Abstract
Alzheimer's disease (AD) is characterized by the presence of neuropathological lesions containing amyloid plaques (APs) and hyperphosphorylated Tau containing neurofibrillary tangles (NFTs) and is associated with neuroinflammation and neurodegeneration. Entorhinal cortex (Brodmann's area 28) is involved in memory associated functions and is one of the first brain areas targeted to form the neuropathological lesions and also severely affected cortical region in AD. Glia maturation factor (GMF), a central nervous system protein and a proinflammatory molecule is known to be up-regulated in the specific areas of AD brain. Our previous immunohistochemical studies using temporal cortex showed that GMF is expressed in the vicinity of APs and NFTs in AD brains. In the present study, we have analyzed the expression of GMF and its association with APs and NFTs in the entorhinal cortex of AD brains by using immunohistochemistry combined with thioflavin-S fluorescence labeling methods. Results showed that GMF immunoreactive glial cells, glial fibrillary acidic protein labeled reactive astrocytes and ionized calcium binding adaptor molecule-1 labeled activated microglia were increased in the entorhinal cortical layers especially at the sites of 6E10 labeled APs and Tau containing NFTs. In conclusion, increased expression of GMF by the glial cells in the entorhinal cortex region, and the co-localization of GMF with APs and NFTs suggest that GMF may play important proinflammatory roles in the pathogenesis of AD.
Keywords: Alzheimer's disease, amyloid plaques, entorhinal cortex, glia maturation factor, neurofibrillary tangles, neuroinflammation
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder affecting 5 million Americans and about 35 million individuals worldwide. Neuropathologically, it is distinguished by the presence of amyloid plaques (APs) and neurofibrillary tangles (NFTs) containing hyperphosphorylated Tau along with neuronal loss and an abundance of reactive glia in the specific areas of the AD brain [1-3]. Tau protein is present as an abnormally phosphorylated form in AD [4-7]. The number of NFTs is closely associated with clinical symptoms in AD patients.
Entorhinal cortex (Brodmann's area 28) is the area where NFTs and APs are first detectable in old age and in AD brains [8]. Entorhinal cortex is involved in memory formation, retrieval, and extinction, as part of circuits that include the hippocampus, the amygdaloid nucleus, and several regions of the neocortex, in particular the prefrontal cortex [8-11]. Entorhinal cortex is one of the regions in the AD brain with the earliest, and most vulnerable and heavily affected region [12,13]. The number of NFTs in the medial temporal lobe structures, including the entorhinal cortex and hippocampal formation, is well correlated with the cognitive memory impairment in AD patients [14,15]. The memory loss is one of the earliest symptoms associated with AD due to the destruction of entorhinal cortex projections to the hippocampal formation, the perforant pathways [9].
Glia maturation factor (GMF) is a brain protein, was first discovered, purified, and sequenced in our laboratory [16-18]. GMF release in excess induces proinflammatory reactions and acts as a prominent mediator of inflammation in the central nervous system (CNS) leading to the death of neurons in the neurodegenerative diseases [19,20]. Previous studies showed that GMF is expressed in the vicinity of APs and NFTs in temporal cortex of human AD brains [3,21]. The entorhinal cortex is severely affected, atrophied and inflamed in AD patients. The inflammatory process may be partially mediated through GMF in the brain. However, the distribution of GMF in entorhinal cortex is not yet clearly studied in AD patients. Therefore, the present study was carried out to demonstrate the expression and the association between GMF expressing glial cells and neuropathological markers such as APs and NFTs distribution in the entorhinal cortex in the AD brains using immunohistochemistry (IHC) staining methods. We report that specific up-regulation of GMF expression in the glial cells is associated with APs and NFTs in the entorhinal cortical layers of AD brain.
