Abstract
Background
Delivery of bone marrow derived stem and progenitor cells to the site of injury is an effective strategy to enhance bone healing. An alternate approach is to mobilize endogenous, heterogeneous stem cells that will home to the site of injury. AMD3100 is an antagonist of the chemokine receptor 4 (CXCR4) that rapidly mobilizes stem cell populations into peripheral blood. Our hypothesis was that increasing circulating numbers of stem and progenitor cells using AMD3100 will improve bone fracture healing.
Methods
A transverse femoral fracture was induced in C57BL/6 mice, after which they were subcutaneously injected for 3 days with AMD3100 or saline control. Mesenchymal stem cells (MSCs), hematopoietic stem and progenitor cells (HSPCs), and endothelial progenitor cells (EPCs) in the peripheral blood and bone marrow were evaluated via flow cytometry, automated hematology analysis, and cell culture 24 hours after injection and/or fracture. Healing was assessed up to 84 days after fracture by histomorphometry and µCT.
Results
AMD3100 injection resulted in higher numbers of circulating MSCs, HSCs, and EPCs. µCT data demonstrated that the fracture callus was significantly larger compared to the saline controls at day 21 and significantly smaller (remodeled) at day 84. AMD3100-treated mice have a significantly higher bone mineral density than saline-treated counterparts at day 84.
Discussion
Our data demonstrate that early cell mobilization had significant positive effects on healing throughout the regenerative process. Rapid mobilization of endogenous stem cells could provide an effective alternative strategy to cell transplantation for enhancing tissue regeneration.
Introduction
Bone marrow contains a variety of stem and progenitor cells that participate in skeletal repair, including mesenchymal stem cells (MSCs) [1], endothelial progenitor cells (EPCs) [2], and hematopoietic stem and progenitor cells (HSPCs) [3]. Each of these cell types has been independently proposed to enhance bone healing [4–6]. EPCs revascularize the injury site and provide access for other types of stem cells to populate the callus [7]; MSCs give rise to chondroblasts and osteoblasts for tissue repair and may have anti-inflammatory properties [4,8,9]; and HSPCs, in addition to re-establishing the local bone marrow, provide precursors to osteoclasts, which are essential for converting cartilage to bone, and ultimately remodeling the callus [6].
In an effort to improve fracture healing, much energy has been directed towards cell-based therapeutics that require the isolation of bone marrow and expansion or concentration of specific stem and progenitor cells ex vivo for subsequent delivery in vivo. Indeed, studies show that vascular infusion of such cells has significant positive effects on bone healing [4,10]. Both transplanted MSCs [4] and endothelial/hematopoietic precursor cells [10] have been shown to enhance bone healing. An alternative to cell transplantation is to rapidly mobilize large numbers of endogenous progenitor cells directly into the peripheral blood that will home to the site of injury and take part in tissue regeneration.
Under normal physiological conditions there are few circulating HSCs and EPCs in peripheral blood [11–15]. While the existence of circulating MSCs in peripheral blood is controversial, adherent fibroblast-like cells with adipogenic and osteogenic capacity have been detected in very low numbers [16,17]. EPC numbers in peripheral blood are significantly increased in association with vascular injury, burns and fracture [7,15,18–21]. Similar to EPCs, data suggests that in response to tissue trauma, bone marrow-derived MSCs and osteogenic progenitors also enter the peripheral blood [22–26]. An emerging strategy is to utilize molecules that interfere with molecular mechanisms that retain stem and progenitor cells in the bone marrow niche, to significantly increase circulating numbers of stem and progenitor cells in peripheral blood and enhance healing. One such molecule is AMD3100, a selective antagonist of chemokine (CXC motif) receptor 4 (CXCR4) [27]. AMD3100 rapidly mobilizes CXCR4+ cells such as HSCs, EPCs, and possibly MSCs into the peripheral blood [28]. Mobilization of these progenitor cells into the peripheral blood occurs because of disruption to SDF-1/CXCR4 interaction, which anchors progenitor cells to their niches [29]. AMD3100 has very few side effects in humans [30] and is already FDA-approved and used in hospitals to mobilize HSCs for bone marrow transplantation [31,32].
Previous studies demonstrate positive effects of mobilizing endogenous stem cells with AMD3100 on bone. Wang et al. demonstrated that 15 daily injections of AMD3100 enhanced healing of critical-sized calvarial defects in mice in as soon as 4 weeks [33]. Another study by McNulty et al., asserts that a single dose of AMD3100, 3 hours after murine bone marrow ablation surgery significantly enhances intramedullary trabecular bone regeneration 21 days later [34]. Another study by Kumar et al. used a combination of insulin-like growth factor-1 and AMD3100 to treat a murine tibial defect and found improvement in bone healing after 8 weeks [35].
In this study, we examined the effect of a brief, 3-day AMD3100 treatment upon mobilization of endogenous stem and progenitor cells into the peripheral blood and its effects upon bone regeneration in a murine femoral fracture model. We expand upon previous studies by simultaneously comparing HSC, MSC, and EPC populations in both the blood and bone marrow from all combinations of non-fractured/fractured and saline/AMD3100 treated mice via flow cytometry. In addition, we evaluated bone healing via callus formation up to 12 weeks after injury by examining both the soft and hard callus during fracture healing with histomorphometry and µCT analysis.
We hypothesized that administration of AMD3100 would increase circulating numbers of HSPCs, EPCs, and MSCs into the peripheral blood and enhance fracture healing.
Materials and Methods
Animals
The total number of animals used is detailed in Table 1. A total of 39 13–14 week old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA) were used for hematological and flow cytometric analyses. Transverse fractures were created in the right femur of 19 animals, 8 of which were administered 2 subcutaneous doses of 5mg/kg AMD3100 (Sigma, St. Louis, MO, USA), the first immediately post surgery and the second 23 hrs later (1hr prior to euthanasia). 7 fractured animals were administered an equal volume of saline carrier at these same timepoints and the remaining 4 fractured animals were not injected. An additional 16 animals that were not fractured received 2 equivalent doses of AMD3100 (n=9) or saline (n=7). Blood and bone marrow were collected from an additional 4 mice with neither injections nor fractures. 5mg/kg AMD3100 results in maximal hematopoietic progenitor cell mobilization in mice [36]. Blood was collected via cardiac puncture in all mice 1 hr after the second injection of saline or AMD3100 and prior to euthanasia.
Table 1.
