Abstract
Adipokines are secreted from adipose tissue, influence energy homeostasis and may contribute to the association between obesity and hypertension. Among 1,897 participants enrolled in the Multi-Ethnic Study of Atherosclerosis, we examined associations between blood pressure and leptin, tumor necrosis factor – α [TNFα], resistin and total adiponectin. The mean age and body mass index was 64.7 years and 28.1 respectively, and 50% were female. After adjustment for risk factors, a 1-standard deviation increment higher leptin level was significantly associated with higher systolic (5.0 mmHg), diastolic (1.9), mean arterial (2.8) and pulse pressures (3.6), as well as a 34% higher odds for being hypertensive (p < 0.01 for all). These associations were not materially different when the other adipokines, as well as body mass index, waist circumference or waist to hip ratio, were additionally added to the model. Notably, the associations between leptin and hypertension were stronger in men, but were not different by race/ethnic group, body mass index or smoking status. Adiponectin, resistin and TNFα were not independently associated with blood pressure or hypertension. Higher serum leptin, but not adiponectin, resistin or TNFα, is associated with higher levels of all measures of blood pressure, as well as a higher odds of hypertension, independent of risk factors, anthropometric measures and other selected adipokines.
Keywords: adipokine, leptin, blood pressure, hypertension, ethnicity
INTRODUCTION
Obesity is a significant and pervasive public health problem. As of 2010, over 35% of the United States adult population was obese, while just under 17% of US children and adolescents were classified as obese.1 Individuals who are overweight or obese are at increased risk for developing metabolic disorders, such as the metabolic syndrome, diabetes and dyslipidemia, which confer a higher risk for cardiovascular diseases.2, 3 Additionally, there is a strong and consistent association between greater adipose tissue and higher levels of blood pressure, as well as incident hypertension.4, 5 Indeed, it is estimated that 70% of hypertension can be attributed to excess body weight due to adiposity.6
The pathogenesis of obesity-associated hypertension is multifactorial. Increased sympathetic nervous system activity, activation of the renin-angiotensin system and volume expansion appear to be central determinants of prolonged elevation of systemic blood pressure.7, 8 In obese states, sympathetic nervous system activity is increased in the renal nerves, which results in increased renal tubular sodium reabsorption and impairs renal-pressure natriuresis resulting in increased blood pressure.9, 10 Importantly, the precise mechanisms driving increases in renal sympathetic nervous system activity have not been elucidated.
Adipokines are cytokines derived from adipose and related tissues that regulate energy utilization primarily by influencing lipid and glucose metabolism in peripheral tissues.11 For example, adiponectin modulates insulin sensitivity by decreasing lipid synthesis and glucose production in the liver, as well as decreasing triglyceride production and increasing free fatty acid oxidation in skeletal muscle. Conversely, leptin regulates energy expenditure by inducing satiety, while also increasing hepatic glucose production and its uptake in skeletal muscle.12 Given the links between obesity and increased blood pressure, as well as adipokines and adipose tissue deposition, we conducted a study to determine whether serum adipokine concentrations were associated with blood pressure, as well as diagnoses of both hypertension and isolated systolic hypertension. We hypothesized that higher leptin or decreasing adiponectin concentrations would be associated with higher blood pressure.
MATERIALS AND METHODS
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of African, Chinese and Hispanic Americans, as well as non-Hispanic Whites. Details about the study design have been published.13 In brief, between July 2000 and August 2002, 6,814 men and women who were 45 to 84 years old and were free of clinically apparent cardiovascular disease (CVD) were recruited from 6 United States communities. Individuals with a history of physician-diagnosed heart attack, angina, heart failure, stroke or TIA, or having undergone an invasive procedure for cardiovascular disease (CABG, angioplasty, valve replacement or pacemaker placement) were excluded from participation. Enrolled participants returned for follow-up clinic examinations on 3 subsequent visits at approximately 18-month intervals. All participants provided written informed consent and the institutional review boards (IRB) at the participating Universities approved the study.