Materials and Methods
Human AD Brains
Temporal lobes from human postmortem rapid brains of AD patients (N=6) and from non-AD brains (n=3) were obtained from the University of Iowa Deeded body program. Temporal lobes were then fixed in 4% paraformaldehyde solution (in 0.1M phosphate buffered saline pH 7.4, PBS; GIBCO, Life Technologies, Auckland, NZ) for 24-48 h. Then the fixed temporal lobe blocks were cut into 50 μm thick sections using a sledge freezing microtome. These sections were collected in PBS and stored in cryostorage solution (Glycerol 30 ml, ethylene glycol 30 ml, 40 ml 0.1M PBS) at -80°C until used for immunohistochemical staining. The neuropathological analysis was performed by using thioflavin-S (Sigma, St. Louis, MO) histochemical staining to detect the presence of AD specific histopathological hallmarks NFTs and APs as we have reported previously [22]. Brains which showed the presence of laminar localization pattern, increased density of Tau containing NFTs, and APs in the hippocampal and temporal cortical regions were confirmed as AD brains [21,23,24]. All the experimental procedures were carried out according to the Institution approved guidelines.
Double Immunohistochemical Staining of GMF with Tau, and GMF with 6E10
Sections containing parahippocampal gyrus (PHG) were removed from cryostorage solution and rinsed in PBS for 10 min and IHC was done as we performed previously [3,5,21,22]. Briefly, the free floating tissue sections were incubated with 0.3% hydrogen peroxide (in PBS) for 20 min at room temperature (RT) to quench the endogenous peroxidase activity if any. Sections were then washed in PBS and then incubated with blocking solution (5% normal goat serum, 3% BSA and 0.1% Triton-X in PBS) for 1 h at room temperature (RT) to block nonspecific staining. Then the sections were incubated with the primary antibodies to GMF (rabbit polyclonal antibody, prepared in our laboratory and used at 1:500 dilutions), Tau (mouse anti-Tau-1 monoclonal antibody; Chemicon International, Temecula, CA; at 1:500 dilutions) mainly for NFTs, 6E10 (Beta Amyloid, 1-16, 6E10 monoclonal antibody; Covance, Dedham, MA; at 1:1000 dilutions) for APs, glial fibrillary acidic protein (GFAP; anti-glial fibrillary acidic protein, clone GA5 monoclonal antibody, Millipore, Temecula, CA; at 1:500 dilutions) in the combinations mentioned above. These antibodies were diluted in blocking buffer and incubated for overnight at 4°C. The sections were then washed in PBS and incubated for 1 h at RT with corresponding biotinylated secondary anti-mouse IgG or anti-rabbit IgG antibodies raised in goat (Vector Laboratories, Burlingame, CA). Then the sections were rinsed again in PBS and developed with an avidin-biotin-complex (ABC) standard staining kit (Vector Laboratories) solution (diluted in PBS) for 1 h at RT. After washing in PBS, the sections were developed with diaminobenzidine (DAB, brown) or SG (blue gray) substrate solution (Vector Laboratories). The sections were rinsed with PBS and then distilled water and mounted on the glass slides and briefly dried. Slides were then dehydrated, cleared and cover slipped with Permount (Fisher Scientific, Pittsburgh, PA). All the washings were carried out in PBS for 3 times and 10 min in each). To quantitate the GMF staining in relation to the number of APs, we have counted 6E10-positive APs, Tau positive NFTs with GMF positive cells in the areas with more number of plaques/NFTs and compared with area with less number of APs/NFTs area in the entorhinal region. All the countings were performed under high magnification in 5-10 different fields and then averaged. The data were presented as the number of APs/NFTs or GMF positive cells/95 mm2. Differences between more APs/NFTs area and less APs/NFTs area were analyzed using unpaired t-test. A p value less than 0.05 was considered statistically significant.
Double Staining with GMF, GFAP, Ionized Calcium Binding Adaptor Molecule-1 (IBA-1) and Thioflavin-S
Sections from the entorhinal cortex were incubated with antibodies to GMF, GFAP (immunoreactive astrocytes; anti-glial fibrillary acidic protein, clone GA5 monoclonal antibody, Millipore) and IBA-1 (Anti- IBA-1, polyclonal antibody, rabbit; Wako Chemicals, Richmond, VA) individually by the IHC method as we described above [3,25]. GMF, GFAP or IBA-1 immunolabeled sections from entorhinal cortex were then counterstained with thioflavin-S to show the association between GMF, GFAP or IBA-1 with NFTs or APs in AD brains.