Animal Numbers per Experiment and Endpoint
| Experiment | Figure | Endpoints | Treatment | Total mice per endpoint |
|||||
|---|---|---|---|---|---|---|---|---|---|
| -no injection -no fracture |
-saline injection -no fracture |
-AMD3100 injection -no fracture |
-no injection -fracture |
-saline injection -fracture |
-AMD3100 injection -fracture |
||||
|
Advia, Flow cytometry |
1, 2, 3 | 24 hours after fracture/ 1 hour after second injection |
4 | 7 | 9 | 4 | 7 | 8 | 39 |
|
Cell Culture Cytometry |
4 | 1 hour after 1st injection |
4 | 3 | 4 | - | - | - | 11 |
|
Histo- morphometry, µCT |
5, 6 | 7 days after fracture |
- | - | - | - | 3 | 3 | 6 |
| 14 days after fracture |
- | - | - | - | 3 | 3 | 6 | ||
| 21 days after fracture |
- | - | - | - | 3 | 3 | 6 | ||
| 42 days after fracture |
- | - | - | - | 6 | 5 | 11 | ||
| 84 days after fracture |
- | - | - | - | 7 | 7 | 14 | ||
For histological and µCT analysis, transverse fractures were created in the right femur of a total of 43 13–14 week old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA). Half of the mice were injected subcutaneously with 5mg/kg AMD3100 once every 24 hours for 3 days, starting approximately 3–5 hours after fracture. The remaining mice were injected with an equal volume of the saline carrier. Mice were euthanized via CO2 asphyxiation followed by cervical dislocation 7, 14, 21, 42, and 84 days after injection and/or surgery.
For ex vivo culture of adherent cells, 11 mice were injected with either 5 mg/kg AMD3100 or saline. One hour later, mice were anesthetized with 1.5–2% isoflurane and 0.5–1 mL of blood was collected via cardiac puncture for adherent cell culture and analysis. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Surgical Procedure
Consistent transverse femoral fractures were created as previously described [37] using the method developed by Bonnarens and Einhorn [38–40] with a modified fracture apparatus [41]. Briefly, mice were injected subcutaneously with 0.05 mg/kg buprenorphine (Hospira Inc., Lake Forest, IL, USA) for analgesia and 1 mL saline subcutaneously 5–10 minutes before surgery and anesthetized with 1.5–2% isoflurane (Minrad, Inc., Bethlehem, PA, USA). A 0.01” diameter straight stainless steel wire pin (Small Parts, Miami Lakes, FL, USA) was inserted into the femoral intramedullary cavity and closed transverse fractures were created by dropping a blunt weight upon the middiaphysis. Mice were radiographed to determine pin positioning and fracture pattern. Mice were injected with 0.05mg/kg buprenorphine every 12 hours after surgery for 48 hours for analgesia. The animals were allowed to bear their full weight and their activity was completely unrestricted post-operatively.
Peripheral Blood and Bone Marrow Cell Isolation
Blood and bone marrow from 40 mice were collected to evaluate subsets of circulating blood cell numbers using an automated hematology analyzer to perform complete blood counts and flow cytometry. 500–1000µl of peripheral blood was collected from anesthetized mice via cardiac puncture into 100 mL of 50mM EDTA (Life Technologies, Carlsbad, CA, USA) to prevent coagulation. Bone marrow was collected immediately after death by isolating the left femur and tibia and removing all soft tissue and all articular surfaces except that of the proximal femur. Bones were then crushed with a mortar and pestle in a solution of 2% new calf serum and 2 mM EDTA in HBSS (all from Life Technologies, Carlsbad, CA, USA) and supernatant from the bone wash was collected, filtered through 70µm nylon mesh, and further processed as described below.
Hematology Analysis and Flow Cytometry
Complete blood counts were performed on 50µl aliquots of peripheral blood using an Advia 120 hematology analyzer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). The remaining peripheral blood was treated with ammonium chloride (Life Technologies, Carlsbad, CA, USA) on ice for 15 minutes to lyse red blood cells and prepare for flow cytometric analyses. For both peripheral blood and bone marrow cells, Live/Dead Fixable Near Infrared Viability Kit (Life Technologies, Carlsbad, CA, USA) was used for gating according to manufacturer instructions with the following modifications. Up to 8×106 cells were resuspended in 100 µL phosphate-buffered saline (PBS) and 0.5 µL of viability dye was added. Samples were incubated at room temperature for 20 minutes, protected from light. After incubation, 5 µL of fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA) was added to bind any remaining dye. The following anti-mouse immunophenotyping antibodies were added as a cocktail and incubated at room temperature for 20 minutes in the dark: Ter119 (APC-Cy7), B220 (APC-C7), CD3 (APC-Cy7), Gr-1 (APC-Cy7), c-kit (Brilliant Violet 421™), CD135 (PE-Cy5), CD29 (Alexa Fluor 700®), CD44 (Brilliant Violet 570™) from Biolegend (San Diego, CA); CD150 (PerCp-eFluor® 710) from eBioscience (San Diego, CA); Flk-1 (PE-Cy7) from BD Pharmingen (San Jose, CA); CD105 (PE) from eBioscience (San Diego, CA, USA); CD34 (FITC) from BD Biosciences ( San Jose, CA, USA); sca-1 (APC) from eBioscience (San Diego, CA, USA). Cells were washed once. A minimum of 106 events per sample was acquired using a LSRII flow cytometer (Becton Dickinson, NJ) and analyzed using FlowJo software (Treestar, Inc., Ashland, OR). A summary of flow cytometry markers for HSPC [42], HSC [43], MSC, and EPC [44–46] types are described in Table 2. Four different combinations of cell surface markers were derived from previous publications [47–50]. The rationale for these choices are discussed in the results section. HSPCs are comprised of hematopoietic progenitor cells as well as smaller numbers of hematopoietic stem cells and are identified by positive staining for c-kit and sca-1 and negative staining for lineage markers (B220, CD3, Ter119, Gr-1). HSCs are a subset of the HSPC population.
Table 2.