At clinic exams 2 and 3 (approximately 2 – 4 years after the baseline visit), a random subsample of 1,970 participants (approximately ½ at each visit) from 5 of the 6 MESA field centers enrolled in an ancillary study to determine the presence and extent of calcified atherosclerosis in the abdominal aorta using computed tomography.14 The images from these scans, and stored venous blood taken contemporaneously, were utilized to determine the associations of both abdominal body composition and serum concentrations of adipokines with subclinical and clinical CVD. The data obtained on these participants comprise the sample for the current study.
Data Collection
At all clinic examinations (including visits 2 and 3 when the abdominal CT scans were conducted), standardized questionnaires were used to obtain sociodemographic, race/ethnicity and health history information. Cigarette smoking was defined as current, former, or never. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist and hip circumferences were measured using a standard flexible tape measure. Resting blood pressure was measured 3 times in seated participants with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon). The calculation of systolic blood pressure, diastolic blood pressure, pulse pressure and mean arterial pressure was based on the average of the second and third readings. Hypertension (HTN) was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or current use of an antihypertensive medication, while isolated systolic hypertension (ISH) was defined as a systolic blood pressure ≥140 mm Hg or current use of an antihypertensive medication and a diastolic blood pressure < 90 mmHg.
Laboratory
At all clinic examinations, total and HDL cholesterol, triglycerides, and glucose levels were measured from blood samples obtained after a 12-hour fast.15 Fasting blood was also assayed for measures of systemic inflammation (CRP, fibrinogen, interleukin-6) and insulin concentration. Dyslipidemia was defined as a total-cholesterol/HDL-cholesterol ratio > 5.0 or if the participant used medication to reduce cholesterol. Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of hypoglycemic medication.
Stored fasting blood samples obtained at clinic visits 2 and 3 were analyzed to provide serum concentrations of leptin, TNFα, resistin and total adiponectin. These adipokines were measured using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Average analytical coefficients of variation across several control samples for these analytes ranged from 6.0–13.0%.
Statistical Analysis
Among the 1,970 potential participants, there were 73 individuals who were missing values for the covariates and were excluded from the analysis, resulting in a final analytic sample of 1,897 participants. Characteristics of the population were determined with a mean and standard deviation for continuous variables, while categorical variables were summarized as a count and percentage of the study population. ANCOVA was used to determine the means of adipokines by hypertension status after adjusting for age, gender and race.
The adipokines were log transformed to reduce skewness and outliers. Pearson correlation coefficients were generated after adjusting for age, gender and race/ethnicity. So that all participants, including those taking blood pressure medications, could be included in the analysis, we utilized censored normal regression to assess the association between adipokines and continuous measures of blood pressure.16, 17 To determine the association between the adipokines and both hypertension and isolated systolic hypertension, we utilized unconditional logistic regression and the continuous and categorical (in quartiles) forms of the adipokine variables. For all regression analyses, the initial model 1 was adjusted for age, gender and race/ethnicity. Models were subsequently adjusted for smoking and education (model 2); then cystatin C, diabetes, dyslipidemia and fibrinogen (model 3); and finally BMI (model 4), waist circumference (model 5) or the waist to hip ratio (model 6), separately.
Multiplicative interactions between each adipokine and both race/ethnicity and body mass index (separately) were assessed. A two-tailed p-value < 0.05 was considered statistically significant and all statistical analyses were conducted using SPSS (Version 19.0; IBM Corp, Armonk, New York, USA) and STATA (Version 12; StataCorp, College Station, TX).
RESULTS
Overall, the mean age of the cohort was 64.7 years and 50% were female. Forty percent were non-Hispanic White, 26% were Hispanic/Latino, 21% were African American and 13% were Chinese American. Over half (54%) of the participants were hypertensive and 44% were taking a blood pressure medication. The mean systolic and diastolic blood pressures, as well as pulse and mean arterial pressures, were 124, 70, 54 and 88 mmHg, respectively. The mean BMI was 28.1 kg/m2 and 31% were obese. Most (46%) were never smokers whereas 43% were former smokers and 11% were current smokers. Forty percent were classified as dyslipidemic, 14% were classified as having diabetes mellitus and 6% had a positive family history of premature CVD. Mean/median (standard deviation) values for adiponectin, leptin, resistin, tumor necrosis factor -α (TNF-α), C-reactive protein (CRP), interleukin-6 (IL6) and fibrinogen, were 20.7/17.4 (13.2) μg/ml, 20.9/13.5 (22.3) ng/ml, 16.3/15.1 (6.8) ng/ml, 5.8/4.6 (9.6) pg/ml, 3.2/1.5 (7.0) mg/l, 2.2/1.9 (7.9) pg/ml and 434/427 (91.5) mg/dl, respectively.
