Abstract
Some researchs have demonstrated that the loss of delta Np63 is associated with aggressive phenotypes and poor prognosis. However, other research indicates that delta Np63 is considered to have oncogenic properties. Delta Np63 overexpression is often observed in association with the oncogenic growth of squamous cell carcinomas and bladder cancer. In this study, we investigated the oncogenic role of delta Np63 in regulating cell adhesion in transitional cell carcinoma of the bladder (TCCB). The cells were stably transfected with the delta Np63 short hairpin RNA (shRNA) plasmid. Immunocytochemistry was performed to determine the knockdown efficiency. Tumour cells were studied for their ability to adhere to vascular endothelial cells. Confocal microscopy was used to analyse the changes in cytoskeletal F-actin. F-actin expression was measured by flow cytometry. Cell invasion ability was assessed using transwell chambers. The delta Np63-silenced tumour cells were shown to adhere more tightly than controls to vascular endothelial cells (P<0.05). The content of F-actin in the delta Np63-silenced cells was enhanced (P<0.05). The Matrigel invasion assays showed that human 5637 bladder cancer cells had a lower degree of motility when transfected with pdelta Np63-shRNA (P<0.05). In conclusion, silencing of the delta Np63 expression can enhance the adhesiveness of 5637 cells by inducing F-actin cytoskeleton production, and it will possibly inhibit the TCCB invasion and metastasis.
Keywords: bladder transitional cell carcinoma, cell adhesion, delta Np63
Introduction
The invasion and metastasis of malignant tumour cells is a complex process that is regulated by multiple factors. In addition to the degree of neovascularisation, change in cell adhesion ability is an important factor in the progression of malignant tumours.1 Tumourigenesis and the development of metastatic disease are accompanied by changes in the expression of cell adhesion molecules. In carcinomas, the cell adhesion molecules that are normally expressed are lost or expressed in a functionally altered form, which allows tumour cells to escape from contact-mediated controls and spread to remote sites. Metastatic potential in solid tumours may also be associated with the expression of new cell adhesion molecules by the tumour cells. These newly expressed adhesion molecules appear to mediate tumour cell interaction with leukocytes and endothelium and may direct dissemination of the tumour cells throughout the body.2 Thus, the abnormal expression of cell adhesion molecules plays a key role in tumour development and is a common feature of malignant tumours.3
The p63 gene is a member of the p53 gene family. It shares a high degree of homology and structural similarity with the p53 protein.4,5 The p63 protein was originally thought to be another tumour suppressor that functioned in a similar capacity to that of p53. p63 has two major isoforms that differ at the amine terminus: TAp63 and delta Np63. TAp63 has a transactivating amino terminal, while delta Np63 has a dominant-negative activating amino terminal.6,7,8,9 In general, the TAp63 isoform behaves like p53 because it transactivates various p53 downstream targets, induces apoptosis and mediates cell cycle control. In contrast, the delta Np63 isoform has been shown to display functions that are opposite to those of the TAp63 isoform and may act as an oncoprotein.8,9,10,11 Delta Np63 overexpression is often observed in squamous cell carcinomas, in which context it is expected to enhance oncogenic growth.9,12 Delta Np63 may function as a dominant negative mediator in p53 tumour suppression.5 It is generally accepted that TAp63 plays a more p53-like role, whereas delta Np63 has an antagonistic or even oncogenic role in the progression of malignant tumours to cancer, including invasion and metastasis in malignant tumours.7,13,14,15 However, research indicates that the loss of delta Np63 is associated with increased invasive and metastatic potential.16,17,18 Our previous study19 demonstrated that delta Np63 was overexpressed in transitional cell carcinoma of the bladder (TCCB) tissues at both the mRNA and protein levels, which suggests that delta Np63 plays an oncogenic role in TCCB cells. Targeting this oncoprotein by using a short hairpin RNA (shRNA)-expressing vector induced the specific silencing of delta Np63 expression. The suppression of delta Np63 expression impairs TCCB tumour growth in vivo and improves the survival of tumour-bearing nude mice. No invasion or metastasis was found in tumour-bearing nude mice.19 Therefore, we speculate that delta Np63 may contribute to invasion or metastasis by affecting cell adhesion ability in TCCB.