Flow Cytometry Stem Cell Markers
| Cell Type | Positive Markers | Negative Markers | Reference: | |
|---|---|---|---|---|
| HSPC | c-kit, sca-1 | B220, CD3, Ter119, Gr-1 | Okada et al., 1992[42] | |
| HSC | c-kit, sca-1, CD150 | B220, CD3, Ter119, Gr-1, CD135 | Kiel et al., 2005 [43] | |
| MSC | A | CD44, CD29, CD105 | B220, CD3, Ter119, Gr-1 | Sung et al., 2008 [47] |
| B | sca-1 | CD31, B220, CD3, Ter119, Gr-1 | Short et al., 2003 [48], 2009 [49] | |
| C | sca-1 | CD31, CD44, B220, CD3, Ter119, Gr-1 | Short et al., 2003[48], 2009[49], Qian et al., 2012[50] |
|
| D | sca-1, CD29, CD105 | CD31, CD34, CD44, B220, CD3, Ter119, Gr-1 |
Sung et al., 2008 [47], Qian et al., 2012 [50] |
|
| EPC | CD34, FLK-1, CD31 | B220, CD3, Ter119, Gr-1, CD44, CD29, CD150, CD135 |
Chakroborty et al., 2008 [44], Kim et al., 2010 [45], Jung et al., 2012 [46] |
Adherent cell culture, staining and quantification
0.5–1 mL of blood was collected via cardiac puncture from 11 mice anesthetized with 1.5–2% isoflurane and injected 1 hr prior with either 5mg/kg AMD3100, an equivalent volume of saline, or not injected. An equal volume of HBSS was added to peripheral blood samples and centrifuged at 300 × g for 15 minutes. The supernatant was removed and the pellet was resuspended in HBSS and centrifuged at 1,000 × g for 5 min. The pelleted fraction was plated in 12-well plates in alpha-MEM media (Invitrogen, Carlsbad, CA) with 20% FBS (Invitrogen, Carlsbad, CA, USA) and 1% Penicillin/Streptomycin (Invitrogen, Carlsbad, CA, USA). After 11 days of culture, adherent cells were rinsed twice with PBS, fixed with ice cold methanol for 10 minutes on ice, stained with 0.5% Crystal Violet (Fisher Scientific, Hampton, NH, USA) solution (25% methanol and 75% water) on a shaker for 10 minutes. Plates were then submerged in water and dried overnight. A transparent 5 × 3 dot grid covering the entire surface of the well was created and placed on the bottom of the well. Using an inverted brightfield microscope (Leica, Buffalo Grove, IN, USA), each dot was placed in the middle of the field of view and all cells in the field were counted. The total number of cells from all 15 fields of view were averaged to calculate the average number of cells per field of view.
µCT Analysis
Femurs were scanned in 4% phosphate buffered formalin at 55 KVP, 145mA, with a voxel size of 12mm, with a volume of interest of 316 mm3, and sampled 5 times (Scanco Medical µCT 35, Brüttisellen, Switzerland). Only slices that contained callus were included in the analysis, in which the outer and inner edges of the callus were contoured manually. As previously described [51], this excludes the original cortical bone from the measurements and the region of interest was only the newly formed callus itself. A fixed, global threshold with the lower limit corresponding to a mineral density of 421 mg HA/cm3 and the upper limit corresponding to 3,000 mg HA/cm3 was used to distinguish mineralized from non-mineralized tissue. Femurs were then evaluated with a Gaussian filter (sigma=0.8, support=1.0) for noise reduction. Calculated parameters included total callus volume (TV), mineralized callus volume (BV), fraction of mineralized callus (BV/TV), bone mineral density (BMD, average attenuation value of bone and non-bone tissue) and tissue mineral density (TMD, average attenuation value of bone tissue only).[52]
Histology
For histology, pins were removed and femurs were extracted and placed into 4% phosphate buffered formalin for 2–3 days at 4°C. During this time the fractured femurs were evaluated by µCT. Then femurs were placed into a 2.3:1 decalcification solution of 10% EDTA and 4% phosphate buffered formalin solution for 10 days at 4°C [53]. Both proximal and distal ends of each femur were cut approximately 2mm away from the edge of the callus with a razor. Femurs were then processed, paraffin-embedded and serial sectioned transversely through the entire fracture callus. Serial sections were taken at 500mm intervals [54]. Slides were then deparaffinized, rehydrated, and stained with H&E and Alcian Blue.
Stereological Measurements of Callus Volume and Compartments
To calculate the percentage of each tissue type in the fracture callus, digital photographs were taken of the whole H&E/Alcian Blue histological sections at 10x. Whole callus volume and the compartment volumes of hyaline cartilage and newly formed/transforming bone were estimated using an established point counting technique using a point grid overlay [55].
Statistical analysis
Advia and flow cytometry (Figure 1, Figure 2, and Figure 3) data were analyzed by one-way ANOVA with Sidak’s multiple comparisons post-test where we pre-selected which columns to compare (Saline vs. AMD3100, Saline Fracture vs. AMD3100 Fracture, Saline vs. Saline Fracture, AMD3100 vs. AMD3100 Fracture). Flow cytometry data (Figure 2 and Figure 3) were normalized to saline injected controls. Outliers in flow cytometry experiments were excluded using the Grubbs' test. Microscope cytometry data (Figure 4) was analyzed by one-way ANOVA with Tukey’s multiple comparison post-test. Histomorphometry data (Figure 5) was analyzed by an unpaired one-tailed Student’s t-test. µCT data (Figure 6) was analyzed by multiple t-tests and corrected for multiple-comparisons using the Holm-Sidak method. Differences were considered significant at p < 0.05. Graphs were made and statistical tests run using GraphPad Prism and Instat (GraphPad Software, Inc. La Jolla, CA, USA).
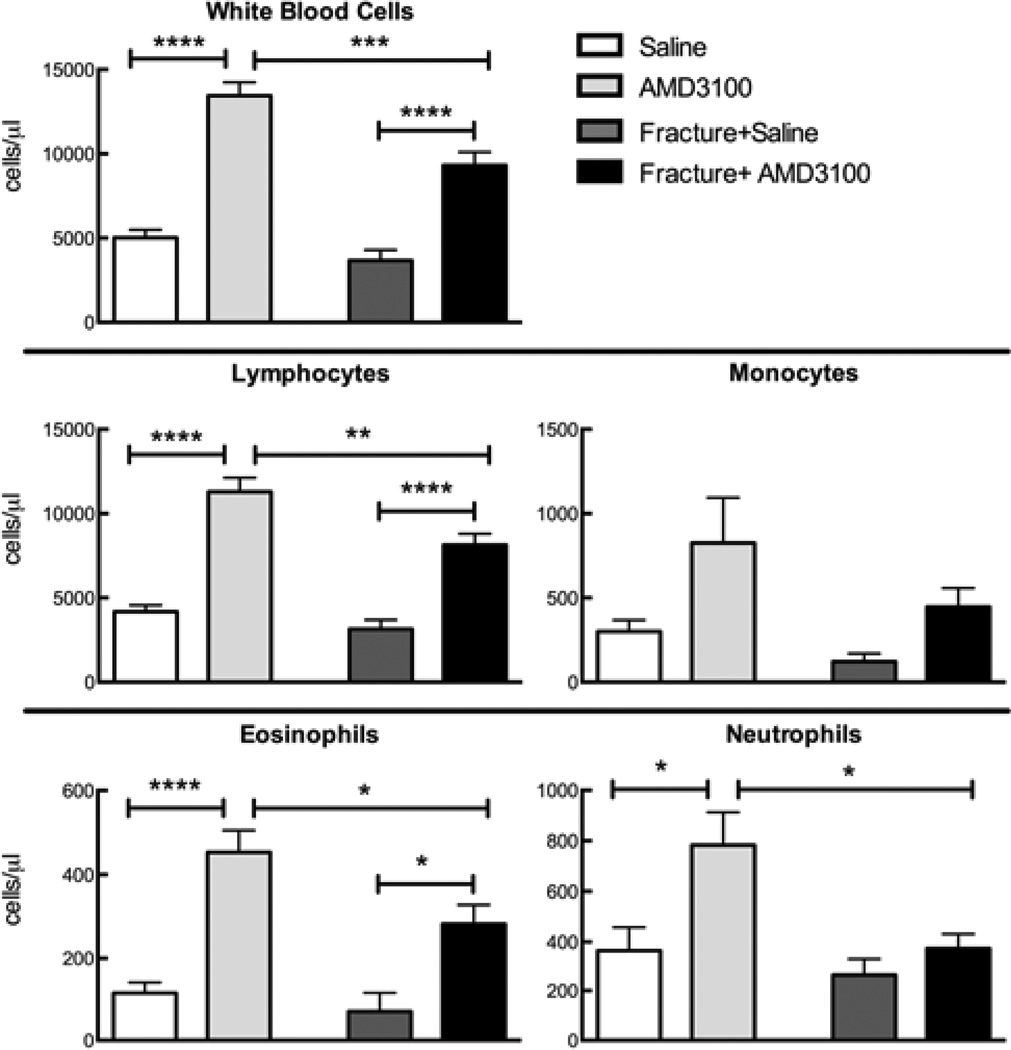
Figure 1.