The characteristics of the study cohort stratified by hypertension status are provided in Table 1. After adjustment for age, sex and race/ethnicity, those who were hypertensive were significantly older, more likely to be African American, to have dyslipidemia, diabetes mellitus or a family history of premature CVD. Those with hypertension also had significantly higher levels of BMI, cystatin-C, fibrinogen, leptin and resistin, but not CRP, IL6 or TNF-α. Adiponectin levels were borderline higher in those without hypertension (p = 0.08).
TABLE 1.
COHORT CHARACTERISTICS
| Characteristic* | HTN (N = 1033) | No HTN (N = 864) | p-value |
|---|---|---|---|
| Age (years) | 67.4 | 61.5 | < 0.01 |
| Gender (Male) | 51.0% | 49.0% | 0.40 |
| Ethnicity | |||
| Caucasian | 40.1% | 40.1% | 0.97 |
| Asian | 10.5% | 15.9% | <0.01 |
| African American | 26.0% | 15.0% | <0.01 |
| Hispanic | 23.4% | 29.0% | <0.01 |
| Education (more than HS) | 63.3% | 65.6% | 0.29 |
| Ever Smoker | 55.0% | 52.5% | 0.90 |
| Body Mass Index (kg/m2) | 29.2 | 26.9 | < 0.01 |
| Systolic Blood Pressure (mmHg) | 131.8 | 114.7 | <0.01 |
| Diastolic Blood pressure (mmHg) | 72.4 | 67.3 | <0.01 |
| Mean Arterial Pressure (mmHg) | 92.2 | 83.1 | <0.01 |
| Pulse Pressure (mmHg) | 59.5 | 47.4 | <0.01 |
| Diabetes Mellitus | 20.7% | 7.0% | < 0.01 |
| Dyslipidemia | 45.2% | 34.5% | < 0.01 |
| Family History of CVD | 10.5% | 0.8% | 0.11 |
| C-reactive Protein (mg/l) | 3.34 | 3.05 | 0.39 |
| Interleukin-6 (pg/ml) | 2.22 | 2.18 | 0.92 |
| Fibrinogen (mg/dl) | 438 | 429 | 0.02 |
| Adiponectin (μg/ml) | 20.2 | 21.3 | 0.076 |
| Leptin (ng/ml) | 24.1 | 17.0 | <0.01 |
| Resistin (ng/ml) | 16.8 | 15.9 | 0.03 |
| Tumor Necrosis Factor - α (pg/ml) | 5.61 | 4.89 | 0.42 |
All adjusted for age, gender, and race (except when it is the variable of interest)
The linear correlations between the blood pressure measures and the natural log transformed values of the different adipokines were modest. With adjustment for age, sex and race, the strongest significant correlations were between leptin and systolic blood pressure (r = 0.14, < 0.01), diastolic blood pressure (r = 0.07, p < 0.01), pulse pressure (r = 0.15, p < 0.01) and mean arterial pressure (r = 0.12, p < 0.01). Although not as strong, adiponectin was significantly and inversely correlated with all measures of blood pressure: SBP: −0.10, < 0.01; DBP: −0.06, < 0.01; PP: −0.08, < 0.0 and MAP: −0.09, < 0.01. Resistin was significantly associated with and pulse pressure (0.05, 0.03) but none of the other blood pressure measures. Tumor necrosis factor -α was not significantly correlated with any of the blood pressure measures.
