ABSTRACT
We evaluated levels of vaginal extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinase (MMP-8) in vaginal secretions in relation to the composition of vaginal bacterial communities and d- and l-lactic acid levels. The composition of vaginal bacterial communities in 46 women was determined by pyrosequencing the V1 to V3 region of 16S rRNA genes. Lactobacilli were dominant in 71.3% of the women, followed by Gardnerella (17.4%), Streptococcus (8.7%), and Enterococcus (2.2%). Of the lactobacillus-dominated communities, 51.5% were dominated by Lactobacillus crispatus, 36.4% by Lactobacillus iners, and 6.1% each by Lactobacillus gasseri and Lactobacillus jensenii. Concentrations of l-lactic acid were slightly higher in lactobacillus-dominated vaginal samples, but most differences were not statistically significant. d-Lactic acid levels were higher in samples containing L. crispatus than in those with L. iners (P < 0.0001) or Gardnerella (P = 0.0002). The relative proportion of d-lactic acid in vaginal communities dominated by species of lactobacilli was in concordance with the proportions found in axenic cultures of the various species grown in vitro. Levels of l-lactic acid (P < 0.0001) and the ratio of l-lactic acid to d-lactic acid (P = 0.0060), but not concentrations of d-lactic acid, were also correlated with EMMPRIN concentrations. Moreover, vaginal concentrations of EMMPRIN and MMP-8 levels were highly correlated (P < 0.0001). Taken together, the data suggest the relative proportion of l- to d-lactic acid isomers in the vagina may influence the extent of local EMMPRIN production and subsequent induction of MMP-8. The expression of these proteins may help determine the ability of bacteria to transverse the cervix and initiate upper genital tract infections.
IMPORTANCE
A large proportion of preterm births (>50%) result from infections caused by bacteria originating in the vagina, which requires that they traverse the cervix. Factors that influence susceptibility to these infections are not well understood; however, there is evidence that matrix metalloproteinase (MMP-8) is known to alter the integrity of the cervix. In this work, we show that concentrations of vaginal extracellular matrix metalloproteinase inducer (EMMPRIN) are influenced by members of the vaginal microbial community and concentrations of d- or l-lactic acid isomers in vaginal secretions. Elevated levels of d-lactic acid and the ratio of d- to l-lactic acid influence EMMPRIN concentrations as well as MMP-8 levels. Thus, isomers of lactic acid may function as signaling molecules that alter host gene expression and influence risk of infection-related preterm birth.
Introduction
The human vagina is home to a wide diversity of microbial organisms. In a recent study in which the vaginal microbial communities of 400 women from four ethnicities were sampled, Ravel et al. (1) found five distinct community types. Four of them, community types I, II, III, and V, are dominated by, but not exclusively composed of, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, respectively, while a greater diversity of taxa are found in community type IV, with a predominance of strict anaerobes (1).
It has been widely accepted that lactic acid-producing bacteria, particularly Lactobacillus species, promote vaginal health by the production of lactic acid, as well as by other less-defined mechanisms. Reducing vaginal pH to about 4 to 5 restricts the growth of potentially harmful bacteria (2). The loss of lactobacilli and overgrowth of other bacterial taxa contribute to the rise of bacterial vaginosis (BV) and aerobic vaginitis (AV). Both conditions are associated with adverse physical symptoms, including vaginal odor, discharge, and pain, as well as increased susceptibility to upper genital tract infections in nonpregnant women and with infection-mediated premature delivery when present during pregnancy (3, 4). The explanation(s) for the loss of lactic acid-producing bacteria, as well as the mechanisms leading to BV- and AV-associated pathology, remains undetermined. It has been noted that of the four principal Lactobacillus species found in vaginal communities of reproductive-age women, L. iners is more often associated with BV (5–7). This suggests that biological characteristics specific to this species may make a vaginal microbial community more susceptible to BV or AV.
Extracellular metalloproteinase inducer (EMMPRIN) or CD147 is a protein present on human host cell membranes and in extracellular fluid. It is a major inducer of matrix metalloproteinase (MMP-8), an enzyme that degrades the extracellular matrix (8). EMMPRIN-induced MMP-8 has been strongly implicated in promoting tumor metastasis (9), controlling endometrial breakdown and regeneration during the menstrual cycle (10), and allowing bacteria to pass through the endocervix (11). In addition, EMMPRIN is an essential cofactor for protein monocarboxylate transporter 1 (MCT-1), which regulates intracellular lactic acid levels (12). Lactic acid is produced by the anaerobic metabolism of glucose and other sugars to provide energy in the form of adenosine triphosphate. Upregulation of EMMPRIN and MCT-1 protects cells from intracellular lactic acid buildup and maintains intracellular pH at a functional level (8).
We have recently reviewed the mechanism leading to lactic acid predominance in the vagina (2). Mammalian cells, including vaginal epithelial cells, produce only the l-lactic acid isomer, with the possible exception of low levels of d-lactate production via the methylglyoxal pathway (13). In contrast, lactic acid-producing bacteria produce both the d- and l-lactic acid isomers (2). We and others have also presented evidence that l-lactic acid, in addition to its role in influencing vaginal acidity, has specific immune properties, such as stimulation of the interleukin 23 (IL-23)/IL-17 T lymphocyte pathway (14, 15), induction of proinflammatory cytokines by vaginal epithelial cells in the presence of a synthetic viral RNA (16), induction of tumor angiogenesis (17), lymphocyte activation (18), and inhibition of bacterial growth (19, 20). However, the role that d-lactic acid plays in the vaginal ecosystem has not been investigated. In this communication, we report the production of lactic acid isomers by the Lactobacillus species that dominate human vaginal microbial communities and define the relationship between communities dominated by these species and the appearance of EMMPRIN and MMP-8 in the vaginal fluid. We hypothesize that these interrelationships influence susceptibility to upper genital tract infection.
