Abstract
The significance and diagnostic value of semen analysis in chronic bacterial prostatitis has been extensively debated and remains controversial. To investigate the diagnostic relevance of semen culture in the bacteriological workup of prostatitis patients, we retrospectively analyzed a clinical database of 696 symptomatic patients. All patients were routinely subjected to a four-glass test, followed by semen culture and analysis. This allowed to dissect from the database three different diagnostic scenarios, and to compare the 'two-glass' pre-/post- massage test and the standard 'four-glass' test with a 'five-glass' test (four-glass plus post-VB3 semen culture). The 'five-glass' test showed 3.6- or 6.5-fold increases in relative sensitivity and lesser reductions (−13.2% or −14.7%) in relative specificity for traditional uropathogens (TUs) compared with the four-glass or two-glass test, respectively. The area under the ROC curve and Jouden's index were increased, whereas positive and negative likelihood ratios were lower than comparators, indicating that the 'five-glass' assay may be superior in confirming the negative outcome of both standard tests. The five-, four-, and two-glass tests detected TUs (Enterobacteriaceae, Enterococci, etc.) in 120, 33, and 20 patients and unusual pathogens (Streptococci, other Gram-positive species, Mycoplasmata, and others) in 130, 56, and 45 patients, respectively. When patients were subjected to pharmacological treatment, including a combination of a fluoroquinolone and a macrolide, no differences in eradication rates were observed between groups diagnosed with different tests, irrespective of pathogen category. Eradication was associated with long-term sign/symptom remission; no significant intergroup differences in sign/symptom scores were observed throughout a 24-month off-therapy follow-up period. In conclusion, our data support the usefulness of semen analysis in the diagnostic workup of prostatitis patients when this test is used to complement the four-glass Meares and Stamey test. Improvement of microbiological assays conveys important diagnostic and therapeutic implications.
Keywords: alfuzosin, azithromycin, chronic bacterial prostatitis, chronic pelvic pain syndrome, ciprofloxacin, Meares and Stamey test, National Institutes of Health Chronic Prostatitis Symptom Index, prostatitis, semen analysis, seminal fluid, Serenoa repens
Introduction
Chronic prostatitis (CP) syndromes have been traditionally classified as bacterial or abacterial 1. Chronic bacterial prostatitis (CBP, category II, National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] Chronic Prostatitis Workshop, 1995) is diagnosed when lower urinary tract (LUT) cultures show an infected prostate gland as the focus of recurrent episodes of bacteriuria 2. Prostatitis syndromes in which pathogens are not isolated fall into the CP/chronic pelvic pain syndrome class (CP/CPPS, category III, NIDDK), with inflammatory (IIIa) or non-inflammatory (IIIb) variants, depending on white blood cell (WBC) counts in patients' specimens.
The aetiology of abacterial prostatitis is mostly unknown; experts hypothesize neurological, psychological, anatomical, or immunological disorders as possible causes of this condition 3, 4.
In some cases, category III syndromes respond favourably to antibiotics. For this reason, antibacterial agents (AA) are currently recommended for the empirical treatment of inflammatory CP/CPPS 5. To explain the positive effect of AA in these cases, some experts indicate the intrinsic anti-inflammatory activity of quinolones as a possible determinant of patients' response 6. Others hypothesize an underlying infection by pathogens (including anaerobes; 7, 8) that are not routinely searched for or detected by conventional techniques 9.
Since 1968, the four-glass assay according to Meares and Stamey 10, 11 has been considered the standard test for detection and localization of pathogens in the LUT. About 10 years ago, a simplified two-glass test was proposed, based on the collection of sole pre-prostatic massage (VB2) and post-massage (VB3) urine samples 12.
Some authors have suggested that segmented tests do not display sufficient sensitivity and may underestimate the prevalence of bacterial prostatitis cases, thus misdirecting the therapeutic approach to the disease 13, 14. In addition to segmented tests, other procedures are adopted in the prostatitis diagnostic workup. As semen contains diluted prostatic material (around 20%–30% of the total volume), together with material from the testes and other genital tract tissues, some authors have suggested the usefulness of semen analysis for the detection of prostatic pathogens. Mobley proposed culture of semen/total ejaculate about 30 years ago, but the efficacy of this assay is still debated 15.
Making reference to the results of a National Institutes of Health-Chronic Prostatitis Collaborative Research Network (NIH-CPCRN) case-control study, Nickel 16 stated that no significant difference was shown in the detection of bacteria in the semen culture of CP/CPPS patients compared with asymptomatic subjects with no evidence of clinical CP/CPPS. In a survey performed in 40 men with category II CBP due to Escherichia coli infection, Weidner and coworkers 17 demonstrated that semen cultures could identify significant bacteriospermia in only about 50% of semen specimens from men with CBP. For this reason, ejaculate culture has not been recommended as a first-line diagnostic assay in men with suspected CBP 18, 19. However, semen analysis has been associated with the standard four-glass assay for the detection of pathogenic bacteria and inflammatory leukocytes in important clinical studies performed by the NIH-CPCRN 20.
Recently, Budía and coworkers 13 have published the results of a study performed on 895 patients who met the consensus criteria for clinical CP/CPPS, showing that semen has higher sensitivity than an expressed prostatic secretion (EPS) sample for the diagnosis of CBP. Thus, culture and microbiological analysis of semen might increase the number of patients diagnosed with bacterial disease and decrease the number of subjects classified by exclusion into category III CP/CPPS.
Currently, semen culture and analysis has been indicated as 'optional' by the consensus meeting of the NIH-CPCRN (Chantilly, VA, USA, 26 March 2002; 21) and has been 'recommended in selected patients' by the International Giessen Consensus (Giessen, Germany, September 2002; 21).
In the context of published recommendations for the evaluation of patients with prostatitis, Nickel 21 stated that although semen analysis and culture do increase the number of patients placed in category II, the importance and relevance of this finding was unknown, because no study showed a significant symptomatic improvement in patients with demonstrated positive semen cultures when treated with antibiotics.
To address these issues, at least in part, we performed a retrospective survey on a database of over 900 patients from a single hospital. The results of this study suggest that semen culture may complement the existing LUT segmented tests to enhance the sensitivity of microbiological analysis in symptomatic prostatitis patients.
Patients and methods
The data presented in this study are the result of a retrospective survey on patients who underwent the non-experimental, diagnostic, and therapeutic protocols routinely adopted in our clinical setting between the years 2000 and 2006; these protocols were based on internationally recommended diagnostic and therapeutic algorithms. Patients provided written consent to processing and anonymous publication of their clinical data. According to Italian bylaws (Determinazione AIFA 20/3/2008, GU 76, 31/3/2008), the Ethical Committee of the Principal Investigator's hospital was notified of the protocol describing the current observational study.
Study design, inclusion criteria, and diagnostic procedures
The present study is based on a retrospective analysis of a database of over 900 patients who were diagnosed in a single primary care urological centre specializing in the treatment of prostatitis syndromes.
Patients between 20 and 59 years old were included in this study if they showed signs and symptoms of category II CBP or category III CP/CPPS at the first visit, according to the NIH criteria (NIDDK Chronic Prostatitis Workshop, Bethesda, MD, USA, Dec 7–8, 1995). Symptoms should have been present for at least 3 months within the 6 months before the first visit. Clinical diagnosis required at least one of the characteristic signs/symptoms of prostatitis (i.e., dysuria, perineal pain or discomfort, testicular pain, painful ejaculation, associated or not with urinary frequency, urinary urgency, hesitance at micturition, urinary retention, or decreased urinary stream, prostate abnormality and/or asymmetry at digital rectal examination [DRE]).
Patients with any of the following conditions were not considered for this study: category I acute bacterial prostatitis; urethral discharge; Chlamydia; therapy with AA or other medications effective at the prostatic level within a 90-day period before entering the study; renal/hepatic/cardiac insufficiency; indwelling catheters; cystostomy; ureterostomy; previous prostatic or pelvic surgery; or neoplasia. To reduce the confounding role of frank hyperplasia in the evaluation of voiding symptoms, life quality, and therapy outcomes, we excluded prostatitis patients presenting a prostatic enlargement exceeding 40 mL.
