Abstract
Small interfering RNA (siRNA) mediated gene silencing has been utilized as a powerful molecular tool to study the functional significance of a specific protein. However, due to transient gene silencing and insufficient transfection efficiency, this approach can be problematic in primary cell culture such as vascular smooth muscle cells. To overcome this weakness, we utilized an adenoviral-encoded microRNA (miRNA)-embedded siRNA “mi/siRNA”-based RNA interference. Here, we report the results of silencing a disintegrin and metalloprotease 17 (ADAM17) in cultured rat vascular smooth muscle cells and its functional mechanism in angiotensin II signal transduction. 3 distinct mi/siRNA sequences targeting rat ADAM17 were inserted into pAd/CMV/V5-DEST and adenoviral solutions were obtained. Nearly 90% silencing of ADAM17 was achieved when vascular smooth muscle cells were infected with 100 multiplicity of infection of each ADAM17 mi/siRNA encoding adenovirus for 3 days. mi/siRNA-ADAM17 but not mi/siRNA-control inhibited angiotensin II-induced epidermal growth factor receptor trans-activation and subsequent extracellular signal-regulated kinase activation and hypertrophic response in the cells. mi/siRNA-ADAM17 also inhibited angiotensin II-induced heparin-binding epidermal growth factor-like factor shedding. This inhibition was rescued with co-infection of adenovirus encoding mouse ADAM17 but not by its cytosolic domain deletion mutant or cytosolic Y702F mutant. As expected, angiotensin II induced tyrosine phosphorylation of ADAM17 in the cells. In conclusion, ADAM17 activation via its tyrosine phosphorylation contributes to heparin-binding epidermal growth factor-like factor shedding and subsequent growth promoting signals induced by angiotensin II in vascular smooth muscle cells. An artificial mi/siRNA-based adenoviral approach appears to be a reliable gene-silencing strategy for signal transduction research in primary cultured vascular cells.
Keywords: ADAM17, Epidermal Growth Factor Receptor, Angiotensin II Type 1 Receptor, Signal Transduction, Vascular Biology
1. Introduction
Angiotensin II (Ang II), the major bioactive hormone of the renin-angiotensin system, has long been implicated in contributing to various cardiovascular diseases such as hypertension, atherosclerosis and heart failure. Within the vasculature, vascular smooth muscle cells (VSMC) primarily express the Ang II type 1 (AT1) receptor, which mediates most of the pathological functions of Ang II [1, 2]. In VSMC, AT1 receptor signal transduction is known to involve many signaling proteins and pathways to mediate Ang II-induced VSMC hypertrophy, proliferation, and/or migration [2, 3]. A disintegrin and metalloproteases (ADAMs) are a family of membrane-anchored, zinc-dependent metalloproteases [4]. Among many ADAMs, ADAM17 is primarily responsible for the ectodomain shedding of several critical substrates including precursors of epidermal growth factor (EGF) receptor ligands [4, 5]. Our data from VSMC permanently over-expressing a catalytically inactive dominant-negative mutant of ADAM17 suggest the importance of this metalloprotease in mediating heparin-binding EGF-like growth factor (HB-EGF) generation and subsequent “trans”-activation of EGF receptor induced by Ang II [6].
Recent studies suggest potential roles of ADAM17 in mediating cardiovascular diseases. ADAM17 expression is enhanced in atherosclerotic lesions in apoE-/- mice and humans [7, 8]. We have shown that over-expression of dominant-negative mutant ADAM17 by adenovirus reduced VSMC hyperplasia in response to arterial injury [9]. ADAM17 is also implicated in hypertensive cardiac hypertrophy in rodents [10]. Moreover, an ADAM17 polymorphism was associated with cardiovascular mortality [11].
Despite these studies, the molecular mechanism by which ADAM17 activity is enhanced in the cardiovascular system such as by Ang II remains largely unclear. However, post-transcriptional modification including Thr735 phosphorylation has been proposed as one potential mechanism by which extracellular stimuli increase ADAM17 activity [12]. Given more than 30 ADAM family members, many of them ubiquitously expressed, the over-expression approach such as with an ADAM17 dominant-negative mutant likely involves adverse effects influencing other ADAMs in the system. The approach is also unsuitable for mechanistic studies to look for the ADAM17 catalytic regulation by Ang II. Whilst small interfering RNA (siRNA) technology offers a way to specifically silence target protein expression, the limitations of siRNA include poor transfection efficiency in primary cultured cells and a transient nature of gene silencing [13]. Viral vector-based siRNA technology in particular, an engineered microRNA (miRNA)-embedded siRNA “mi/siRNA” approach, should offer the flexibility to select various potent promoter drivers and a high transduction efficiency to specifically silence proteins [14-16] with a long half life such as ADAM17 [17] in primary cultured cells.