Because the function of different isoforms of delta Np63 in bladder cancer remains controversial, we sought to investigate the role of delta Np63 in human bladder cancer cell adhesion and its mechanism by using small interference RNA targeting delta Np63 in human bladder cancer 5637 cells.
Materials and methods
Cell lines and plasmid
The 5637 human TCCB cell line was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 medium (Gibco, Shanghai, China) supplemented with 10% (v/v) foetal bovine serum (FBS; Sijixin Inc., Beijing, China) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C with 5% (v/v) CO2. A pair of effective shRNAs targeting human delta Np63 mRNA were selected from among those used for preliminary experiments. The pair of shRNAs forms a structure consisting of two 19-bp stems targeting delta Np63 mRNA, a 9-bp loop and a short polyA sequence. Two oligonucleotides (forward: 5′-GATCCGTGCCCAGACTCAATTTAGTTTCAAGACGACTAAATTGAGTCTGGGCATTTTTTGTCGACA-3′ and reverse: 5′-AGCTTGTCGACAAAAAATGCCCAGACTCAATTTAGTCGTCTTGAAACTAAATTGAGTCTGGGCACG-3′) were synthesized and ligated directly into BamHI and HindIII linearized genesil-1 plasmid (Jingsai Inc., Wuhan, China). The resultant delta Np63-shRNA expression construct, pdelta Np63-shRNA, was confirmed using PstI+SalI double digestions and by DNA sequencing. The negative control plasmid, termed pdelta Np63-cRNA, was inserted equidistant from the following two oligonucleotides: forward: 5′-GATCCGACTTCATAAGGCGCATGCTTCAAGACGGCATGCGCCTTATGAAGTCTTTTTTGTCGACA-3′ and reverse: 5′-AGCTTGTCGACAAAAAAGACTTCATAAGGCGCATGCCGTCTTGAAGCATGCGCCTTATAAGTCG-3′.
Sensitivity of the 5637 cells to G418
G418 (hygromycin) is an aminoglycoside antibiotic used in molecular genetic tests. It is the dominant selection reagent that was used most often to obtain stably transfected cells. Because the plasmid contains a gene coding for resistance to aminoglycoside antibiotics, only successful transfection would result in cell survival. The surviving 5637 cells were seeded and cultured into 12-well plates and selected in the presence of different concentrations of G418 (Zhongshang Inc., Beijing, China) ranging from 100 to 1000 µg ml−1. The cell culture medium was changed every 3 days and changed to normal medium with G418 on day 14. Cells were then cultured for 7 days in normal medium without G418. The optimal concentration of G418 was chosen as the lowest concentration in a given 12-well plate without any living non-transfected cells, which was observed by fluorescence microscopy. The mean results of three experiments are reported here.
Cell transfection and selected stable, positive cell clones
For transfection, 5637 cells were seeded in 24-well plates at 1×106 cells per well and allowed to grow overnight to approximately 80% confluence. Cells were transfected with the mixture of 0.4 µg of pdelta Np63-shRNA, 0.4 µg pdelta Np63-cRNA or 0.4 µl of phosphate-buffered saline (PBS) and 10 µl of Effectene transfection reagent (Qiagen, Shanghai, China) in 600 µl of fresh 1640 culture medium. Thirty-six hours after transfection, the cell culture medium was changed to fresh 10% (v/v) FBS with 400 µg ml−1 G418, and the medium was changed every 3 days. After 21 days, cultures of clones that were positive for pdelta Np63-shRNA or pdelta Np63-cRNA as well as a PBS group (transfected without plasmid) were maintained. Forty-eight hours after transfection, the cells were harvested for immunocytochemistry as described below. The mean results of three experiments are reported here.