RESULTS
The bacterial taxa present in the vaginal communities of 46 women were identified by classification of partial 16S rRNA gene sequences amplified from vaginal swab samples. The total numbers of phyla and Lactobacillus species detected are shown in Table 1. Lactobacilli dominated the vaginal communities of 33 of the 46 women (71.3%). At the species level, L. crispatus (37.0%) and L. iners (26.1%) were most prevalent in all but four of the lactobacillus-dominant vaginal communities. Gardnerella was the dominant genus in eight (17.4%) of the women, Streptococcus was the dominant genus in four (8.7%) women, and Enterococcus was the dominant genus in one woman. The mean ages of women with (31.0 ± 6.6 years) or without (32.9 ± 6.3 years) Lactobacillus-dominated communities did not differ. There was no association between community type and parity or gravidity.
TABLE 1 .
Composition of vaginal bacterial communitiesa
| Taxon | No. (%) of subjects (prevalence) |
No. (%) of communities (dominance) |
|---|---|---|
| Lactobacillus | 40 (88.9) | 33 (71.3) |
| L. crispatus | 21 (46.7) | 17 (37.0) |
| L. iners | 19 (42.2) | 12 (26.1) |
| L. jensenii | 12 (26.7) | 2 (4.3) |
| L. gasseri | 8 (17.8) | 2 (4.3) |
| Gardnerella | 11 (24.4) | 8 (17.4) |
| Streptococcus | 8 (17.8) | 4 (8.7) |
| Prevotella | 8 (17.8) | 0 |
| Dialister | 6 (13.3) | 0 |
| Atopobium | 5 (11.1) | 0 |
| E. coli/Shigella | 4 (8.9) | 0 |
| Finegoldia | 4 (8.9) | 0 |
| Peptoniphilus | 4 (8.9) | 0 |
| Sneathia | 3 (6.7) | 0 |
| Megasphaera | 3 (6.7) | 0 |
| Veillonella | 3 (6.7) | 0 |
| Coriobacter | 2 (4.4) | 0 |
| Anaerococcus | 2 (4.4) | 0 |
| Ureaplasma | 2 (4.4) | 0 |
| Corynebacterium | 2 (4.4) | 0 |
| Staphylococcus | 1 (2.2) | 0 |
| Enterococcus | 1 (2.2) | 1 (2.2) |
| Peptostreptococcus | 1 (2.2) | 0 |
| Acidovorax | 1 (2.2) | 0 |
| Acinetobacter | 1 (2.2) | 0 |
| Cloacibacterium | 1 (2.2) | 0 |
| Diaphorobacter | 1 (2.2) | 0 |
The compositions of vaginal bacterial communities of 46 women were determined by analysis of 16S rRNA gene sequences as described in Materials and Methods. Dominance refers to the number of instances in which a taxon was the largest fraction of a community.
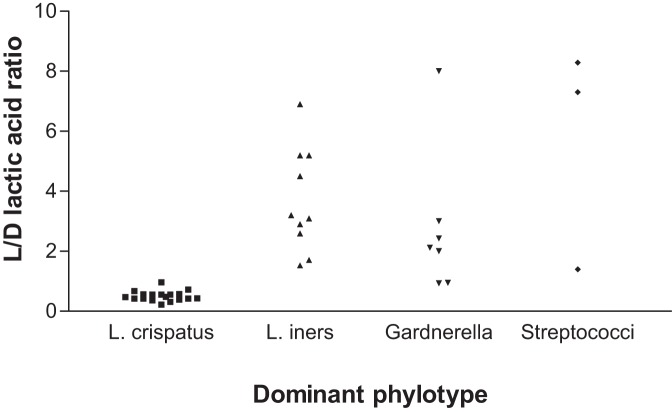
The concentrations of d- and l-lactic acid in women with different vaginal community types are shown in Table 2. The median concentration of l-lactic acid was lower in women with vaginal communities dominated by Gardnerella, Streptococcus, or Enterococcus than in women where lactobacilli dominated. However, due perhaps to low numbers, the only significant difference in l-lactic acid concentration was between L. iners and Gardnerella (P = 0.0105). In marked contrast, the median concentration of d-lactic acid was significantly higher in women whose vaginas were dominated by L. crispatus than in those dominated by L. iners (P < 0.0001) or Gardnerella (P = 0.0002). The concentration of l-lactic acid in women whose vaginas were dominated by L. iners, Gardnerella, and Streptococcus greatly exceeded the level of d-lactic acid, as evidenced by the median ratio of l- to d-lactic acid being greater than 2 (Table 2). This ratio was 0.48 when L. crispatus was dominant. The ratio of l- to d-lactic acid in the individual samples is shown in Fig. 1.
TABLE 2 .