Database analysis allowed the identification of a cohort of 696 patients who met the above-mentioned criteria.
Published recommendations and internationally acknowledged guidelines were followed in the diagnosis of prostatitis 21. Examination of history included a review of past and present symptoms, focusing on previous genitourinary diseases and infections, and on past and present medications. The severity of CP symptoms was scored using an Italian validated translation of the NIH Chronic Prostatitis Symptom Index (NIH-CPSI) 22. The physical examination included complete abdominal examination, focusing on the external genitalia and perineum. Consistency and irregularity of the prostate gland were evaluated by DRE. Pelvic and transrectal ultrasound was used for prostatic and bladder diagnostic imaging. Urine peak flow rate (Qmax), percent bladder voided volume (%BVV), and post-micturition residual urine (by sovrapubic ultrasound) were measured in all patients.
Microbiological tests included, sequentially, (i) pre-VB1 urethral swab analysis and culture, followed by (ii) a four-glass LUT segmented localization test according to Meares and Stamey 10 and (iii) post-VB3 semen analysis and culture. Before each test, patients were required to cleanse thoroughly their hands and glans penis with an antiseptic soap. The samples were tested for the presence and load of traditional uropathogens (TUs; Pseudomonas aeruginosa, Klebsiella spp., Proteus spp., E. coli, other members of the Enterobacteriaceae family, Enterococcus faecalis, Staphylococcus aureus) 23 and 'unusual pathogens' (UPs; definition by Skerk et al. 24): Streptococcus spp., Haemophylus spp., Citrobacter spp., Coryneforms, Ureaplasma urealyticum, Mycoplasma spp., and others). When a patient showed at least a TU together with an UP in the context of a multiple infection localized at the prostatic level, he was considered positive for class II CBP and placed in the TU group. For diagnosis of bacterial infection of the prostate, colony counts in EPS/VB3 were required to be at least 10-fold greater than those assessed on VB1/VB2. In the case of detection of multiple pathogens in VB1, VB2, EPS, VB3, and semen samples, in order to exclude accidental contamination, localizations were confirmed by repeating the tests after 7 days.
Inflammatory WBCs were counted on four-glass specimens and semen using the more stringent composite criteria (10+ in EPS, 5+ in VB3) described by Schaeffer et al. 20.
Comparative analysis of microbiological diagnostic tests in CBP
Diagnostic scenarios
For comparative studies, the results of the above-described microbiological tests were collected on a single database and used to dissect the following diagnostic scenarios:
—the VB2 and VB3 samples were used to diagnose bacterial prostatitis according to the two-glass pre-massage and post-massage tests 12;
—the VB1, VB2, EPS, and VB3 samples were used to diagnose bacterial prostatitis according to the four-glass Meares and Stamey test 10, 11;
—the VB1, VB2, EPS, VB3, and semen samples were used for pathogen detection within a complemented assay denominated 'five-glass test'.
Comparative parameters
Comparison between diagnostic assays is typically performed by calculating the sensitivity (Se) and specificity (Sp) of 'competing' tests. In a published report describing the results of a comparative analysis between segmented tests for diagnosis of CP/CPPS, 2 × 2 contingency tables were used to compare the positivity/negativity of the four-glass test with the positivity/negativity of the two-glass test 25, and to calculate the relative sensitivity and specificity. A similar approach was described by Zegarra Montes et al. 26. We have adopted the same procedure in the current study by generating 2 × 2 tables to compare the five-glass test with the four-glass test or the two-glass test.
Variations in sensitivity imply a natural trade-off with the specificity of a given assay, thus hampering the comparison between diagnostic tests. Therefore, grouping sensitivity and specificity within a single index or parameter, such as (i) the area under receiver operating characteristic curve (AUROCC), (ii) Youden's index (j), or (iii) the likelihood ratio (LR), represents a more useful procedure for such a comparison. In 2000, Biggerstaff 27 described a graphic system of diagnostic accuracy allowing simple, immediate comparison between two dichotomous diagnostic tests by graphic visualization of the following three parameters:
—The AUROCC 28: In a binary setting (e.g., in the case of dichotomous tests indicating a positive or negative outcome of an LUT segmented assay), the true positive rate (sensitivity) and the false positive rate (100-specificity) of the test define a single cutoff value on the receiver operating characteristic (ROC) graph, and the AUROCC at the cutoff point is simply the area under the trapezoid generated by the cutoff value on the ROC graph (i.e., the average between sensitivity and specificity ([Se+Sp]/2) 29.
—Youden's index: This comprises specificity and sensitivity of a given assay in a single equation (j = Se−[1−Sp]). 'j' ranges between 0 for a worthless test and 100 for an ideal test and is considered the best measure in terms of regret in a decision theoretical context 27. In the Biggerstaff graph, j is the intercept on the vertical axis of a line with slope 1 (Figure 1), passing through the single cutoff point representing a given dichotomous test 27.
Figure 1.
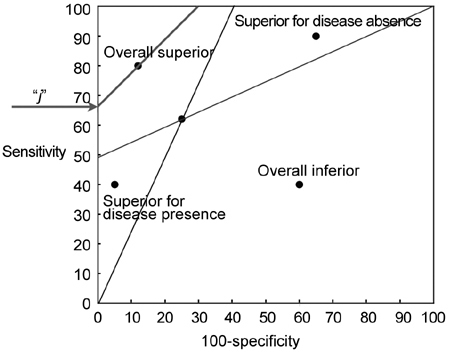
Likelihood ratio (LR) graph: regions of comparison 27. The slopes (tangents) of the lines connecting the cutoff point of a 'gold standard' comparator test to points (0, 0) and (100, 100) represent the LR+ and the LR−, respectively. The LR+ and LR− lines divide the chart into four areas. Depending on the position of a competitor test in these areas, it can be defined as overall superior to the gold standard, overall inferior, superior for confirming the absence of the disease under investigation, or superior for confirming the presence of the same disease. Youden's index (j) is the intercept on the vertical axis of a line whose slope is 1, passing through the cutoff point.
—Positive (LR+) and negative (LR−) likelihood ratios (LR+ = Se/[1−Sp]; LR− = [1−Se]/Sp; for definition see Zegarra Montes et al. 26): These can also be extrapolated from the ROC graph and represent the slopes of the lines connecting a cutoff point to the origin of the axes (point [0, 0]) or to the point (100, 100), respectively (Figure 1 27).
As indicated by Biggerstaff 27, LR+ and LR− lines relative to a 'gold standard' test divide the ROC plot into four areas: depending on the specific area in which a 'competitor' test is positioned in the chart, this can be classified as overall superior to the gold standard assay, and as overall inferior or superior for confirming the absence or the presence of a given disease 27. A model graph showing these areas is reproduced in Figure 1.
Effect of pharmacological treatment in patients diagnosed with different localization tests
Study design
At time point T0, after complete clinical and microbiological assessments, patients received a full course of combination pharmacological therapy. On completion of treatment (one or two cycles), patients were subjected to a complete diagnostic protocol, including microbiological and clinical evaluations. Patients showing microbiological persistence after 6 weeks of treatment were subjected to a second course of therapy, according to a protocol published earlier 30. T1 was defined as the time point at which, after a first or second course of combination therapy, microbiological eradication was achieved, or, in the case of lack of eradication after a second cycle of therapy, pathogen persistence was observed. At time points T2, T3, T4, T5 and T6–6, 12, 18, 24, and 30 months after time point T1, respectively–patients were subjected to complete clinical evaluations (visits, instrumental analysis, questionnaires). Microbiological analyses were repeated in patients showing symptom relapse during follow-up.
The same design was applied to the study performed on a subset of patients with negative four-glass cultures and positive semen localization of uropathogens, as described in Section 3.3.