Blood cell counts (Advia 120) from fractured and non-fractured mice, injected with AMD3100 or saline, 23 and 1 hour prior to euthanasia. Fractures were induced 24 hours before euthanasia. Bars represent mean ± SEM, n=4–9. Stars represent statistical significance. *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001.
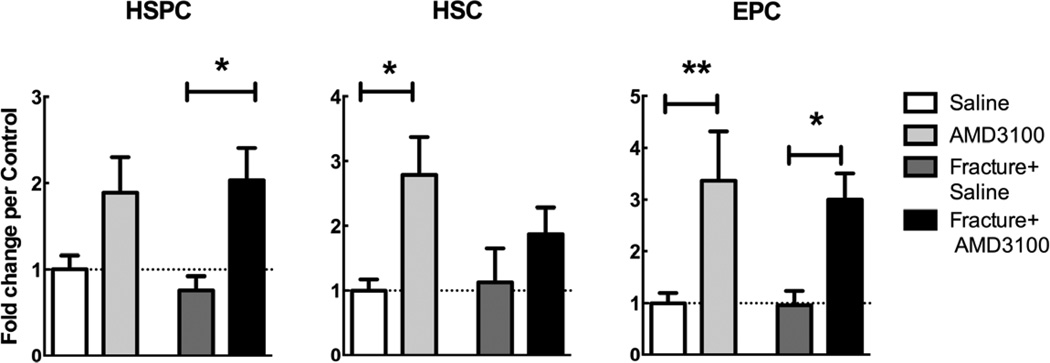
Figure 2.
Flow cytometry counts of HSPCs, HSCs, and EPCs (as defined in Table 2), reported in fold change versus the saline control. Blood was collected from fractured and non-fractured mice injected with AMD3100 or saline 23 and 1 hour prior to euthanization. Fractures were induced 24 hours before euthanasia. Mean ± SEM, n=4–8; *=p<0.05, **=p<0.01
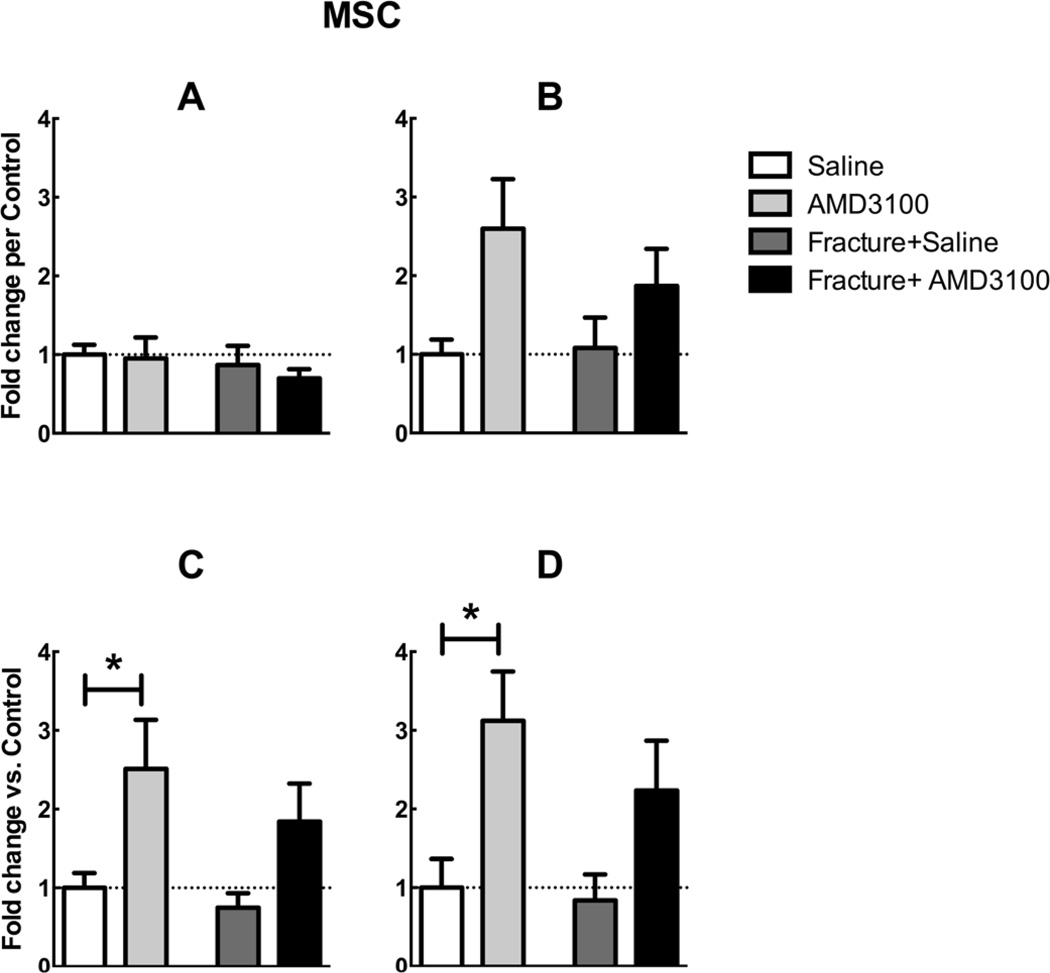
Figure 3.