Lactic acid isomers in the vagina of women with different dominant bacterial communities
| Dominant species | No. of samples | Median concn (range) |
||
|---|---|---|---|---|
| d-Lactic acid (mM) | l-Lactic acid (mM) | l/d-Lactic acid ratio | ||
| L. crispatus | 17 | 0.32 (0.26–4.80)a | 0.57 (0.08–2.78) | 0.48 (0.22–0.98)b |
| L. iners | 12 | 0.06 (<0.02–1.36) | 0.57 (0.26–3.10)c | 3.15 (1.53–6.90) |
| L. gasseri | 2 | 2.92 (0.23–5.60) | 2.23 (0.16–4.30) | 0.73 (0.69–0.76) |
| L. jensenii | 2 | 0.45 (<0.02–0.89) | 0.85 (0.55–1.15) | 2.02 (1.29–2.75) |
| Gardnerella | 8 | 0.07 (<0.02–1.29) | 0.17 (0.03–2.00) | 2.43 (0.93–26.0) |
| Streptococcus | 4 | 0.03 (<0.02–0.49) | 2.20 (0.14–4.06) | 7.30 (1.40–8.29) |
| Enterococcus | 1 | 0.03 | 0.08 | 2.67 |
P < 0.0001 versus L. iners d-lactic acid; P = 0.0002 versus Gardnerella d-lactic acid.
P < 0.0001 versus L. iners and Gardnerella l/d-lactic acid ratio; P = 0.0015 versus Streptococcus l/d-lactic acid ratio.
P = 0.0105 versus Gardnerella l-lactic acid.
FIG 1 .
Association of dominant vaginal bacteria and the ratio of l- to d-lactic acid in vaginal fluid. The composition of vaginal bacterial communities was determined by analysis of 16S rRNA gene sequences. The species of Lactobacillus and other genera present that dominated (constituted the highest proportions) each community was identified. The concentrations of d- and l-lactic acid in each vaginal sample were determined by enzymatic assays. Regression coefficients and P values are reported in the text.
The relationships between vaginal concentrations of EMMPRIN and levels of l-lactic acid, d-lactic acid, and MMP-8 are shown in Fig. 2. Vaginal EMMPRIN concentrations show positive statistical correlation to the concentrations of l-lactic acid (Spearman r = 0.6580, P < 0.0001) and MMP-8 (Spearman r = 0.5875, P < 0.0001). However, there was no association between EMMPRIN and d-lactic acid concentration (Spearman r = 0.2273, P = 0.1244). Vaginal EMMPRIN concentrations were also positively correlated to the relative ratio of l- to d-lactic acid in the vagina (P = 0.0060).
FIG 2 .
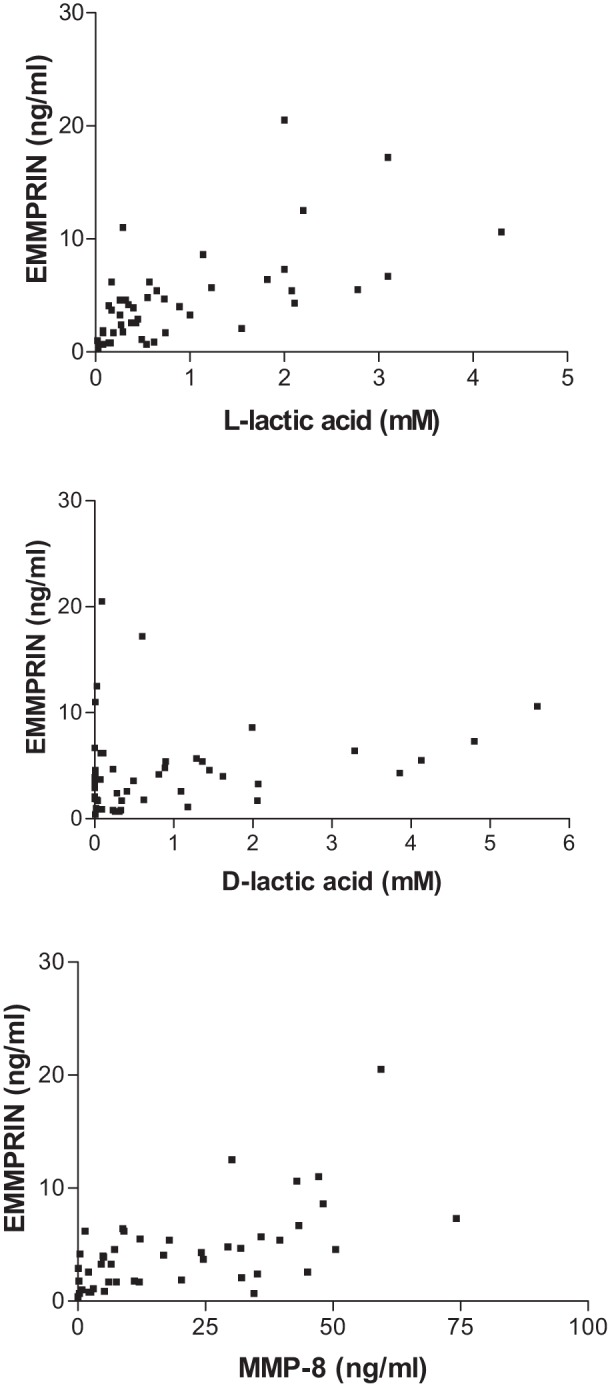
Association of EMMPRIN with d- and l-lactic acid and MMP-8 in vaginal fluid. Vaginal fluids were assayed for EMMPRIN and MMP-8 by ELISA, and d-lactic acid and l-lactic acid were determined by enzymatic assays. Associations between concentrations of EMMPRIN and l-lactic acid (top), d-lactic acid (middle), and MMP-8 (bottom) were analyzed by the Spearman rank correlation test. Regression coefficients and P values are reported in the text.