In this study, we made adenoviral vectors encoding engineered mi/siRNAs to target rat ADAM17 and significantly suppressed ADAM17 expression in rat VSMC. We showed that silencing ADAM17 expression using this adenovirus inhibited Ang II-mediated HB-EGF shedding and subsequent growth promoting signal transduction. We then tested our hypothesis that Ang II activates ADAM17 via tyrosine phosphorylation by silencing endogenous rat ADAM17 with ADAM17 mi/siRNA adenovirus in VSMC and replacing it with a mouse Y702F ADAM17 mutant.
2. Materials and methods
2.1. Reagents
HEK293A cell line and all cloning materials were purchased from Invitrogen. Ang II was purchased from Sigma-Aldrich. FuGene 6 was purchased from Roche. Antibodies against Tyr204-phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2), ERK2, and ADAM17 (sc-7383, sc-154, and sc-13973) were purchased from Santa Cruz Biotechnology. Antibody for Tyr1068-phosphorylated EGF receptor (44788G) was purchased from Invitrogen. Antibody for GAPDH (MRB374) was purchased from Millipore. Antibody for ADAM9 (M61420) was purchased from BD Biosciences. Antibody for phospho-tyrosine (05-321) was purchased from Millpore. Antibody for phospho-serine/threonine (61-8100) was purchased from Zymed.
2.2. Generation of Recombinant Adenoviruses
Replication-incompetent recombinant adenoviruses for RNA interference expressing engineered pre-miRNA encoding murine miR-155 stem loop and embedded siRNAs targeting rat ADAM17 mRNA were constructed using the BLOCK-iT™ Adenoviral RNAi Expression System (Invitrogen) according to the manufacturer's instructions. In this system, the virally encoded construct contains, in order: 21 nucleotide antisense target sequence, 19 nucleotide miR-155 derived loop sequence and sense target sequence nucleotides 1-8 and 11-21 (Supplementary Fig. A1). This sequence forms the basis of the synthetic “siRNA-embedded pre-miRNA” and contains the correct stem loop structure. This cassette is flanked by pri-miRNA sequence based on native miR-155. The entire expression cassette will form a stem-loop precursor/pri-miRNA and be processed by Drosha and Dicer to form pre-miRNA and the mature mi/siRNA, respectively. The mi/siRNA would then be incorporated into RNA-induced silencing complex (RISC) for cleavage of target mRNA [18]. Three distinct 21mer oligonucleotides encoding for the mi/siRNAs perfectly complementary to coding regions of rat ADAM17 mRNA (Accession: NM_020306GI: 9945329) were designed using the Invitrogen BLOCK-iT™ RNAi online designer program and were subsequently cloned into the pcDNA™ 6.2-GW/EmGFP-miR vector. The regions of rat ADAM17 coding sequences matching the mi/siRNAs, their homologies to mouse and rat ADAM17, and a Blast search results covering rat gene coding regions as well as 3’UTRs are shown in Supplemental Fig. A2. Note that the silencing system of mi/siRNAs is mechanistically distinct from endogenous miRNAs, which reduce expression of multiple targets via interaction to target 3’UTRs with imperfect complementarity [19]. For convenience, we abbreviated the miRNA-embedded ADAM17 mi/siRNA as miA17. These sequences included: miA17-94 to target residue 94-114 of ADAM7 mRNA: 5’-CTT GAG AAG CTT GAT TCT TTG-3’, miA17-450 to target residue 450-470 of ADAM7 mRNA: 5’-GCC ACT TTG GAG GTT TGT TAA-3’, and miA17-724 to target residue 724-744 of ADAM7 mRNA: 5’-GGA GAA GAG AGC ACT ACT ACA-3’. The pcDNA™6.2-GW/EmGFP-miR control plasmid (Invitrogen) with a 21mer sense target sequence: 5’-GTC TCC ACG CGC AGT ACA TTT-3’, which is predicted not to target any known mammalian gene, was used as a scramble control (referred to as miCon). Adenoviruses encoding these mi/siRNAs were generated using the ViraPower™ Adenoviral Expression System (Invitrogen) according to the manufacturer's instructions to produce crude adenoviral stocks. Adenoviral vectors encoding wild-type mouse ADAM17 (wtADAM17), its cytoplasmic domain deletion mutant (1-699) and Y702F mutant were created using the mammalian expression vectors as the template [9, 20]. Viral titers were calculated as previously described [21] and are expressed in units of multiplicity of infection (MOI).
