 (s, 3 H), 1.26 (s, 12 H), 1.02 ppm (t, J=7.5 Hz, 3 H); 13C NMR (125 MHz, CDCl3): δ=171.1, 151.1, 147.9, 139.8, 129.1, 116.6, 83.3, 63.0, 52.9, 37.5, 27.0, 25.2, 23.8, 13.4 ppm; HRMS (ESI): calc. for C21H31BNaO6 [M+Na]+: 413.2110; found: 413.2113.
Typical experimental procedure for palladium-catalyzed oxidative borylating carbocyclization of allenyne 1 to boronate 5: 1 a (52.7 mg, 0.20 mmol) and 1.0 mL of THF were added to a mixture of B2pin2 (66.2 mg, 0.26 mmol), BQ (24.0 mg, 0.22 mmol), Pd(OAc)2 (1.0 mg, 0.004 mmol), and BF3⋅Et2O (6 μL, 0.04 mmol) at RT. The reaction was stirred at 50 °C for 20 h. After the reaction was complete, as monitored by TLC, evaporation and column chromatography on silica gel (pentane/ethyl acetate=10/1) afforded 5 a (59.6 mg, 77 %) as a liquid; 1H NMR (400 MHz, CDCl3): δ=5.55 (d, J=1.6 Hz, 1 H), 5.34–5.23 (m, 1 H), 3.716 (s, 3 H), 3.715 (s, 3 H), 3.19–3.17 (m, 2 H), 1.68 (d, J=7.2 Hz, 3 H), 1.18 (s, 12 H), 1.17 (s, 3 H), 1.13 ppm (s, 3 H); 13C NMR (100 MHz, CDCl3): δ=199.1, 171.5, 171.3, 153.8, 122.9, 107.1, 91.2, 83.1, 63.5, 52.7, 36.5, 25.0, 24.7, 24.5, 23.9, 23.8, 14.8 ppm; HRMS (ESI): calcd for C21H31BNaO6 [M+Na]+: 413.2110; found: 413.2103.
Supplementary material
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- 1.Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 2a.Brown HC, Rao BCS. J. Am. Chem. Soc. 1959;81:6434. For selected references, see. [Google Scholar]
- 2b.Ishiyama T, Ahiko T, Miyaura N. J. Am. Chem. Soc. 2002;124:12414. doi: 10.1021/ja0210345. [DOI] [PubMed] [Google Scholar]
- 2c.Kennedy JWJ, Hall DG. J. Am. Chem. Soc. 2002;124:11586. doi: 10.1021/ja027453j. [DOI] [PubMed] [Google Scholar]
- 2d.Maleczka RE, Jr, Shi F, Holmes D, Smith MR., III J. Am. Chem. Soc. 2003;125:7792. doi: 10.1021/ja0349857. [DOI] [PubMed] [Google Scholar]
- 3a.Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. For reviews, see. [Google Scholar]
- 3b.Suzuki A. J. Organomet. Chem. 1999;576:147. [Google Scholar]
- 3c.Miyaura N. Top. Curr. Chem. 2002;219:248. [Google Scholar]
- 3d.Miyaura N. In: Metal-Catalyzed Cross-Coupling Reactions. de Meijere A, Diederich F, editors. Weinheim: Wiley-VCH; 2004. pp. 41–123. [Google Scholar]
- 4a.Ishiyama T, Miyaura N. Chem. Rec. 2004;3:271. doi: 10.1002/tcr.10068. For selected reviews, see. [DOI] [PubMed] [Google Scholar]
- 4b.Miyaura N. Top. Curr. Chem. 2002;219:11. [Google Scholar]
- 5a.Brown HC, Rao BCS. J. Am. Chem. Soc. 1959;81:6423. For selected references for the Brown hydroboration, see. [Google Scholar]
- 5b.Brown HC, Rao BCS. J. Am. Chem. Soc. 1959;81:6428. [Google Scholar]
- 5c.Clay JM, Vedejs E. J. Am. Chem. Soc. 2005;127:5766. doi: 10.1021/ja043743j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. For a review of borylation of C–H bonds, see: for recent selected reports of transition-metal-catalyzed C–H borylations, see. [DOI] [PubMed] [Google Scholar]
- 6b.Ishiyama T, Isou H, Kikuchi T, Miyaura N. Chem. Commun. 2010;46:159. doi: 10.1039/b910298a. [DOI] [PubMed] [Google Scholar]
- 6c.Yamazaki K, Kawamorita S, Ohmiya H, Sawamura M. Org. Lett. 2010;12:3978. doi: 10.1021/ol101493m. [DOI] [PubMed] [Google Scholar]
- 6d.Selander N, Willy B, Szabó KJ. Angew. Chem. 2010;122:4145. doi: 10.1002/anie.201000690. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2010;49:4051. [Google Scholar]
- 6e.Itoh H, Kikuchi T, Ishiyama T, Miyaura N. Chem. Lett. 2011;40:1007. [Google Scholar]