Leptin
After adjustment for age, gender and race/ethnicity, a 1-unit increase in the natural log of leptin was associated with a 5.9 mmHg higher SBP (p < 0.01), which decreased to 5.0 mmHg (p < 0.01) with additional adjustment for education, smoking, cystatin-C, diabetes, dyslipidemia and fibrinogen (Table 2). The association was further attenuated, but remained statistically significant, with adjustment for BMI (2.3 mmHg, p <0.01), waist circumference (2.6 mmHg, p < 0.01) or the waist to hip ratio (3.7 mmHg, p < 0.01). The findings were similar for diastolic blood pressure, mean arterial pressure and pulse pressure (see Table 2). When leptin was categorized into quartiles (Q1: < 5.6, Q2: 5.6 – 13.5, Q3: 13.5 – 28.3, Q4: > 28.3), and even after full adjustment (i.e. model 4), each successive quartile was significantly associated with higher SBP, DBP, MAP and PP (Figure 1). Notably, the associations appeared particularly strong for the highest quartile and there was a significant reduction in the magnitudes of the associations with adjustment for BMI.
TABLE 2.
CHANGES IN DIFFERENT MEASURES OF BLOOD PRESSURE WITH HIGHER CONCENTRATIONS OF SELECTED ADIPOKINES
| Leptin | Adiponectin | Resistin | TNF - α | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | SBP | DBP | MAP | PP | SBP | DBP | MAP | PP | SBP | DBP | MAP | PP | SBP | DBP | MAP | PP |
| 1 | 5.9* | 2.3* | 3.3* | 4.4* | −5.2* | −1.7* | −2.8* | −4.0* | 4.5* | 1.2 | 2.1° | 4.1* | 1.6 | 0.9 | 1.0 | 1.1 |
| 2 | 5.9* | 2.3* | 3.3* | 4.3* | −5.1* | −1.7* | −2.7* | −3.9* | 4.7* | 1.3 | 2.2° | 4.3* | 1.7 | 0.9 | 1.1 | 1.2 |
| 3 | 5.0* | 1.9* | 2.8* | 3.6* | −4.6* | −1.4° | −2.4* | −3.4* | 1.5 | 0.0 | 0.5 | 1.7 | −0.4 | 0.2 | 0.0 | 0.6 |
| 4 | 2.3* | 1.3* | 1.6* | 1.3° | −1.7 | −0.5 | −0.9 | −1.3 | 1.0 | −0.1 | 0.2 | 1.2 | −0.5 | 0.2 | 0.0 | 0.7 |
| 5 | 2.8* | 1.6* | 1.9* | 1.6° | −1.9 | −0.6 | −1.0 | −1.3 | 1.1 | −0.1 | 0.3 | 1.3 | −0.6 | 0.1 | −0.1 | −0.8 |
| 6 | 3.7* | 1.5* | 2.2* | 2.6* | −2.2 | −0.6 | −1.1 | −1.6 | 1.4 | −0.4 | 0.4 | 1.5 | −0.6 | 0.1 | −0.1 | −0.8 |
Changes in measures of blood pressure are for 1-unit increment of the natural log (ln) of the adipokine;
p < 0.01,
p < 0.05
Model 1: age, gender, race
Model 2: model 1 + smoking, income
Model 3: model 2 + creatinine, diabetes, dyslipidemia, fibrinogen
Model 4: model 3 + body mass index
Model 5: model 3 + waist circumference
Model 6: model 3 + waist to hip ratio
FIGURE 1. ASSOCIATIONS BETWEEN QUARTILES OF LEPTIN AND DIFFERENT MEASURES OF BLOOD PRESSURE.