Results
GMF Expression in the Entorhinal Cortex Specifically at the Sites of APs and NFTs of AD Brain
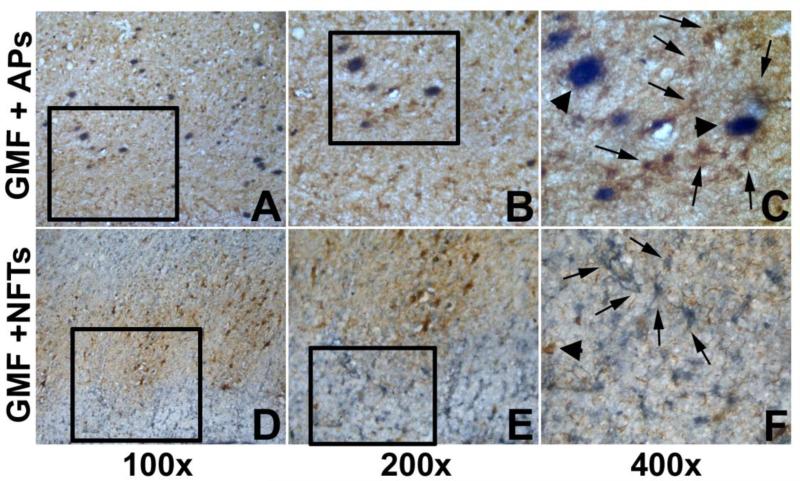
We have performed double IHC for GMF with 6E10 for APs, as well as GMF with Tau for NFTs in AD brains. Entorhinal cortex showed an increase in the expression of GMF staining (brown color, arrows) in the GMF immunoreactive cells in and around APs (gray-blue, arrow head) in Figure 1A,B,C. GMF-positive (gray-blue, arrows) and Tau-positive NFTs (brown, arrow head) were shown in Fig. 1D,E,F. GMF immunoreactive astrocytes were numerous in the entorhinal cortex of AD brains. Double-immunolabeling with antibodies to GMF and Tau confirmed that GMF-positive astrocytes (resembling morphology of reactive astrocytes) in the entorhinal cortex of AD brain (Fig. 1D,E,F).
Fig. 1.
GMF up-regulation in the entorhinal cortex specifically at the sites of APs (A,B, C) and NFTs (D,E,F) in AD brain. Double immunohistochemistry depicting APs (with 6E10 antibody) in blue-gray color (s in A,B,C, arrow heads) and GMF-positive glial cells in brown color (arrows A,B,C) in the entorhinal cortex of AD brains. Note, GMF-positive astrocytes were localized near the 6E10-positive APs (C). Double immunostaining of GMF and Tau antibody showing the association of GMF (blue-gray color; arrows in F) and Tau-immunoreactive NFTs (brown color in D,E,F, arrow head) in the entorhinal cortex of AD brain. Note the double labeling of GMF immunoreactive glial cells and Tau-immunostained NFTs, and astrocytes present near to that exhibit GMF-positive staining (D,E,F).