The genome sequences of the four principal species of Lactobacillus found in the vaginal microbiome show differences in the potential for producing d- and l-lactic acid based on the presence or absence of d- and l-lactate dehydrogenase (Table 3). While the genomes of L. crispatus and L. gasseri contained two copies of l-lactate dehydrogenase and one copy of d-lactate dehydrogenase, the genome of L. jensenii has the inverse, two copies of d-lactate dehydrogenase and only one copy of l-lactate dehydrogenase. Surprisingly, the gene coding for d-lactate dehydrogenase is completely absent from the genome of L. iners UPII 60-B.
TABLE 3 .
Species and strains of Lactobacillus used in this studya
| Lactobacillus species | Original strain designation | BEI designation | Genome size (Mb) | No. of copies per genome |
|
|---|---|---|---|---|---|
| LLD | DLD | ||||
| L. crispatus | MV-1A-US | HM-637 | 2.17 | 2 | 1 |
| L. crispatus | JV-V01 | HM-103 | 2.07 | 2 | 1 |
| L. gasseri | MV-22 | HM-644 | 1.93 | 2 | 1 |
| L. gasseri | SV-16A-US | HM-642 | 1.99 | 2 | 1 |
| L. gasseri | SJ-9E-US | HM-641 | 1.78 | 2 | 1 |
| L. iners | UPII 60-B | HM-131 | 1.32 | 2 | 0 |
| L. jensenii | SJ-7A-US | HM-639 | 1.68 | 1 | 2 |
| L. jensenii | 1153-3-CHN | HM-640 | 1.62 | 1 | 2 |
| L. jensenii | JV-V16 | HM-105 | 1.60 | 1 | 2 |
| L. jensenii | 269-3 | HM-645 | 1.69 | 1 | 2 |
BEI, Biodefense and Emerging Infections Resources; LLD, l-lactate dehydrogenase; DLD, d-lactate dehydrogenase.
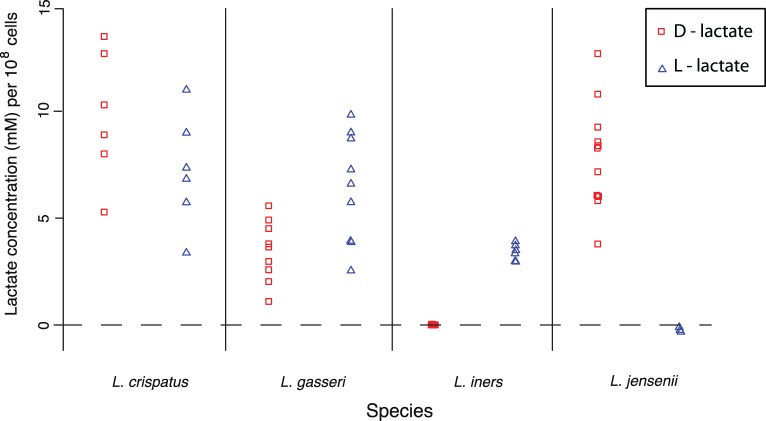
Growth of pure cultures of several Lactobacillus species under in vitro conditions allowed us to test if d- and l-lactic acid were in fact being produced as expected (Fig. 3 and Table 4). Both L. gasseri and L. crispatus produced both isomers. l-Lactic acid production by L. jensenii was below the level of detection. A similar situation was observed for the production of d-lactic acid by L. iners. However, for this species, the lack of detection of this isomer constitutes corroborative evidence that this species may in fact be unable to produce d-lactic acid.
FIG 3 .
Concentration of d- and l-lactate produced in axenic cultures of different Lactobacillus species. A value of zero indicates that the isomer was not detected in the samples analyzed.
TABLE 4 .
Production of d- and l-lactic acid isomers in axenic cultures of lactobacilli
| Species | Lactate isomer | No. of strainsa | Concn (mM)/108 cellsb |
|
|---|---|---|---|---|
| Mean | SD | |||
| L. crispatus | d | 2 | 9.78 | 3.06 |
| l | 2 | 7.21 | 2.63 | |
| L. gasseri | d | 3 | 3.46 | 1.46 |
| l | 3 | 6.39 | 2.56 | |
| L. iners | d | 1 | 0.01 | 0.01 |
| l | 1 | 3.40 | 0.39 | |
| L. jensenii | d | 4 | 7.74 | 2.45 |
| l | 4 | ND | ND | |
Lactic acid concentration was estimated in triplicate for each strain, except for L. iners, where concentration was estimated for six independent biological replicates of the same strain.
ND, the concentration of lactic acid was not determined.