- 6f.Ros A, Estepa B, López-Rodríguez R, Álvarez E, Fernández R, Lassaletta JM. Angew. Chem. 2011;123:11928. doi: 10.1002/anie.201104544. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:11724. [Google Scholar]
- 6g.Kawamorita S, Miyazaki T, Ohmiya H, Iwai T, Sawamura M. J. Am. Chem. Soc. 2011;133:19310. doi: 10.1021/ja208364a. [DOI] [PubMed] [Google Scholar]
- 6h.Dai H-X, Yu J-Q. J. Am. Chem. Soc. 2012;134:134. doi: 10.1021/ja2097095. [DOI] [PubMed] [Google Scholar]
- 6i.Xiao B, Li Y-M, Liu Z-J, Yang H-Y, Fu Y. Chem. Commun. 2012;48:4854. doi: 10.1039/c2cc31737k. [DOI] [PubMed] [Google Scholar]
- 7a.Suginome M. Chem. Rec. 2010;10:348. doi: 10.1002/tcr.201000029. For a review of catalytic carboborations, see. [DOI] [PubMed] [Google Scholar]
- 7b.Daini M, Suginome M. J. Am. Chem. Soc. 2011;133:4758. doi: 10.1021/ja200856t. [DOI] [PubMed] [Google Scholar]
- 7c.Raducan M, Alam R, Szabó KJ. Angew. Chem. 2012;124:13227. doi: 10.1002/anie.201207951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:13050. [Google Scholar]
- 8a.Burns AR, González JS, Lam HW. Angew. Chem. 2012;124:10985. [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:10827. [Google Scholar]
- 8b.Ito H, Toyoda T, Sawamura M. J. Am. Chem. Soc. 2010;132:5990. doi: 10.1021/ja101793a. [DOI] [PubMed] [Google Scholar]
- 8c.Miura T, Takahashi Y, Murakami M. Org. Lett. 2008;10:1743. doi: 10.1021/ol800380t. [DOI] [PubMed] [Google Scholar]
- 8d.Camelio AM, Barton T, Guo F, Shaw T, Siegel D. Org. Lett. 2011;13:1517. doi: 10.1021/ol200157x. [DOI] [PubMed] [Google Scholar]
- 8e.Gong W, Singidi RR, Gallucci JC, RajanBabu TV. Chem. Sci. 2012;3:1221. [Google Scholar]
- 8f.Singidi RR, Kutney AM, Gallucci JC, RajanBabu TV. J. Am. Chem. Soc. 2010;132:13078. doi: 10.1021/ja105939v. [DOI] [PubMed] [Google Scholar]
- 8g.Onozawa S, Hatanaka Y, Choi N, Tanaka M. Organometallics. 1997;16:5389. [Google Scholar]
- 8h.Onozawa S, Hatanaka Y, Tanaka M. Chem. Commun. 1997:1229. [Google Scholar]
- 9a.Marco-Martínez J, López-Carrillo V, Buñuel E, Simancas R, Cárdenas DJ. J. Am. Chem. Soc. 2007;129:1874. doi: 10.1021/ja0685598. [DOI] [PubMed] [Google Scholar]
- 9b.Pardo-Rodríguez V, Buñuel E, Collado-Sanz D, Cárdenas DJ. Chem. Commun. 2012;48:10517. doi: 10.1039/c2cc34468h. [DOI] [PubMed] [Google Scholar]
- 10a.Marco-Martínez J, Buñuel E, Muñoz-Rodríguez R, Cárdenas DJ. Org. Lett. 2008;10:3619. doi: 10.1021/ol801424m. [DOI] [PubMed] [Google Scholar]
- 10b.Marco-Martínez J, Buñuel E, López-Durán R, Cárdenas DJ. Chem. Eur. J. 2011;17:2734. doi: 10.1002/chem.201001124. [DOI] [PubMed] [Google Scholar]
- 11.Pardo-Rodríguez V, Marco-Martínez J, Buñuel E, Cárdenas DJ. Org. Lett. 2009;11:4548. doi: 10.1021/ol9017694. [DOI] [PubMed] [Google Scholar]
- 12a.Löfstedt J, Franzén J, Bäckvall J-E. J. Org. Chem. 2001;66:8015. doi: 10.1021/jo0157324. [DOI] [PubMed] [Google Scholar]
- 12b.Franzén J, Bäckvall J-E. J. Am. Chem. Soc. 2003;125:6056. doi: 10.1021/ja029505a. [DOI] [PubMed] [Google Scholar]
- 12c.Piera J, Närhi K, Bäckvall J-E. Angew. Chem. 2006;118:7068. doi: 10.1002/anie.200602421. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2006;45:6914. [Google Scholar]
- 12d.Piera J, Persson A, Caldentey X, Bäckvall J-E. J. Am. Chem. Soc. 2007;129:14120. doi: 10.1021/ja075488j. [DOI] [PubMed] [Google Scholar]
- 12e.Johnston EV, Karlsson EA, Lindberg SA, Åkermark B, Bäckvall J-E. Chem. Eur. J. 2009;15:6799. doi: 10.1002/chem.200900980. [DOI] [PubMed] [Google Scholar]