GMF Expression, Astrocyte and Microglia Activations, and its Association with APs and NFTs in the Entorhinal Cortex of AD Brain as Assessed by Thioflavin-S Staining
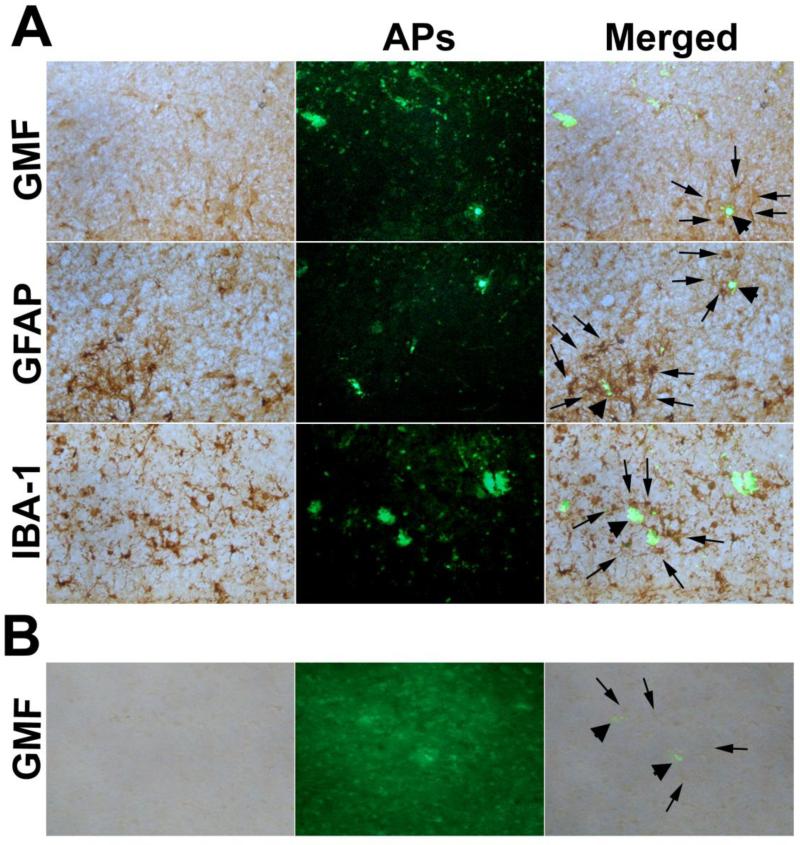
GMF immunoreactivity (brown, arrows, merged) and thioflavin-S histochemical staining (green) in entorhinal cortex were shown in Fig. 2 A,B. The accumulation of GMF immunoreactive cells (arrows, top panel) was observed around the thioflavin-S stained APs (green, merged, top panel, arrow head). The entorhinal cortex, where we found an increased expression of GMF, is one of the known cortical areas most severely devastated in AD. A large number of APs and NFTs were observed in the entorhinal cortex. GMF is highly expressed at the sites of APs and NFTs in the entorhinal cortex of AD brain. We observed numerous GMF-immunoreactive astrocytes clustering in and around APs. GMF staining and Thioflavin-S staining revealed GMF and APs co-localization in the entorhinal cortex (arrows, merged) of AD brains.
Fig. 2.
Double-labeling of GMF, GFAP and IBA-1 with Thioflavin S staining in the entorhinal cortex of AD brains (A). Tissue sections of entorhinal cortex were double labeled with GMF (brown) and thioflavin-S (green). Reactive astrocytes and activated microglia were stained for the expression of GFAP (brown) and IBA-1(brown), respectively by ABC staining method using DAB substrate. Thioflavin-S staining shows APs (green, arrow head). In the merged picture, we show APs (arrow heads) were surrounded by GMF immunoreactive glial cells (top panel; arrows) or astrocytes (middle panel, arrows) or microglia (lower panel arrows) in the entorhinal cortex of AD brains. We have also stained GMF (arrows) and Thioflavin-S stained APs (arrow head) in the entorhinal cortex of non-AD brains (B) for comparison with AD brains. Original magnifications: (x200).
Astrocytes and its Association with APs in the Entorhinal Cortex of AD Brain
The association between GFAP-labeled astrocytes with thioflavin-S stained APs (arrow heads) and NFTs in the entorhinal cortex of AD was shown in Fig. 2A (middle panel).Astrocytes were found in the vicinity of AP and few APs were surrounded by a cluster of astrocytes (arrows, merged).
Microglia and its Association with APs in the Entorhinal Cortex of AD Brain
Activated microglia, as detected by IBA-1 immunoreactivity was strongly associated with APs (thioflavin-S staining) (arrows, merged) found in the entorhinal cortex. Activated microglia was integrated with the core of the APs (Fig. 2A, bottom panel, arrow head).Figure 2B shows GMF (arrows) immunostaining and Thioflavin-S stained APs (arrow heads) in non-AD control brain to compare with AD brains.