Immunocytochemistry
Cells were fixed with chilled acetone at 4 °C for 30 min, followed by incubation for 10 min with 3% (v/v) hydrogen peroxide in methanol. Goat serum was used to block nonspecific binding. The fixed and blocked cells were incubated overnight at 4 °C with a 1∶200 dilution of a mouse monoclonal anti-delta Np63 antibody (Santa Cruz, Beijing, China). Cells were washed three times, and an appropriate biotinylated-conjugated secondary antibody (Santa Cruz) was applied for 15 min at a 1∶150 dilution, at 37 °C. Cells were washed three times and incubated with streptavidin-horseradish peroxidase (Zhongshan Golden Bridge Inc., Beijing, China) for 15 min at 37 °C. Horseradish peroxidase substrate diaminobenzidine solution was added for 15 s; the staining was stopped by washing the coverslip repeatedly with water. The cells were counterstained with hematoxylin. The mean results of three experiments are reported here.
Adhesion between 5637 cells and umbilical venous endothelial cells
Human umbilical venous endothelial cells (HUVECs) were provided as a gift by the Department of Physiology of Chongqing Medical University. Then, 0.1 ml (5×104 HUVEC cells per ml) of cells was seeded into 96-well plates. When the HUVEC cells grew to confluence, 0.1 ml (5×104 bladder cancer cells per ml) of cells (pdelta Np63-shRNA-, pdelta Np63-cRNA- and PBS-treated) were added to the 96-well plate and cultured for 30 min at 37 °C. Non-adherent cells were removed. Then, 100 µl of Rose Bengal was added to each plate for 5 min, followed by the addition of 100 µl of 95% alcohol and 100 µl of PBS to the plate for 30 min at room temperature. The absorbance (A) at 570 nm was measured for each group. Notably, HUVEC cells form a monolayer, to which cells transfected with pdelta Np63-shRNA, pdelta Np63-cRNA or PBS were added for the investigation of cell adhesion ability. Rose Bengal staining was used as an index of cell adhesion ability. The mean results of three experiments are reported here.
F-actin morphology and quantitative observations of 5637 cells
pdelta Np63-shRNA-, pdelta Np63-cRNA- and non-transfected 5637 cells at l×105 cells per ml×0.2 ml were seeded onto coverslips in 24-well plates. The coverslips were taken out when cells were seeded; cell populations then grew to cover 70%–80% of each coverslip. The coverslips were fixed by 3.7% formaldehyde for 10 min at room temperature and stored at −20 °C in acetone for 5 min. Then, 20 µl (2 µg ml−1) of tetramethylrhodamine isothiocyanate phalloidin (Sigma, Shanghai, China) were added to each coverslip and incubated for 25 min in the dark. The coverslips were washed in deionized water and mounted. Fluorescence signals were visualized using confocal microscopy.
For flow cytometry, 0.2 ml of pdelta Np63-shRNA-, pdelta Np63-cRNA- and non-transfected cells (at l×105 cells per ml) were seeded onto 24-well plates. Tetramethylrhodamine isothiocyanate phalloidin was added to each well after the cells had grown to confluence. The relative quantity of F-actin was determined based on the fluorescence intensity at 540 nm as detected using flow cytometry. At least 1000 cells were counted and analysed. The mean results averaged over three experiments are reported as fluorescence intensity per 10 000 cells.
Cell invasion
Cell invasion was assessed using transwell chambers with 8 µm pore-size membranes coated with Matrigel (Collaborative Research, Bedford, MA, USA) that were placed in 24-well plates. Cells (5×104) were loaded into the upper segment of the chambers and allowed to traverse the membrane for 24 h. The upper segment of the chamber contained 5% (w/v) FBS; the lower chamber contained 10% (w/v) FBS. After the cells migrate from a low to a high concentration of serum, any non-migratory cells were removed. The invaded cells were harvested by trypsinizing the lower surface of the membrane and were collected and seeded into a new 24-well plate, and 500 µl of 0.1% gentian violet was added. The membrane was then removed after 30 min and washed with PBS. The invasive activity was then quantified by counting the number of cells that had invaded 10 randomly selected microscopic fields (>40 cells per field). Each group was analysed in triplicate, and the mean was reported.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA), version 13.0 for Windows. Non-parametric ANOVA was performed to compare the differences among more than two parameters. Statistical significance in this study was set at P<0.05. All reported P values are two-sided.