DISCUSSION
Vaginal EMMPRIN has at least two roles: the regulation of intracellular lactic acid levels in host cells and the induction of MMP-8 as well as other matrix metalloproteinases (8). The former is essential to prevent a decrease in intracellular pH and acid-mediated cell death. The second role is less well understood, but it may be part of the periodic regeneration of the epithelial layers. Regulation of MMP-8 production by controlling EMMPRIN levels may be essential to avoid its reaching a level capable of degrading the endocervical barrier that prevents vaginal microorganisms from ascending to the upper genital tract. Breeching of this barrier could lead to an upper genital tract infection in both pregnant and nonpregnant women. In the former situation, it could increase susceptibility to infection-related preterm birth.
l-Lactic acid is produced by both vaginal epithelial cells and bacteria, while d-lactic acid is almost exclusively a bacterial product. Both are products of glucose metabolism that occurs in the anaerobic vaginal environment. The l-lactic acid isomer can be actively transported into and out of epithelial cells by an MCT-1- and EMMPRIN-mediated mechanism (8). Our in vitro assays show significant variation in the production of d- and l-lactic acid by the different Lactobacillus species (Fig. 1). This suggests that depending on the dominant species of Lactobacillus present, different concentrations of d- and l-lactic acid may be present in the vaginal environment. In analyses of the vaginal microbiome, we demonstrated that vaginal concentrations of l-lactic acid were similar regardless of the predominant bacterial taxa present in the vagina. The only significant difference noted was between communities dominated by L. iners and those dominated by Gardnerella. This is consistent with the notion that vaginal epithelial cells contribute l-lactic acid to the vaginal milieu isomer in a manner that is relatively independent of the vaginal bacterial populations present. In marked contrast, d-lactic acid levels were strongly associated with the predominance of L. crispatus in the vagina. The detection of low levels of d-lactic acid in the absence of this Lactobacillus was probably due to its production by lactic acid-producing bacteria that were present at low levels in some women.
EMMPRIN concentrations in vaginal secretions were positively correlated to the concentration of l-lactic acid in the vagina. The most likely explanation for this observation is that l-lactic acid induction of EMMPRIN production is necessary to regulate intracellular lactic acid concentrations and maintain cell viability. Vaginal epithelial cells (21) and uterine fibroblasts (22) have been shown to produce EMMPRIN. In addition, the vaginal concentrations of EMMPRIN and MMP-8 were also correlated, suggesting that MMP-8 may be induced as a secondary consequence of this induction. In contrast, vaginal EMMPRIN levels were not correlated to the concentration of d-lactic acid in the vagina. Thus, d-lactic acid is not an EMMPRIN inducer in the female lower genital tract. While the concentration of d-lactic acid by itself was unrelated to vaginal EMMPRIN levels, we found that an increase in the concentration of d-lactic acid relative to the l-lactic acid level correlated significantly to a decrease in EMMPRIN concentration. Since vaginal EMMPRIN is an inducer of MMP-8, the ratio of d- to-l-lactic acid in the vagina would also influence MMP-8 production at this site. Two of the major bacterial species associated with bacterial vaginosis, namely Gardnerella vaginalis and L. iners, appear to be either very poor d-lactic acid producers or capable of readily degrading this isomer. This provides a plausible mechanism for the observation of an association between vaginal MMP-8 levels and bacterial vaginosis (23). In a previous study, we demonstrated that in the presence of a synthetic double-stranded mimic of viral RNA, l-lactic acid strongly facilitated proinflammatory cytokine production by an in vitro-cultured vaginal epithelial cell line (16). Others have shown that l-lactic acid possesses distinct immunological properties and is not merely an end product of anaerobic glycolysis (14–18). Thus, steady-state levels of both lactic acid isomers appear to have distinct multiple roles in the vaginal milieu.
We hypothesize that the paucity or absence of vaginal d-lactic acid results in elevated local EMMPRIN levels and, thus, high concentrations of MMP-8. This facilitates breakdown of the extracellular matrix and allows bacterial migration past the endocervix and into the uterus. If the woman is pregnant, this will increase susceptibility to infection-related preterm birth. In addition, MMP-8 by itself has been implicated in rupture of the fetal membranes (24). In nonpregnant women, it will facilitate upper genital tract infections, resulting in endometritis, salpingitis, and/or oophoritis. Based on this mechanism, and our prior studies on l-lactic acid, it might be advantageous to provide exogenous d-lactic acid to the vagina of women with an elevated vaginal pH that is indicative of a loss of lactobacilli.
It has been suggested that vaginal microbial communities dominated by L. iners may be more susceptible to the incidence of bacterial vaginosis. In this study, we show that women with communities dominated by this species have lower concentrations of d-lactic acid. Our in vitro studies and an analysis of the genome also show that this species seems to be unable to produce d-lactic acid. The association between the reduced levels of d-lactic acid in communities dominated by L. iners and the presence of high concentrations of MMP-8 is in concordance with suggestions that these communities are more susceptible to BV (5–7). It is therefore possible that the incidence of this condition, and possibly others stemming from sexually transmitted infection, is associated specifically with a lower concentration of d-lactic acid in the vaginal communities.