2.3. VSMC culture and adenoviral infection
VSMC were prepared from the thoracic aorta of male Sprague Dawley rats (~350 g) by the explant method and cultured as previously described [22]. Prior to infection, VSMC from passage 3 to 10 at 80~90% confluence in culture wells were made quiescent by incubation with serum-free medium for 2-3 days. VSMC were infected with adenovirus as described [23] with modification to include 3% FuGENE6 to enhance infection efficiency as reported in other cell lines [24]. To avoid any potential phenotypic alteration, VSMC have been renewed every 2-3 months and VSMC from frozen stock were never used.
2.4. Plasmid transfection in HEK293 A cells
Rat ADAM17 cDNA was cloned by a PCR reaction from rat VSMC mRNAs and subcloned into pcDNA3 IRES DsRed vector. C-terminus HA tag was included by PCR reactions. The pcDNA3 rat ADAM17-HA DsRed vector (1.25 μg) and pcDNA™ 6.2-GW/EmGFP-miR vector encoding ADAM17 mi/siRNAs (3.75 μg) or control mi/siRNA (3.75 μg) were co-transfected to 5×105 HEK293A cells seeded on 6 well plate using 1% lipofectamin in 1 mL optiMEM medium for 3 h under manufacture's instruction. After the transfection, 1 mL DMEM with 10% fetal bovine serum was added and the cells were incubated for 2 days.
2.5. Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as previously described [22]. Cell lysates or immunoprecipitation lysates were subjected to SDS PAGE gel electrophoresis and transferred to nitrocellulose membranes by electrophoresis. Membranes were incubated with primary antibodies as indicated at 4°C overnight, exposed to horseradish peroxidase conjugated secondary antibodies for 1-2 h and proteins of interest were detected using chemiluminescent substrate (Thermo Scientific) according to manufacturer's instructions.
2.6. Hypertrophy assay
To assess Ang II-induced VSMC hypertrophy directly, we measured cell protein accumulation [21], but did not use a radiolabeled leucine incorporation assay in order to avoid unnecessary use of a radioactive compound. Consistent with a highly cited past report [25], in VSMCs derived from 12 week-old Sprague-Dawley rats, 72 h Ang II incubation in serum-free DMEM resulted in increases in cell protein without any significant change in cell proliferation/viability [21]. To measure cell protein accumulation, VSMCs grown on 12-well plates were incubated with serum-free DMEM for 2 days and infected with adenovirus in serum-free DMEM for 3 days or incubated with or without 100 nM Ang II for 3 days. After aspiration of the medium, cells were washed twice with ice-cold Hanks balanced salt solution, and the total amount of cellular protein was measured as previously described [21].
2.7. HB-EGF shedding assay
Ang II-mediated HB-EGF shedding was quantified in VSMC using an alkaline-phosphatase-tagged HB-EGF encoding adenovirus (HB-EGF-ALP) as previously described [26]. Following serum starvation, VSMC were co-infected with adenoviral vectors encoding HB-EGF-ALP, control or ADAM17 mi/siRNAs together with wild type mouse ADAM17, a mutant mouse ADAM17, or control GFP where specified. 3 days after infection, cells were stimulated with Ang II for 60 min. Secreted HB-EGF was measured using a colorimetric alkaline phosphatase activity assay as previously described [26].
2.8. Statistical analysis
Data are presented as mean±SD. Groups were compared using ANOVA followed by Tukey post hoc test, student t test, or paired t test. The null hypothesis was rejected when p<0.05.
3. Results
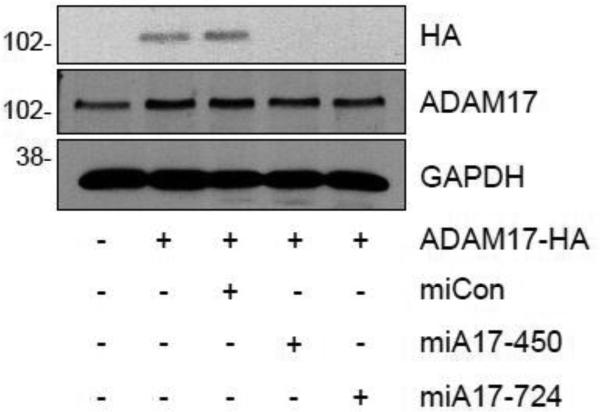
To test the ability of the ADAM17 targeting mi/siRNAs to reduce rat ADAM17 expression, HEK293A cells were co-transfected with an expression vector encoding HA-tagged rat ADAM17 and either ADAM17 mi/siRNA or the control mi/siRNA vector for 2 days. miA17-450, miA17-724 (Fig. 1) and miA17-94 (data not shown) but not mi/siRNA control inhibited exogenously introduced rat ADAM17-HA expression almost completely in HEK293A cells, whereas none of the mi/siRNAs affect the expression of endogenous human ADAM17 in the cells.