Pharmacological treatment
Starting from time point T0, patients received an oral once-daily dose of 500 mg ciprofloxacin, associated with a single daily dose of 500 mg azithromycin, administered during the first three consecutive days at the beginning of each week of treatment with ciprofloxacin, as described earlier 30, 31, 32. An alpha-blocker (Alfuzosin ER, 10 mg day−1) was administered for voiding/obstructive signs and symptoms due to mechanical/inflammatory/proliferative alterations. Herbal extracts and supplements (Serenoa repens 340 mg twice daily, lycopene, selenium) were added to the medication regime.
Combination therapy 30 included a daily dose of the alpha-adrenoceptor blocker alfuzosin (10 mg, extended-release formulation) 33 and a S. repens extract (640 mg day−1). This therapeutic regimen was administered for 6 weeks to all patients. In agreement with a published therapeutic algorithm 34, patients were treated with the alpha-blocker for the first 6 months of the follow-up period (time frame: T1–T2), to which the S. repens extract was also associated.
The same therapeutic scheme was administered to patients with negative four-glass cultures and positive semen localization of uropathogens (described in Section 3.3).
Response evaluation
Response was graded as follows, in agreement with the definitions and pathogen loads indicated by Naber et al. 35:
—Eradication, defined as a causative organism absent (bacterial load: < 103 colony forming units [CFU] mL−1) after completion of therapy (T1 time point).
—Persistence, defined as a causative organism present (bacterial load: ≥ 103 CFU mL−1) after completion of therapy.
—Superinfection, defined as a causative organism eradicated (bacterial load: < 103 CFU mL−1), with the appearance of a different pathogen (bacterial load: ≥ 103 CFU mL−1) after completion of therapy.
Statistics
χ2 test was used for contingency table and eradication rate comparisons.
Intragroup differences in NIH-CPSI questionnaire scores before/after therapy and during follow-up were analysed using Wilcoxon's signed rank test. Intragroup differences between Qmax or %BVV levels before/after therapy and during follow-up were measured using a paired, two-tailed t-test. Intergroup differences in NIH-CPSI scores were analysed using the Mann–Whitney/Wilcoxon rank sum test.
Intergroup variance at single time points was analysed by ANOVA (Qmax, %BVV) or the Kruskal–Wallis test (NIH-CPSI scores). The XLStatistics 5.71 program (© Rodney Carr; http://www.deakin.edu.au/~rodneyc/) was used for statistical analysis of data.
Results
Addition of semen cultures enhances the sensitivity of standard segmented tests for prostatic localization of uropathogens
Retrospective analysis of bacteriological data in 696 patients showing signs and symptoms of prostatitis and meeting restrictive selection criteria resulted in three different diagnostic scenarios: (i) a two-glass PPMT, in which only VB2 and VB3 samples were taken into account for uropathogen detection, (ii) a full four-glass test, comprising VB1, VB2, EPS, and VB3 samples, and (iii) a 'five-glass' test, joining a four-glass test with post-VB3 ejaculate culture and analysis.
The patients' records were reviewed for detection of TUs or UPs.
Table 1 lists the number of patients with positive single or multiple localizations of TU or UP.
Table 1. Microbiological isolates per patient, detected with five-, four-, or two-glass tests.
| Number of patients with indicated isolate(s) | |||
|---|---|---|---|
| Traditional uropathogens | Five-glass | Four-glass | Two-glass |
| Enterococcus spp. (single isolate): | 48 | 7 | 4 |
| + E. coli | 7 | 3 | 1 |
| + Proteus spp. | 1 | – | – |
| + Enterobacter spp. | 1 | – | – |
| + Streptococcus spp. | 4 | – | – |
| + Streptococcus spp. + C. albicans | 1 | – | – |
| + Ureaplasma urealyticum | 2 | – | – |
| + Haemophilus parainf. | 1 | – | – |
| + Staphylococcus spp. (coagulase-negative) | 1 | – | – |
| + Morganella morganii | 2 | – | – |
| Escherichia coli | 22 | 9 | 6 |
| + Streptococcus spp. | 3 | 1 | – |
| + Streptococcus spp. + U. urealyticum | 1 | 1 | – |
| + Streptococcus spp. + M. hominis | 1 | – | – |
| + M. hominis + U. urealyticum | 1 | – | – |
| Proteus spp. | 8 | 5 | 4 |
| + E. faecalis + U. urealyticum | 1 | – | – |
| Klebsiella spp. | 4 | 2 | 2 |
| + C. albicans | 1 | – | – |
| + E. faecalis + E. coli | 1 | – | – |
| + E. faecalis + Citrobacter spp. | 1 | – | – |
| + E. faecalis + Corynebacterium spp. | 1 | 1 | 1 |
| Staphylococcus aureus | 3 | 2 | 1 |
| Pseudomonas aeruginosa | 1 | 2 | 1 |
| + Corynebacterium spp. | 1 | – | – |
| Enterobacter spp. | 1 | – | – |
| + Streptococcus spp. | 1 | – | – |
| Total | 120 | 33 | 20 |
| Unusual pathogens | Five-glass | Four-glass | Two-glass |
| Streptococcus spp. | 47 | 22 | 19 |
| + H. parainfluenzae | 1 | 1 | 1 |
| + C. albicans | 2 | – | – |
| Ureaplasma urealyticum | 21 | 7 | 7 |
| + H. parainfluenzae | 1 | 1 | 1 |
| + C. albicans | 2 | – | – |
| Haemophilus parainfluenzae | 15 | 6 | 6 |
| + N. gonorrhoeae | 1 | 1 | 1 |
| Corynebacterium spp. | 10 | 7 | 6 |
| Staphylococcus spp. (coagulase-negative) | 9 | 7 | – |
| Morganella morganii | 7 | – | – |
| Citrobacter spp. | 4 | 1 | 1 |
| Mycoplasma hominis + U. urealyticum | 3 | 2 | 2 |
| Gardnerella vaginalis | 3 | – | – |
| Neisseria gonorrhoeae | – | 1 | – |
| Candida albicans | 3 | 1 | 1 |
| Total | 130 | 56 | 45 |
Table 2 compares the total number of patients showing positive or negative microbiological localizations on the basis of different diagnostic tests, stratified by class of pathogen. A two-glass or four-glass test yielded 20 (2.9%) or 33 (4.7%) positive localizations for TUs, respectively. As a consequence, 676 (two-glass) and 663 (four-glass) patients of our cohort of 696 symptomatic subjects were classified into the abacterial group. With the five-glass test, the number of patients with positive cultures for TUs increased to 120 (17.2% of 696 patients) (i.e., 6-fold or 3.6-fold) compared with the two- or four-glass tests, respectively. A similar trend was observed in the UP group.
Table 2. Laboratory results in 696 symptomatic patients.
| Test | Traditional uropathogens |
Unusual pathogens |
||||
|---|---|---|---|---|---|---|
| Five-glass | Four-glass | Two-glass | Five-glass | Four-glass | Two-glass | |
| Positive localization | 120 | 33 | 20 | 130 | 56 | 45 |
| Negative localization | 576 | 663 | 676 | 566 | 640 | 651 |
| Total | 696 | 696 | 696 | 696 | 696 | 696 |
| Prevalence, positive tests (%) | 17.24 | 4.74 | 2.87 | 18.67 | 8.04 | 6.46 |
Results of the χ2 test applied to 2 × 2 contingency tables comparing the five-glass test with the four-glass or two-glass tests (Table 3) served to reject the null hypothesis that differences in segmented tests would not affect test outcomes (P < 0.0001 for all comparisons).
