Abstract
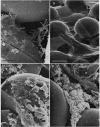
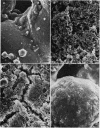
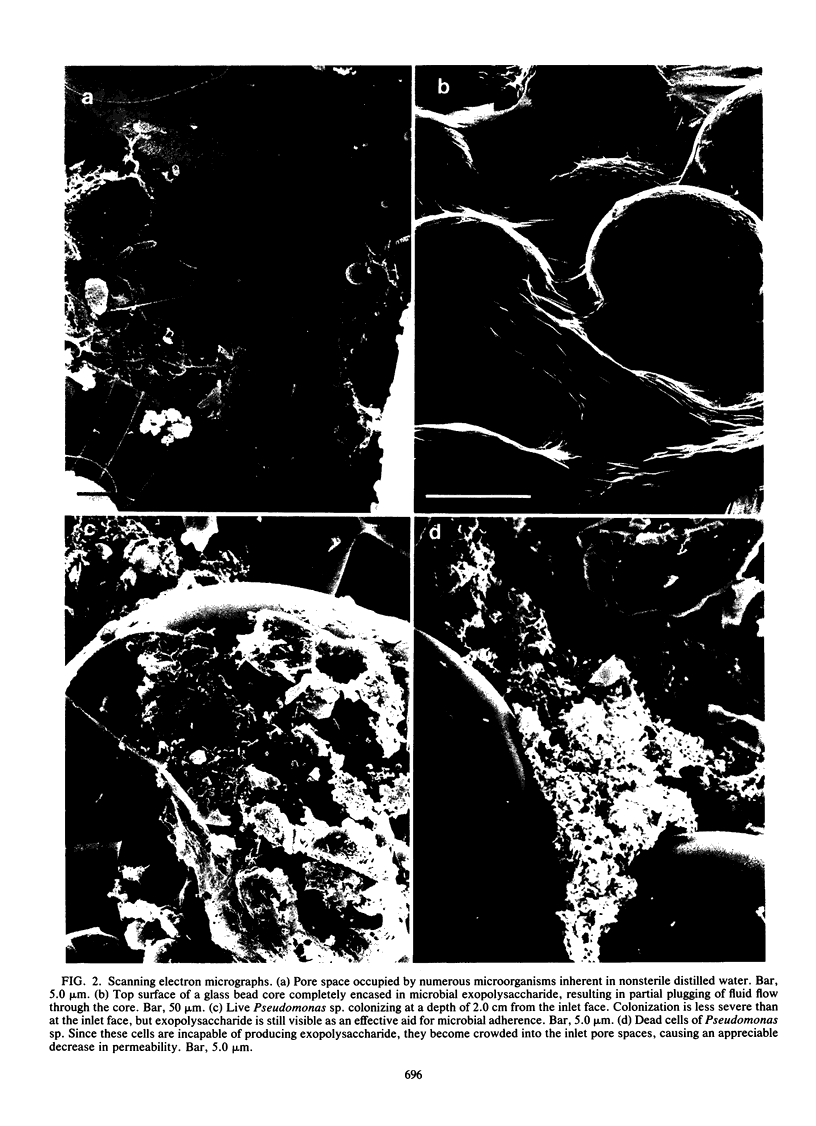
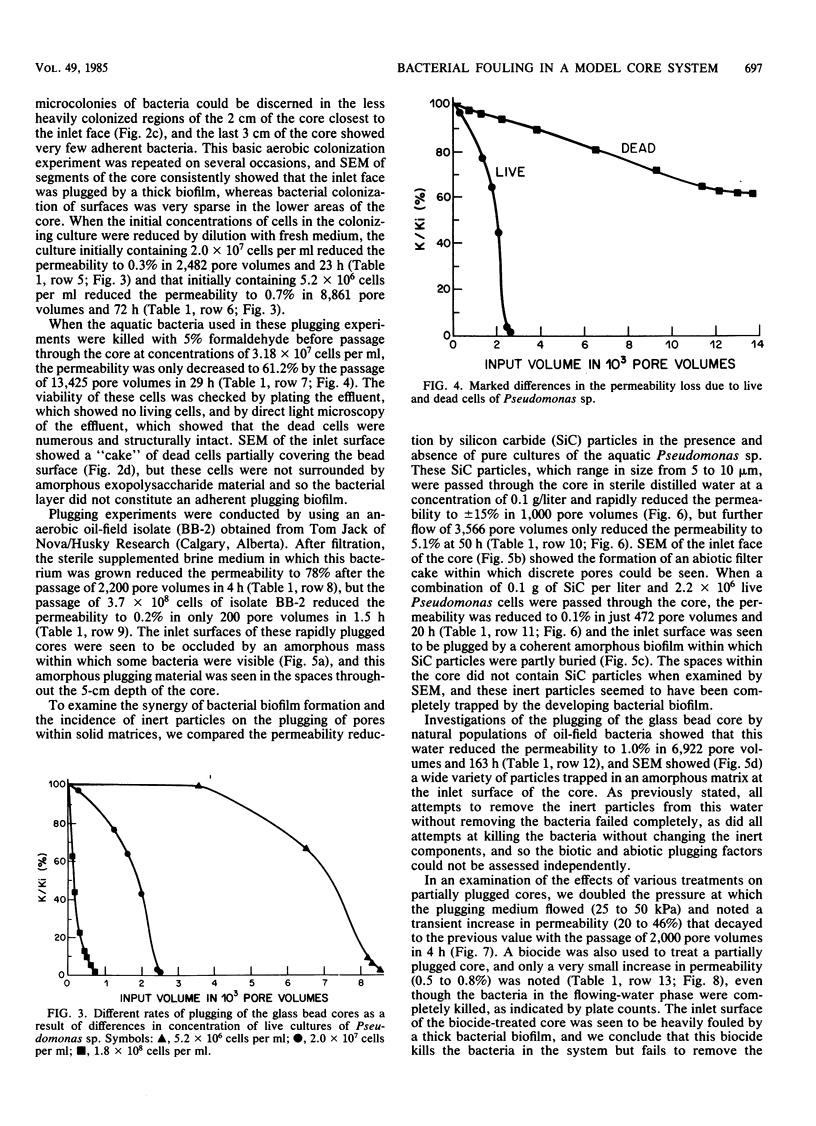
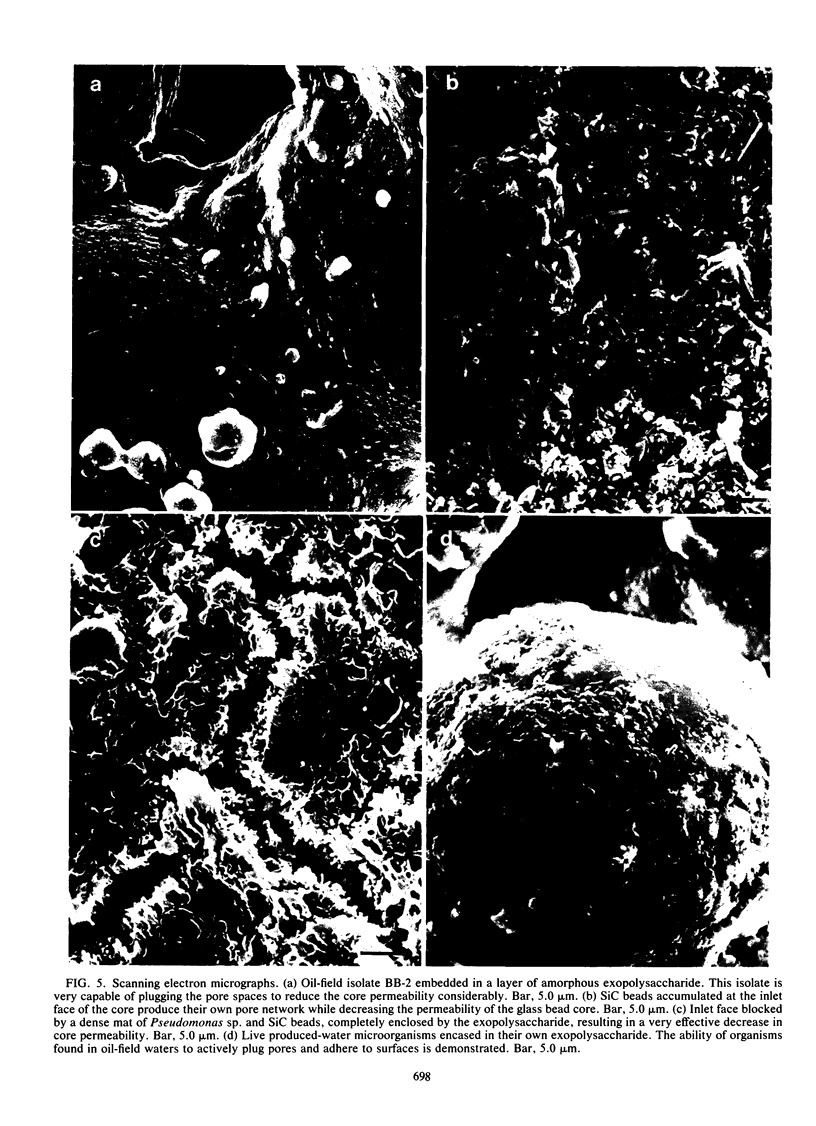
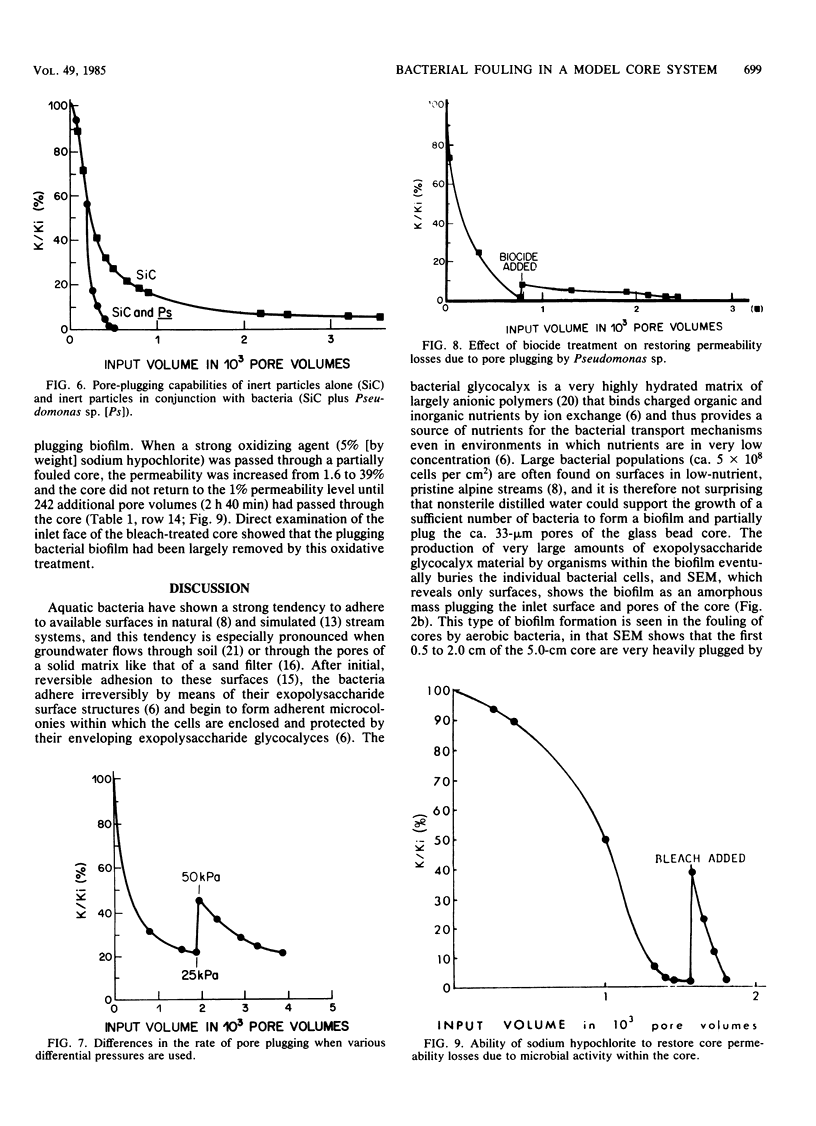
We have used a sintered glass bead core to simulate the spaces and surfaces of reservoir rock in studies of the bacterial plugging phenomenon that affects waterflood oil recovery operations. The passage of pure or mixed natural populations of bacteria through this solid matrix was initially seen to promote the formation of adherent bacterial microcolonies on available surfaces. Bacteria within these microcolonies produced huge amounts of exopolysaccharides and coalesced to form a confluent