Fig. 1.
Silencing of exogenously expressed rat ADAM17-HA by ADAM17 mi/siRNAs. HEK293A cells were co-transfected with HA-tagged rat ADAM17 and ADAM17 mi/siRNA constructs or control mi/siRNA. Cell lysates were collected after 2 days and Western blot analysis was performed. Representative blots are shown from duplicated experiments.
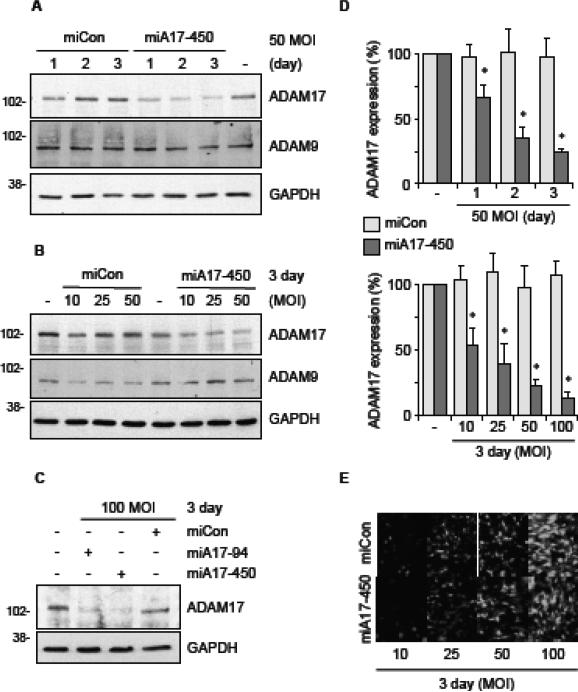
To verify silencing by the adenovirus encoding rat ADAM17 mi/siRNAs in physiologically relevant cells, primary cultured rat VSMC were infected with adenovirus vectors encoding ADAM17 mi/siRNAs or the control mi/siRNA at various concentrations and durations. In addition, 3% FuGene6 was added to each infection to enhance infection efficiency of adenovirus as assessed by transduction efficiency of encoded green fluorescent protein (GFP) (Supplementary Fig. B1). GAPDH and ADAM9 detection were used as loading controls and to check the specificity of silencing. Relatively linear time and concentration dependencies of ADAM17 silencing were observed between 1 to 3 days and 10 to 100 MOI of the ADAM17 mi/siRNA adenovirus infection. Nearly 90% silencing of ADAM17 was achieved when VSMC were infected with 100 MOI of any of the ADAM17 mi/siRNA encoding adenovirus but not the control mi/siRNA adenovirus for 3 days (Fig. 2 and Supplementary Fig. B2-B4).
Fig. 2.
Silencing of endogenous ADAM17 in rat VSMC by adenovirus encoding ADAM17 mi/siRNAs. VSMC were infected with adenovirus expressing control mi/siRNA or ADAM17 mi/siRNA (A & B: A17-450, C: A17-94 & A17-450) at MOI ranging from 10-100 for a period of 1-3 days. Cell lysates were analyzed by immunoblotting with antibodies as indicated. Representative blots are shown from triplicated experiments. D, Densitometry analysis of the data (miCon & miA17-450) from triplicated experiments (mean ± SD, * p <0.05 compared with miCon). E, GFP expression was analyzed by fluorescent microscopy. Representative results are shown from triplicated experiments.