Table 3. 2 × 2 contingency tables of positive and negative traditional uropathogen or unusual pathogen localization distribution when the 5-glass, 4-glass or 2-glass tests were applied to 696 patients with chronic prostatitis signs and symptoms.
| Traditional uropathogens | Four-glass, negative | Four-glass, positive | Total | |
| Five-glass, negative | 576 | 0 | 576 | |
| Five-glass, positive | 87 | 33 | 120 | |
| Total | 663 | 33 | 696 | |
| Two-glass, negative | Two-glass, positive | Total | ||
| Five-glass, negative | 574 | 2 | 576 | |
| Five-glass, positive | 102 | 18 | 120 | |
| Total | 676 | 20 | 696 | |
| Unusual pathogens | Four-glass, negative | Four-glass, positive | Total | |
| Five-glass, negative | 566 | 0 | 566 | |
| Five-glass, positive | 74 | 56 | 130 | |
| Total | 640 | 56 | 696 | |
| Two-glass, negative | Two-glass, positive | Total | ||
| Five-glass, negative | 563 | 3 | 566 | |
| Five-glass, positive | 88 | 42 | 130 | |
| Total | 651 | 45 | 696 |
Contingency tables allowed the calculation of relative sensitivity and specificity between five-glass and four-glass or two-glass tests. Table 4 shows that—for any class of pathogen—the five-glass test was characterized by a marked increase in sensitivity and a lesser decrease in specificity compared with both four- and two-glass tests.
Table 4. Comparison of relative sensitivity and specificity between the five-glass and the four-glass or two-glass tests.
| Traditional uropathogens | Unusual pathogens | χ2 | P-value | ||
|---|---|---|---|---|---|
| Sensitivity |
Five-glass | 100.0 | 100.0 | 2.71 | 0.099 |
| Four-glass | 27.5 | 43.1 | |||
| Ratio | 3.6 | 2.3 | – | – | |
| Five-glass | 96.9 | 93.3 | 5.52 | 0.018 | |
| Two-glass | 15.0 | 32.3 | |||
|
Ratio |
6.5 |
2.9 |
– |
– |
|
| Specificity | Five-glass | 86.8 | 88.4 | 0.008 | 0.930 |
| Four-glass | 100.0 | 100.0 | |||
| Ratio | 0.9 | 0.8 | – | – | |
| Five-glass | 84.9 | 86.5 | 0.009 | 0.925 | |
| Two-glass | 99.6 | 99.5 | |||
| Ratio | 0.8 | 0.9 | – | – |
Sensitivity and specificity allowed the calculation of three comparative parameters: the AUROCC, Youden's index (j), and LR.
In the case of five-glass vs. four-glass comparison for detection of TUs, the AUROCC was 93.4 for the five-glass test and 63.7 for the four-glass test, and Youden's index was 86.9 for the five-glass test and 27.5 for the four-glass test (Figure 2, panel A). When the five-glass test was compared with the two-glass test (panel B), the values of AUROCC were 90.9 and 57.3, respectively; j was 81.8 for the five-glass test and 14.6 for the two-glass test. Likelihood ratios of tests are also shown in Figure 2. LR+ relative to two-glass or four-glass tests was higher than the corresponding values shown by the five-glass test. The LR− of the five-glass test was lower than the corresponding values shown by both the two-glass and the four-glass assays. In this case, the five-glass test performs better than the four- or two-glass tests because an LR− closer to the unit indicates that a diagnostic assay cannot discriminate between diseased and non-diseased subjects showing a negative test.
Figure 2.
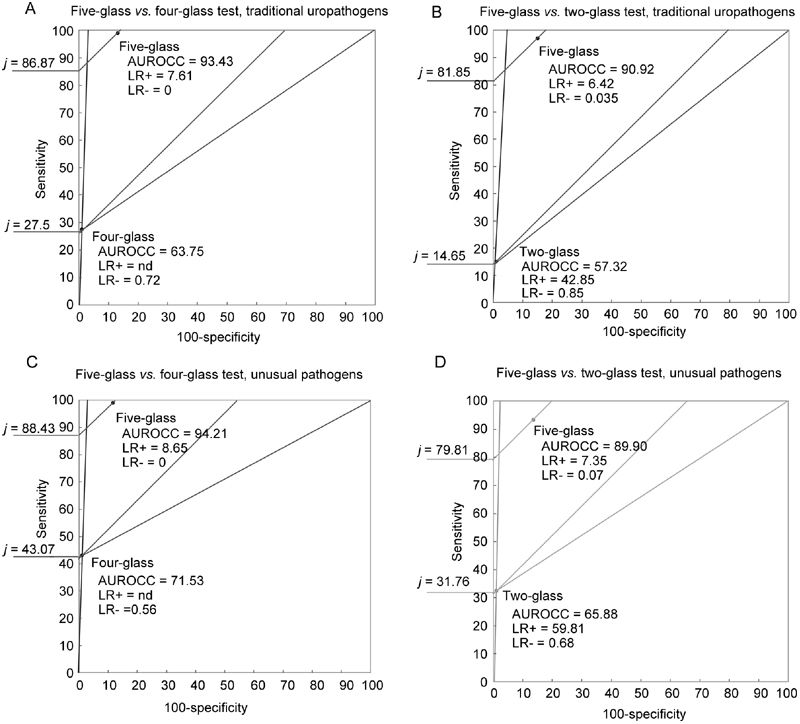
Likelihood ratio graphs comparing the four-glass Meares and Stamey test with the five-glass test and the two-glass PPMT with the five-glass test for the detection of traditional uropathogens or unusual pathogens. AUROCC, area under receiver operator characteristic curve; j, Youden's Index; LR+, likelihood ratio for a positive test; LR−, likelihood ratio for a negative test.
Differences between test outcomes were highly significant in the case of UPs (Table 3; P < 0.0001 for the null hypothesis for all comparisons, χ2 test), and the five-glass test showed increased sensitivity compared with the two standard comparators (Table 4). The parameters of grouping sensitivity and specificity (j, AUROCC) were also higher for the five-glass test compared with the four- or two-glass tests, and likelihood ratios followed a trend similar to that observed for the TU group (Figure 2, panels C, D).
Effect of pharmacological treatment of patients with positive localizations detected by a four-glass, a two-glass, or a five-glass assay
As shown in Table 2, the five-, four-, or two-glass tests allowed the localization of TUs in 120, 33, and 20 symptomatic patients, respectively, whereas UPs were detected in 130, 56, and 45 patients, respectively.
The effect of a protocol of combination therapy including a fluoroquinolone and a macrolide, associated with an alpha-adrenoceptor blocker and a S. repens extract 30, 31, 32, was evaluated in these six groups of patients. Table 5 lists the eradication rates stratified by pathogen class and diagnostic test. The χ2 test revealed no differences between eradication rates (P > 0.8 for all comparisons), confirming the null hypothesis that different diagnostic tests would not affect the eradication rates of isolated pathogens.
Table 5. Eradication rates after one or two cycles of therapy, stratified by class of pathogen and test.
| Test | Traditional uropathogens |
Unusual pathogens |
||||
|---|---|---|---|---|---|---|
| Five-glass | Four-glass | Two-glass | Five-glass | Four-glass | Two-glass | |
| Eradication after 1 cycle of treatment (%) | 90 (75) | 27 (81.8) | 17 (85) | 105 (80.7) | 46 (82.1) | 37 (82.2) |
| Eradication after 2 cycles of treatment (% of persistent cases) | 22 (73.3) | 5 (83.3) | 3 (100) | 21 (84) | 9 (100) | 7 (100) |
| Total eradicated patients (% of total patients) | 112 (93.3) | 32 (96.9) | 20 (100) | 126 (96.9) | 55 (98.2) | 44 (97.7) |
| Total patients | 120 | 33 | 20 | 130 | 56 | 45 |
| χ2 | 0.018a | 0.005b | 0.04c | 0.003a | 0.001b | 0.002c |
| P-value | 0.89a | 0.93b | 0.84c | 0.95a | 0.97b | 0.98c |
Five-glass vs. four-glass.
Four-glass vs. two-glass.
Five-glass vs. two-glass.
Figure 4 shows the long-term effect of therapy on voiding signs (i.e., Qmax [panel A] and % BVV [panel B]) in patients showing TU or UP localizations by the five-, four-, or two-glass tests. The proportion of drop-outs at late follow-up (T5–T6) was about 15% in all patient groups, irrespective of age, severity of the disease, diagnostic test, or pathogen.
Figure 4.