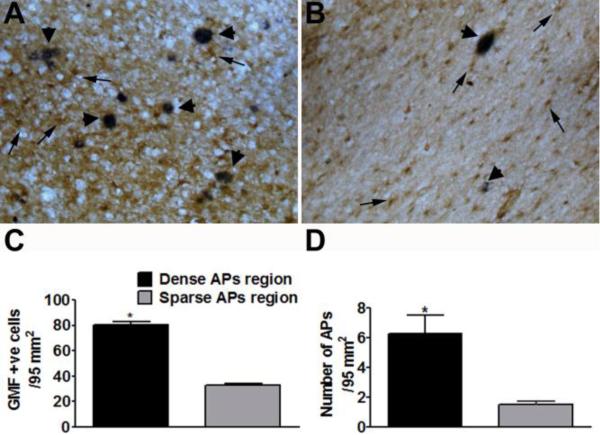
GMF up-regulation at the Sites of APs in the Entorhinal Cortex of AD Brain
APs, as detected by the presence of blue-gray 6E10 immunoreactions, were found throughout the entorhinal cortex of AD brains. GMF (brown, arrows) was highly expressed at sites of APs in the entorhinal cortex. Double immunostaining with GMF and 6E10 shows reactive astrocytes and APs (blue-gray color, arrow head) in the entorhinal cortex of AD brains (Fig. 3A,B). Figure 3A and B shows dense APs region and sparse APs region, respectively. Following staining, we then counted the number of GMF-positive cells in relation to the density of APs. We report that the number of GMF-positive cells (Fig. 3C) were significantly (p<0.05) higher in the area where the density of APs are more (Fig. 3D) in the entorhinal cortex in AD brains.
Fig. 3.
Double immunostaining of GMF (brown color) and APs with 6E10 antibody (blue-gray color in A and B, arrows) in the entorhinal cortex in AD brains. (A) Shows more plaques associated with more GMF-positive cells and (B) shows less APs associated with less GMF-positive cells in the entorhinal cortex. Original magnification: x200. GMF-positive cells in relation to the density of APs were counted under the microscope. The number of GMF-positive cells (C) were significantly (p<0.05) higher in the area where the density of APs are also dense (D) in the entorhinal cortex in AD brains. The data were presented as mean ± SEM, n=3, *p<0.05.
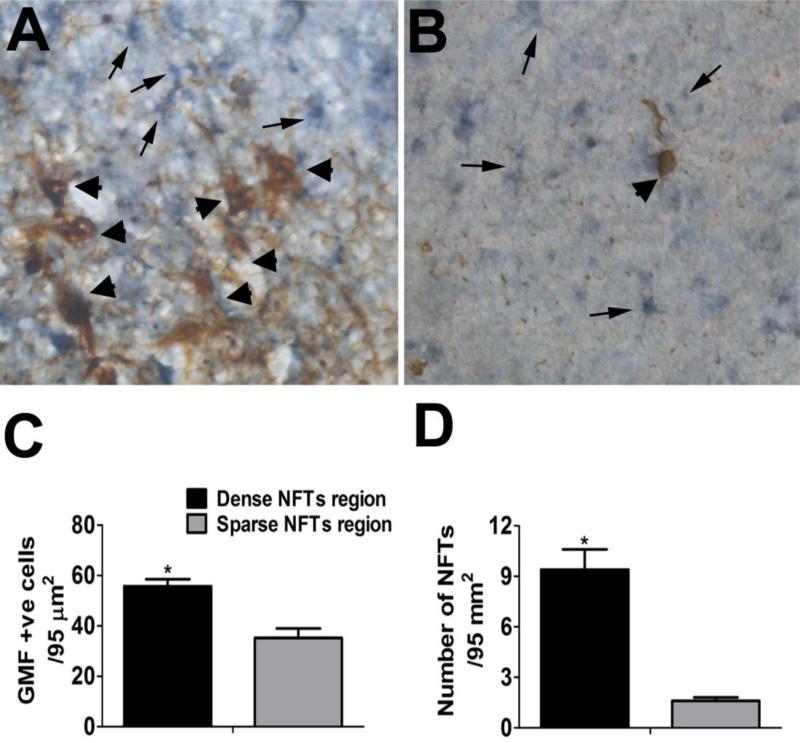
GMF up-regulation at the sites of NFTs in the entorhinal cortex of AD brain
NFTs were detected with Tau immunoreactions (brown, arrow heads) and were found throughout the entorhinal cortex of AD brains. GMF (blue-gray, arrows) was highly expressed at sites of NFTs in the entorhinal cortex. Double immunostaining with GMF (blue-gray) and Tau shows reactive astrocytes near NFTs in the entorhinal cortex of AD brains (Fig. 4A,B). Similarly, Figure 4A and B shows dense NFTs region and sparse NFTs region, respectively in the entorhinal cortex region. Further, we also counted the number of GMF-positive cells (Fig. 4C) and were significantly (p<0.05) higher in the area where the density of NFTs were more (Fig. 4D) in the entorhinal cortex of AD brains.