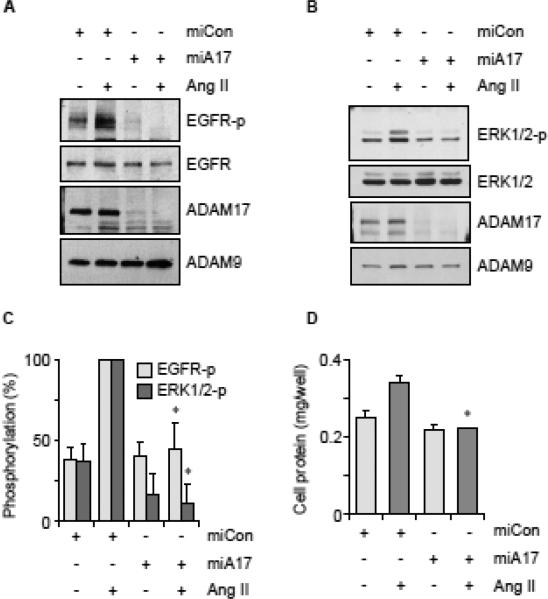
To ascertain that the ADAM17 mi/siRNA inhibits downstream ADAM17 function, Ang II-mediated EGF receptor trans-activation and ERK1/2 phosphorylation were evaluated in VSMC infected with the mi/siRNA adenovirus. Knockdown of ADAM17 in VSMC by adenovirus encoding miA17-450 significantly reduced Ang II-mediated EGF receptor and ERK1/2 activation in VSMC (Fig. 3A, 3B and 3C). Wild-type mouse ADAM17 rescued Ang II-induced EGF receptor trans-activation inhibited by miA17-450 (Supplementary Fig. B5). Ang II-induced protein accumulation in VSMC was also inhibited by miA17-450 compared with control adenovirus (Fig. 3D). Neither, the adenovirus nor Ang II affected cell number in these conditions.
Fig. 3.
Ang II-induced downstream signal transduction was inhibited by adenovirus encoding ADAM17 miRNA. VSMC were infected with adenovirus encoding miCon or miA17-450 at 100 MOI for 3 days and stimulated with 100 nM Ang II for 2 min (A) or 10 min (B). Cell lysates were analyzed by immunoblotting with antibodies as indicated. Representative blots are shown from triplicated experiments. C, Densitometry analysis of the blots from triplicated experiments (mean ± SD, * p <0.05 compared with stimulated control). D, After infection with adenovirus encoding miA17-450 or miCon (100 MOI), VSMCs were stimulated by AngII (100 nM) for 3 days. Cell protein accumulation was measured. Data are mean ± SD of 4 experiments. *p<0.05 compared with stimulated control.
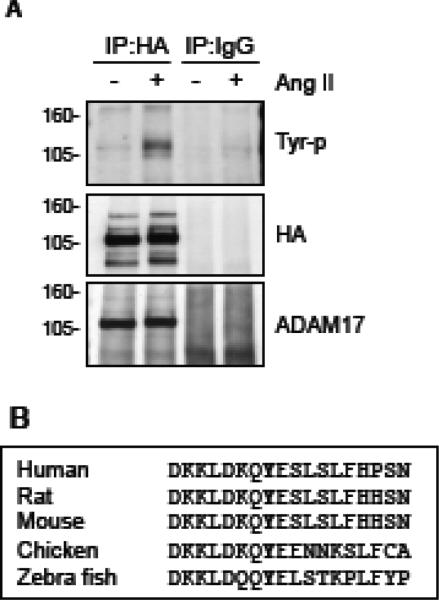
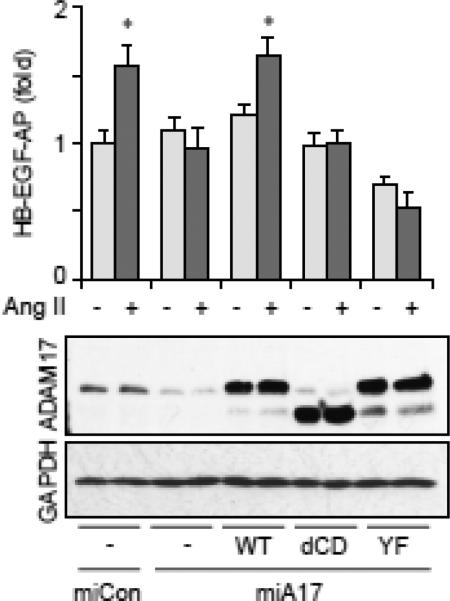
To understand the mechanism by which Ang II increases ADAM17 activity, we assessed ADAM17 phosphorylation with exogenously introduced HA-tagged ADAM17. This is because we have not yet found any reliable antibody against ADAM17 to immunoprecipitate rat ADAM17 sufficiently for phosphorylation analysis. VSMC expressing HA-tagged wild-type mouse ADAM17 were stimulated with Ang II and ADAM17 phosphorylation was analyzed with anti-phospho-tyrosine or phosphoserine/threonine antibody upon immunoprecipitation. Ang II-induced tyrosine phosphorylation of ADAM17 was detectable at 1 min, maximal at 2 min and declined at 5 min (Fig. 4A and Supplementary Fig. B6), but no serine/threonine phosphorylation was observed (Data not shown). Tyr702 is the sole cytosolic tyrosine residue of ADAM17 with highly conserved surrounding sequences (Fig. 4B). To test if ADAM17 Tyr702 phosphorylation is required for ADAM17 activation by Ang II, we took advantage of the stringent selectivity of mi/siRNA silencing. As shown in Fig. 5, we were able to replace endogenous rat ADAM17 with mouse wild-type or mutant ADAM17 in rat VSMC. Wild-type mouse ADAM17 rescued Ang II-induced HB-EGF shedding inhibited by miA17-450. By contrast, replacement of rat ADAM17 with mouse ADAM17 Y702F mutant or cytoplasmic domain deletion mutant could not reconstitute the Ang II response despite having expression levels comparable to the wild-type. These data suggest that Ang II activates ADAM17 via its cytoplasmic Tyr702 phosphorylation in VSMC.