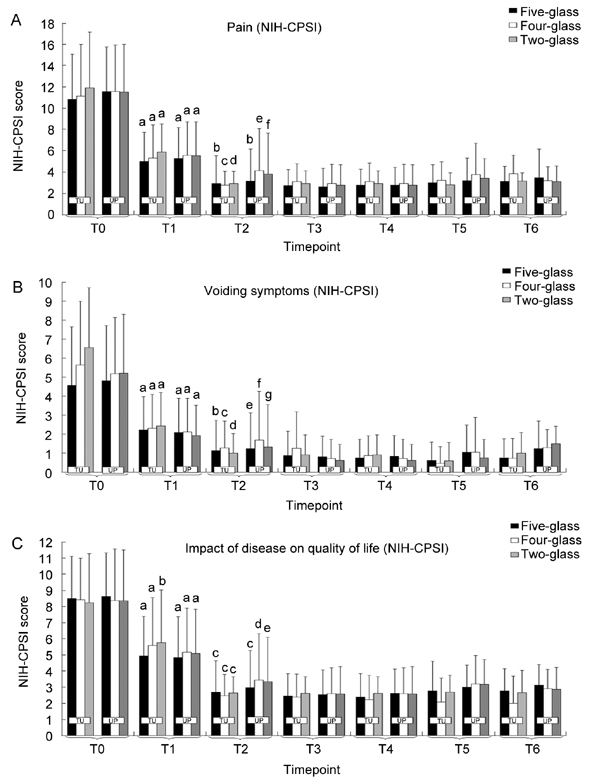
Pain, voiding, and life quality impact domains of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) assessed in patients showing positive localizations of traditional uropathogens (TU) or unusual pathogens (UP) using the five-glass (black bars), four-glass (white bars), or two-glass (shaded bars) tests. Patients were subjected to one or two cycles of combination therapy with ciprofloxacin, azithromycin, alfuzosin, and S. repens. At T1 antibacterial agents were suspended and the remaining drugs were continued for a period of 6 months (T1–T2). The follow-up at the end of therapy was extended for 24 months (T6). (A): aP < 0.0001 vs.T0; bP < 0.001 vs. T1; cP = 0.00016 vs. T1; dP = 0.00019 vs. T1; eP = 0.091 vs. T1; fP = 0.07 vs. T1. Wilcoxon signed rank test. Intergroup differences at each time point: P > 0.1 (TU and UP together); P > 0.1 (TU only); P > 0.1 (UP only). Kruskal–Wallis test. (B): aP < 0.0001 vs. T0; bP < 0.0001 vs. T1; cP = 0.0008 vs. T1; dP = 0.004 vs. T1; eP = 0.0003 vs. T1; fP = 0.89 vs. T1; gP = 0.83 vs. T1. Wilcoxon signed rank test. Intergroup differences at each time point: P > 0.1 (TU and UP together); P > 0.1 (TU only); P > 0.1 (UP only). Kruskal–Wallis test. (C): aP < 0.0001 vs. T0; bP = 0.002 vs. T0; cP < 0.0001 vs. T1; dP = 0.004 vs. T1; eP = 0.009 vs. T1. Wilcoxon signed rank test. Intergroup differences at each time point: P > 0.1 (TU and UP together); P > 0.1 (TU only); P > 0.1 (UP only). Kruskal–Wallis test.
Combination therapy induced a marked, significant improvement of both parameters in all studied groups that showed good homogeneity of response and an absence of intergroup differences at each time point analysed (P > 0.1 [ANOVA] for all groups, divided or not by pathogen class, at all time points; Figure 3).
Figure 3.
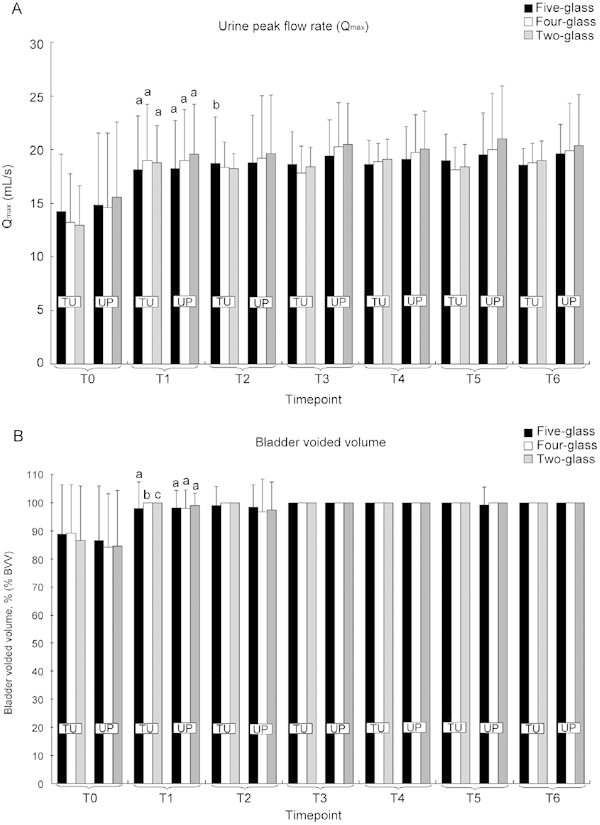
Voiding parameters in symptomatic patients showing positive localizations of traditional uropathogens (TU) or unusual pathogens (UP) using the five-glass (black bars), four-glass (white bars), or two-glass (shaded bars) tests. Patients were subjected to one or two cycles of combination therapy with ciprofloxacin, azithromycin, alfuzosin, and S. repens. At microbiological eradication (time point T1), antibacterial agents were suspended and the remaining drugs were continued for a period of 6 months (T1–T2). The follow-up at the end of therapy was extended for 24 months, up to time point T6. (A): Time course of urine peak flow rate (Qmax). aP < 0.0001 vs. T0; bP = 0.046 vs. T1. Paired t-test. Intergroup differences at each time point (ANOVA): P > 0.1 (TU and UP together); P > 0.1 (TU only); P > 0.1 (UP only). (B): Time course of post-micturition percent bladder voided volume (% BVV). aP < 0.0001 vs. T0; bP = 0.005 vs. T0; cP = 0.01 vs. T0. Paired t-test. Intergroup differences at each time point (ANOVA): P > 0.1 (TU and UP together); P > 0.1 (TU only); P > 0.1 (UP only).
Similar results were obtained by analysing the three domains of the NIH-CPSI questionnaire: marked reductions in pain, voiding symptoms, and the impact of the disease on patients' quality of life were observed at T1, were further enhanced at time point T2, and were extended throughout the entire 24-month follow-up period, up to time point T6 (Figure 4). For all NIH-CPSI scores, no intergroup differences were measured (P > 0.1, Kruskal–Wallis) for all groups at all time points, stratified or not by pathogen class (Figure 4).
Eradication of TUs localized in the semen sample of patients with a negative four-glass assay is associated with improvement of prostatitis signs and symptoms
To further evaluate the relevance of uropathogen localization in semen samples, we identified—within the population of 696 subjects described above—patients showing positive semen cultures for TUs or UPs, but negative VB1, VB2, EPS, and VB3 samples. These symptomatic patients would have been classified by exclusion in the category III CP/CPPS group on the basis of a negative four-glass test 36.
In these patients, analysis of WBC counts showed that 65.7% and 71.6% of patients with a TU (N = 74) or an UP (N = 87) in semen only, respectively, had more than 10 WBC per HPF in EPS or VB3 specimens. No differences between these proportions were revealed by the χ2 test (P = 0.753), thus indicating similar inflammatory findings in both TU or UP groups.
From this patient population, we selected 64 subjects showing a single positive localization of the five uropathogens most widely accepted as causative agents of category II CBP (i.e., E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus faecalis) 37.
Patients were subjected to a 6-week course of combination pharmacological treatment, described in Section 2. At time point T1, the AA were discontinued and in 46 patients showing microbiological eradication (72.2%) the two remaining drugs were administered for a period of 6 months. In the remaining 18 cases, persistence of the causative pathogens was observed, and patients were subjected to a second cycle of combination therapy 30. Therapy was effective in all 18 patients who showed microbiological eradication. AA were then discontinued in this group, and the remaining drugs were administered for 6 months. At time point T2, 6 months after microbiological eradication, all 64 patients were off therapy for all drugs administered in the current protocol.