Results
The optimal G418 selection concentration for 5637 cells
All parental 5637 cells were dead 4 days after being selected by G418 concentrations over 500 µg ml−1, while the parental cells were dead at 7 days after the application of G418 at 400 µg ml−1. There were a few surviving cells at G418 concentrations of 200 and 300 µg ml−1. Therefore, 400 µg ml−1 was selected as the lethal concentration, and 200 µg ml−1 was used as the selective concentration.
Selected stable, positive cell clones
The 5637 cells were transfected with pdelta Np63-shRNA or pdelta Np63-cRNA and selected in G418 at 200 µg ml−1. The stable cell clones also expressed green fluorescent protein (GFP) and could be observed by fluorescence microscopy. There was no GFP in the PBS control group. Stable positive cell clones were those that grew in medium of 200 µg ml−1 G418 (Figure 1).
Figure 1.
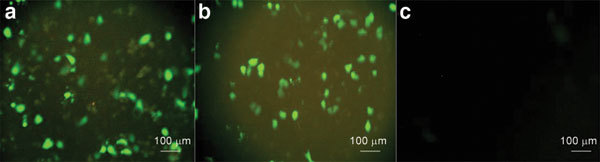
Stable cell growth of GFP cells was monitored by fluorescence microscopy. (a) pdelta Np63-shRNA group; (b) pdelta Np63-cRNA group; (c) PBS group. The stable cell clone grows with GFP which could be observed by fluorescence microscope (a, b). There is no GFP in group of PBS group (c). GFP, green fluorescent protein; PBS, phosphate-buffered saline; shRNA, short hairpin RNA.
Silencing of delta Np63 protein expression by delta Np63 shRNA
The 5637 TCCB cell line inherently expresses high levels of delta Np63. The expression of delta Np63 protein was detected 48 h post-transfection by immunocytochemistry. The nuclei of delta Np63-positive cells were stained brown. The nuclei of cells transfected with PBS or the control vector were strongly stained, while the nuclei of cells transfected with pdelta Np63-shRNA were only weakly stained.
5637 cell adhesion after the transfection of delta Np63 shRNA
The absorbances of pdelta Np63-shRNA-, pdelta Np63-cRNA- and PBS-treated cells were 0.182±0.012, 0.110±0.002 and 0.105±0.004, respectively (Figure 2). The level in pdelta Np63-shRNA-transfected cells was significantly higher than that in the pdelta Np63-cRNA and PBS groups(p<0.05). These findings suggest that silencing the expression of delta Np63 may increase the ability of 5637 cells to adhere to HUVEC cells.
Figure 2.
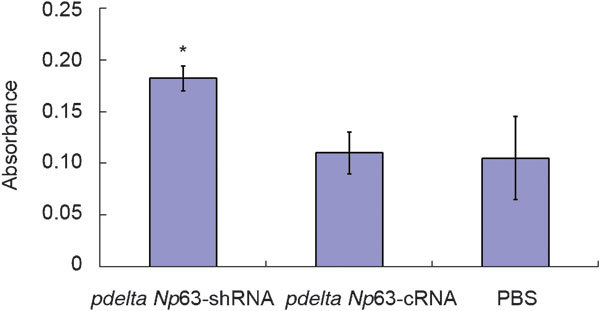
Absorbance of Rose Bengal as index of cell adhesion. *P<0.05, compared with NP63-cRNA and PBS groups, respectively. PBS, phosphate-buffered saline.