Flow cytometry counts of MSCs defined using 4 different sets of surface markers (as defined in Table 2), reported in fold change versus the saline control. Blood was collected from fractured and non-fractured mice injected with AMD3100 or saline 23 and 1 hour prior to euthanasia. Fractures were induced 24 hours before euthanasia. Mean ± SEM, n=4–8; *=p<0.05.
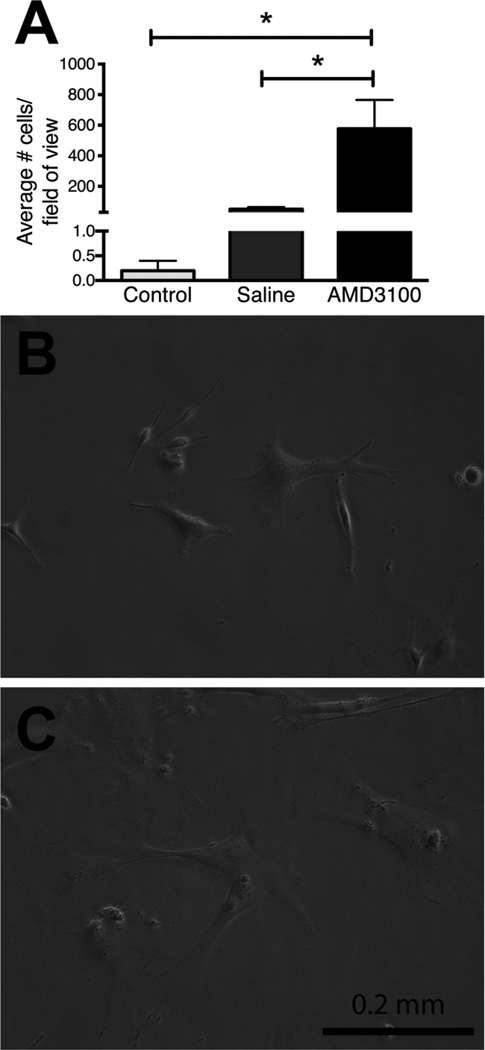
Figure 4.
Peripheral blood from AMD3100 and saline treated mice was cultured as described in the methodology for 11 days. (A) Mean adherent cell number per field of view ± SEM, n=3–4 * = p<0.05. Significantly more adherent cells were cultured from peripheral blood derived from AMD3100 treated mice than saline and no-injection controls. The morphology of the adherent cells cultured from mobilized peripheral blood (B) was similar to that of plastic adherent cells cultured from bone marrow (C).
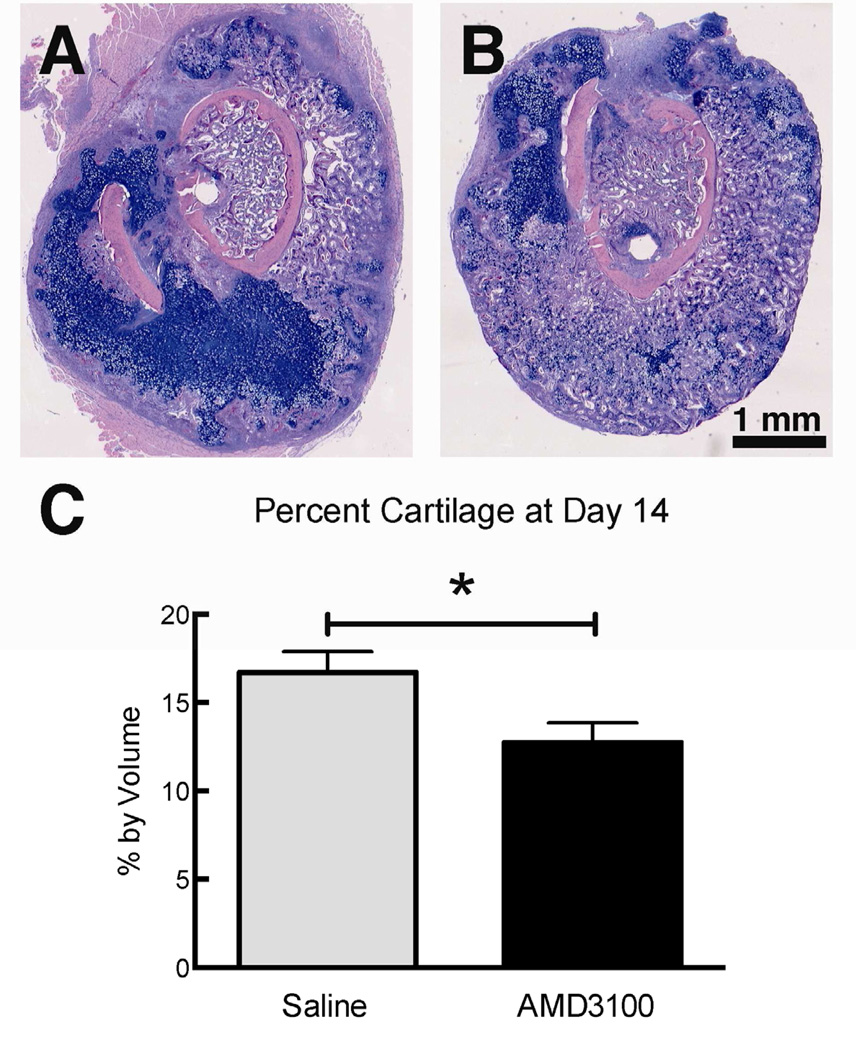
Figure 5.
Transverse section with H&E/Alcian Blue staining through a 14-day-old fracture callus of a mouse injected with saline vehicle (A) or 5mg/kg AMD3100 (B) once a day for the first three days following fracture. (C) Bar indicate the mean percentage by volume ± SEM of hyaline cartilage in the fracture callus determined via stereology from serial histological sections (n=3), * = p<0.05.