- 12f.Persson AKÅ, Bäckvall J-E. Angew. Chem. 2010;122:4728. [Google Scholar]
- Angew. Chem. Int. Ed. 2010;49:4624. for a DFT calculation for allene attack on a PdII center, see. [Google Scholar]
- 12g.Karlsson EA, Bäckvall J-E. Chem. Eur. J. 2008;14:9175. doi: 10.1002/chem.200801294. [DOI] [PubMed] [Google Scholar]
- 13a.Deng Y, Bäckvall J-E. Angew. Chem. 2013;125:3299. [Google Scholar]
- Angew. Chem. Int. Ed. 2013;52:3217. [Google Scholar]
- 13b.Deng Y, Bartholomeyzik T, Persson AKÅ, Sun J, Bäckvall J-E. Angew. Chem. 2012;124:2757. doi: 10.1002/anie.201107592. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:2703. [Google Scholar]
- 13c.Jiang T, Persson AKÅ, Bäckvall J-E. Org. Lett. 2011;13:5838. doi: 10.1021/ol202451f. [DOI] [PubMed] [Google Scholar]
- 13d.Persson AKÅ, Jiang T, Johnson MT, Bäckvall J-E. Angew. Chem. 2011;123:6279. doi: 10.1002/anie.201008032. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:6155. [Google Scholar]
- 13e.Jiang M, Jiang T, Bäckvall J-E. Org. Lett. 2012;14:3538. doi: 10.1021/ol301551x. [DOI] [PubMed] [Google Scholar]
- 14a.Yip K-T, Yang D. Org. Lett. 2011;13:2134. doi: 10.1021/ol2006083. For selected recent oxidative carbocyclization reactions by other groups, see. [DOI] [PubMed] [Google Scholar]
- 14b.Matsuura BS, Condie AG, Buff RC, Karahalis GJ, Stephenson CRJ. Org. Lett. 2011;13:6320. doi: 10.1021/ol202881q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14c.Wu T, Mu X, Liu G. Angew. Chem. 2011;123:12786. doi: 10.1002/anie.201104575. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:12578. [Google Scholar]
- 14d.Mu X, Wu T, Wang H, Guo Y, Liu G. J. Am. Chem. Soc. 2012;134:878. doi: 10.1021/ja210614y. [DOI] [PubMed] [Google Scholar]
- 14e.Zhu R, Buchwald SL. Angew. Chem. 2012;124:1962. [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:1926. [Google Scholar]
- 14f.Wei Y, Deb I, Yoshikai N. J. Am. Chem. Soc. 2012;134:9098. doi: 10.1021/ja3030824. [DOI] [PubMed] [Google Scholar]
- 15a.Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318. doi: 10.1021/cr068006f. For selected reviews involving palladium-catalyzed oxidative carbocyclizations, see. [DOI] [PubMed] [Google Scholar]
- 15b.Dénès F, Pérez-Luna A, Chemla F. Chem. Rev. 2010;110:2366. doi: 10.1021/cr800420x. [DOI] [PubMed] [Google Scholar]
- 15c.Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]
- 15d.Deng Y, Persson AKÅ, Bäckvall J-E. Chem. Eur. J. 2012;18:11498. doi: 10.1002/chem.201201494. [DOI] [PubMed] [Google Scholar]
- 16.Larsson JM, Szabó KJ. J. Am. Chem. Soc. 2013;135:443. doi: 10.1021/ja309860h. [DOI] [PubMed] [Google Scholar]
- 17. A small normal secondary isotope effect will affect the value.
- 18.Simmons EM, Hartwig JF. Angew. Chem. 2012;124:3120. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:3066. [Google Scholar]
- 19. At the end of the reaction the starting material ratio was estimated to 1:1.2 and the product ratio was 2.43:1. Therefore, the isotope effect calculated from the product ratio and the change of the starting material ratio is approximately 2.43×[(1+1.2)/2]=2.7 (see the Supporting Information)
- 20. A normal secondary deuterium isotope affects the ratio to some extent.
- 21. For another possible mechanism involving a pallada(IV)cyclopentene intermediate[13a,b] (see the Supporting Information)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