Levels and morphology of F-actin in 5637 cells
Phalloidin specifically binds to F-actin. F-actin can be visualized by fluorescence microscopy after incubation with tetramethylrhodamine isothiocyanate phalloidin. The staining is red and distributed to the cytoskeleton and cell processes. The microfilaments had enhanced fluorescence in the pdelta Np63-shRNA group as compared to the control group and followed the cell's polarity, suggesting that protein levels of F-actin are increased in pdelta Np63-shRNA-transfected cells. Conversely, in the pdelta Np63-cRNA and PBS groups, the F-actin staining was diffuse and irregular (Figure 3). The mean fluorescence intensities in the pdelta Np63-shRNA-, pdelta Np63-cRNA- and non-transfected groups were 60.71%±8.25%, 7.00%±1.01% and 8.50%±1.26%, respectively (Figure 4). The levels in the pdelta Np63-shRNA-transfected group were significantly higher than those in the other two groups(p<0.05). These results further confirm that silencing the expression of delta Np63 increases F-actin expression in 5637 cells and thus could enhance the polymerisation of F-actin.
Figure 3.
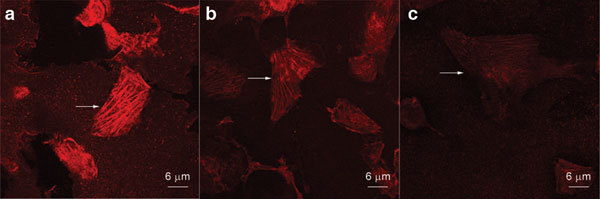
Morphology of F-actin observation in 5637 cell. (a) pdelta Np63-shRNA group; (b) pdelta Np63-cRNA group; (c) PBS group. The protein level of F-actin in pdelta Np63-shRNA is obviously increased (a). But in pdelta Np63-cRNA and PBS groups, the F-actin is diffusion, brightness of microfilament is lower, cell polarity disappears, and cell arrangement is crumbly and irregular (b, c). PBS, phosphate-buffered saline; shRNA, short hairpin RNA.
Figure 4.
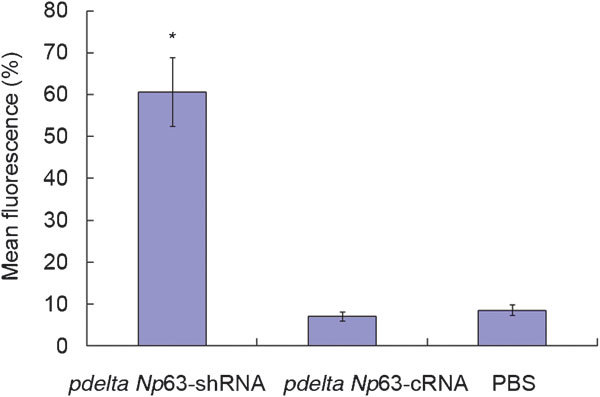
Quantitative of F-actin fluorescence intensity in each group. *P<0.05, compared with pdelta NP63-cRNA and PBS groups, respectively. PBS, phosphate-buffered saline.
Cell invasion
The Matrigel invasion assays showed that 5637 cells in the pdelta Np63-shRNA group had a lower degree of motility than those in either the pdelta Np63-cRNA group or the PBS group. The invasion ability of pdelta Np63-shRNA cells (12.30±4.16 cells per field) was lower than that of cells in the pdelta Np63-cRNA group (23.27±8.23 cells per field, P<0.05) or PBS group (24.34±4.27 cells per field, P<0.05) (Figure 5).
Figure 5.