Fig. 4.
Ang II stimulates tyrosine phosphorylation of ADAM17 in VSMC. A, VSMC infected with adenovirus encoding HA-tagged wild-type ADAM17 were stimulated with 100 nM Ang II for 2 min. Cell lysates were immunoprecipitated with anti-HA antibody or control IgG. Antibodies against phospho-tyrosine, HA and ADAM17 were used for immunoblotting in top, middle and bottom panels, respectively, for the samples immunoprecipitated with anti-HA antibody or control IgG. Representative blots are shown from triplicated experiments. B, Comparison of the amino acid sequence of the cytoplasmic domain of ADAM17 containing the conserved Tyr702 in various species.
Fig. 5.
Requirement of ADAM17 Tyr702 phosphorylation for ADAM17 activation by AngII. Rat VSMC were infected with adenoviruses (50 MOI) encoding HB-EGF-AP and miA17-450 targeting rat ADAM17 or non-targeting miCon (100 MOI) together with wild type mouse ADAM17, cytoplasmic domain deletion mutant (dCD), Y702F mutant (YF) or control GFP (10 MOI) for 3 days and HB-EGF shedding assay was performed with or without 100 nM Ang II stimulation (n=3, *p<0.05). The cell lysates were analyzed by immunoblotting as indicated.
4. Discussion
Our data presented here demonstrated that the artificial mi/siRNA-based adenoviral approach is a reliable gene-silencing strategy for signal transduction research in primary cultured VSMC. ADAM17 was found to be a major sheddase for HB-EGF generation contributing to the EGF receptor trans-activation induced by Ang II in VSMC. These data are in line with our past publication utilizing a catalytically inactive mutant of ADAM17 as a dominant-negative mutant [6] and further demonstrates the requirement of ADAM17 for ERK1/2 activation and hypertrophy induction by Ang II in VSMC.
All three candidate sequences of ADAM17 mi/siRNAs were able to silence both exogenously introduced rat ADAM17 in HEK293A cells and endogenous ADAM17 in rat VSMC. We could not see any significant difference in the silencing abilities among the three mi/siRNAs. It should be noted that miA17-450 target sequence has only one mismatch to that of human ADAM17, whereas no interference of human ADAM17 was recognized supporting the strict selectivity of the mi/siRNA silencing. Based on these observations, we believe that the presence of other potential mi/siRNA ADAM17 targets is unlikely. In addition, the targeting sequences were designed to avoid potential sequences that are homologous to other mRNA sequences in the target species. We have conducted our own Blast search for each ADAM17 mi/siRNA sequence covering coding regions as well as 3’UTRs and the highest homology was seen in miA17-94 to S1pr3 3’UTR (NM_001271143) with 5 nucleotide mismatch (supplemental Fig. A3). No other ADAMs were found to have more than 12 nucleotide match to the ADAM17 mi/siRNA 21mers. The mi/siRNA specificity was also validated by inclusion of ADAM9 blots.