Fig. 4.
Double immunostaining of GMF (blue-gray) and NFTs with Tau antibody (brown in A and B) in the entorhinal cortex in AD brains. (A) More NFTs (brown, arrow heads) are associated with more GMF-positive cells (blue-gray, arrows). (B) Shows less NFTs associated with less GMF-positive cells in the entorhinal cortex. Original magnification: x400. GMF-positive cells in relation to the density of NFTs were counted under the microscope. The number of GMF-positive cells (C) were significantly (p<0.05) higher in the area where the density of NFTs are also dense (D) in the entorhinal cortex in AD brains. The data were presented as mean ± SEM, n=3, *p<0.05.
Discussion
The present study demonstrates the up-regulation of GMF expression in the entorhinal cortex of AD brain. Further, we report that GMF up-regulation is increased specifically at the sites of APs and hyperphosphorylated Tau containing NFTs, the pathological hallmarks of AD. We found that GMF immunoreactive glial cells are present in all the layers of the entorhinal cortex. In the present study, we investigated the expression of GMF in the entorhinal cortex of AD brains and the morphological associations between GMF reactive glial cells and NFTs and APs by using single and double-immunolabeling IHC methods. We have performed thioflavin-S histochemistry and the double IHC for GMF and Aβ (with 6E10-antibody), as well as GMF and hyperphosphorylated Tau.
Previous reports indicate that entorhinal cortex is affected in the early stages of AD pathogenesis [10,11,15,26-29]. Entorhinal cortex is an important region in the medial temporal lobe and is involved in the memory associated functions. Memory deficit is a common feature observed in the AD patients; therefore investigation of GMF expression in the entorhinal cortex in terms of its proinflammatory role associated with the neuronal death seems very important. Cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) play critical role in inflammatory processes of AD [30]. GMF, the brain protein may augment the effect of other proinflammatory molecules in the pathogenesis of AD. NFTs are seen in the entorhinal cortex, limbic and neocortex over the course of clinical progression of AD. NFTs are associated with synapse as well as neuronal loss, suggesting that the process of NFT formation is strongly associated with brain dysfunctions [31] such as in AD. GMF has been reported to be expressed in the human brain regions [3,21]. Due to normal aging processes and in the presence of AD, the entorhinal cortex and hippocampus are reported to be independently and highly affected. Both these regions have direct connectivity and play important role in the memory associated functions [9,32,33]. Anatomical abnormalities have been observed in these regions, in the very early stages of AD pathogenesis [15,33-35]. Imaging studies have shown atrophy in the entorhinal cortex as well as in the hippocampus of very early stages of AD pathogenesis characterized by confusion and other memory related disorders [32,36-38]. The expression and distribution of GMF within the CNS has been previously reported using IHC staining procedures [39,40]. In the rat brain, GMF mRNA and protein are reported to be widely expressed in the spinal cord, cerebellum, midbrain, basal ganglia, sensory/ motor cortex, entorhinal cortex and hippocampus [41].
