Abstract
Stem cells are defined by their capacity for both self-renewal and directed differentiation; thus, they represent great promise for regenerative medicine. Historically, stem cells have been categorized as either embryonic stem cells (ESCs) or adult stem cells (ASCs). It was previously believed that only ESCs hold the ability to differentiate into any cell type, whereas ASCs have the capacity to give rise only to cells of a given germ layer. More recently, however, numerous studies demonstrated the ability of ASCs to differentiate into cell types beyond their tissue origin. The aim of this review was to summarize contemporary evidence regarding stem cell availability, differentiation, and more specifically, the potential of these cells in the diagnosis and treatment of erectile dysfunction (ED) in both animal models and human research. We performed a search on PubMed for articles related to definition, localisation and circulation of stem cells as well as the application of stem cells in both diagnosis and treatment of ED. Strong evidence supports the concept that stem cell therapy is potentially the next therapeutic approach for ED. To date, a large spectrum of stem cells, including bone marrow mesenchymal stem cells, adipose tissue-derived stem cells and muscle-derived stem cells, have been investigated for neural, vascular, endothelial or smooth muscle regeneration in animal models for ED. In addition, several subtypes of ASCs are localized in the penis, and circulating endogenous stem cells can be employed to predict the outcome of ED and ED-related cardiovascular diseases.
Keywords: adipose tissue-derived stem cells, bone marrow stem cells, erectile dysfunction, mesenchymal stem cells, stem cells
Introduction
Stem cells are by definition capable of self-renewal, meaning that they can make exact copies of themselves indefinitely, and of differentiation into a variety of phenotypes.1, 2 They have been shown to be able to functionally regenerate damaged tissues, depending on the stimuli or signals that they receive.1, 2 When a stem cell divides, both daughter cells have the potential either to remain stem cells or to become a more specialized type of cell, such as a muscle cell, a red blood cell or a brain cell. Stem cells are classified into totipotent, pluripotent, multipotent and unipotent in a hierarchal order based on the number of cell lineages to which they may potentially differentiate.3 Totipotent stem cells, like the zygote and its offspring cells of the morula, have the greatest differentiation potential and are capable of forming cells of the ectoderm, mesoderm and endoderm.4 Pluripotent stem cells give rise to the three germ layers, but not to extra-embryonic tissues. Embryonic stem cells (ESCs), isolated from the inner cell mass of blastocysts,5 are the most widely acknowledged example of pluripotent cells.6 Multipotent stem cells, such as haematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) , isolated from the developing germ layer and their descended adult organs, are capable of self-renewal, and they can differentiate into any cell type within their germ layer.4 Unipotent cells are progenitor cells or precursor cells with a limited capacity for self-renewal, and they can differentiate into only one defined cell type, such as epithelial cells.4, 7
Harvesting ESCs requires the destruction of human embryos and has raised significant ethical and political concerns. These barriers have prompted the search for alternative stem cell sources, including amniotic fluid-derived stem cells and adult stem cells (ASCs).7 ASCs are gaining popularity, as researchers are finding a more extensive differentiation potential than was previously thought to exist, with some studies demonstrating pluripotency of certain ASCs.8, 9
Erectile dysfunction (ED) is defined as the inability to attain and maintain an erection with sufficient rigidity to permit satisfactory sexual intercourse.10 Vascular diseases, which are correlated with smoking, aging, hyperlipidemia, diabetes and hypertension, are major causes of ED. Injury to the cavernous nerves during pelvic surgery, such as radical prostatectomy, comprises an appreciable number of ED cases as well.6 Other causes of ED include direct trauma to the genitals, endocrine disorders, and fibrosis of the penile vasculature and corporal smooth muscle.11 The aetiological theme linking both vasculogenic and neurogenic ED is the loss of normal cellular function or death of the cells themselves.
While oral pharmacotherapies, such as phosphodiesterase-5 inhibitors (PDE5is), have clear benefits, their actions are necessarily ephemeral, and treatment is relatively costly.6 Furthermore, they do not provide a cure, and a number of patients have tissue damage that is so extensive that the response to either oral or local pharmacotherapy is minimal. Consequently, researchers have been investigating stem cells as a substitute therapeutic strategy. Compared with other fields, the application of cell-based therapy for ED is relatively new.12 The potentially curative nature of stem cells recently prompted a number of studies with varying stem cell populations and strategies.6 Currently, it appears to be one of the most studied potential future treatments of ED.
Stem cell sources, their location and circulation
Stem cells and sources
Currently, there are three ways to obtain a pluripotent stem cell, including ESC isolation, somatic cell nuclear transfer and somatic cell reprogramming. In 1981, pluripotent ESCs were first discovered in the inner cell mass of the mouse embryo.13 Their ability to differentiate into many cell types aroused tremendous hope for their potential application in cell therapy and regenerative medicine. However, as stated above, ESCs present an ethical burden. To overcome this limitation, new stem cell technologies, such as the induction of pluripotency by somatic cell nuclear transfer and reprogramming, have been established.7 Over the past few years, the successful generation of induced pluripotent stem cells (iPSCs) brought about new hope for regenerative therapies.14 Many groups have now shown that somatic cells can be reprogrammed by overexpression of variable sets of few transcription factors in cells that share various characteristics with ESCs.14, 15, 16, 17 To date, several novel reprogramming protocols are available,18, 19, 20, 21, 22 which have broadened the spectrum of cell types and species involved in iPSCs generation. Similar to ESCs, iPSCs also show the potential for differentiation into numerous cell types. However, the degree of molecular similarity between iPSCs and ESCs has not been completely elucidated. To date, it is not clear whether these small differences are the result of interexperiment variability or whether reprogramming of somatic cells generates a state that is unique to ESCs.23 Thus, iPSCs seem to hold promise for regeneration medicine once ascribed solely to ESC.