Technically, inclusion of a cationic liposome, FuGene6, appears to be effective for enhancing the adenoviral infection in cultured VSMC. Cationic polymer and lipids such as poly L-lysine and lipofectin have been reported to enhance adenoviral transduction in vitro as well as in vivo [24, 27]. However, 3 days post infection were needed to see maximum silencing of ADAM17 while significant expression of the vector encoded GFP signal was observable 2 days post infection in the present study. This is likely due to a relatively long half-life (~24h) of ADAM17 protein as previously reported [17]. The adenoviral synthetic mi/siRNA gene silencing has also been utilized in neonatal rat cardiac myocytes [28-31] and human umbilical vein endothelial cells [32]. The adenoviral mi/siRNA approach seems to be a reliable new strategy to silence specific proteins in primary cultured cells in which standard transfection of siRNA appears ineffective.
Moreover, this strategy seems useful to replace endogenous target protein with the mutant or wild type from other species as demonstrated in a recent [33] and the present studies. As such, physiologically relevant data such as structure activity relationship can be obtained by expressing exogenous mutant protein at a physiological level, instead of over-expressing mutant in order to compete with wild type function. Requirement of Thr735 phosphorylation for ADAM17 activation by various agonists in distinct cell systems have been demonstrated [34, 35]. ADAM17 T735A but not Y702F showed dominant-negative activity with over-expression [35, 36]. However, Phorbol ester-induced EGF receptor ligand shedding was rescued with the ADAM17 cytoplasmic domain deletion mutant in ADAM17 null fibroblasts [20]. Our data is the first to show the critical role of ADAM17 Tyr702 phosphorylation for its activation. Thus, ADAM17 activation mechanisms may be cell and agonist specific. Our previous studies demonstrated that the metalloprotease-dependent EGF receptor trans-activation by Ang II requires Gq-mediated intracellular Ca2+ elevation [21, 22] and a membrane microdomain (caveolae/lipid rafts) [26], but not a tyrosine kinase, Src [37] or PYK2 [38]. Further research is needed to identify the putative ADAM17 tyrosine kinase in VSMC utilized by AngII as well as in vivo relevance of ADAM17 in mediating cardiovascular diseases associated with enhanced Ang II activities.
5. Conclusions
ADAM17 was found to be a major sheddase for HB-EGF contributing to the growth promoting signals induced by Ang II in VSMC. Activation of ADAM17 by Ang II required ADAM17 Tyr702 phosphorylation. An engineered mi/siRNA-based adenoviral RNA interference approach appears to be a reliable gene-silencing strategy for signal transduction research in primary cultured VSMC. Our data complements past and present studies that suggest ADAM17 may be an important therapeutic target in cardiovascular diseases. It will be interesting to test this concept in a model of cardiovascular diseases in vivo with a viral vector encoding this artificial ADAM17 targeting mi/siRNA such as in adeno-associated virus backbone, which may lead a novel gene therapy development.
Supplementary Material
Highlights.
Adenoviruses encoding ADAM17 targeting miRNA-embedded siRNA were created.
The adenovirus silenced ADAM17 expression in vascular smooth muscle cells.
The adenovirus inhibited angiotensin II-induced signal transduction in the cells.
The adenovirus inhibited angiotensin II-induced hypertrophy of the cells.
Requirement of ADAM17 phosphorylation for catalytic activation was presented.
Acknowledgements
This work was supported in part by National Institute of Health Grants, HL076770 (S.E.) and by American Heart Association Established Investigator Award, 0740042N (S.E.) and American Heart Association Postdoctoral Fellowship, 11POST7800000 (A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none declared
References
- 1.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- 2.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–28. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 3.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 4.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 5.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, et al. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–7. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 7.Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, et al. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187:82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Oksala N, Levula M, Airla N, Pelto-Huikko M, Ortiz RM, Jarvinen O, et al. ADAM-9, ADAM-15, and ADAM-17 are upregulated in macrophages in advanced human atherosclerotic plaques in aorta and carotid and femoral arteries--Tampere vascular study. Ann Med. 2009;41:279–90. doi: 10.1080/07853890802649738. [DOI] [PubMed] [Google Scholar]