High proportions of lactobacilli are often equated with vaginal health. This is typically attributed to production of lactic acid that acidifies the vaginal environment. The resulting low vaginal pH is purported to preclude or restrict the growth of other microorganisms and thereby reduce the occurrence of population alterations that predispose to disease (20). While vaginal pH undoubtedly does contribute to maintaining microbial homeostasis at this site, it remains to be determined how vaginal health is maintained in adolescents and menopausal women who lack high numbers of lactobacilli (25–27) or in the >25% of reproductive-age women whose vaginal communities are not dominated by Lactobacillus (1). In each of these groups, a vaginal pH of >4.5, the generally accepted upper limit of normal in women with a lactobacillus-dominated bacterial community, is usually present (1, 25–27). The findings of the present study on a potential role of lactate isomers as signaling molecules that influence specific gene expression and previous reports delineating the immunological properties of lactic acid (14) suggest that steady-state levels of d- and l-lactic acid exert multiple influences on the composition and activity of the vaginal milieu, distinct from acidification. These lactic acid-dependent mechanisms might help explain, at least in part, why Homo sapiens is the only host species known in which Lactobacillus spp. typically dominate vaginal communities (28–31).
We further posit that products of bacterial species other than lactobacilli also contribute in some humans as well as in other host species to the maintenance of host vaginal health. Other low-molecular-weight metabolites, including various short-chain fatty acids, produced by nonlactobacillus microbial vaginal inhabitants may also influence host gene expression, especially when these species predominate. We find this to be an attractive hypothesis in part because vaginal bacterial species have coevolved with their hosts over time, and it seems likely that they collectively provide benefits to the host as part of a complex mutualistic relationship. Moreover, this moves away from the perhaps overly simplistic notion that lactobacilli are “good” because they lower the vaginal pH and all other species are inconsequential at best or opportunistic pathogens at worst. This hypothesis is eminently testable and worthy of being investigated in the future.
Limitations of our study are the relatively low number of women evaluated and the fact that some women in the study population were being evaluated for vulvodynia. While we see no observable differences between women with or without vulvodynia in any of the parameters evaluated in this investigation, and there is no prior evidence to suggest alterations in vaginal communities or vaginal fluid components in women with this condition, it remains a possibility that unidentified variables might have influenced our findings. However, the distribution of Lactobacillus species and the proportion of women with and without Lactobacillus-dominated vaginal biotypes were identical to what we observed in previous studies of healthy reproductive-age women (1, 32). Nevertheless, further investigations on larger populations of women, including pregnant women and those with or without bacterial vaginosis or aerobic vaginitis, are essential to validate the present observations.
MATERIALS AND METHODS
Subjects.
The study population consisted of 46 reproductive-age white women (ages 20 to 43). Sixteen of the subjects were undergoing routine yearly examinations, while 30 were being evaluated for vulvodynia. Bacteriological findings in vaginal samples from women with or without vulvodynia were equivalent, and there were no differences in vaginal concentrations of lactic acid isomers, EMMPRIN, or MMP-8 in women with similar biotypes. Therefore, data were combined for this analysis. All women were in good health with no clinical signs or symptoms indicative of either bacterial vaginosis or aerobic vaginitis. In addition, the women had no history of immune or endocrine disorders. Subjects all refrained from vaginal intercourse for at least 1 week prior to sample collection. This study was approved by the Institutional Review Board at Weill Cornell Medical College, and all subjects gave informed consent.
Vaginal samples.
Samples for determination of vaginal bacterial communities were collected by the woman’s physician using the Copan ESwabs sample collection system (Fisher Scientific, Pittsburgh, PA) and frozen at −80°C. The swabs were shipped to the University of Idaho for microbiological analysis of community composition as described previously by pyrosequencing and classification of the V1 to V3 region of bacterial 16S ribosomal RNA gene sequences (1). Vaginal secretions were obtained by rotating a cotton swab against the posterior vaginal wall and releasing the contents into 1.0 ml sterile phosphate-buffered saline. The samples were centrifuged to remove particulate components and the supernatants frozen in aliquots at −80°C until assayed as described below.
Assays.
Concentrations of d- and l-lactic acid were measured colorimetrically using the EnzyChrom l-lactate and EnzyChrom d-lactate kits from BioAssay Systems (Hayward, CA). The levels of MMP-8 (R&D Systems, Minneapolis, MN) and EMMPRIN (R&D Systems) in vaginal secretions were determined in duplicate with commercial enzyme-linked immunosorbent assay (ELISA) kits (Fig. 3). Values were converted to ng/ml or mM by reference to a standard curve that was generated in parallel to the test samples. The lower limit of sensitivity was 0.02 mM for l- and d-lactic acid, 39 pg/ml for EMMPRIN, and 62.5 pg/ml for MMP-8.
Bacterial strains and genome sequences.
The following bacteria were obtained from BEI Resources (http://www.beiresources.org) and used to determine the production of d- and l-lactic acid in axenic cultures: L. crispatus MV-1A-US, L. crispatus JV-V01, L. gasseri MV-22, L. gasseri SV-16A-US, L. gasseri SJ-9E-US, L. iners UPII 60-B, L. jensenii SJ-7A-US, L. jensenii 1153-3-CHN, L. jensenii JV-V16, L. jensenii 269-3 (Table 3). The strains of lactobacilli were grown on MRS-NYC III (MNC; 10 g/liter proteose peptone, 10 g/liter beef extract, 5 g/liter yeast extract, 5 g/liter NaCl, 0.1 g/liter MgSO4, 0.05 g/liter MnSO4, 2 g/liter K2HPO4, 20 g/liter glucose, 100 ml/liter fetal bovine serum) medium supplemented with 1.5% agar, and then individual colonies were used to inoculate 5 ml of MNC broth. All cultures were incubated at 37°C in an atmosphere with 5% CO2. Once the cultures had reached stationary phase, we determined the cell number by measuring culture optical density at 600 nm and converting this to CFU/ml by using a previously determined standard curve. In addition, 1-ml aliquots of cultures were transferred to microcentrifuge tubes and centrifuged at 4,500 × g for 3 min. Five hundred microliters of supernatant was collected, transferred to clean tubes, and stored for up to a week at −20°C. Batches of samples were thawed, and the concentrations of d- and l-lactic acid isomers were measured according to the protocol described above.