To avoid ethical dilemmas, ASCs have been given increasing attention. ASCs tend to be tissue specific, self-renewing populations of cells that can differentiate into cell types associated with the organ system in which they reside.24, 25, 26 While they used to be considered multipotent at the most, a continuously expanding body of literature suggests that certain ASCs appear to possess pluripotent differentiation capacity.2 ASCs are easily identified in tissues with high cell turnover. Within the last decade, many niches of ASCs were discovered in many tissues, such as the brain, liver, skin, skeletal muscle, the gastrointestinal tract, adipose tissue, pancreas, eye, blood and dental pulp.24, 25, 26, 27, 28 Among them, the most notable exception to the tissue specificity of ASCs are the MSCs, which are defined as (multipotent) MSCs. They are capable of differentiating in vitro into various mesenchymal/mesodermal cells and differentiating developmentally if injected into a blastocyst.26 MSCs have been extensively tested and proven effective in preclinical studies, and they currently are being tested in US FDA-approved clinical trials for the treatment of myocardial infarction, stroke, meniscus injury, limb ischemia, graft-versus-host disease and autoimmune disorders.29 Although the mechanisms are not completely clear, MSCs are known to secrete a broad range of cytokines and growth factors that have both paracrine and autocrine effects on damaged tissues. MSCs have been isolated from bone marrow, adipose tissue, skeletal muscle, dental pulp and cord blood.30, 31, 32, 33, 34 Among them, the most studied are bone marrow-derived stem cells (BMSCs), which are derived from bone marrow stroma (hence their name). In the early 1960s, BMSCs were first determined to be responsible for marrow reconstitution due to their ability to renew themselves and their ability to differentiate into various cell types.35, 36 Recently, MSCs derived from the stromal vascular fraction of adipose tissue, termed as adipose tissue-derived stem cells (ADSCs), represent an abundant and easily accessible source of stem cells.37 While bone marrow is obtainable in the gram range by a painful marrow aspiration procedure, adipose tissue can be obtained in the range of hundreds of grams with a minimally invasive procedure. The possibility of harvesting hundreds of grams of adipose tissue excludes the need for MSC isolation and culture steps and allows for the direct re-injection of the stromal vascular fraction during the same surgical procedure in which they were harvested. ADSCs bear a strong resemblance to BMSCs, as demonstrated by the expression of common cell surface markers, similar gene expression profiles and similar differentiation potentials. Therefore, it is reasonable to expect that ADSCs will become the preferred choice of ASCs for future clinical applications.37
Localisation of ASCs in their native tissue—stem cell niche
The stem cell niche is a microenvironment that maintains stem cells in a quiescent state. After tissue injury, the niche promotes either self-renewal or differentiation to form new tissues.38 Several components of this microenvironment regulate stem cell characteristics within the niche: cell–cell interactions between stem cells and other stem cells or neighbouring differentiated cells; interactions of stem cells with adhesion molecules and the extracellular matrix; the presence and active secretion of a multitude of cytokines and growth factors; and oxygen tension and other physiochemical determinants. The stem cell niche is often located in the perivascular space of various tissues, thus providing direct access to the systemic circulation into which endogenous stem cells are recruited during tissue injury. In spite of the perivascular location of the niche, this microenvironment appears to be in a continuous state of relative hypoxia.39, 40 For example, the mesenchymal stem cell niche is exposed to oxygen tension as low as 2%.40 The latter finding has driven researchers to culture stem cells in hypoxic conditions, thereby replicating their physiological environment. It has been shown that various stem cells may benefit from these culture conditions, replicating the niche in vitro: there have been observations of increased growth factor secretion and increased engraftment potential after hypoxic culturing.41
The niche supports stem cell activity through complex but not completely known mechanisms, including: (i) the secretion of soluble factors, such as growth factors and cytokines;41 (ii) a strong interaction and self-renewal potential between stem cells and their niches; for example, hair follicle bulge stem cells expanded in culture and grafted into nude mice generated new bulge niches;42 and (iii) the activation of a variety of signals and cytokines when stem cell reinforcements are needed for tissue repair or regeneration, transforming the niche from a resting place to a command centre.43, 44, 45
There are two classic types of stem cell niches based on their anatomic relationship to stem cells:38 the stromal type and the epithelial type:
Stromal type. Niche cells reside in close proximity to their respective stem cell pools. For example, many HSCs and BMSCs reside along the endosteal surface of trabecular bone in close proximity to both bone-forming osteoblasts and vascular endothelial cells (ECs)46, 47, 48 (Figure 1a), the latter of which may facilitate mobilisation of stem cells from bone marrow into the circulation and their return to these niches.49 During the last few years, the vascular wall has been considered as an important reservoir for different types of stem and progenitor cells;50, 51 in addition, many kinds of stem cells, including ADSCs, HSCs, neural stem cells (NSCs), intestinal stem cells and spermatogonial stem cells,37, 52, 53, 54, 55 have been discovered by culturing stromal vascular fractions from their respective native tissue. Generally, perivascular cells, including pericytes in capillaries and adventitial cells around larger vessels, are considered to be able to originate multilineage mesodermal progenitor cells (Figure 1c).50
Epithelial type. The most typical example of this type resides within a specialized region of the outer root sheath of the hair follicle and is known as the follicular bulge stem cell niche. The stem cells in this niche could give rise to new matrix cells located at the base of the hair and adjacent to the dermal papilla that support hair growth56 (Figure 1b).
Figure 1.
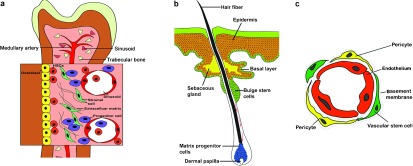
Stem cells niches. (a) Stromal type. HSCs are found adjacent to sinusoidal blood vessels as well as at or near the endosteum. Ostoblasts and mesenchymal progenitors have been proposed to produce factors that regulate HSC maintenance. (b) Epithelial type. Stem cells located within the bulge region of the hair follicle give rise to new matrix cells located at the base of the hair and adjacent to the dermal papilla that supports hair growth. Bulge cells can also help maintain the sebaceous gland and the epidermal stem cells. (c) The vascular wall as a reservoir for stem and progenitor cells. Capillaries are constructed by endothelial cells lining the lumen and pericytes, which cover the endothelial tube. Vascular wall-resident endothelial progenitor cells, HSCs and MSCs, are localized in the subendothelial space and are probably located between or around pericytes. HSCs, haematopoietic stem cells; MSCs, mesenchymal stem cells.
Trafficking of exogenously administered stem cells
In the clinical setting, the administration route of stem cells depends on the anatomy and the extent of damage of the involved tissue or organ, offering a choice between two approaches: direct local implantation versus systemic intravascular administration.57 Local implantation is an invasive procedure that could also disrupt the highly complex and delicate microenvironment, causing inflammation and multifocality of many disorders.57 Therefore, systemic diseases rely on vascular delivery of stem cells. However, the therapeutic efficacy depends on ensuring that sufficient stem cells home to the bone marrow or mobilize from the bone marrow to the damaged area. Homing is thought to be a multistep process that involves: (i) signalling and chemoattraction by chemokines; (ii) activation and attachment to adhesion molecules on the endothelial cell surface; and (iii) transendothelial migration of the stem cells into the diseased tissue.
Chemokines are a family of small cytokines. Their name is derived from their ability to induce directed chemotaxis in nearby responsive cells. Some chemokines are considered pro-inflammatory and can be induced during an immune response to recruit cells of the immune system to a site of infection. While most chemokines are important mediators of inflammation, it was recently discovered that chemokine secretion in sites of tissue damage or inflammation can be used to direct regenerative therapy.58 More specifically, certain types of stem cells have been shown to express mRNA and a large number of chemokine-receptors. Various groups have shown expression of the chemokine stromal cell-derived factor 1α (CXCL12) in myocardial infarction;59 CX3CL1, CCL2, CCL3 and CCL5 in peripheral neural injury;60 and CCL7 in rat models of birth injury and stress urinary incontinence.61 Recent data indicate that CXCL12 may play a role in trafficking ADSCs towards the major pelvic ganglion in rats with an injured cavernous nerve.62 In our laboratory, we have performed a comparative analysis between ADSCs and BMSCs and were able to show that these two types of MSCs express a similar panel of chemokine receptors. We identified expression of mRNA for 12 of the currently known 21 chemokine receptors, and found that these receptors were located both on the cell surface as well as intracellularly, suggesting that chemokine receptors are recycled in these stem cells, as has been previously shown in various immune cells (unpublished data). What the exact role of these chemokine receptors is in homing towards sites of tissue injury and what effects this stem cell recruitment has on the host tissue remains largely unknown and is the focus of intense investigation in stem cell biology.
After the recruitment of stem cells towards the injured tissue by chemoattraction, the activation of lymphocyte function-associated antigen 1, very late antigen 4/5 and CD44, cytoskeleton rearrangement, membrane type 1-matrix metalloproteinase activation and the secretion of matrix metalloproteinase-2/9 play important roles in the interaction between stem cells and endothelial cells.58, 59, 60, 61, 62, 63 Rolling and firm adhesion is followed by transendothelial migration across the physical endothelium/extracellular matrix barrier.