Figures 5 and 6 show the trends of Qmax, %BVV, and NIH-CPSI scores assessed before treatment (T0), at microbiological eradication (T1), and throughout the follow-up period (T2–T6). Patients showing semen clearance underwent a marked improvement of all measured parameters and scores at the end of combination treatment with AA (T0–T1), extending throughout the entire follow-up period, beyond the 6-month therapy with alfuzosin and S. repens.
Figure 5.
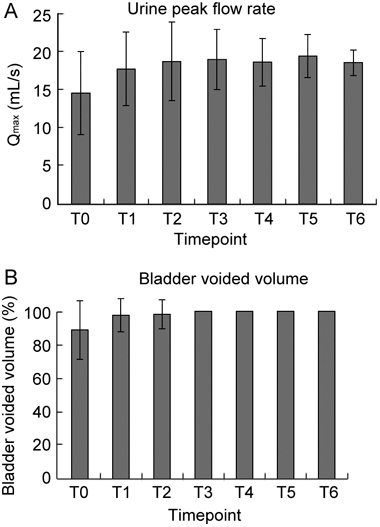
Voiding parameters in 64 patients showing positive semen cultures for traditional uropathogens (TU) but negative VB1, VB2, EPS, and VB3 samples. Patients underwent one or two courses of combination pharmacological therapy, described under Patients and methods. At microbiological eradication (T1), the antibacterial agents were discontinued and the remaining compounds were administered for a period of 6 months, up to time point T2. (A): Urine peak flow rate (Qmax); T1 vs. T0, P = 0.0022; T2 vs. T0, P = 0.00013. (B): Bladder percent voided volume; T1 vs. T0, P = 0.0056; T2 vs. T0, P < 0.0001. Paired t-test.
Figure 6.
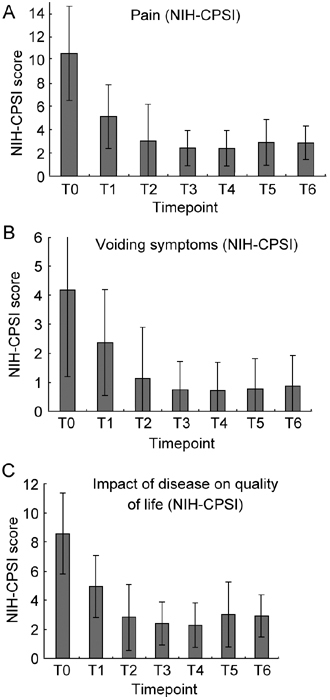
National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) scored in 64 patients showing positive semen cultures for uropathogens, but negative VB1, VB2, EPS, and VB3 samples (four-glass test). (A): Values at T1 and T2 are significantly different from T0 and T1, respectively (P < 0.0001 in both cases). (B): T1 vs. T0, P = 0.00103; T2 vs. T1 or T0, P < 0.0001. (C): impact of the disease on patients' quality of life; T1 vs. T0 and T2 vs. T1 or T0, P < 0.0001. Wilcoxon signed rank test.
If patients were stratified according to the microbiological outcome of combination therapy after a single cycle of treatment, the group (n = 46) showing pathogen eradication exhibited at T1 a mean pain score of 5.28 ± 2.71 compared with a value of 10.22 ± 4.27 at T0 (−48.33%, P < 0.0001, Mann–Whitney/Wilcoxon). The group of 18 patients showing persistence of causative pathogens after a first unsuccessful cycle of therapy exhibited a mean score of 6.83 ± 2.77 (score at T0 = 11.16 ± 3.68), indicating a lesser pain reduction (−38.79%) than the former group. The differences between pain scores in the two groups, which were not significant at T0 (P = 0.44, Mann–Whitney/Wilcoxon), became significant after a single cycle of therapy (P = 0.044, Mann–Whitney/Wilcoxon), and lost significance when semen bacterial clearance was achieved in both groups (P = 0.13, Mann–Whitney/Wilcoxon). No differences were recorded between the two groups when Qmax, %BVV, and NIH-CPSI voiding and life quality scores were compared (data not shown).
Discussion
Some authors have proposed that failure to detect bacterial infection in a number of prostatitis patients responding symptomatically to antibacterial treatment is because of the insufficient sensitivity of currently available LUT segmented tests 13. Recently, a number of published studies focused on semen culture and analysis in CP patients 13, 26, 38, 39. These reports demonstrated the efficacy of semen analysis in detecting pathogens that cause CBP. However, with one exception, Reference 39, in these studies semen analysis was investigated as an alternative to prostatic massage in the context of a modified four-glass test 26 or as an alternative to traditional segmented tests as a whole 13. Our study indicates that, rather than representing a diagnostic alternative, semen culture and analysis can complement traditional four- or two-glass assays to detect an increased number of pathogens. As shown by Zegarra Montes and coworkers 26, when used alone, semen analysis may misdiagnose a fraction of symptomatic patients. As EPS/VB3 cultures may in some cases evidence pathogens not detected in the ejaculate, we believe that a four-glass test and semen analysis should be combined in the framework of a sequential five-glass test.
In this context, collection of the ejaculate after the VB3 sample may be advantageous, because it can generate a washout of the prostate and thus complement the prostatic massage, especially in the absence of EPSs. The contraction of prostatic stromal smooth muscle fibres during the emission phase of ejaculation may in fact provide a propulsive force facilitating the extrusion of prostatic material and pathogens, mobilized by the prostatic massage. This is shown in Table 1: addition of semen to a four-glass test allowed the detection of largely increased numbers of Enterococcus spp., E. coli, Staphylococcus, and Streptococcus spp. isolates, in agreement with the findings of a CPCRN study 20.
In their report, Zegarra Montes and coworkers 26 admitted that a limitation of their study was not having followed the response of their symptomatic patients to antibiotic treatment. Indeed, a relationship between the mere isolation of a pathogen and its actual causative role in prostatitis can be better defined by investigating the clinical evolution of the syndrome after eradication of the detected pathogen.
In this respect and within the limits of an observational study, we have shown that the increased population of patients showing positive localization by the five-glass test responded to pharmacological treatment in a manner not statistically different from that of patients diagnosed by means of traditional segmented assays. Thus, symptomatic patients classified as affected by bacterial prostatitis based on the five-glass, four-glass, or two-glass assays showed homogeneous responses to therapy, irrespective of the pathogen class and diagnostic test adopted. Such homogeneous response points to a common aetiology of the condition of all diagnosed patients (i.e., bacterial infection).
It is important to underline that the improvement of Qmax, % BVV, and CPSI scores was extended beyond the 6-month period of prolonged therapy with alpha-blockers and S. repens extracts. Therefore, sustained remission from signs and symptoms of CP cannot be attributed to the transient effect of these agents, but is more likely due to permanent eradication of the pathogens localized at the prostatic level by the different tests. However, this does not exclude a role of these agents in rapid symptom relief, even before microbiological eradication.
The reliability of semen as a diagnostic sample is further supported by our subset analysis performed on patients showing uropathogens in semen but not in VB1, VB2, EPS, or VB3. These symptomatic patients, who were negative to the Meares–Stamey test and should have been classified by exclusion into the CP/CPPS class, showed sustained remission by clinical prostatitis signs and symptoms at the end of antibacterial treatment and, importantly, long after suspension of alpha-blocker therapy. Moreover, NIH-CPSI scores in this cohort of patients suggest that eradication of uropathogens specifically localized in semen samples may be associated with pain reduction, but that this symptom persists when semen clearance fails, although voiding symptoms were improved in non-eradicated patients, probably thanks to the effect of alpha-adrenoceptor blockers.