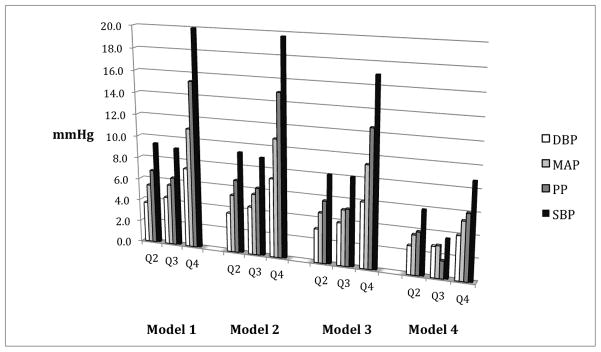
Referent Category = Quartile 1; Q2 = 2nd Quartile, Q3 = 3rd Quartile, Q4 = 4th Quartile
SBP = Systolic Blood Pressure, DBP = Diastolic Blood Pressure, MAP = Mean Arterial Pressure, PP = Pulse Pressure
Model 1: age, gender, race
Model 2: model 1 + smoking, education
Model 3: model 2 + cystatin C, diabetes, dyslipidemia, fibrinogen
Model 4: model 3 + body mass index
All values significant at p < 0.05
After adjustment for age, gender and race/ethnicity, a 1-standard deviation (SD) increase in leptin was associated with a 53% higher odds for the presence of hypertension (p < 0.01) [Table 3]. This association was not changed with additional adjustment for smoking and education, but was attenuated to 34% (p < 0.01) when cystatin-C, diabetes, dyslipidemia and fibrinogen were added to the model and then became non-significant when BMI was also included (1.09, p = 0.22). The results were similar when BMI was replaced by waist circumference or the waist to hip ratio (separately). Notably, when leptin was categorized into quartiles, there was a graded relationship between each successively higher quartile and the odds for the presence of hypertension (Figure 2). Specifically, with adjustment for age, gender and race/ethnicity and compared to the lowest quartile (Q1), the second (Q2), third (Q3) and fourth (Q4) quartiles were associated with 1.93, 2.25 and 3.88 higher odds for hypertension (p < 0.01 for all). With adjustment for all of the variables listed above, including BMI, these odds ratios were moderately attenuated (1.60, 1.60 and 2.04, respectively) but remained statistically significant (p < 0.01 for all). The findings were similar when BMI was replaced by waist circumference or the waist to hip ratio. All of the findings described above were similar when ISH was the outcome variable.
TABLE 3.
THE ODDS OF HYPERTENSION AND ISOLATED SYSTOLIC HYPERTENSION FOR SELECTED ADIPOKINES
| Model | Leptin | Adiponectin | Resistin | TNF - α | ||||
|---|---|---|---|---|---|---|---|---|
| HTN | ISH | HTN | ISH | HTN | ISH | HTN | ISH | |
| 1 | 1.53* | 1.53* | 1.10 | 1.14° | 0.89° | 0.86° | 0.96 | 0.95 |
| 2 | 1.53* | 1.53* | 1.06 | 1.11° | 0.89° | 0.86° | 0.95 | 0.94 |
| 3 | 1.34* | 1.34* | 0.97 | 1.01 | 0.92 | 0.90 | 0.90 | 0.88 |
| 4 | 1.09 | 1.12 | 0.97 | 1.01 | 0.99 | 0.96 | 0.91 | 0.88 |
Odds Ratios are for 1-standard deviation increment of the adipokine;
p < 0.01,
p < 0.05
Model 1: age, gender, race
Model 2: model 1 + smoking, income
Model 3: model 2 + creatinine, diabetes, dyslipidemia, fibrinogen
Model 4: model 3 + body mass index
FIGURE 2. ODDS OF HYPERTENSION BY QUARTILE OF LEPTIN.