Similar processes play a role in homing of stem cells towards their niche from the circulation. Chemoattraction, mainly by CXCL12, adhesion to the endothelium and transendothelial migration are comparable to those processes in injured tissues. In the last stage, stem cells finalize their ‘homing' by anchorage to their specialized niches in the extravascular space of the endosteum region and at perivascular sites.60
Reawakening and mobilisation of endogenous stem cells
The development of techniques to reawaken and mobilize endogenous pools of stem cells would represent progress in the field of regenerative medicine. In general, the recruitment of HSCs from bone marrow into blood is termed ‘mobilisation'.64 This is a complex mechanism that has not yet been clarified. The haematopoietic niche of the bone marrow expresses a broad array of cell surface receptors, such as the CXC chemokine receptors CXCR4 and CXCR2, lymphocyte function-associated antigen 1, very late antigen 4 and glycoprotein CD44, among others.65 Data from a number of preclinical models showed that the inhibition of these receptor–ligand interactions resulted in enhanced progenitor cell mobilisation.66, 67, 68 Among them, CXCL12/CXCR4 interactions may play key role in the regulation of the routine and active egress of progenitor and maturing cells from the marrow into the blood.64 Plerixafor, a novel agent that disrupts the CXCR4–CXCL12 bond, which is the primary haematopoietic stem cell anchor in the bone marrow, has recently been US FDA-approved for mobilizing HSCs from the bone marrow. Another common mobilisation strategy in current clinical applications includes the use of cytokines alone or in conjunction with chemotherapy.65 The currently available cytokines include granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, and erythropoietin and recombinant methionyl human stem cell factor.65
Penile stem cells (PSCs)
Many types of ASCs have been reported in different tissues. In this review, we will focus on the male erectile organ, the penis. As an organ composed of multiple types of tissues, the penis itself contains a variety of stem cells. To date, few types of penile stem cells have been isolated or identified.
Foreskin stem cells
Two types of foreskin stem cells have been isolated to date, including skin-derived progenitors (SKPs) and MSCs. A new and unique multipotent progenitor cell population from adult mammalian dermis, termed SKPs, has been isolated and expanded from rodent and human skin and differentiated into both neural and mesodermal progeny.69, 70 SKPs did not apparently produce keratinocytes, distinguishing them from epidermal stem cells. These progenitor cells have been reported to reside in the dermal papillae of hair follicles.71 When the skin cells of neonatal and adult rodents were dissociated to single cells and grown in suspension culture in the presence of the mitogens fibroblast growth factor (FGF) and epidermal growth factor, floating spheres of proliferating cells were generated.69 These spheres were positive for nestin, a filament mainly expressed in neural and skeletal muscle progenitors. Moreover, differentiation of SKPs in vitro resulted in the generation of separate subpopulations of cells expressing neuronal, glial, smooth muscle and adipocyte markers. In 2005, SKPs from neonatal human foreskin tissue were identified,72 and they showed similar biological characteristics to the animal cells mentioned above. Because human neonatal foreskin does not contain hair follicles, the presence of SKPs in foreskin provides evidence for an extrafollicular SKP niche.72 However, the difficulty of reliably isolating and expanding comparable populations of SKPs from both whole hairless and hairy adult human skin suggests that this extrafollicular niche may be significantly limited after the neonatal period in humans.70, 71
Meanwhile, MSCs were also obtained from low-temperature preserved human foreskin biopsies by adherent culture.73 These cells could differentiate into mesodermal lineages, including adipocytes, osteocytes and myocytes.74 MSCs were antigenically distinct from SKPs, and when grown under the same conditions, the MSCs grew adherently (plastic adherence is one of the three hallmarks of MSCs), while SKPs grew as floating spheres.
Tunica albuginea stem cells
Vernet and colleagues75 investigated whether cultures of cells from normal tunica albuginea and Peyronie's disease plaques undergo osteogenesis, express markers of stem cells, and originate other cell lineages via processes modulated by transforming growth factor-β1. They found that both cultures express the stem cell marker CD34 and the SMCs markers smoothelin and transgelin. Cells expressing CD34 and Sca-1 were also found in vivo in the normal tunica albuginea, as well as in the corpora cavernosa.76 They were identified as potential endogenous stem cells because they are likely the ones that, in the in vitro models, undergo multiple lineage differentiation, and in the Peyronie's disease plaque, they may convert into myofibroblasts and osteoblasts. In addition, shaft penile tissue sections from the rat and wild type mouse were immunostained for Oct-4, an ESC marker.77 Results showed that Oct-4+ cells were detected in tunical and corporal tissues and that they could differentiate into SMCs, myofibroblasts and cardiomyocytes. This is the first report of the isolation and characterisation of embryonic-like endogenous stem cells in penile tissues.
Perivascular stem cells
Although perivascular stem cells have been extracted from multiple organs, such as bone marrow, dental pulp, placenta, fat and umbilical cord,51 the penis, as a part of a systematic circulation tree, has not yet received attention in this regard. We hypothesize that a ubiquitous reserve of multilineage progenitor cells in the capillaries, veins and arteries of the penis exists.
Endothelial progenitor cells (EPCs)
ED, cardiovascular disease and male hypogonadism share the common denominator of endothelial dysfunction, which has a well-established role in the pathogenesis of both atherosclerosis and plaque instability.78, 79, 80 One of the most exciting discoveries in vascular biology came in 1997 with the apparent detection of bone marrow-derived EPCs,81 which have the capacity to migrate into the peripheral circulation and to differentiate into mature ECs, thus providing a circulating pool of the cells that may contribute to ongoing endothelial repair.82 This process, called vasculogenesis,83 is impaired in ED, as documented by low levels of circulating EPCs.82 Meanwhile, at least one research group believes that EPCs are native inhabitants of certain blood vessel walls, and it would be instructive to determine whether EPCs are embedded within the penile vasculature and can be stimulated to repair dysfunctional penile ECs.84
Recently, several groups demonstrated that in ED patients, the reduced level of EPCs could be restored by administration of PDE5i,85, 86 such as vardenafil and tadalafil, resulting in an effective vasculoprotection and prevention of the initiation and progression of endothelial dysfunction. The mechanisms remain unknown; however, a possible hypothesis involves the presence of PDE5 in bone marrow that, when inhibited, may magnify the local effect of nitric oxide, thus leading to the mobilisation of stem and progenitor cells.85
Application of stem cells for predicting ED
EPCs show a wide heterogenic antigenic profile, including expression of CD34, CD133 and KDR. CD34 is an adhesion molecule expressed on HSCs and is typically considered a marker of immaturity; CD133 is a surface antigen of unknown function that identifies more immature progenitor cells than CD34 alone; and KDR represents the type 2 vascular endothelial growth factor (VEGF) receptor and indicates early endothelial differentiation.87
There are several types of circulating EPCs related to ED. In particular, circulating levels of CD34+CD133+ cells were recently shown to be decreased in patients with ED with or without cardiovascular risk factors,82 while CD34+KDR+ cells showed no relationship with ED.88
Meanwhile, increased levels of osteocalcin+ EPCs could be a predictive marker of subsequent coronary artery disease as well as ED.89 An increasing number of authors have described a population of EPCs expressing osteocalcin, a typical osteo-related protein.90 Carlo's group89 investigated the correlation between osteocalcin+ EPCs and cavernous atherosclerotic lesions in ED patients. They found that osteocalcin+ EPC levels increased significantly with cavernous atherogenesis progression.