We also determined if genomes of these species encoded d-lactate dehydrogenase, l-lactate dehydrogenase, or both. The protein sequences of all strains tested in vitro, as well as their identifiers, were downloaded from PATRIC (26). We then searched for identifiers that contained the words “lactate dehydrogenase,” and the numbers of identifiers for d-lactate dehydrogenase and l-lactate dehydrogenase for each strain were recorded.
We performed a BLASTp (protein) search to compare the protein sequences annotated as either d- or l-lactate dehydrogenase of the strains used to the sequences of the genes originally characterized as coding for l-lactate dehydrogenase (33) and d-lactate dehydrogenase (34) to make sure that the protein sequences were correctly annotated. Except for the strains L. gasseri SV-16A-US and L. gasseri SJ-9E-US, all sequences annotated as the lactate dehydrogenases matched significantly to the originally characterized sequences. For these two L. gasseri strains, we found no protein sequences annotated as either d- or l-lactate dehydrogenase in the NCBI protein database. We therefore downloaded the protein sequences from PATRIC (35) and again used BLASTp to compare these sequences to the original sequences. We found that these sequences were significantly similar as well.
Statistics.
Differences in concentrations of l- and d-lactic acid and the l/d-lactic acid ratio in vaginal secretions between women with different kinds of vaginal communities were analyzed with the Mann-Whitney test, since the values were not normally distributed. The association between d- and l-lactic acid, EMMPRIN, and MMP-8 concentrations in vaginal secretions was analyzed by the Spearman rank correlation test. Because we performed multiple comparisons with each data set, we applied a Bonferroni correction and considered significant a P value of <0.0024.
ACKNOWLEDGMENTS
The technical assistance of Hannah Mossop from Weill Cornell and Sanqing Yuan, Roxana Hickey, and Elizabeth Domreis from the University of Idaho is gratefully acknowledged. Matt Settles from the University of Idaho provided bioinformatic support for analysis of pyrosequencing data and classification of partial 16S rRNA gene sequences.
Footnotes
Citation Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. 2013. Influence of vaginal bacteria and d- and l-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio 4(4):e00460-13. doi:10.1128/mBio.00460-13.
REFERENCES
- 1. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl 1):4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. 2011. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 204:e1–e5 [DOI] [PubMed] [Google Scholar]
- 3. Donders G. 2010. Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: a review. Obstet. Gynecol. Surv. 65:462–473 [DOI] [PubMed] [Google Scholar]
- 4. Donders G, Bellen G, Rezeberga D. 2011. Aerobic vaginitis in pregnancy. BJOG 118:1163–1170 [DOI] [PubMed] [Google Scholar]
- 5. Tamrakar R, Yamada T, Furuta I, Cho K, Morikawa M, Yamada H, Sakuragi N, Minakami H. 2007. Association between lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect. Dis. 7:128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wertz J, Isaacs-Cosgrove N, Holzman C, Marsh TL. 2008. Temporal shifts in microbial communities in nonpregant African-American women with and without bacterial vaginosis. Interdiscip. Perspect. Infect. Dis. 2008:181253 PubMed; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. 2009. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus and/or L. iners are more conductive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 9:116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iacono KT, Brown AL, Greene MI, Saouaf SJ. 2007. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 83:283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. 2008. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-P13K-Ca2+ pathways. Cell. Mol. Life Sci. 65:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braundmeier AG, Fazleabas AT, Lessey BA, Guo H, Toole BP, Nowak RA. 2006. Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium. J. Clin. Endocrinol. Metab. 91:2358–2365 [DOI] [PubMed] [Google Scholar]
- 11. Rahkonen L, Rutanen EM, Unkila-Kallio L, Nuutila M, Nieminen P, Sorsa T, Paavonen J. 2009. Factors affecting matrix metalloproteinase-8 levels in the vaginal and cervical fluids in the first and second trimester of pregnancy. Hum. Reprod. 24:2693–2702 [DOI] [PubMed] [Google Scholar]
- 12. Wilson MC, Meredth D, Fox JE, Manoharan C, Davies AJ, Halestrap AP. 2005. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4; the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J. Biol. Chem. 280:27213–27221 [DOI] [PubMed] [Google Scholar]
- 13. Ewaschuk JB, Naylor JM, Zello GA. 2005. d-Lactate in human and ruminant metabolism. J. Nutr. 135:1619–1625 [DOI] [PubMed] [Google Scholar]
- 14. Witkin SS, Alvi S, Bongiovanni AM, Linhares IM, Ledger WJ. 2011. Lactic acid stimulates interleukin-23 production by peripheral blood mononuclear cells exposed to bacterial lipopolysaccharide. FEMS Immunol. Med. Microbiol. 61:153–158 [DOI] [PubMed] [Google Scholar]