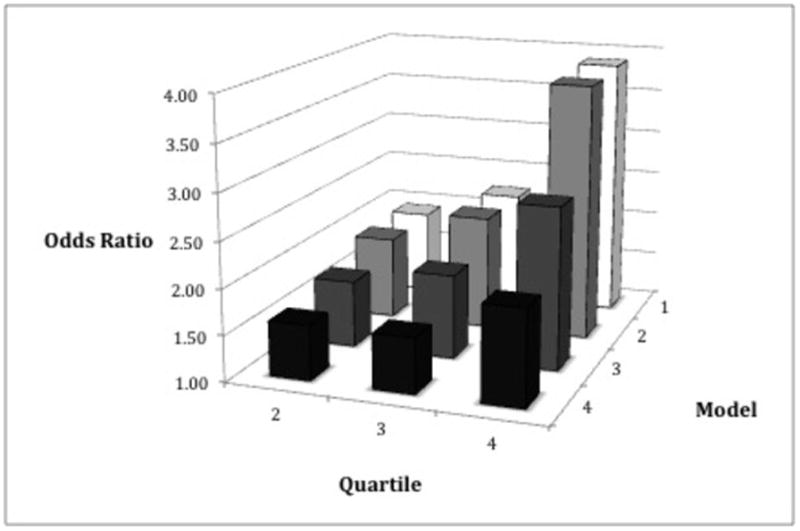
Reference Group = First quartile of leptin
Model 1: age, gender, race
Model 2: model 1 + smoking, education
Model 3: model 2 + cystatin C, diabetes, dyslipidemia, fibrinogen
Model 4: model 3 + body mass index
All values significant with p < 0.01
As the distribution of leptin has been shown to differ significantly by gender across different ethnic groups18, we conducted analyses stratified by this variable. After full adjustment and among men, a 1-unit increment in the natural log of leptin was associated with 1.6, 1.1, 1.2 and 0.8 mmHg higher SBP, DBP, MAP and PP levels respectively, while, among women, this same increment in leptin was associated with 2.3, 1.3, 1.6 and 1.4 mmHg higher SBP, DBP, MAP and PP levels. The odds for hypertension were somewhat higher in men (1.26 vs. 1.10). We then examined the same associations but using gender specific quartiles of leptin (Table 4). Among women, there was a stepwise increase in the strength of the association between leptin and all of the measures of blood pressure, as well as the odds for hypertension. For example, women in the 2nd, 3rd and 4th quartiles of leptin had systolic blood pressure values that were 0.0, 2.4 and 5.2 mmHg higher respectively, compared to those in the 1st quartile. Conversely, among men, the strongest association for the different measures of blood pressure was for those in the third quartile of leptin, while those in the highest quartile had the highest odds for being classified as hypertension. Despite the differences in the shape of the associations for men and women, the magnitudes where somewhat similar between these two groups, with the exception of the odds for hypertension, which were higher among men.
TABLE 4.
GENDER SPECIFIC DIFFERENCES IN SELECTED MEASURES OF BLOOD PRESSURE BY QUARTILES OF LEPTIN
| Quartile | Effect Sizes of Blood Pressure Differences or Relative Odds of Hypertension | ||||
|---|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | MAP (mmHg) | PP (mmHg) | HTN (Odds Ratio) | |
| Men | |||||
| 2 | 3.4 | 1.8 | 2.2 | 2.3 | 2.0° |
| 3 | 6.4° | 3.2° | 4.1° | 3.9° | 2.3° |
| 4 | 3.4 | 2.9 | 2.8 | 1.6 | 2.4° |
| Women | |||||
| 2 | 0.0 | 2.0 | 1.2 | −1.7 | 1.1 |
| 3 | 2.4 | 1.9 | 1.9 | 1.2 | 1.2 |
| 4 | 5.2 | 3.2 | 3.6 | 3.2 | 1.7 |
p < 0.05
Quartile 1 (gender specific) is the reference group
Values of Leptin by Quartile in Men: Q1: < 3.3, Q2: 3.3 – 7.1, Q3: 7.1 – 14.3, Q4: > 14.3 ng/ml
Values of Leptin by Quartile in Women: Q1: < 12.9, Q2: 12.9 – 25.1, Q3: 25.1 – 42.8, Q4: > 42.8 ng/ml
Adjusted for age, race, body mass index, smoking, income, diabetes, dyslipidemia, creatinine, fibrinogen
There were no significant interactions between leptin and race/ethnicity, BMI or smoking status.
Total Adiponectin
As shown in Table 2, after adjustment for age, gender and race/ethnicity, higher total adiponectin was associated with significantly lower values of SBP, DBP, MAP and PP (p < 0.01 for all). These associations remained significant after additional adjustment for education, smoking, diabetes, dyslipidemia, cystatin-C and fibrinogen, but not BMI. Similarly, compared to the lowest quartile (Q1: < 11.8, Q2: 11.8 – 17.4, Q3: 17.4 – 26.3, Q4: > 26.3), the highest quartile of adiponectin was significantly associated with significantly lower levels of all of the blood pressure measures, but the associations became not statistically significant when BMI was added to the models (data not shown).