We should note that decreased levels of EPCs are not specific indicators for ED. Cardiovascular risk factors are known to decrease EPCs with a subsequent increase of cardiovascular events and cardiovascular deaths.91 Thus, circulating levels of EPCs are thought to be a link between cardiovascular risk factors and endothelial dysfunction, with a potential influence on erectile function.
Application of stem cells as a therapy of ED
Current pharmacological agents for vasculogenic ED, such as PDE5i, lack efficacy in treating ED patients with advanced diabetes or patients suffering from ED following radical prostatectomy.92, 93 In the latter condition, Wallerian degeneration of the nerve and target apoptosis and fibrosis in the corpus cavernosum result in an irreversible loss of function.6, 94 Stem cell-based therapy has been proposed in the management of ED by complete replacement of lost or damaged cells or by protecting threatened host cells via immunomodulatory effects, the provision of trophic factors or gene delivery.95 In recent years, a number of reports related to the progress of stem cell-based ED therapy have been published. Different therapeutic forms of stem cells have been developed, including multiple sources of stem cells or progenitor cells, gene-transfected stem cells, stem cell lysates and stem cells seeded on tissue matrices (Table 1).
Table 1. Application of stem cells for the therapy of erectile dysfunction.
| SCs type | SCs form | Model system | Study duration | Functional results | Histology and molecular results |
|---|---|---|---|---|---|
| Rat BMSCs102 | Cell (gene modified with eNOS) | Aged rats | 3 weeks | Enhanced ICP during CN electrostimulation. Better enhancement when combined with eNOS gene therapy | Enhanced eNOS expression, and cGMP levels. Transplanted cells exhibited ECs and SMCs markers |
| Human BMSC103 | Cell | Young adult rats | 2 weeks | No functional testing. | Transplanted cells exhibited ECs and SMCs markers |
| Rat BMSCs106 | Cell (isolated by p75 NGF receptor) | Young adult rats with CN crush | 4 weeks | Enhanced ICP during CN electrostimulation. Better enhancement for p75-derived BMSCs | p75-derived BMSCs secreted significantly more basic NGF than control group |
| Rat BMSCs107, 109 | Cell (gene modified with VEGF) | Young adult rats with DM I | 4 weeks | Enhanced ICP during CN electrostimulation. Better enhancement when combined with VEGF gene therapy | Increased content of smooth muscle and endothelium in corporal cavernosum. Transplanted cells exhibited ECs and SMCs markers |
| Rat MDSCs115 | Cell | Young adult rats with CN transaction | 2 weeks, 4 weeks | Enhanced ICP during CN electrostimulation | Increased percent area of PGP 9.5 staining |
| Mouse MDSCs78 | Cell | Young adult rats and aged rats | 2 weeks, 4 weeks | Enhanced ICP during CN electrostimulation in aged rats (2 weeks) and young adult rats (4 weeks) | Increased content of smooth muscle in corporal cavernosum. Transplanted cells exhibited SMCs markers |
| Rabbit MDSC114 | ACCMs seeded with MDSCs | Young adult rabbits | 2 months, 4 months, 6 months | No functional testing | Cells expressing ECs and SMCs markers and better arranged growth were prevalent |
| Rat EPCs132 | Cell (gene modified with VEGF) | Young adult rats with DM I | 3 weeks | Enhanced ICP during CN electrostimulation | Enhanced neovascularisation in the corpora cavernosum. Transplanted cells exhibited ECs markers |
| Human UCBSCs 134 | Cell | Senior human with DM II | 11 months | Regained morning erections in some patients. Increased rigidity, but was insufficient for penetration | No relevant testing |
| Rat ADSCs123 | Cell | Young adult rats | 4 weeks | No functional testing | Injected ADSCs were localized to the sinusoid endothelium. Some Transplanted cells exhibited ECs markers |
| Rat ADSCs126 | Cell | Young adult rats with hyperlipid-emia | 4 weeks | Enhanced ICP during CN electrostimulation | Increased content of smooth muscle and endothelium in corporal cavernosum, and nNOS-positive nerve fibres in penile dorsal nerves |
| Rat ADSCs127 | Cell | Adult ZDF rats with DM II | 4 weeks | Enhanced ICP during CN electrostimulation | Increased nNOS staining area in penile dorsal nerves. Inhibition of apoptosis in corpus cavernosum |
| Rat ADSCs129 | Cell and cell lysate | Young adult rats with CN crush | 4 weeks | Enhanced ICP during CN electrostimulation | Increased content of smooth muscle in corporal cavernosum, and nNOS-positive nerve fibres in penile dorsal nerves. Inhibition of fibrosis and apoptosis |
| Human fetal NCSCs137 | Cell | Young adult rats | 2 weeks | No functional testing | Transplanted cells exhibited ECs and SMCs markers |
| Rat fetal BDSCs136 | Cell | Young adult rats | 6 weeks | No functional testing | Transplanted cells exhibited SMCs markers or VEGF |
| Rat GRPCs140 | Cell | Young adult rats with spinal cord injury | 12 weeks | Preserved full and partial erectile events per 24 h | GRPCs survived, differentiated and formed extensive transplants that were well integrated with host tissue |
| Rat neural ESCs141 | Cell | Young adult rats with CN crush | 12 weeks | Enhanced ICP during CN electrostimulation | Increased neurofilament staining in the MPG and penile dorsal nerves. Improved morphological characteristics of nerve fibres in the corpora cavernosum |
Abbreviations: ACCMs, acellular corporal collagen matrices; ADSCs, adipose tissue derived stem cells; BDSCs, brain-derived stem cells; BMSCs, bone marrow mesenchymal stem cells; CN, cavernous nerve; DM I, diabetes mellitus type I; DM II, diabetes mellitus type 2; ECs, endothelial cells; eNOS, endothelial nitric oxide synthase; EPCs, endothelial precursor stem cells; ESCs, embryonic stem cells; GRPCs, glial restricted progenitor cells; ICP, intracavernous pressure; MDSCs, muscle-derived stem cells; MPG, major pelvic ganglia; NCSCs, neural crest stem cells; nNOS, neuronal nitric oxide synthase; SCs, stem cells; SMCs, smooth muscle cells; UCBSCs, umbilical cord blood stem cells. PGP9.5, protein gene product 9.5; NGF, nerve growth factor; VEGF, vascular endothelial growth factor; ZDF, Zucker diabetic fatty.
While some of the more extravagant claims are excessively optimistic, there is clear validity to the notion that stem cells may lead to novel and potentially curative therapies. Nonetheless, clinical application is a long way off. Prior to clinical use of stem cells, it will be necessary to thoroughly investigate the malignant potential of stem cells, especially for ESCs. Although ASCs seem to be more stable than ESCs and are not as prone to forming tumours, many studies have observed that ASCs can also form malignant tumours when transplanted in vivo.96 Another consideration is the rejection of allogeneic stem cells by the host immune system. 97 The use of ASCs and tissues derived from the patient's own ASCs would mean that the cells are less likely to be rejected by the immune system. This represents a significant advantage, as immune rejection can be circumvented only by continuous administration of immunosuppressive drugs, and the drugs themselves may cause deleterious side effects. To date, nine different types of stem cells have been reported in the therapy of ED in the laboratory.
BMSCs
The preclinical applications of BMSCs in treating ED were initially based on their capacity to home to damaged tissues, differentiate into the necessary mature phenotypes, and their amenability to genetic manipulation.6, 98
In 2003, Deng et al.99 demonstrated successful adenoviral gene transfer of endothelial nitric oxide synthase (eNOS) to ex vivo expanded rat BMSCs. The transfected BMSCs expressed high levels of eNOS that persisted in culture for more than 21 days. The cells retained their multipotential differentiation capability after transduction. Moreover, intracavernous injection of eNOS-BMSCs increased the expression of eNOS in the corpus cavernosum and improved erectile function in aged rats.