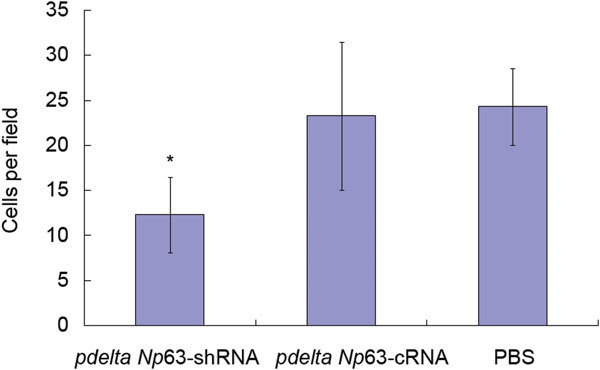
Number of migrating cells by Matrigel invasion assays. *P<0.05, compared with pdelta NP63-cRNA and PBS groups, respectively. PBS, phosphate-buffered saline.
Discussion
Despite research findings that the loss of delta Np63 is associated with increased invasive and metastatic potential in preclinical models,20 the expression of p63 in bladder tumour cells was still higher than that in normal cells. Nevertheless, there is still some research indicating that delta Np63 has oncogenic properties. First, delta Np63 overexpression is often observed in squamous cell carcinomas and increases the associated level of oncogenic growth.12,21,22 Second, delta Np63 can function as a dominant-negative factor that inhibits p53 tumour suppression.6 Third, delta Np63 overexpression induces the nuclear accumulation of β-catenin and activates β-catenin signalling, which promotes cell proliferation.13 However, the role of delta Np63 in TCCB is poorly defined, and the mechanisms underlying the effect of delta Np63 remain to be determined. These discrepant results may be related to gene polymorphisms and to the particular isoform of delta Np63. Our previous study19 has demonstrated that delta Np63 transcription and translation are increased in human TCCB tumour tissues. To further explore the functions of delta Np63 in TCCB, we utilized RNA interference technology to induce delta Np63 gene silencing. The ability of RNA interference to silence individual gene expression with a high degree of specificity, an effect mediated by shRNA, represents a unique opportunity to study gene function.23 Plasmid and viral vectors can be used to produce shRNA using the polymerase III promoter and then to achieve efficient and stable gene silencing.24,25 In this study, we constructed a delta Np63 shRNA expression plasmid, which achieved efficient and specific delta Np63 gene silencing in the 5637 human TCCB cell line following transfection in vitro.
We found that when delta Np63 expression was silenced, the ability of 5637 cells to adhere to HUVEC cells was increased significantly. Levels of F-actin and microfilament density (which followed cell polarity) were elevated in the pdelta Np63-shRNA group. F-actin is one of the major components of the cytoskeleton and plays an important role in many cell functions, including the regulation of cell shape, motility, secretion, intracellular transport, endocytosis and exocytosis, and cell adhesion.26,27,28 The phenotypic changes resulting from transformation are reflected as distinct alterations in the cytoskeleton.29,30 The tumour cells that traverse the blood vessels to form metastases must pass through endothelial cells and the subendothelial basement membrane. This process also involves adhesion. The reduced expression of tumour cell adhesion molecules may decrease cell adhesion, thereby permitting metastasis. Conversely, increased 5637 cell adhesion to HUVEC cells and increased levels of F-actin can inhibit tumour metastasis.31
Delta Np63 may function as an oncogene in the TCCB progression of invasion and metastasis by downregulating F-actin expression. The actin cytoskeleton is altered during tumour cell transformation. The most notable change involves restructuring of the F-actin skeleton. F-actin has been used in human breast cancer and T-lymphatic cell carcinoma as a sensitive marker for the early detection of tumour progression.32,33 Our results are consistent with Kao's research on human bladder cancer cells. When F-actin expression is increased, F-actin aligns with the cell's polarity, which results in reduced tumour metastasis.34,35,36 Increases in the density of F-actin result in loose arrangements that are not aligned with cell polarity and result in increased tumour metastasis. Therefore, delta Np63 may be a new drug target for TCCB therapy, especially in efforts to target invasion and metastasis. Delta Np63 shRNA may be a novel and promising approach for silencing delta Np63 expression. Furthermore, it is noteworthy that the migration and invasion capacities of the 5637 cells were reduced after delta Np63 was silenced, which suggests that the delta Np63 gene is essential for TCCB cell motility and invasion.
