Abstract
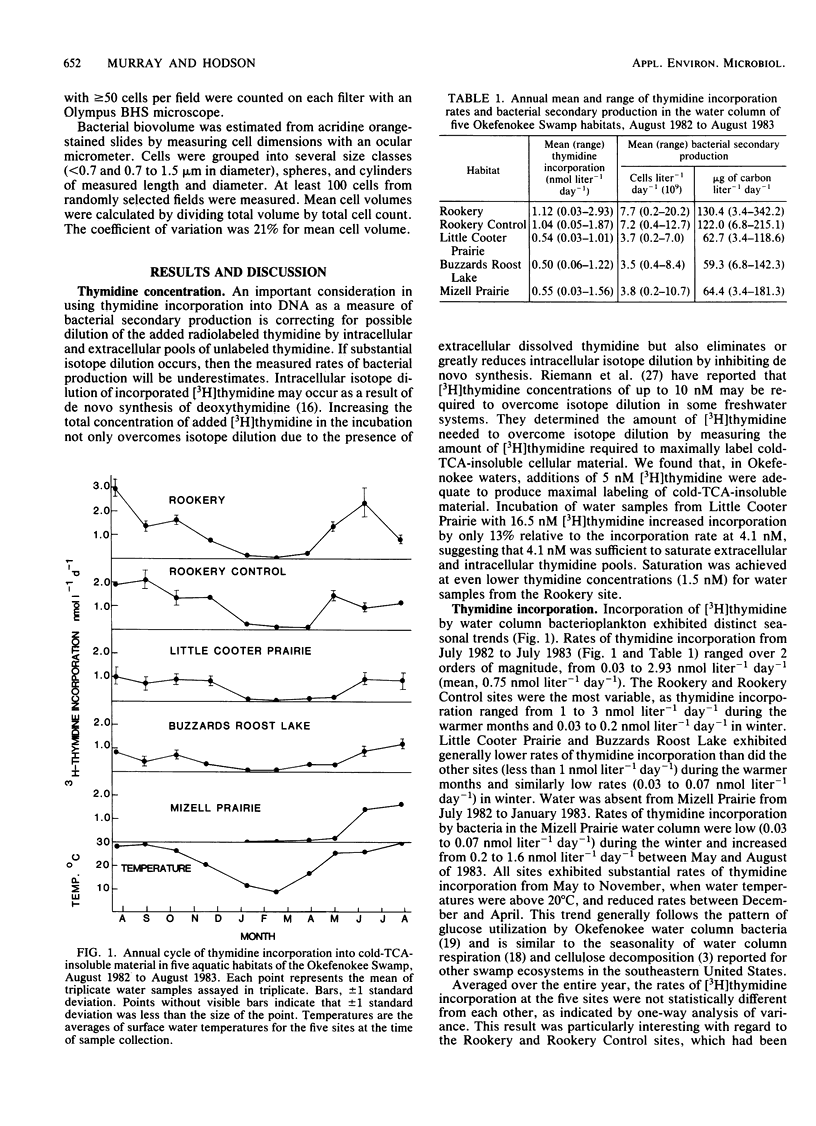
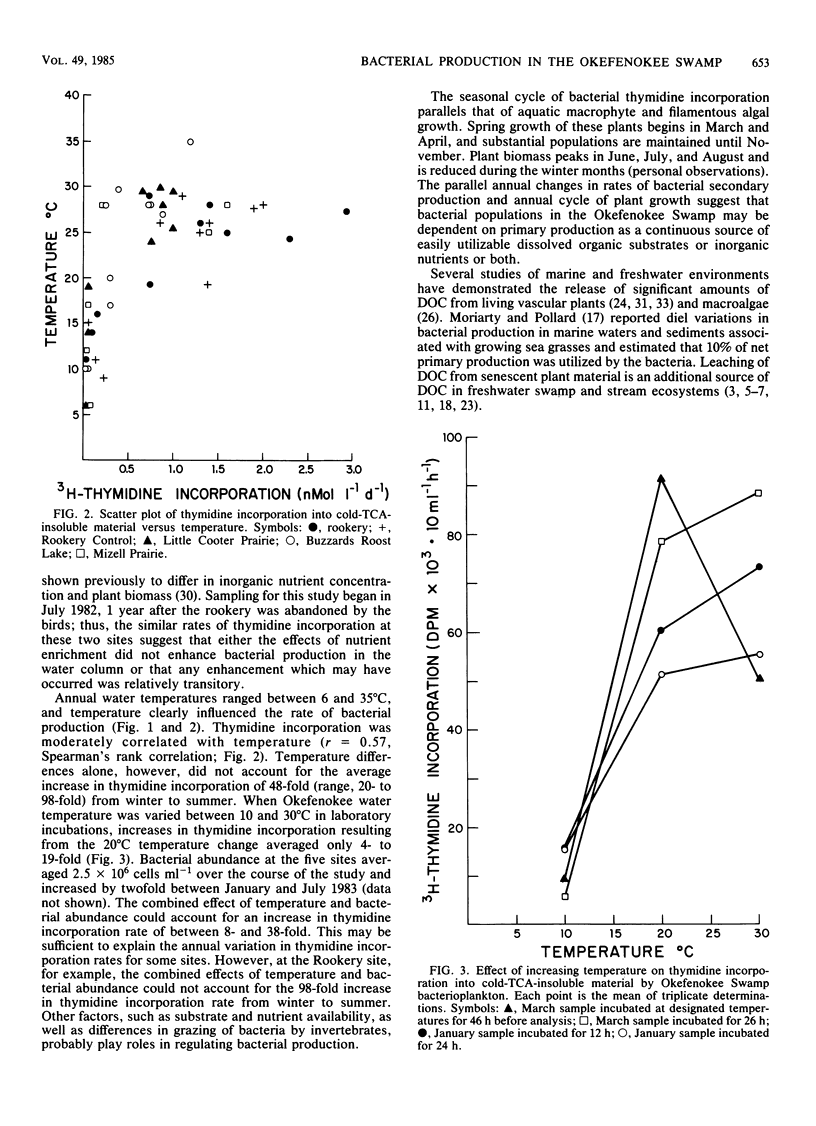
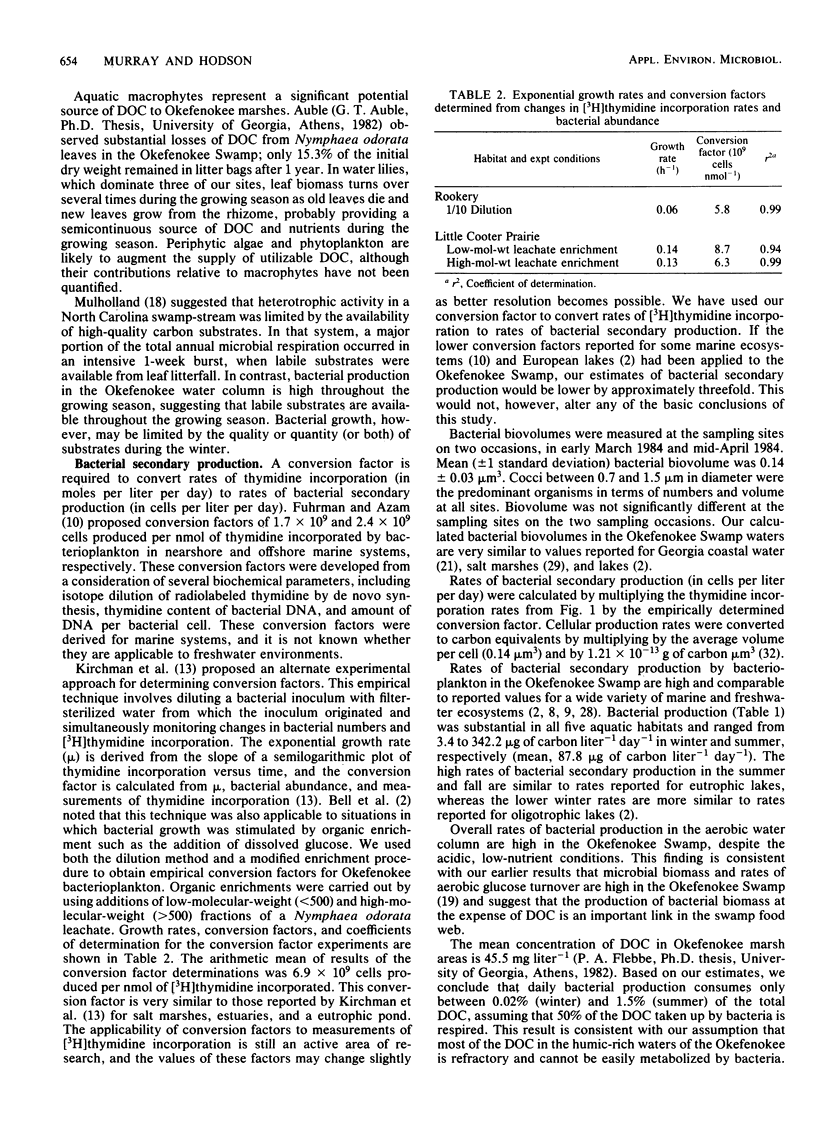
Rates of bacterial secondary production by free-living bacterioplankton in the Okefenokee Swamp are high and comparable to reported values for a wide variety of marine and freshwater ecosystems. Bacterial production in the water column of five aquatic habitats of the Okefenokee Swamp was substantial despite the acidic (pH 3.7), low-nutrient, peat-accumulating character of the environment. Incorporation of [3H]thymidine into cold-trichloroacetic acid-insoluble material ranged from 0.03 to 2.93 nmol liter−1 day−1) and corresponded to rates of bacterial secondary production of 3.4 to 342.2 μg of carbon liter−1 day−1 (mean, 87.8 μg of carbon liter−1 day−1). Bacterial production was strongly seasonal and appeared to be coupled to annual changes in temperature and primary production. Bacterial doubling times ranged from 5 h to 15 days and were fastest during the warm months of the year, when the biomass of aquatic macrophytes was high, and slowest during the winter, when the plant biomass was reduced. The high rates of bacterial turnover in Okefenokee waters suggest that bacterial growth is an important mechanism in the transformation of dissolved organic carbon into the nutrient-rich bacterial biomass which is utilized by microconsumers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Microbial biomass and utilization of dissolved organic matter in the okefenokee swamp ecosystem. Appl Environ Microbiol. 1984 Apr;47(4):685–692. doi: 10.1128/aem.47.4.685-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B., Søndergaard M. Measurements of diel rates of bacterial secondary production in aquatic environments. Appl Environ Microbiol. 1984 Apr;47(4):632–638. doi: 10.1128/aem.47.4.632-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer P. N., Srinivasan S., Chopra P. S., Martin J. G., Lucas T., Burrowes C. B., Sauvage L. Electrochemistry of thrombosis--an aid in the selection of prosthetic materials. J Biomed Mater Res. 1970 Mar;4(1):43–55. doi: 10.1002/jbm.820040106. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