In 2007, it was shown that rat BMSCs were able to reverse age-associated ED both with and without eNOS transfection through mechanisms involving increased penile eNOS, improved eNOS/cGMP signalling, and apparent differentiation into penile cells expressing endothelial and smooth muscle markers.100 It also has been suggested that human BMSCs are capable of differentiating toward ECs and SMCs in the corpus cavernosum of rats.101 However, the efficacy of these cells for the treatment of penile erectile function should be confirmed in immunocompromised animals because human stem cells are not compatible in non-immunocompromised animals. In addition, although a broad differentiation potential of BMSCs has been observed, it may also be explained as the result of spontaneous fusion of host and donor cells;102, 103 therefore, it is difficult to prove the differentiation.
In 2010, Kendirci and colleagues104 showed that transplantation of BMSCs isolated with p75 nerve growth factor (NGF) receptor into the rat penis could rescue erectile function following cavernous nerve injury. The authors suggested that these effects were mediated by FGF, NGF, brain-derived neurotrophic factor (BDNF), VEGF and insulin-like growth factor 1 (IGF-1) secreted by the stem cells. In a rat model of diabetes mellitus type 1, Qiu et al.105 indicated that transplantation of BMSCs restored erectile function by increasing the content of endothelium and smooth muscle in the corporal cavernosum, though they did not study the paracrine action of transplanted BMSCs or fusion between BMSCs and host smooth muscle cells or endothelial cells. For age-associated ED, BMSC transplantation improved erectile function during a long follow-up by improving eNOS/cGMP signalling and, potentially, by also differentiation into ECs and SMCs.106 In 2011, a study suggested that VEFG-gene-modified BMSCs resulted in enhanced regeneration of smooth muscle and endothelium in the corpora cavernosa of type I diabetic rats.107 The efficacy and safety of BMSC treatment over a long duration needs to be investigated. Unexpected side effects of VEGF caused by angiogenesis, such as angioma formation, may occur in a clinical trial.108 Thus, the possible side effects of VEGF need to be assessed before a clinical trial is conducted.
It is worth emphasizing that BMSCs may not simply act by replacing lost or damaged cells. The provision of trophic factors may protect threatened cells from disease or stimulate proliferation of host progenitors. Meanwhile, they could act independently to alter the immune response to limit damage and promote repair and regeneration; thus, their immunomodulatory effects have been employed clinically in the treatment of graft-versus-host disease.109 It remains controversial which mechanism plays the main role; however, an increasing number of observations support the hypothesis that BMSCs exert their effects on the host tissue by paracrine mechanisms.104 The beneficial effects of BMSCs in other animal models have been observed within 3 days following transplantation, a time frame that is too small to allow for engraftment and differentiation.104 Additionally, and perhaps most striking, the beneficial effects of BMSCs have been replicated using cell-free lysates and conditioned BMSC culture medium.110, 111
Muscle-derived stem cells (MDSCs)
MDSCs are ASCs found in muscle tissues. They are easily isolated from autologous muscle biopsies, and they pose low immunogenic and carcinogenic risks. Their enzymatic and non-enzymatic antioxidant capacity provides them a critical property for MDSC survival post-transplantation.112 This superior capacity supports their survival advantages.
In 2006, MDSCs were first injected into the corpora cavernosum to treat ED in rats with bilateral cavernous nerve injury.113 Although the increase in PGP 9.5 neuronal staining suggested that the MDSCs protected the penile nerve from atrophy after cavernous nerve transaction, the mechanisms need to be interpreted in the following studies.
In 2008, the ability of MDSCs to convert into SMCs after implantation into the corpora cavernosum of aged rats was reported.76 Exogenous MDSCs were able to correct ED, and endogenous cells also expressed stem cell markers in the control group, suggesting that exogenous stem cell implantation as well as endogenous stem cell modulation may be viable therapeutic approaches for ageing-related ED. Similar to BMSCs, when using the rat as a host, a truly allogenic source of MDSCs has to be tested to eliminate the need for pharmacological immunosuppression. Moreover, the effects of MDSCs were comparatively short-lived, and the considerable stimulation of erectile function achieved at 2 weeks was reduced at 4 weeks. Therefore, additional research needs to be conducted to improve the survival of MDSCs and ensure that the salutary functional effects could be prolonged for at least 3–6 months to exclude transient amelioration.
In 2010, acellular corporal collagen matrices seeded with MDSCs were implanted within the albuginea of rabbits.114 Histological analyses of the explants at all time points in the experimental group showed more cells and improved arranged growth compared to the control group. Alpha-smooth muscle actin and eNOS-positive cells were more prevalent in the experimental group. As the authors mentioned in the text, oxygen and nutrition were not available to MDSCs in the deeper regions of the acellular corporal collagen matrices because of their complex structure, resulting in some death of the transplanted MDSCs. Thus, more suitable scaffold materials should be explored.
ADSCs
ADSCs isolated from the stromal vascular fraction of adipose tissue have been recently identified and investigated for their multiple differentiation properties.115 ADSCs share similar properties of other stem cells, such as the ability to divide and renew themselves over long periods of time and to differentiate into specialized cells. They are much easier and safer to obtain in large quantities than BMSC,116 and, therefore, they appear to be a better choice for clinical application for ED regeneration medicine.
In 2006, Ning et al.117 reported that ADSCs could be induced by isobutylmethylxanthine to differentiate into neuron-like cells via the IGF-1 signalling pathway.118 The significance of these studies is that ADSCs possess the potential to treat degenerative neurological diseases, including neurogenic ED.2
In 2008, a pilot experiment was conducted that demonstrated that ADSCs improved the erectile function in the rat following bilateral cavernous nerve crush injury.119 Several possible mechanisms underlying the treatment are proposed, including cell incorporation and differentiation into native tissue cells versus paracrine signalling and stimulation of the host tissue to regenerate, for instance, by secretion of growth factors such as IGF-1 and GDF-5.116, 120
In 2009, Ning et al.121 first reported that ADSCs could differentiate into ECs in the penis and that this differentiation was mediated by FGF2 signalling. Previously published experimental procedures for the differentiation of ADSCs into ECs generally employed culture media that contained VEGF, IGF or FGF2, and VEGF was assumed to be the responsible factor.122 Interestingly, vitamin C was also found to be essential for endothelial differentiation of ADSCs. Its role is most likely to promote the maintenance of a healthy growth environment for endothelial cells by protecting them from oxidative stress.123
In 2010, a surge of reports concerning the application of ADSCs as a therapy for ED was published. First, ADSCs were injected into the penis to examine their effects on a rat model of hyperlipidemia-associated ED; elevated erectile function was demonstrated after ADSCs transplantation.124 An increase in neuronal nitric oxide synthase (nNOS)-positive nerve fibres and ECs was observed in the experimental group compared to the control animals. The authors indicated that the underlying mechanisms involved cytokine and growth factor secretion rather than stem cell differentiation. However, they failed to indicate the specific growth factors. Another critical question is the long-term safety of ADSCs with respect to the possibility of tumour formation. Investigation of the long-term fate of ADSCs is required before human trials could be considered, although the lack of ADSCs in tissue sections observed for a 28-day follow-up period implies that the potential of these cells to persist and undergo malignant transformation is limited.124 Second, Garcia and co-workers125 observed improved erectile function in type-2 diabetic rats after autologous ADSC injection. This observation was based on decreased numbers of apoptotic cells in the corpus cavernosum, increased numbers of sinusoid ECs and increased expression of nNOS in the penile nerves. Because only a few of the prelabelled ADSCs were observed within the corporal tissue of the treatment group, the authors suggested that the treatment effect of the ADSCs may not be through direct transformation into local cell types, but rather, via a more ‘indirect' mechanism, whereby ADSCs improve the extracellular environment and local tissue function within the treatment area. The biggest shortcoming of this study is that the authors did not assess the local retention or net survival of the injected ADSCs. How to improve local retention of the transplanted ADSCs is a challenge for cellular therapy. Improved local retention could lead to greater improvement in erectile function after treatment. The use of bioabsorbable PLGA microspheres is a well-established means by which to improve local retention, survival and possibly the therapeutic effect of transplanted cells.126 Third, ADSCs and cell-free lysates derived from ADSCs were injected into the penis in a rat model of cavernous nerve injury, resulting in a significant recovery of erectile function in both treatments compared with the control group.127 In the treated rats, nNOS and smooth muscle content were preserved, and less fibrosis occurred in the corpus cavernosum. The underlying mechanisms involved neuron preservation and cytoprotection by inhibition of apoptosis by releasing intracellular preformed substances or by active secretion of certain biomolecules. However, the authors failed to provide direct evidence to support the effects of a paracrine pathway. Moreover, further investigations should be aimed to identify the proteomic characteristics of the cell-free lysate. At last, CXCL5, which is secreted in much larger amounts by ADSCs than by control cells (penile smooth muscle cells), 128 was discovered to be capable of enhancing chemoattraction and angiogenesis, 129 promoting rat major pelvic ganglia neurite outgrowth, and activating the JAK/STAT pathway in cultured Schwann cells.128 CXCL5 may thus contribute to ADSCs' therapeutic efficacy in cavernous nerve injury-induced ED.