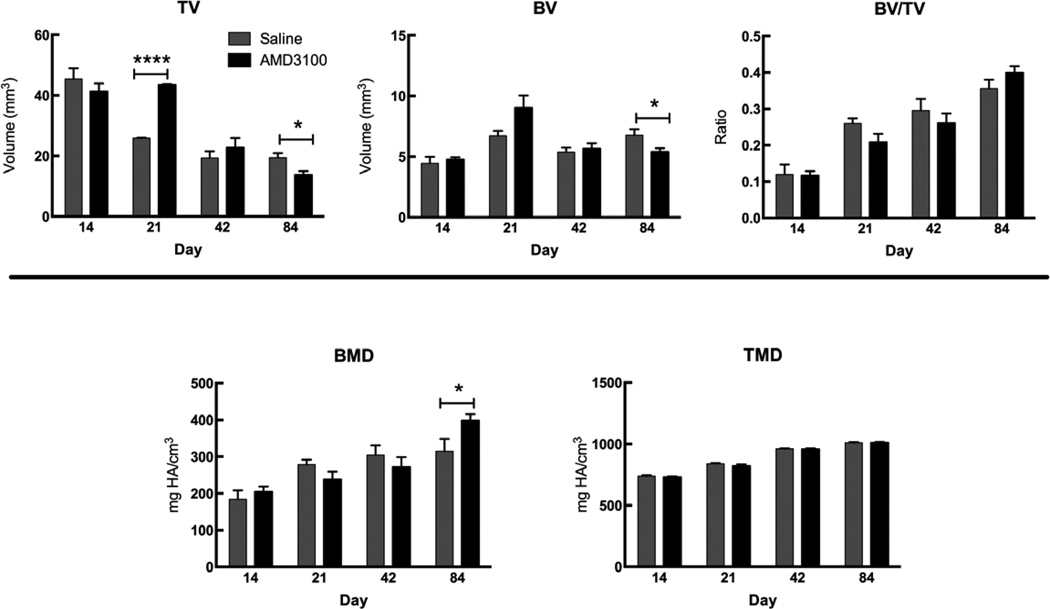
Figure 6.
µCT evaluation of total callus volume (TV), mineralized bone volume (BV), BV/TV, BMD, and TMD of the whole and mineralized portions of the callus. Bars represent mean ± SEM, n=3–7; * = p<0.05, **** = p<0.0001). At day 21, the AMD3100-treated mice had a significantly larger TV and BV than the control group. However, by day 84, the AMD3100-treated mice had a significantly smaller TV and BV, indicating a faster remodeling. In addition, there was a significant increase in the bone mineral density of the whole callus in the AMD3100-treated animals at day 84, indicating a stronger, more mineralized bone.
Results
Hematology Analysis
Injection of saline alone in both fractured and non-fractured animals had no effect on circulating white blood cell numbers when compared to non-injected animals (data not shown). For clarity we report saline injected controls only in the results. Mice treated with AMD3100 had a significantly greater number of circulating white blood cells, lymphocytes, and eosinophils than their saline-treated fractured or non-fractured controls (Figure 1). AMD3100 treatment only significantly increased circulating neutrophil number in non-fractured mice (Figure 1). AMD3100 administration did not affect circulating numbers of platelets and basophils and no changes were observed in the hematocrit, red blood cell number, and hemoglobin with the exception of a significant reduction in all three between saline treated control mice and animals treated with AMD3100 who had sustained a fracture (data not shown). Interestingly, fractured mice treated with AMD3100 had significantly fewer total white blood cells, including lymphocytes, eosinophils, and neutrophils than the non-fractured AMD3100-treated mice.
Flow Cytometry
Cell subsets were defined according to criteria outlined in Table 2. AMD3100 significantly increased circulating numbers of HSCs and EPCs in peripheral blood in non-fractured mice compared to saline injected controls (Figure 2). AMD3100 also significantly increased circulating numbers of HSPCs and EPCs in fractured animals (Figure 2).
Although the International Society for Cellular Therapy published a list of markers to minimally define MSCs [56], more recent studies have utilized alternate sets of MSC antibody markers. For this reason we analyzed our MSC flow cytometry data according to four different marker sets outlined in several different studies [47–50] (Table 2). AMD3100 significantly increased circulating numbers of MSCs as defined by criteria in Table 2, C and D in non-fractured mice compared to saline injected controls (Figure 3 C, D). There was no significant difference between any groups when MSCs were defined according to Table 2,A (Figure 3A). One-way ANOVA revealed significant differences between groups with MSCs defined according to Table 2,B, with the difference between saline and AMD3100 non-fractured mice approaching significance (p=0.05, Figure 3B). The extent of AMD3100’s mobilizing effect was similar in both fractured and non-fractured mice. In addition, the administration of AMD3100 and/or fracture appeared to have no effect on bone marrow stem cell populations (data not shown).
Adherent Cell Culture
A significantly greater number of plastic adherent cells were cultured from the peripheral blood of animals one hour after AMD3100 injection, compared to saline injected animals and control animals (no injection; Figure 4A). Plastic adherent cells formed colonies and had similar morphology to bone marrow-derived MSCs (Figure 4B,C). In rats, these plastic adherent cells have been shown to differentiate into adipocytes, chondrocytes, and osteoblasts, indicating that they were MSCs [57].
Histomorphometric Analysis and mCT
AMD3100-treated mice had significantly less cartilage in the callus at day 14 compared to saline-treated controls measured using histomorphometry (Figure 5).
At day 21, mice treated with AMD3100 had a significantly larger callus volume and an increase in bone volume that approached significance (p=0.1) than the saline-treated mice (Figure 6). By day 84, however, the effects of AMD3100 on callus size are reversed. AMD3100 treated mice have a significantly smaller callus volume and bone volume than their saline-treated counterparts indicating faster remodeling of the fracture callus to the original bone size. In addition, AMD3100-treated mice have a significantly higher BMD than saline-treated counterparts at day 84.
Discussion
In this study we show that AMD3100 increased HSPC, EPC, and MSC numbers in the peripheral blood and increased the number of adherent MSC-like cells in peripheral blood cultures. It is well established that AMD3100 mobilizes HSPCs to the peripheral blood, and is now being used clinically in combination with G-CSF to mobilize HSPCs in patients with non-Hodgkin’s lymphoma and multiple myeloma [28,58]. There is evidence that other CXCR4-positive cells, such as MSCs and EPCs, may also be mobilized by AMD3100 inhibition of CXCR4, though Levesque et al. theorizes that MSCs and EPCs may be more difficult to mobilize than HSPCs simply because they are larger and adhere more strongly to their niche [29].