In summary, our results confirm that standard tests may underestimate the number of patients showing a symptomatic bacterial infection at the prostatic level, because the five-glass test detected about fourfold or sixfold more TUs when compared with the four- or two-glass tests, respectively. In several cases (n = 35), semen contained the same pathogens found in EPS/VB3 samples and confirmed the localization evidenced by the standard tests and, hence, the diagnosis of type II CBP. In other cases, semen analysis alone allowed localization of pathogens in symptomatic patients, which would have been classified into the category III CP/CPPS on the basis of negative results of the four-glass test 36 (i.e., in 87 patients in the TU group and in 74 patients in the UP group).
Besides TUs or UPs investigated in the present study, other organisms that are not analysed in daily clinical and laboratory routine may represent important aetiological determinants of prostatic infection. Imirzalioglu et al. 40 have shown that innovative molecular and chromatography techniques, associated with bioinformatic analysis, may reveal fastidious and anaerobic bacteria that have been shown to represent an important fraction (around 22%) of causative pathogens in urinary tract infections.
Nevertheless, because infection by TUs was demonstrated in only 17.2% of symptomatic patients by the five-glass procedure, our study confirms non-bacterial aetiology as the primary cause of CP syndromes.
Analysis of different diagnostic scenarios allowed us to build contingency tables and compare the relative sensitivity and specificity between five-glass and four-glass or two-glass tests. Table 4 shows that—for any class of pathogen—compared with either four- or two-glass tests, the five-glass test was characterized by a marked increase in sensitivity, associated with a lesser decrease in specificity.
Increased sensitivity implies a natural trade-off with the specificity of a given test, thus hampering the comparison between diagnostic assays. Therefore, grouping sensitivity and specificity within a single index or parameter, such as (i) the AUROCC, (ii) Youden's index (j), or (iii) LR, represents a more appropriate approach to comparison of the tests. The display system according to Biggerstaff 27, which includes grouping all these parameters in a single comparative graph, showed that the five-glass assay falls into the area classifying a test as superior to a standard comparator for "confirming the absence of disease" (Figure 3). However, this classification is applied when sensitivity and specificity values are calculated from 2 × 2 contingency tables comparing the positivity/negativity of a test with the presence or absence of disease 27. As in our case–as shown by Nickel et al. 25 and Zegarra Montes et al. 26–comparison is made with positivity/negativity of a reference standard test, the five-glass assay may be appropriately described as "superior for confirming the negative outcome" of the comparator four-glass or two-glass test. In our case, superiority is due to the lower LR− shown by the five-glass test, relative to both standard tests. In other words, semen analysis can complement standard tests to increase their negative predictive value (NPV) (e.g., NPV5-glass = 0.99 vs. NPV2-glass = 0.86). As the diagnosis of category III CP/CPPS is based on exclusion of demonstrable prostatic infection 36, superiority of the five-glass test in confirming the negative outcomes of four- or two-glass tests may be interpreted in terms of an increased power in the discrimination between category II CBP and category III CP/CPPS. The therapeutic implications of increased diagnostic power are of notable importance because enhanced accuracy in differential diagnosis between bacterial and abacterial prostatitis can greatly enhance the quality of therapeutic protocols, direct the choice of specific drugs for effective antibacterial therapy, decrease the need for empirical treatment, and reduce the emergence of chemoresistance caused by the inappropriate use of antibiotics.
Figure 3 shows that similar comparative patterns can be observed for TUs or UPs, mostly represented by Gram-positive bacteria and Mycoplasmata. Discussing the lack of reproducibility of Gram-positive localizations in segmented tests, Krieger and coworkers 41 suggested that these bacteria might represent intermittently shed, non-pathogen organisms transiently colonizing the LUT. Indeed, several authors suggest that lactobacilli, coryneforms, streptococci, or other Gram-positive bacteria may survive in the prostate as non-pathogenic commensals (reviewed in Reference 42). However, we and others have documented, in the past and also in this paper, that eradication of uncommon pathogens (mostly Gram-positive bacteria) in symptomatic patients is associated with remission of CP signs and symptoms in a manner not statistically different from the response of patients showing TU localization 32, 37. In particular, we have performed a comparative analysis of microbiological and clinical responses to the same combination therapy protocol described in this paper in 104 symptomatic patients showing evidence of prostatic infection by TUs or by UPs 32. The TU and UP groups showed a good correlation between eradication rates and clinical success rates, supporting the view that organisms other than the traditionally recognized uropathogens may play a role in prostatitis 32. This is in agreement with data more recently shown by Nickel and Xiang 37.
A major limitation of segmented tests is represented by the likelihood of urine or semen to collect contaminating urethral organisms. In a key review article in 1981, Stamey 11 stated that EPS or ejaculate samples might contain such organisms, which can mislead the diagnosis of an infection at the prostatic level. Indeed, the viscosity of semen and the mechanical force applied during masturbation may result in detachment from the urethral walls of organisms colonizing the more distal tract of the urethra. In their context, Stamey's observations were meant to stress the limitations of the VB1 sample in detecting the presence of urethral contaminants. To reduce ambiguity in the interpretation of EPS or semen cultures, Stamey 11 proposed to attempt urethral clearance with oral penicillin or nitrofurantoin prior to segmented tests. In daily clinical practice, such a procedure is unlikely to be adopted and can be misleading, due to the increasing fraction of chemoresistant commensals and pathogens. A urethral swab, sterilely collected before the emission of VB1, might be a reasonable alternative to complement VB1 (under investigation), provided that swabs do not allow quantitative measurement of bacterial loads.
In conclusion, our data indicate that the presence of pathogens in semen is likely to be indicative of an underlying prostatic infection if semen is analysed in the frame of a complete five-glass test. In this context, semen culture and analysis may represent an important component of the diagnostic workup in patients showing clinical signs and symptoms of CP.
The limits of our study lay in its retrospective, observational design. From a database of patients subjected to a panel of microbiological assays, we simulated the diagnostic scenarios of different segmented tests. Moreover, patients included in our treatment protocol showed signs and laboratory findings of CBP: thus, we do not know to what extent patients with CPPS without microbiological localization would have responded to combination therapy (this is currently under investigation). Ideally, distinct groups of randomized patients should be subjected to different segmented tests and to subsequent single-agent antibacterial treatment.
Acknowledgments
We are indebted to all patients included in this study. We thank medical assistants Ms Monica Bertocchi, Ms Teresa Rossoni, Ms Giusi Meli, and Ms Maria Soledad Valle Mena (Hospital: "Istituti Clinici di Perfezionamento, via Don Orione 2, Milano 20131, Italy), and to database keeper Ms. Marta Conti (University of Insubria, via A da Giussano 12, Busto A.21052, Italy).