EPCs
While EPCs have been evaluated for predicting vasculogenic ED as discussed above, there are few reports regarding the use of EPCs to treat ED. In 2010, Gou's team 130 investigated intracavernosal injection of EPCs overexpressing VEGF. The results showed that modified EPCs could restore the erectile function of rats with diabetic ED by enhancing the expression of VEGF, facilitating the process of neovascularisation and incorporating into the endothelium to improve the function of ECs.
Umbilical cord blood stem cells (UCBSCs)
Umbilical cord blood is an important source of stem cells. UCBSCs are deemed to be the newest and youngest among the stem cells. They also avoid the debates surrounding ESCs because they can be obtained without destroying an embryo. Moreover, UCBSCs do not possess mutations in DNA, which are commonly found in ASCs.131 In 2010, intracavernous transplantation of human UCBSCs was reported to improve erectile function and blood glucose levels in patients with diabetic ED without immune suppression.132 This study is the first clinical report describing the use of unmodified stem cell therapy for the treatment of diabetic ED. However, the exact mechanisms were not elucidated and may involve as-yet-unidentified humoral factors generated by the stem cells themselves. Moreover, the total cell number that was injected in the report was quite small relative to the unit body weights compared with other studies, and the rates of implantation and differentiation of stem cells in the corpus cavernosa during the follow-up remained unknown.
Brain-derived stem cells (BDSCs)
Previous work showed the remarkable plasticity of central nervous system-derived stem cells; they have the capacity to give rise to large, flat smooth muscle-like cells that express phenotypic characteristics of SMCs.133 In 2009, Song et al.134 injected foetal BDSCs labelled with green fluorescent protein into penile tissue. Six weeks later, there was evidence of transdifferentiation of BDSCs into penile SMCs as the differentiated cells (30%–40%) expressed smooth muscle markers. Although the demonstration of pluripotency and confirmation of the expression of smooth muscle markers confirm that BDSCs are worthy of further examination as a potential resource for penile smooth muscle regeneration, clinical application will be limited regarding the source of these cells.
Neural crest stem cells (NCSCs)
NCSCs are the progenitor cells of cell types constituting the peripheral nervous system, including neurons, Schwann cells and adrenal chromaffin cells as well as SMCs.135 NCSCs are closely related to neural tube cells, as both are descendants of the same higher order stem cells.136 Importantly, similar to ASCs, intraspinal administration of NCSCs did not result in the formation of tumours or teratomas.137 Many studies have shown that transplantation of NCSCs could induce the regeneration of connective tissues, SMCs, skeletal muscle and endothelium. In 2008, the feasibility of using human NCSCs for corpus cavernosum repair and potential differentiation of NCSCs into penile cavernous sinus ECs and SMCs was investigated.135 Histological analysis indicated that transplanted NCSCs could possibly differentiate into ECs and SMCs, as shown by their expression of cell type-specific markers. However, we do not know whether NCSCs-based cell therapy could compromise penile erectile function in vivo in animals. Furthermore, the differentiation of human NCSCs is unconvincing before excluding the effects of fusion between transplanted cells and host cells. The critical limitation of NCSCs in clinical application, similar to BDSCs, is the lack of a sufficient source of these donor cells.
Glial restricted progenitor cells (GRPCs)
In 2011, Nout et al.138 delivered GRPCs into a rat model of spinal cord injury, and they found that those animals regained some autonomic functions, such as a similar number of erectile events per 24 h compared to the control group, implying that GRPCs have potential therapeutic efficacy in patients with ED associated with spinal cord injury.
ESCs
Although ESCs demonstrate powerful differentiation potency, research has lagged due to legal and ethical considerations. In 2004, ESCs transfected with BDNF that had differentiated along a neuronal cell line were injected into the corpus cavernosum and found to improve cavernous nerve regeneration and functional erectile status after bilateral crush injury in the rat.139 Stem cells may present a cellular substrate at the lesion site to support axonal extension. In addition, stem cells may not require a prolonged presence to function; their mechanism of action may be through growth factor expression (BDNF, NGF and neurotrophin-3),140 inhibition of demyelination and as an initial lattice of cellular substrate.
Perspectives
Stem cells, especially the ASCs, are regarded as candidates for the diagnosis and treatment of ED due to many factors. Stem cells possess the potential to undergo long-term proliferation, self-renewal and multipotent differentiation, and they can serve as a vehicle for the release of neurotrophins, such as nerve growth factor, to repair damage caused by diabetes or pelvic surgery.
In addition to using stem cells for treatment of ED, stem cells could be used as a predictor for the diagnosis and progress of ED.
It is worth noting that except for the application of exogenous stem cells, reawakening and mobilizing endogenous stem cells could allow the prevention and treatment of ED in the near future.
Of note, while most of the literature cited in this review concerns animal studies, increasing interest is being given to translation of the observed results into clinical applications of stem cells. Positive and encouraging results have been obtained with the use of BMSC, MDSC and ADSC in various diseases.
With growing evidence from basic research on the use of stem cells for treatment of ED, in 2010, the first clinical trial was conducted in Korea.132 In this study, morning erection was restored within one month in three patients and within 3 months in six patients. However, despite having increased penile rigidity, none of the patients was able to achieve vaginal penetration unless aided by taking sildenafil before coitus. In 2011, another clinical trial was carried out in France with intracavernous BMSCs injected into patients with ED post-prostatectomy. The transplanted stem cells were autologous intracavernous bone marrow-mononucleated cells containing mainly MSCs, EPCs and HSCs (http://www.clinicaltrials.gov/ct2/show/NCT01089387?term=stem±cell±and±ED&rank=1). As stated above, a significant proportion of preclinical studies investigating the effects of stem cells on ED concern cavernous nerve injury-induced ED, a condition that we observe almost solely after pelvic surgery for malignancies. The application of stem cells in this condition poses the risk of interaction between the stem cells and prostate tumour cell lines,141 and it is difficult to predict the effects of this interaction in vivo. Thus, while animal studies are promising, translation to clinical application requires further study on how various types of stem cells and their secretions interact with tumour cells.