AMD3100 injection has been shown to significantly increase the number of EPCs in murine peripheral blood [28,59]. EPC mobilization is dose dependent with AMD3100’s effect plateauing at the 5mg/kg dose with maximal EPC mobilization 1 hour after injection [59]. As MSCs are also CXCR4-positive, we hypothesized that they, too, would be mobilized by AMD3100. The in vitro expansion of MSCs, primarily from bone marrow, has helped define their characteristic surface makers. However, differences in isolation and culture have made comparing studies difficult. Although a list of markers to minimally define human MSCs was published in 2006 [56], there remains no consistent use of surface antigens to date. One marker, CD44, that has been routinely used to define both human and mouse MSCs, was found to be absent in freshly isolated bone marrow, but appears later during cultivation [50]. As such, we analyzed our flow cytometry data in multiple ways to examine the mouse MSC population in freshly isolated peripheral blood. The data regarding the ability of AMD3100 to mobilize MSCs is contradictory. Pitchford et al. did not detect significant MSC-like cells in blood mobilized with 5 mg/kg of AMD3100 after 1 hour using colony forming unit assays as an end point [28]. However, in this study we detected an increase in circulating MSCs via flow cytometry and cultured a significantly greater number of plastic adherent cells with MSC morphology from the peripheral blood of AMD3100-treated mice than from control mice. Our results are supported by McNulty et al, who observed a 40-fold increase in peripheral blood MSCs 1 hour after a single injection of 5 mg/kg AMD3100 [34].
Data in the literature suggest that stem and progenitor cell populations in the peripheral blood increase in response to injury. Eghbali-Fatourechi et al. noted that circulating osteoprogenitor cell numbers increased at least 4-fold in a patient who had sustained a severe fracture 20 days earlier [60]. Another study by Ma et al. demonstrated that peripheral EPC numbers significantly increased in patients within 48 hours after fracture and peaked around 3 days after injury [61]. In light of these and similar studies we had expected to see increased numbers of circulating cells in fractured mice and that this response would be enhanced in the presence of AMD3100 mobilization. Interestingly, we observed no increase in peripheral blood cells following fracture. In addition, AMD3100-treated mice with fractures had significantly fewer circulating white blood cells, lymphocytes, eosinophils, and neutrophils than the non-fractured AMD3100-treated mice. It is possible that cells are indeed mobilized in response to fracture and that this is enhanced by AMD3100 administration, but that the presence of a traumatic fracture in the body causes cells to rapidly marginate and migrate to the site of injury, thus reducing circulating cell numbers in the peripheral blood. In studies using labeled MSCs injected intravenously, cells have been imaged in the lung and bone defects as early as 24 hours [4,8,62]. Further studies are required to understand the regulation of stem and progenitor cell proliferation following mobilization and traumatic injury.
Though significant changes occurred in the soft portion of the callus by day 14, our µCT data did not show any changes in TV, BV, BMD, or TMD. Newly formed and transforming bone may not yet have mineralized to a degree that is above our set bone threshold for detection by µCT. By µCT, AMD3100 treated mice had a significantly larger callus at day 21 compared to saline treated controls. In other studies intravenous infusion of cultured MSCs resulted in improved fracture healing as evidenced by a significantly larger callus volume, new bone volume and improved biomechanical properties.[4] Similarly, in another model, intravenous transplantation of human circulating CD34+ cells (endothelial/hematopoietic progenitor cells) cells contributed to fracture healing by enhancing vasculogenesis and osteogenesis [10]. Local transplantation of EPCs has also improved bone regeneration in non-healing fractures and segmental defects [63–65]. The number of MSCs present at the fracture site may have a direct effect on the number of osteoblasts present to form new bone. If osteoblast activity is already at its optimal rate, the only way to accelerate healing is to guide more stem cells to the site of injury [66]. By day 84 AMD3100 treated animals had a smaller callus with a significantly higher BMD than the saline-treated controls. Our data demonstrate that early cell mobilization had significant and positive effects on bone healing throughout the regenerative process.
In this study we used AMD3100, a selective antagonist of CXCR4, which disrupts the anchorage of CXCR4+ cells in the SDF-1 rich bone marrow and promotes mobilization. In addition to its role in retaining stem cells in their niches there are studies that indicate that the CXCR4/SDF-1 pathway is a key stimulus for stem cells to home to sites of tissue damage, including bone injury. For example the periosteum in healing bone grafts expresses SDF-1 and new bone formation is inhibited by administration of anti-SDF-1 neutralizing antibody, chemical inhibition of the CXCR4/SDF-1 axis, and in mice with genetically reduced SDF-1 and CXCR4 expression [67]. In another study, only CXCR4+ MSCs delivered intravenously were able to home to the site of fracture [4]. Osturu et al found that osteoprogenitor cells were mobilized into the peripheral blood as a result of tissue injury, and migrated to bone forming sites by chemo-attraction to SDF-1 and differentiated into osteoblasts [68].
A possible limitation of our study is that while AMD3100 may effectively mobilize our cell populations of interest, it may also disrupt cell homing to the site of damage. In our own studies we found that administration of AMD3100 twice daily for the duration of the healing process negatively altered bone healing in this same fracture model [37]. In this current study, however, we administered AMD3100 only 3 times during the first 3 days after healing. AMD3100 has a short half life of 0.9 hours (in rats) and it is likely that AMD3100 is cleared from the system relatively quickly [30]. The positive effects on bone healing demonstrated herein suggest that the effects of AMD3100 on cell mobilization may have overcome any potential negative effects of AMD3100 on cell homing to the fracture site.
In summary, we report that AMD3100 administration mobilized multiple populations of stem and progenitor cells purported to play significant roles in bone healing. We also demonstrate positive effects on callus size and bone mineral density during bone healing. Our data support earlier literature that demonstrate positive effects of AMD3100 treatment on healing cranial bone defects [33] and intramembranous bone formation following bone marrow ablation [34]. Positive effects of AMD3100 on tissue healing are strong proof of concept that cell mobilization is an appropriate therapeutic target. However, given the pivotal role of SDF-1/CXCR4 axis in cell homing, using AMD3100 alone may not be the optimal mobilizing agent for this purpose. Interestingly, AMD3100 administered in combination with insulin-like growth factor-1 was shown to effectively promote healing in a segmental bone defect [35]. Further studies are required to understand the interaction of AMD3100 with other cytokines and growth factors, refine the dosing of AMD3100 or identify alternate molecular targets to promote cell mobilization.