References
- Drach GW, Fair WR, Meares EM, Stamey TA. Classification of benign diseases associated with prostatic pain: prostatitis or prostatodynia. J Urol. 1978;120:266. doi: 10.1016/s0022-5347(17)57135-9. [DOI] [PubMed] [Google Scholar]
- Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282:236–7. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- Schaeffer AJ. Clinical practice. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med. 2006;355:1690–8. doi: 10.1056/NEJMcp060423. [DOI] [PubMed] [Google Scholar]
- Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: state of the art. Scand J Immunol. 2007;66:217–27. doi: 10.1111/j.1365-3083.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- Wagenlehner FM, Weidner W, Naber KG. Therapy for prostatitis, with emphasis on bacterial prostatitis. Expert Opin Pharmacother. 2007;8:1667–74. doi: 10.1517/14656566.8.11.1667. [DOI] [PubMed] [Google Scholar]
- Labro MT. Antibiotics as anti-inflammatory agents. Curr Opin Investig Drugs. 2002;3:61–8. [PubMed] [Google Scholar]
- Nagy E, Szöke I, Török L, Pajor L. The role of anaerobic bacteria in prostatitis. Adv Exp Med Biol. 2000;485:289–99. doi: 10.1007/0-306-46840-9_38. [DOI] [PubMed] [Google Scholar]
- Kermes K, Punab M, Lõivukene K, Mandar R. Anaerobic seminal fluid micro-flora in chronic prostatitis/chronic pelvic pain syndrome patients. Anaerobe. 2003;9:117–23. doi: 10.1016/S1075-9964(03)00085-4. [DOI] [PubMed] [Google Scholar]
- Domingue GJ, Sr, Hellstrom WJ. Prostatitis. Clin Microbiol Rev. 1998;11:604–13. doi: 10.1128/cmr.11.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meares EM, Stamey TA. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5:492–518. [PubMed] [Google Scholar]
- Stamey TA. Prostatitis. J R Soc Med. 1981;74:22–40. doi: 10.1177/014107688107400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC. The Pre and Post Massage Test (PPMT): a simple screen for prostatitis. Tech Urol. 1997;3:38–43. [PubMed] [Google Scholar]
- Budía A, Luis Palmero J, Broseta E, Tejadillos S, Benedicto A, et al. Value of semen culture in the diagnosis of chronic bacterial prostatitis: a simplified method. Scand J Urol Nephrol. 2006;40:326–31. doi: 10.1080/00365590600748247. [DOI] [PubMed] [Google Scholar]
- McNaughton Collins M, Fowler FJ, Jr, Elliott DB, Albertsen PC, Barry MJ. Diagnosing and treating chronic prostatitis: do urologists use the four-glass test. Urology. 2000;55:403–7. doi: 10.1016/s0090-4295(99)00536-1. [DOI] [PubMed] [Google Scholar]
- Mobley DF. Semen cultures in the diagnosis of bacterial prostatitis. J Urol. 1975;114:83–5. doi: 10.1016/s0022-5347(17)66949-0. [DOI] [PubMed] [Google Scholar]
- Nickel JC. Classification and diagnosis of prostatitis: a gold standard. Andrologia. 2003;35:160–7. doi: 10.1046/j.1439-0272.2003.00557.x. [DOI] [PubMed] [Google Scholar]
- Weidner W, Ludwig M, Braehler E, Schiefer HG. Outcome of antibiotic therapy with ciprofloxacin in chronic bacterial prostatitis. Drugs. 1999;58:103–6. doi: 10.2165/00003495-199958002-00021. [DOI] [PubMed] [Google Scholar]
- Weidner W, Anderson RU. Evaluation of acute and chronic bacterial prostatitis and diagnostic management of chronic prostatitis/chronic pelvic pain syndrome with special reference to infection/inflammation. Int J Antimicrob Agents. 2008;31 Suppl 1:S91–5. doi: 10.1016/j.ijantimicag.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Weidner W, Wagenlehner FM, Marconi M, Pilatz A, Pantke KH, et al. Acute bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: andrological implications. Andrologia. 2008;40:105–12. doi: 10.1111/j.1439-0272.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer AJ, Knauss JS, Landis JR, Propert KJ, Alexander RB, et al. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J Urol. 2002;168:1048–53. doi: 10.1016/S0022-5347(05)64572-7. [DOI] [PubMed] [Google Scholar]
- Nickel JC. Recommendations for the evaluation of patients with prostatitis. World J Urol. 2003;21:75–81. doi: 10.1007/s00345-003-0328-1. [DOI] [PubMed] [Google Scholar]
- Giubilei G, Mondaini N, Crisci A, Raugei A, Lombardi G, et al. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol. 2005;47:805–11. doi: 10.1016/j.eururo.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, et al. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003;170:818–22. doi: 10.1097/01.ju.0000082252.49374.e9. [DOI] [PubMed] [Google Scholar]
- Skerk V, Krhen I, Schonwald S, Cajic V, Markovinovic L, et al. The role of unusual pathogens in prostatitis syndrome Int J Antimicrob Agents 200424Suppl 1): S53–6. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Shoskes D, Wang Y, Alexander RB, Fowler JE, Jr, et al. How does the pre-massage and post-massage 2-glass test compare to the Meares-Stamey 4-glass test in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2006;176:119–24. doi: 10.1016/S0022-5347(06)00498-8. [DOI] [PubMed] [Google Scholar]
- Zegarra Montes LR, Sanchez Mejia AA, Loza Munarriz CA, Celis Gutierrez E. Semen and urine culture in the diagnosis of chronic bacterial prostatitis. Int Braz J Urol. 2008;34:30–40. doi: 10.1590/s1677-55382008000100006. [DOI] [PubMed] [Google Scholar]
- Biggerstaff BJ. Comparing diagnostic tests: a simple graphic using likelihood ratios. Stat Med. 2000;19:649–63. doi: 10.1002/(sici)1097-0258(20000315)19:5<649::aid-sim371>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- Gardner IA, Greiner M. Receiver-operating characteristic curves and likelihood ratios: improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol. 2006;35:8–17. doi: 10.1111/j.1939-165x.2006.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Magri V, Trinchieri A, Pozzi G, Restelli A, Garlaschi MC, et al. Efficacy of repeated cycles of combination therapy for the eradication of infecting organisms in chronic bacterial prostatitis. Int J Antimicrob Agents. 2007;29:549–56. doi: 10.1016/j.ijantimicag.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Magri V, Trinchieri A, Montanari E, Del Nero A, Mangiarotti B, et al. Reduction of PSA values by combination pharmacological therapy in patients with chronic prostatitis: implications for prostate cancer detection. Arch Ital Urol Androl. 2007;79:84–92. [PubMed] [Google Scholar]
- Magri V, Trinchieri A, Ceriani I, Marras E, Perletti G. Eradication of unusual pathogens by combination pharmacological therapy is paralleled by improvement of signs and symptoms of chronic prostatitis syndrome. Arch Ital Urol Androl. 2007;79:93–8. [PubMed] [Google Scholar]
- Barbalias GA, Nikiforidis G, Liatsikos EN. Alpha-blockers for the treatment of chronic prostatitis in combination with antibiotics. J Urol. 1998;159:883–7. [PubMed] [Google Scholar]
- Wagenlehner FM, Naber KG. Current challenges in the treatment of complicated urinary tract infections and prostatitis. Clin Microbiol Infect. 2006;12 Suppl 3:67–80. doi: 10.1111/j.1469-0691.2006.01398.x. [DOI] [PubMed] [Google Scholar]
- Naber K, European Lomefloxacin Prostatitis Study Group Lomefloxacin versus ciprofloxacin in the treatment of chronic bacterial prostatitis. Int J Antimicrob Agents. 2002;20:18–27. doi: 10.1016/s0924-8579(02)00067-5. [DOI] [PubMed] [Google Scholar]
- Nickel JC. Alpha-Blockers for the Treatment of Prostatitis-Like Syndromes. Rev Urol. 2006;8 Suppl 4:S26–S34. [PMC free article] [PubMed] [Google Scholar]
- Nickel JC, Xiang J. Clinical significance of nontraditional bacterial uropathogens in the management of chronic prostatitis. J Urol. 2008;179:1391–5. doi: 10.1016/j.juro.2007.11.081. [DOI] [PubMed] [Google Scholar]
- Ivanov IB, Kuzmin MD, Gritsenko VA. Microflora of the seminal fluid of healthy men and men suffering from chronic prostatitis syndrome. Int J Androl. 2008;31:1–6. doi: 10.1111/j.1365-2605.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Mazzoli S. Conventional bacteriology in prostatitis patients: microbiological bias, problems and epidemiology on 1686 microbial isolates. Arch Ital Urol Androl. 2007;79:71–5. [PubMed] [Google Scholar]
- Imirzalioglu C, Hain T, Chakraborty T, Domann E. Hidden pathogens uncovered: metagenomic analysis of urinary tract infections. Andrologia. 2008;40:66–71. doi: 10.1111/j.1439-0272.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- Krieger JN, Ross SO, Limaye AP, Riley DE. Inconsistent localization of gram-positive bacteria to prostate-specific specimens from patients with chronic prostatitis. Urology. 2005;66:721–5. doi: 10.1016/j.urology.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Weidner W, Ludwig M. Common organisms in urogenital infections with special emphasis on prostatitis. Eur Urol Suppl. 2003;2:15–8. [Google Scholar]