In the present study, we have observed an increased number of reactive astrocytes and activated microglia specifically at the sites of increased GMF expression with increased APs and NFTs in entorhinal cortex of AD brains. As speculated in our previous studies, astrocytes and microglia may be increased and or activated as an immune response to the presence of APs and NFTs, and these reactions may initiate the critical inflammatory reaction cascades of events in the early stages of AD [3,21]. Moreover, amyloid deposition is known to provoke the phenotypic activation of microglia and their expression of proinflammatory molecules in AD [42]. APs contain Aβ peptide and are surrounded by infiltrated microglia and astrocytes. These glial cells can release various proinflammatory and neurotoxic molecules causing neuronal degeneration [43]. Our present results suggest a close relationship between astrocytes, microglia, APs and NFTs with degenerating neurons in the entorhinal cortex of AD patients. Previous study have suggested that microglial activation may be manipulated to prevent/delay neurodegeneration and promote neuroprotection in AD through new therapeutic target [44]. NFTs containing hyperphosphorylated Tau is known to be involved in the synaptic loss, and granular Tau is involved in the neuronal loss in AD patients [31]. Increased number of NFTs along with GMF-mediated inflammatory responses in the entorhinal cortex may contribute for the memory loss observed in AD patients. We have observed the high expression of GMF with increased numbers as well as activated astrocytes and microglia in entorhinal cortex of AD patients. However, the changes in the entorhinal cortex alone may not be responsible for all the memory impairment in AD patients [9] as suggested previously. We have also observed that GMF immunoreactive glial cells are strongly associated with APs. APs and NFTs may attract and activate both the astrocytes and microglia, which may further increase the GMF level at these sites. This close relationship between GMF and APs in the entorhinal cortex of AD brain suggests that GMF may be a critical proinflammatory mediator in the early stages of the initiation of AD pathogenesis. Memory impairment in AD seems to be in part, related to glial cell activation in addition to synaptic dysfunction. In accord with our previous results, the present study also supports a close relationship between GMF and APs.
In conclusion, the increased expression of GMF by the glial cells in the entorhinal cortex region, and the co-localization of GMF and thioflavin-S stained NFTs and APs suggest that GMF may play important proinflammatory roles in the pathogenesis of AD.
Acknowledgments
Research support: This work was supported by the Department of Veterans Affairs Merit Review award (to A.Z.) and by the National Institute of Neurological Disorders and Stroke grants NS073670 (to A.Z.)
References
- 1.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 2.Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta neuropathologica. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A. Expression of glia maturation factor in neuropathological lesions of Alzheimer's disease. Neuropathol Appl Neurobiol. 2012;38:572–581. doi: 10.1111/j.1365-2990.2011.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lace G, Savva GM, Forster G, de Silva R, Brayne C, et al. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain. 2009;132:1324–1334. doi: 10.1093/brain/awp059. [DOI] [PubMed] [Google Scholar]
- 5.Thangavel R, Van Hoesen GW, Zaheer A. The abnormally phosphorylated tau lesion of early Alzheimer's disease. Neurochem Res. 2009;34:118–123. doi: 10.1007/s11064-008-9701-1. [DOI] [PubMed] [Google Scholar]
- 6.Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimer's Dis. 2012;2012:731526. doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, et al. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevilaqua LR, Rossato JI, Bonini JS, Myskiw JC, Clarke JR, et al. The role of the entorhinal cortex in extinction: influences of aging. Neural plasticity. 2008;2008:595282. doi: 10.1155/2008/595282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hoesen GW, Hyman BT, Damasio AR. Entorhinal cortex pathology in Alzheimer's disease. Hippocampus. 1991;1:1–8. doi: 10.1002/hipo.450010102. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Isla T, Price JL, McKeel DW, Jr., Morris JC, Growdon JH, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Hof PR, Bussiere T, Gold G, Kovari E, Giannakopoulos P, et al. Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropath Exp Neur. 2003;62:55–67. doi: 10.1093/jnen/62.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Porchet R, Probst A, Bouras C, Draberova E, Draber P, et al. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer's disease. Proteomics. 2003;3:1476–1485. doi: 10.1002/pmic.200300456. [DOI] [PubMed] [Google Scholar]