Conclusions
In conclusion, our results demonstrate that targeting delta Np63 expression, through the use of a shRNA-expressing vector, induced the specific silencing of delta Np63 gene expression. The suppression of delta Np63 expression may upregulate F-actin in TCCB in vivo and thereby change the cytoskeletal phenotype. When delta Np63 expression was silenced among TCCB cells, the capacity for cell adhesion was increased, while levels of cell motility and invasion ability were reduced. Studies at the molecular level suggest that F-actin may be one of the genes targeted by delta Np63.
Author contributions
YFH, DYT, ZJY, ZKY, XHW, CLL and WT conducted the experiments described in the study. The cell culture experiments were finished by YFH and DYT. The plasmid was constructed by XHW and CLL. Statistical analysis was conducted by WT and ZJY. All authors read and approved the final version of the manuscript.
Acknowledgments
This study was supported by the Research Development Foundation of Health Bureau of Chongqing (No. 2011-2-103), National Natural Science Foundation of China (No. 81000002 ) and the New Teacher Fund for Doctor Station, Ministry of Education of China (No. 20115503120012).
The authors declare no competing financial interests.
References
- Francavilla C, Maddaluno L, Cavallaro U. The functional role of cell adhesion molecules in tumor angiogenesis. Semin Cancer Biol. 2009;19:298–309. doi: 10.1016/j.semcancer.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Gloushankova NA. Changes in regulation of cell–cell adhesion during tumor transformation. Biochemistry (Mosc) 2008;73:742–50. doi: 10.1134/s000629790807002x. [DOI] [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in neoplastic disease. Int J Clin Lab Res. 1992;22:69–72. doi: 10.1007/BF02591399. [DOI] [PubMed] [Google Scholar]
- Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–19. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Schmale H, Bamberger C. A novel protein with strong homology to the tumor suppressor p53. Oncogene. 1997;15:1363–7. doi: 10.1038/sj.onc.1201500. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. doi: 10.1038/35043127. [DOI] [PubMed] [Google Scholar]
- Augustin M, Bamberger C, Paul D, Schmale H. Cloning and chromosomal mapping of the human p53-related KET gene to chromosome 3q27 and its murine homolog ket to mouse chromosome 16. Mamm Genome. 1998;9:899–902. doi: 10.1007/s003359900891. [DOI] [PubMed] [Google Scholar]
- Osada M, Ohba M, Kawahara C, Ishioka C, Kanamaru R, et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat Med. 1998;4:839–43. doi: 10.1038/nm0798-839. [DOI] [PubMed] [Google Scholar]
- Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, et al. Delta Np63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res. 2003;63:2351–7. [PubMed] [Google Scholar]
- Ratovitski EA, Patturajan M, Hibi K, Trink B, Yamaguchi K, et al. p53 associates with and targets delta Np63 into a protein degradation pathway. Proc Natl Acad Sci USA. 2001;98:1817–22. doi: 10.1073/pnas.98.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA. 2000;97:5462–7. doi: 10.1073/pnas.97.10.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patturajan M, Nomoto S, Sommer M, Fomenkov A, Hibi K, et al. Delta Np63 induces beta-catenin nuclear accumulation and signaling. Cancer Cell. 2002;1:369–79. doi: 10.1016/s1535-6108(02)00057-0. [DOI] [PubMed] [Google Scholar]
- Park CK, Oh YH. Expression of p63 in reactive hyperplasias and malignant lymphomas. J Korean Med Sci. 2005;20:752–8. doi: 10.3346/jkms.2005.20.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohn M, Zhang S, Chen X. p63alpha and Delta Np63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene. 2001;20:3193–205. doi: 10.1038/sj.onc.1204427. [DOI] [PubMed] [Google Scholar]