- 9.Takaguri A, Kimura K, Hinoki A, Bourne AM, Autieri MV, Eguchi S. A disintegrin and metalloprotease 17 mediates neointimal hyperplasia in vasculature. Hypertension. 2011;57:841–5. doi: 10.1161/HYPERTENSIONAHA.110.166892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, et al. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–82. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 11.Morange PE, Tregouet DA, Godefroy T, Saut N, Bickel C, Rupprecht HJ, et al. Polymorphisms of the tumor necrosis factor-alpha (TNF) and the TNF-alpha converting enzyme (TACE/ADAM17) genes in relation to cardiovascular mortality: the AtheroGene study. J Mol Med (Berl) 2008;86:1153–61. doi: 10.1007/s00109-008-0375-6. [DOI] [PubMed] [Google Scholar]
- 12.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–89. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal V. Improving the efficiency of RNA interference in mammals. Nat Rev Genet. 2004;5:355–65. doi: 10.1038/nrg1323. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau RL, Martins I, Davidson BL. Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther. 2009;17:169–75. doi: 10.1038/mt.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13212–7. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–92. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago-Josefat B, Esselens C, Bech-Serra JJ, Arribas J. Post-transcriptional up-regulation of ADAM17 upon epidermal growth factor receptor activation and in breast tumors. J Biol Chem. 2007;282:8325–31. doi: 10.1074/jbc.M608826200. [DOI] [PubMed] [Google Scholar]
- 18.Du G, Yonekubo J, Zeng Y, Osisami M, Frohman MA. Design of expression vectors for RNA interference based on miRNAs and RNA splicing. The FEBS journal. 2006;273:5421–7. doi: 10.1111/j.1742-4658.2006.05534.x. [DOI] [PubMed] [Google Scholar]
- 19.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Science's STKE : signal transduction knowledge environment. 2007;2007:re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 20.Le Gall SM, Maretzky T, Issuree PD, Niu XD, Reiss K, Saftig P, et al. ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J Cell Sci. 2011;123:3913–22. doi: 10.1242/jcs.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsu H, Higuchi S, Shirai H, Eguchi K, Suzuki H, Hinoki A, et al. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–75. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–6. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 23.Takayanagi T, Bourne AM, Kimura K, Takaguri A, Elliott KJ, Eguchi K, et al. Constitutive stimulation of vascular smooth muscle cells by angiotensin II derived from an adenovirus encoding a furin-cleavable fusion protein. Am J Hypertens. 2012;25:280–3. doi: 10.1038/ajh.2011.221. [DOI] [PubMed] [Google Scholar]
- 24.Ro S, Hwang SJ, Ordog T, Sanders KM. Adenovirus-based short hairpin RNA vectors containing an EGFP marker and mouse U6, human H1, or human U6 promoter. Biotechniques. 2005;38:625–7. doi: 10.2144/05384RN01. [DOI] [PubMed] [Google Scholar]
- 25.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–56. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 26.Takaguri A, Shirai H, Kimura K, Hinoki A, Eguchi K, Carlile-Klusacek M, et al. Caveolin-1 negatively regulates a metalloprotease-dependent epidermal growth factor receptor transactivation by angiotensin II. J Mol Cell Cardiol. 2011;50:545–51. doi: 10.1016/j.yjmcc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda K, Ooboshi H, Chu Y, Fasbender A, Davidson BL, Welsh MJ, et al. Cationic polymer and lipids enhance adenovirus-mediated gene transfer to rabbit carotid artery. Stroke. 1998;29:2181–8. doi: 10.1161/01.str.29.10.2181. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231–6. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn C, Frank D, Will R, Jaschinski C, Frauen R, Katus HA, et al. DYRK1A is a novel negative regulator of cardiomyocyte hypertrophy. J Biol Chem. 2009;284:17320–7. doi: 10.1074/jbc.M109.006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem. 2009;284:29735–45. doi: 10.1074/jbc.M109.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Will RD, Eden M, Just S, Hansen A, Eder A, Frank D, et al. Myomasp/LRRC39, a heart- and muscle-specific protein, is a novel component of the sarcomeric M-band and is involved in stretch sensing. Circ Res. 2010;107:1253–64. doi: 10.1161/CIRCRESAHA.110.222372. [DOI] [PubMed] [Google Scholar]
- 32.Wittchen ES, Aghajanian A, Burridge K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small Gtpases. 2011;2:65–76. doi: 10.4161/sgtp.2.2.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031–44. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfa Cisse M, Sunyach C, Slack BE, Fisher A, Vincent B, Checler F. M1 and M3 muscarinic receptors control physiological processing of cellular prion by modulating ADAM17 phosphorylation and activity. J Neurosci. 2007;27:4083–92. doi: 10.1523/JNEUROSCI.5293-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66. doi: 10.1016/j.molcel.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakashima H, Frank GD, Shirai H, Hinoki A, Higuchi S, Ohtsu H, et al. Novel role of protein kinase C-delta Tyr 311 phosphorylation in vascular smooth muscle cell hypertrophy by angiotensin II. Hypertension. 2008;51:232–8. doi: 10.1161/HYPERTENSIONAHA.107.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, et al. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1831–6. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.