Acknowledgements
We thank Carol Oxford at the UC Davis Flow Cytometry Shared Resource for technical assistance and Dr. Peta Hitchens for assistance with statistical analyses. This work was funded by Project S-10-62Y supported by the AO Foundation, NIAMS R21AR061604 (CEY) and a private grant from Dick and Carolyn Randall.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre A, Planell JA, Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun. 2010;400:284–291. doi: 10.1016/j.bbrc.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 3.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nature reviews. Immunology. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 4.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto T, Kuroda R, Mifune Y, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43:434–439. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Matos MA, Tannuri U, Guarniero R. The effect of zoledronate during bone healing. J Orthop Traumatol. 2010;11:7–12. doi: 10.1007/s10195-010-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto T, Mifune Y, Kawamoto A, et al. Fracture induced mobilization and incorporation of bone marrow-derived endothelial progenitor cells for bone healing. J Cell Physiol. 2008;215:234–242. doi: 10.1002/jcp.21309. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Padmanabhan P, Ray P, et al. Stem cell-mediated accelerated bone healing observed with in vivo molecular and small animal imaging technologies in a model of skeletal injury. J Orthop Res. 2009;27:295–302. doi: 10.1002/jor.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert opinion on biological therapy. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto T, Kawamoto A, Kuroda R, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. The American journal of pathology. 2006;169:1440–1457. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abkowitz JL, Robinson AE, Kale S, et al. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 12.Wright DE, Wagers AJ, Gulati AP, et al. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RA, Pietrzak DC, Scicchitano MS, et al. Detection and characterization of circulating endothelial progenitor cells in normal rat blood. Journal of pharmacological and toxicological methods. 2009;60:263–274. doi: 10.1016/j.vascn.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 15.Lee DY, Cho TJ, Kim JA, et al. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone. 2008;42:932–941. doi: 10.1016/j.bone.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsov SA, Mankani MH, Gronthos S, et al. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis research. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature medicine. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 20.Fox A, Smythe J, Fisher N, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. The British journal of surgery. 2008;95:244–251. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 21.Laing AJ, Dillon JP, Condon ET, et al. Mobilization of endothelial precursor cells: systemic vascular response to musculoskeletal trauma. J Orthop Res. 2007;25:44–50. doi: 10.1002/jor.20228. [DOI] [PubMed] [Google Scholar]
- 22.Mansilla E, Marin GH, Drago H, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplantation proceedings. 2006;38:967–969. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768–774. doi: 10.1136/hrt.2005.069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai K, Vasanji A, Drazba JA, et al. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 25.Alm JJ, Koivu HM, Heino TJ, et al. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res. 2010;28:1634–1642. doi: 10.1002/jor.21167. [DOI] [PubMed] [Google Scholar]
- 26.Khosla S, Eghbali-Fatourechi GZ. Circulating cells with osteogenic potential. Ann N Y Acad Sci. 2006;1068:489–497. doi: 10.1196/annals.1346.022. [DOI] [PubMed] [Google Scholar]
- 27.De Clercq E. The bicyclam AMD3100 story. Nature reviews. Drug discovery. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 28.Pitchford SC, Furze RC, Jones CP, et al. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Levesque JP, Winkler IG, Larsen SR, Rasko JE. Mobilization of bone marrow-derived progenitors. Handb Exp Pharmacol. 2007:3–36. doi: 10.1007/978-3-540-68976-8_1. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 32.Ratajczak MZ, Kim C. The use of chemokine receptor agonists in stem cell mobilization. Expert opinion on biological therapy. 2012;12:287–297. doi: 10.1517/14712598.2012.657174. [DOI] [PubMed] [Google Scholar]
- 33.Wang XX, Allen RJ, Jr., Tutela JP, et al. Progenitor cell mobilization enhances bone healing by means of improved neovascularization and osteogenesis. Plast Reconstr Surg. 2011;128:395–405. doi: 10.1097/PRS.0b013e31821e6e10. [DOI] [PubMed] [Google Scholar]
- 34.McNulty MA, Virdi AS, Christopherson KW, et al. Adult Stem Cell Mobilization Enhances Intramembranous Bone Regeneration: A Pilot Study. Clin Orthop Relat Res. 2012 doi: 10.1007/s11999-012-2357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50:1012–1018. doi: 10.1016/j.bone.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. The Journal of experimental medicine. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toupadakis CA, Wong A, Genetos DC, et al. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J Orthop Res. 2012 doi: 10.1002/jor.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 39.Manigrasso MB, O'Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18:687–695. doi: 10.1097/00005131-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Manigrasso MB, O'Connor JP. Comparison of fracture healing among different inbred mouse strains. Calcif Tissue Int. 2008;82:465–474. doi: 10.1007/s00223-008-9144-3. [DOI] [PubMed] [Google Scholar]
- 41.Marturano JE, Cleveland BC, Byrne MA, et al. An improved murine femur fracture device for bone healing studies. J Biomech. 2008;41:1222–1228. doi: 10.1016/j.jbiomech.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 43.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Chakroborty D, Chowdhury UR, Sarkar C, et al. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–1389. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H, Cho HJ, Kim SW, et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung SY, Choi JH, Kwon SM, et al. Decursin inhibits vasculogenesis in early tumor progression by suppression of endothelial progenitor cell differentiation and function. J Cell Biochem. 2012;113:1478–1487. doi: 10.1002/jcb.24085. [DOI] [PubMed] [Google Scholar]
- 47.Sung JH, Yang HM, Park JB, et al. Isolation and characterization of mouse mesenchymal stem cells. Transplantation proceedings. 2008;40:2649–2654. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Short B, Brouard N, Occhiodoro-Scott T, et al. Mesenchymal stem cells. Archives of medical research. 2003;34:565–571. doi: 10.1016/j.arcmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Short BJ, Brouard N, Simmons PJ. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol Biol. 2009;482:259–268. doi: 10.1007/978-1-59745-060-7_16. [DOI] [PubMed] [Google Scholar]
- 50.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem. 2012;287:25795–25807. doi: 10.1074/jbc.M112.339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan EF, Mason ZD, Chien KB, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 53.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 54.Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res. 2005;20:1715–1722. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- 55.Howard C, Reed MG. Unbiased Stereology. New York: Garland Science/BIOS Scientific Publishers; 2005. [Google Scholar]
- 56.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 57.Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 58.Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319–326. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 59.Yin Y, Huang L, Zhao X, et al. AMD3100 mobilizes endothelial progenitor cells in mice, but inhibits its biological functions by blocking an autocrine/paracrine regulatory loop of stromal cell derived factor-1 in vitro. Journal of cardiovascular pharmacology. 2007;50:61–67. doi: 10.1097/FJC.0b013e3180587e4d. [DOI] [PubMed] [Google Scholar]
- 60.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, et al. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 61.Ma XL, Sun XL, Wan CY, et al. Significance of circulating endothelial progenitor cells in patients with fracture healing process. J Orthop Res. 2012 doi: 10.1002/jor.22134. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S, Wan C, Ramaswamy G, et al. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mifune Y, Matsumoto T, Kawamoto A, et al. Local delivery of granulocyte colony stimulating factor-mobilized CD34-positive progenitor cells using bioscaffold for modality of unhealing bone fracture. Stem Cells. 2008;26:1395–1405. doi: 10.1634/stemcells.2007-0820. [DOI] [PubMed] [Google Scholar]
- 64.Atesok K, Li R, Stewart DJ, Schemitsch EH. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J Orthop Res. 2010 doi: 10.1002/jor.21083. [DOI] [PubMed] [Google Scholar]
- 65.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 66.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 68.Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]