- 15. Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, Inoue N. 2008. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 180:7175–7183 [DOI] [PubMed] [Google Scholar]
- 16. Mossop H, Linhares IM, Bongiovanni AM, Ledger WJ, Witkin SS. 2011. Influence of lactic acid on endogenous and viral RNA-induced immune mediator production by vaginal epithelial cells. Obstet. Gynecol. 118:840–846 [DOI] [PubMed] [Google Scholar]
- 17. Végran F, Boidot R, Michiels C, Sonveaux P, Feron O. 2011. Lactate influx through the endothelial cell monocarboxylate transporter MCT-1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71:2550–2560 [DOI] [PubMed] [Google Scholar]
- 18. Murray CM, Hutchinson R, Bantick JR, Belfield GP, Benjamin AD, Brazma D, Bundick RV, Cook ID, Craggs RI, Edwards S, Evans LR, Harrison R, Holness E, Jackson AP, Jackson CG, Kingston LP, Perry MW, Ross AR, Rugman PA, Sidhu SS, Sullivan M, Taylor-Fishwick DA, Walker PC, Whitehead YM, Wilkinson DJ, Wright A, Donald DK. 2005. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 1:371–376 [DOI] [PubMed] [Google Scholar]
- 19. Alakomi HL, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. 2000. Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Hanlon DE, Moench TR, Cone RA. 2011. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 11:200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mossop H, Linhares IM, Zhou X, Bongiovanni AM, Forney L, Ledger WJ, Witkin SS. 2012. Effect of lactic acid on vaginal EMMPRIN production: implications for altered vaginal microbiota-related pathology. Reprod. Sci. 19(Suppl):395A [Google Scholar]
- 22. Braundmeier AG, Dayger CA, Mehrotra P, Belton RJ, Jr, Nowak RA. 2012. EMMPRIN is secreted by human uterine epithelial cells in microvesicles and stimulates metalloproteinase production by human uterine fibroblast cells. Reprod. Sci. 19:1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diaz-Cueto L, Cuica-Flores A, Ziga-Cordero F, Ayala-Mendez JA, Tena-Alavez G, Dominguez-Lopez P, Cuevas-Antonio R, Arechavaleta-Velasco F. 2006. Vaginal matrix metalloproteinase levels in pregnant women with bacterial vaginosis. J. Soc. Gynecol. Investig. 13:430–434 [DOI] [PubMed] [Google Scholar]
- 24. Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, Jun JK. 2001. An elevated amniotic fluid matrix metalloproteinase -8 level at the time of midtrimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am. J. Obstet. Gynecol. 185:1162–1167 [DOI] [PubMed] [Google Scholar]
- 25. Larsen B, Goplerud CP, Petzold CR, Ohm-Smith MJ, Galask RP. 1982. Effect of estrogen treatment on the genital tract flora of postmenopausal women. Obstet. Gynecol. 60:20–24 [PubMed] [Google Scholar]
- 26. Ginkel PD, Soper DE, Bump RC, Dalton HP. 1993. Vaginal flora in postmenopausal women: the effect of estrogen replacement. Infect. Dis. Obstet. Gynecol. 1:94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alvarez-Olmos MI, Barousse MM, Rajan L, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL., Jr. 2004. Vaginal lactobacilli in adolescents: presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sex. Transm. Dis. 31:393–400 [DOI] [PubMed] [Google Scholar]
- 28. Noguchi K, Tsukumi K, Udono T, Urano T. 2004. Normal vaginal flora in chimpanzees (Pan troglodytes): qualitative and quantitative study. Comp. Med. 54:705–712 [PubMed] [Google Scholar]
- 29. Rivera AJ, Stumpf RM, Wilson B, Leigh S, Salyers AA. 2010. Baboon vaginal microbiota: an overlooked aspect of primate physiology. Am. J. Primatol. 72:467–474 [DOI] [PubMed] [Google Scholar]
- 30. Rivera AJ, Frank JA, Stumpf R, Salyers AA, Wilson BA, Olsen GJ, Leigh S. 2011. Differences between the normal vaginal bacterial community of baboons and that of humans. Am. J. Primatol. 73:119–126 [DOI] [PubMed] [Google Scholar]
- 31. Spear GT, Gilbert D, Sikaroodi M, Doyle L, Green L, Gillevet PM, Landay AL, Veazey RS. 2010. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retroviruses 26:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, Koenig SSK, Fu L, Ma Z, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Dynamics of the human vaginal microbiota. Sci. Transl. Med. 4:ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim SF, Baek SJ, Pack MY. 1991. Cloning and nucleotide sequence of the Lactobacillus casei lactate dehydrogenase gene. Appl. Environ. Microbiol. 57:2413–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taguchi H, Ohta T. 1991. d-Lactate dehydrogenase is a member of the d-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, sequencing, and expression in Escherichia coli of the d-lactate dehydrogenase gene of Lactobacillus plantarum. J. Biol. Chem. 266:12588–12594 [PubMed] [Google Scholar]
- 35. Gillespie JJ, Wattam AR, Cammer SA, Gabbard JL, Shukla MP, Dalay O, Driscoll T, Hix D, Mane SP, Mao C, Nordberg EK, Scott M, Schulman JR, Snyder EE, Sullivan DE, Wang C, Warren A, Williams KP, Xue T, Yoo HS, Zhang C, Zhang Y, Will R, Kenyon RW, Sobral BW. 2011. Patric: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect. Immun. 79:4286–4298 [DOI] [PMC free article] [PubMed] [Google Scholar]