plugging biofilm that eventually caused a >99% decrease in core permeability. Aerobic bacteria developed a plugging biofilm on the inlet face of the core, facultative anaerobes plugged throughout the core, and dead bacteria did not effectively plug the narrow (33-μm) spaces of this solid matrix because they neither adhered extensively to surfaces nor produced the extensive exopolysaccharides characteristic of living cells. The presence of particles in the water used in these experiments rapidly decreased the core permeability because they became trapped in the developing biofilm and accelerated the plugging of pore spaces. Once established, cells within the bacterial biofilm could be killed by treatment with a biocide (isothiazalone), but their essentially inert carbohydrate biofilm matrix persisted and continued to plug the pore spaces, whereas treatment with 5% sodium hypochlorite killed the bacteria, dissolved the exopolysaccharide biofilm matrix, and restored permeability to these plugged glass bead cores.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL R., NEVO Z. EFFECT OF BACTERIAL POLYSACCHARIDE ACCUMULATION ON INFILTRATION OF WATER THROUGH SAND. Appl Microbiol. 1964 May;12:219–223. doi: 10.1128/am.12.3.219-223.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall K. C., Stout R., Mitchell R. Selective sorption of bacteria from seawater. Can J Microbiol. 1971 Nov;17(11):1413–1416. doi: 10.1139/m71-225. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]