- Urist MJ, Di Como CJ, Lu ML. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002;161:1199–206. doi: 10.1016/S0002-9440(10)64396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga F, Kawakami S, Kumagai J, Takizawa T, Ando N, et al. Impaired delta Np63 expression associates with reduced beta-catenin and aggressive phenotypes of urothelial neoplasms Br J Cancer 20031088: 740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga F, Kawakami S, Fujii Y, Saito K, Ohtsuka Y, et al. Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res. 2003;9:5501–7. [PubMed] [Google Scholar]
- He YF, Wu XH, Tang W, Tian DY, Luo CL, et al. Impaired delta Np63 expression is associated with poor tumor development in transitional cell carcinoma of the bladder. J Korean Med Sci. 2008;23:825–32. doi: 10.3346/jkms.2008.23.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MJ, Di Como CJ, Lu ML, Charytonowicz E, Verbel D, et al. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002;161:1199–206. doi: 10.1016/S0002-9440(10)64396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa R, Yang A, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Investig Dermatol. 1999;113:1099–105. doi: 10.1046/j.1523-1747.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Karni-Schmidt O, Castillo-Martin M, Shen TH, Gladoun N, Domingo-Domenech J, et al. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. Am J Pathol. 2011;178:1350–60. doi: 10.1016/j.ajpath.2010.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. RNA interference in functional genomics and medicine. J Korean Med Sci. 2003;18:309–18. doi: 10.3346/jkms.2003.18.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filleur S, Courtin A, Ait-Si-Ali S, Guglielmi J, Merle C, et al. siRNA-mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogenic thrombospondin-1 and slows tumor vascularization and growth. Cancer Res. 2003;63:3919–22. [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Wilda M, Fuchs U, Wossmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi) Oncogene. 2002;21:5716–24. doi: 10.1038/sj.onc.1205653. [DOI] [PubMed] [Google Scholar]
- Wessells NK, Spooner BS, Ash JF, Bradley MO, Luduena MA, et al. Microfilaments in cellular and development process. Science. 1971;171:135–43. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin and actin binding proteins: a critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Friedman E, Venderame M, Winawer S, Pollack R. Actin cytoskeletal organization loss in the benign-to-malignant tumor transition in cultured human colonie epithelial cells. Cancer Res. 1984;44:3040–50. [PubMed] [Google Scholar]
- Federico C, Luciana B, Luisa T, Guelfa C, Franca T, et al. Cytoskeleton organization is aberrantly rearranged in the cells of B chronic lymphocytic leukemia and hairy cell leukemia. Blood. 1986;67:233–9. [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in neoplastic disease. Int J Clin Lab Res. 1992;22:69–72. doi: 10.1007/BF02591399. [DOI] [PubMed] [Google Scholar]
- Mukai M, Imamura F, Ayaki M, Shinkai K, Iwasaki T, et al. Inhibition of tumor invasion and metastasis by a novel lysophosphatidic acid (cyclic LPA) Int J Cancer. 1999;81:918–22. doi: 10.1002/(sici)1097-0215(19990611)81:6<918::aid-ijc13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Shestakova EA, Wyckoff J, Jones J, Singer RH, Condeelis J. Correlation of beta-actin messenger RNA localization with metastatic potential in rat adenocarcinoma cell lines. Cancer Res. 1999;59:1202–5. [PubMed] [Google Scholar]
- Rao JY, Hemstreet GP 3 rd, Hurst RE, Bonner RB, Min KW, et al. Cellular F-actin levels as a marker for cellular transformation: correlation with bladder cancer risk. Cancer Res. 1991;51:2762–7. [PubMed] [Google Scholar]
- Honda K, Yamada T, Endo R, Ino Y, Gotoh M, et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998;140:1383–93. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha MS, Chang IH. Significance of age and comorbidity as prognostic indicators for patients with bladder cancer. Asian J Androl. 2010;12:766–74. doi: 10.1038/aja.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