Furthermore, it remains largely unknown what the fate of the majority of stem cells is after injection into experimental animals. As investigators focus on penile changes after cellular therapy, the effects that this therapy has on other, non-diseased organ tissues is still a mystery in the field of sexual medicine. This issue warrants further investigations before translating these results and applying these cell types in human patients.
Conclusions
A broad range of stem cell sources has been investigated preclinically and has shown a high potential in the treatment and prevention of ED of various origins. While preclinical animal data are highly promising, attention is needed to elucidate the mechanisms of action of stem cell therapy for ED and the potential adverse events associated with stem cell application before the translation to clinical application is attempted.
The authors declare no competing financial interests.
References
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–98. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, et al. Recent advances in andrology-related stem cell research. Asian J Androl. 2008;10:171–5. doi: 10.1111/j.1745-7262.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–55. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Becker C, Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007;51:1217–28. doi: 10.1016/j.eururo.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Strong TD, Gebska MA, Champion HC, Burnett AL, Bivalacqua TJ. Stem and endothelial progenitor cells in erection biology. Int J Impot Res. 2008;20:243–54. doi: 10.1038/sj.ijir.3901635. [DOI] [PubMed] [Google Scholar]
- Yamzon JL, Kokorowski P, Koh CJ. Stem cells and tissue engineering applications of the genitourinary tract. Pediatr Res. 2008;63:472–7. doi: 10.1203/PDR.0b013e31816a704a. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Vrana KE, Hipp JD, Goss AM, McCool BA, Riddle DR, et al. Nonhuman primate parthenogenetic stem cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11911–6. doi: 10.1073/pnas.2034195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Impotence NIH Consensus Conference: impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- Burnett AL. Erectile dysfunction. J Urol. 2006;175:S25–31. doi: 10.1016/S0022-5347(05)00309-5. [DOI] [PubMed] [Google Scholar]
- Wessells H, Williams SK. Endothelial cell transplantation into the corpus cavernosum: moving towards cell-based gene therapy. J Urol. 1999;162:2162–4. doi: 10.1016/S0022-5347(05)68152-9. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Yu X, Hui N, Liu S. Application of iPS in assisted reproductive technology: sperm from somatic cells. Stem Cell Rev. 2011;7:714–21. doi: 10.1007/s12015-011-9236-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Presnell SC, Petersen B, Heidaran M. Stem cells in adult tissues. Semin Cell Dev Biol. 2002;13:369–76. doi: 10.1016/s1084952102000939. [DOI] [PubMed] [Google Scholar]
- Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–27. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–46. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22–6. [PubMed] [Google Scholar]
- Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–8. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- McCulloch EA, Till JE. Proliferation of hemopoietic colony-forming cells transplanted into irradiated mice. Radiat Res. 1964;22:383–97. [PubMed] [Google Scholar]
- Till JE, McCulloch EA, Siminovitch L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci USA. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Garcia M, Ning H, Banie L, Guo YL, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer JC. Primer and interviews: the dynamic stem cell niche. Dev Dyn. 2011;240:737–43. doi: 10.1002/dvdy.22566. [DOI] [PubMed] [Google Scholar]
- Becerra J, Santos-Ruiz L, Andrades JA, Mari-Beffa M. The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev. 2011;7:248–55. doi: 10.1007/s12015-010-9195-5. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–64. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–5. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–15. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–6. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–9. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- Ergun S, Tilki D, Klein D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal. 2011;15:981–95. doi: 10.1089/ars.2010.3507. [DOI] [PubMed] [Google Scholar]
- Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503–6. doi: 10.1016/j.stem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–6. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaldoyanidi S. Directing stem cell homing. Cell Stem Cell. 2008;2:198–200. doi: 10.1016/j.stem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–7. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- Wright N, Hidalgo A, Rodriguez-Frade JM, Soriano SF, Mellado M, et al. The chemokine stromal cell-derived factor-1 alpha modulates alpha 4 beta 7 integrin-mediated lymphocyte adhesion to mucosal addressin cell adhesion molecule-1 and fibronectin. J Immunol. 2002;168:5268–77. doi: 10.4049/jimmunol.168.10.5268. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home. Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–96. [PubMed] [Google Scholar]
- Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107:79–86. doi: 10.1182/blood-2005-05-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, et al. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003;2003:419–37. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–95. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–88. [PubMed] [Google Scholar]
- Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM, et al. Synergistic mobilization of hemopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient mice. Blood. 2001;97:1282–8. doi: 10.1182/blood.v97.5.1282. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tajima F, Ishiga K, Yamazaki H, Oshimura M, et al. Soluble c-kit receptor mobilizes hematopoietic stem cells to peripheral blood in mice. Exp Hematol. 2004;32:390–6. doi: 10.1016/j.exphem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, et al. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–8. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008;26:163–72. doi: 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- Bartsch G, Yoo JJ, de Coppi P, Siddiqui MM, Schuch G, et al. Propagation, expansion, and multilineage differentiation of human somatic stem cells from dermal progenitors. Stem Cells Dev. 2005;14:337–48. doi: 10.1089/scd.2005.14.337. [DOI] [PubMed] [Google Scholar]
- Chen FG, Zhang WJ, Bi D, Liu W, Wei X, et al. Clonal analysis of nestin− vimentin+ multipotent fibroblasts isolated from human dermis. J Cell Sci. 2007;120:2875–83. doi: 10.1242/jcs.03478. [DOI] [PubMed] [Google Scholar]
- Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, et al. Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biol Reprod. 2005;73:1199–210. doi: 10.1095/biolreprod.105.041038. [DOI] [PubMed] [Google Scholar]
- Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, et al. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156–64. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- Vernet D, Heydarkhan S, Kovanecz I, Lue YH, Rajfer J, et al. Characterization of endogenous stem cells from the mouse penis that express an embryonic stem cell gene and undergo differentiation into several cell lineages. J Urol. 2009;181 Suppl:43. [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, et al. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–14. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero A. New immunophenotype of blood endothelial progenitor cells and endothelial microparticles in patients with arterial erectile dysfunction and late onset hypogonadism. J Androl. 2011;32:509–17. doi: 10.2164/jandrol.110.011643. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Foresta C, Caretta N, Lana A, Cabrelle A, Palu G, et al. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005;17:288–90. doi: 10.1038/sj.ijir.3901311. [DOI] [PubMed] [Google Scholar]
- Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11:18–25. doi: 10.1007/s10157-006-0448-1. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- Foresta C, Ferlin A, de Toni L, Lana A, Vinanzi C, et al. Circulating endothelial progenitor cells and endothelial function after chronic Tadalafil treatment in subjects with erectile dysfunction. Int J Impot Res. 2006;18:484–8. doi: 10.1038/sj.ijir.3901465. [DOI] [PubMed] [Google Scholar]
- Foresta C, Caretta N, Lana A, de Toni L, Biagioli A, et al. Relationship between vascular damage degrees and endothelial progenitor cells in patients with erectile dysfunction: effect of vardenafil administration and PDE5 expression in the bone marrow Eur Urol 2007511411–7.discussion 1417–9. [DOI] [PubMed] [Google Scholar]
- Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, et al. Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27:2247–55. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- Baumhakel M, Werner N, Bohm M, Nickenig G. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J. 2006;27:2184–8. doi: 10.1093/eurheartj/ehl202. [DOI] [PubMed] [Google Scholar]
- Foresta C, de Toni L, Biagioli A, Ganz F, Magagna S, et al. Increased levels of osteocalcin-positive endothelial progenitor cells in patients affected by erectile dysfunction and cavernous atherosclerosis. J Sex Med. 2010;7:751–7. doi: 10.1111/j.1743-6109.2009.01520.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–57. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Kendirci M, Bejma J, Hellstrom WJ. Update on erectile dysfunction in prostate cancer patients. Curr Opin Urol. 2006;16:186–95. doi: 10.1097/01.mou.0000193407.05285.d8. [DOI] [PubMed] [Google Scholar]
- Aversa A, Bruzziches R, Vitale C, Marazzi G, Francomano D, et al. Chronic sildenafil in men with diabetes and erectile dysfunction. Expert Opin Drug Metab Toxicol. 2007;3:451–64. doi: 10.1517/17425255.3.3.451. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cadavid NF, Rajfer J. Molecular pathophysiology and gene therapy of aging-related erectile dysfunction. Exp Gerontol. 2004;39:1705–12. doi: 10.1016/j.exger.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Strong TD, Gebska MA, Burnett AL, Champion HC, Bivalacqua TJ. Endothelium-specific gene and stem cell-based therapy for erectile dysfunction. Asian J Androl. 2008;10:14–22. doi: 10.1111/j.1745-7262.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- Jeong JO, Han JW, Kim JM, Cho HJ, Park C, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–7. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Zhong JF. Twisting immune responses for allogeneic stem cell therapy. World J Stem Cells. 2009;1:30–5. doi: 10.4252/wjsc.v1.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Ma A, Wang T, Han K, Liu Y, et al. Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch. 2006;453:43–52. doi: 10.1007/s00424-006-0117-y. [DOI] [PubMed] [Google Scholar]
- Deng W, Bivalacqua TJ, Chattergoon NN, Hyman AL, Jeter JR, Jr, et al. Adenoviral gene transfer of eNOS: high-level expression in ex vivo expanded marrow stromal cells. Am J Physiol Cell Physiol. 2003;285:C1322–9. doi: 10.1152/ajpcell.00141.2003. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278–90. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- Song YS, Lee HJ, Park IH, Kim WK, Ku JH, et al. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007;19:378–85. doi: 10.1038/sj.ijir.3901539. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–8. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, et al. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560–6. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Lin H, Wang Y, Yu W, Chen Y, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2010;8:427–36. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz MT, El-Haggar S, Mostafa T, Atta H, Fouad H, et al. Effect of mesenchymal stem cell penile transplantation on erectile signaling of aged rats. Andrologia. 2010;42:187–92. doi: 10.1111/j.1439-0272.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, Sun C, Yu W, Lin H, Sun Z, et al. Combined strategy of mesenchymal stem cells injection with VEGF gene therapy for the treatment of diabetes associated erectile dysfunction J Androl 2011. e-pub ahead of print 10 February doi:10.2164/jandrol.110.012666. [DOI] [PubMed]
- Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis. Nat Med. 2000;6:1102–3. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–6. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143:577–81. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drowley L, Okada M, Beckman S, Vella J, Keller B, et al. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther. 2010;18:1865–73. doi: 10.1038/mt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, de Miguel F, Usiene I, Kwon D, Yoshimura N, et al. Injection of skeletal muscle-derived cells into the penis improves erectile function. Int J Impot Res. 2006;18:329–34. doi: 10.1038/sj.ijir.3901434. [DOI] [PubMed] [Google Scholar]
- Ji C, Min F, Liang W, Chen Y, Pan S, et al. Construction of tissue-engineered corpus cavernosum with muscle-derived stem cells and transplantation in vivo. BJU Int. 2010;107:1638–46. doi: 10.1111/j.1464-410X.2010.09695.x. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, de Ugarte DA, Huang JI, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Banie L, Ning H, Bella AJ, Lin CS, et al. Potential of adipose-derived stem cells for treatment of erectile dysfunction. J Sex Med. 2009;6 Suppl 3:320–7. doi: 10.1111/j.1743-6109.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–8. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- Ning H, Lin G, Fandel T, Banie L, Lue TF, et al. Insulin growth factor signaling mediates neuron-like differentiation of adipose-tissue-derived stem cells. Differentiation. 2008;76:488–94. doi: 10.1111/j.1432-0436.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony J, Bella T, Lin G, Phonsombat S, Lin CS, et al. Non-cell line induced autologous adult adipose tissue derived stem cells enhance recovery of erectile function in the rat following bilateral cavernous nerve crush injury Sex Med Soc North Am Meet 200868Abstract. [Google Scholar]
- Fandel TM, Bella AJ, Lin G, Tantiwongse K, Lin CS, et al. Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2008;5:1866–75. doi: 10.1111/j.1743-6109.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- Ning H, Liu G, Lin G, Yang R, Lue TF, et al. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–79. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMuzio P, Tulenko T. Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg. 2007;45 Suppl A:A99–103. doi: 10.1016/j.jvs.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J . 2002;16:1102–4. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391–400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, et al. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Seo SW, Choi CY, Kim BS. Porous poly(lactic-co-glycolic acid) microsphere as cell culture substrate and cell transplantation vehicle for adipose tissue engineering. Tissue Eng Part C Methods. 2008;14:25–34. doi: 10.1089/tec.2007.0290. [DOI] [PubMed] [Google Scholar]
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–40. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang R, Wang Z, Lin G, Lue TF, et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437–46. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ning H, Banie L, Wang G, Lin G, et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with chemoattractant and angiogenic properties. Biochem Biophys Res Commun. 2010;402:560–4. doi: 10.1016/j.bbrc.2010.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X, He WY, Xiao MZ, Qiu M, Wang M, et al. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl. 2010;13:332–8. doi: 10.1038/aja.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rube CE, Fricke A, Widmann TA, Furst T, Madry H, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahk JY, Jung JH, Han H, Min SK, Lee YS. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010;8:150–60. [PubMed] [Google Scholar]
- Tsai RY, McKay RD. Cell contact regulates fate choice by cortical stem cells. J Neurosci. 2000;20:3725–35. doi: 10.1523/JNEUROSCI.20-10-03725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Mehta N, Sheh B, Saljooque F, U HS, et al. Transdifferentiation of rat fetal brain stem cells into penile smooth muscle cells. BJU Int. 2009;104:257–62. doi: 10.1111/j.1464-410X.2009.08352.x. [DOI] [PubMed] [Google Scholar]
- Song YS, Lee HJ, Park IH, Lim IS, Ku JH, et al. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction BJU Int 2008102220–4.discussion 224. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Mayer-Proschel M, Rao MS. A common neural progenitor for the CNS and PNS. Dev Biol. 1998;200:1–15. doi: 10.1006/dbio.1998.8913. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M, Schnell L, Grim M, Hu YF, Schneider R, et al. Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol Cell Neurosci. 2006;32:67–81. doi: 10.1016/j.mcn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nout YS, Culp E, Schmidt MH, Tovar CA, Proschel C, et al. Glial restricted precursor cell transplant with cyclic adenosine monophosphate improved some autonomic functions but resulted in a reduced graft size after spinal cord contusion injury in rats. Exp Neurol. 2011;227:159–71. doi: 10.1016/j.expneurol.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–9. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–29. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lin G, Yang R, Banie L, Wang G, Ning H, et al. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010;70:1066–73. doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]