- 14.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropath E#xp Neur. 2009;68:774–784. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson PT, Dimayuga J, Wilfred BR. MicroRNA in Situ Hybridization in the Human Entorhinal and Transentorhinal Cortex. Front Hum Neurosci. 2010;4:7. doi: 10.3389/neuro.09.007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim R, Miller JF, Zaheer A. Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci USA. 1989;86:3901–3905. doi: 10.1073/pnas.86.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim R, Zaheer A, Lane WS. Complete amino acid sequence of bovine glia maturation factor beta. Proc Natl Acad Sci USA. 1990;87:5233–5237. doi: 10.1073/pnas.87.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan R, Zaheer A, Jaye M, Lim R. Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem. 1991;57:483–490. doi: 10.1111/j.1471-4159.1991.tb03777.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaheer A, Sahu SK, Wu Y, Haas J, Lee K, et al. Diminished cytokine and chemokine expression in the central nervous system of GMF-deficient mice with experimental autoimmune encephalomyelitis. Brain Res. 2007;1144:239–247. doi: 10.1016/j.brainres.2007.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaheer A, Zaheer S, Thangavel R, Wu Y, Sahu SK, et al. Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res. 2008;1208:192–203. doi: 10.1016/j.brainres.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaheer S, Thangavel R, Sahu SK, Zaheer A. Augmented expression of glia maturation factor in Alzheimer's disease. Neurosci. 2011;194:227–233. doi: 10.1016/j.neuroscience.2011.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thangavel R, Van Hoesen GW, Zaheer A. Posterior parahippocampal gyrus pathology in Alzheimer's disease. Neurosci. 2008;154:667–676. doi: 10.1016/j.neuroscience.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A. Modular and laminar pathology of Brodmann's area 37 in Alzheimer's disease. Neuroscience. 2008;152:50–55. doi: 10.1016/j.neuroscience.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A. Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer's disease. Neurosci. 2009;160:427–433. doi: 10.1016/j.neuroscience.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaheer S, Thangavel R, Wu Y, Khan MM, Kempuraj D, et al. Enhanced expression of glia maturation factor correlates with glial activation in the brain of triple transgenic Alzheimer's disease mice. Neurochem Res. 2013;38:218–225. doi: 10.1007/s11064-012-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebral cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 29.Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, et al. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takashima A. Tau aggregation is a therapeutic target for Alzheimer's disease. Curr Alzheimer Res. 2010;7:665–669. doi: 10.2174/156720510793611600. [DOI] [PubMed] [Google Scholar]
- 32.Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 33.Apostolova LG, Lu PH, Rogers S, Dutton RA, Hayashi KM, et al. 3D mapping of mini-mental state examination performance in clinical and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:224–231. doi: 10.1097/01.wad.0000213857.89613.10. [DOI] [PubMed] [Google Scholar]
- 34.Scher AI, Xu Y, Korf ES, White LR, Scheltens P, et al. Hippocampal shape analysis in Alzheimer's disease: a population-based study. NeuroImage. 2007;36:8–18. doi: 10.1016/j.neuroimage.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009;73:1899–1905. doi: 10.1212/WNL.0b013e3181c3f293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer's disease. Prog Brain Res. 2007;163:741–753. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- 37.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 38.Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. Neurosci. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim R, Hicklin DJ, Miller JF, Williams TH, Crabtree JB. Distribution of immunoreactive glia maturation factor-like molecule in organs and tissues. Brain Res. 1987;430:93–100. doi: 10.1016/0165-3806(87)90179-9. [DOI] [PubMed] [Google Scholar]
- 40.Wang BR, Zaheer A, Lim R. Polyclonal antibody localizes glia maturation factor beta-like immunoreactivity in neurons and glia. Brain Res. 1992;591:1–7. doi: 10.1016/0006-8993(92)90971-b. [DOI] [PubMed] [Google Scholar]
- 41.Zaheer A, Fink BD, Lim R. Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem. 1993;60:914–920. doi: 10.1111/j.1471-4159.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 42.Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepherd CE, Grace EM, Mann DM, Halliday GM. Relationship between neuronal loss and ‘inflammatory plaques’ in early onset Alzheimer's disease. Neuropathol Appl Neurobiol. 2007;33:328–333. doi: 10.1111/j.1365-2990.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 44.Tan B, Choi RH, Chin TJ, Kaur C, Ling EA. Manipulation of microglial activity as a therapy for Alzheimer's disease. Front Biosci. 2012;4:1402–1412. doi: 10.2741/s342. [DOI] [PubMed] [Google Scholar]