A 1-SD increment in adiponectin was associated with significantly lower odds for hypertension after adjustment for age, gender, race/ethnicity, smoking and education (OR = 0.89, p = 0.03). However, this association was no longer significant when diabetes, dyslipidemia, cystatin-C and fibrinogen were added to the model (0.92, p = 0.15). Also, and unlike the case with leptin, different quartiles of adiponectin were not significantly associated with hypertension after adjustment. The results were consistent when ISH was the outcome variable.
Resistin and Tumor Necrosis Factor -α
There were no consistent significant associations between either resistin or TNF -α (as continuous or quartile variables) and the different measures of blood pressure, HTN or ISH.
DISCUSSION
In this study of a large, multi-ethnic population-based cohort from multiple sites across the United States, higher leptin concentrations were associated with higher levels of systolic, diastolic, mean and pulse pressures, as well as diagnoses of both hypertension and isolated systolic hypertension, independent of relevant covariates including body mass index or other measures of body morphology. Indeed, after multivariable adjustment, those with leptin concentrations in the highest quartile had over a 2-fold higher odds of hypertension. On the other hand, adiponectin was associated with lower levels of blood pressure and odds for hypertension or ISH, but these associations were not independent of other risk factors and, in particular, adiposity. Finally, neither resistin nor tumor necrosis factor -α were consistently associated with the different measures of blood pressure or hypertension. Taken together, these results suggest that leptin may be influencing blood pressure levels by a mechanism distinct from adiposity and other relevant risk factors while the same cannot be said for adiponectin and both resistin and tumor necrosis factor – α do not appear to significantly influence blood pressure.
Of note, the associations between leptin and higher blood pressure, as well as being classified as hypertensive, were independent of risk factors, total body adiposity and other selected measures of adiposity-associated inflammation. Additionally, the results of our study suggest that gender may modify the association between leptin and blood pressure. While the magnitudes of the associations were similar for the different continuous measures of blood pressure (i.e. SBP, DBP, MAP, PP), compared to women, higher leptin levels in men were associated with significantly higher odds for being classified with hypertension. In contrast, the associations were not significantly different by ethnicity, level of adiposity (by BMI categories) or smoking status.
A potential mechanism by which obesity may increase blood pressure is via increased sympathetic activity due to hyperleptinemia.19 Animals with diet-induced obesity, as well as most obese humans, are hyperleptinemic.20 Leptin is a cytokine secreted by adipose tissue that acts on the hypothalamus to decrease food intake via appetite suppression, while also increasing energy expenditure through sympathetic nervous system activity in the kidneys, adrenals and brown adipose tissue (BAT).19, 21, 22 Notably, there is a differential effect of leptin on BAT and the renal sympathetic nervous system activity. Specifically, increased blood pressure attenuates the effect of leptin on renal sympathetic fibers but does not affect its effect on fibers innervating BAT. Conversely, hypothermia augments the effect of leptin on BAT but not on renal sympathetic nerves.23, 24 These data suggest that the effect of leptin on renal sympathetic fibers is involved in the regulation of hemodynamics whereas its effect on BAT sympathetic nervous system activity is associated with temperature homeostasis.
Additionally, several studies have reported that the administration of leptin by various methods increases natriuresis without affecting renal blood flow, glomerular filtration rate or potassium excretion, suggesting that the natriuretic effect of leptin results from inhibiting tubular Na+ reabsorption.25–27 Indeed, both Shek28 and Kuo29 have demonstrated that leptin infusions resulting in concentrations similar to those observed in obese individuals increases mean arterial pressure while renal Na+ excretion is not changed. The importance of leptin as a regulator of sodium and volume is further supported by recent investigations showing that leptin expression in adipose tissue is directly proportional to dietary sodium, a response that would be expected for mechanisms regulating sodium balance.30
Due to the consistent and robust associations between leptin and blood pressure/hypertension, the results of our study provide strong support for the hypothesis that leptin is relevant to the kidney’s regulation of blood pressure. This finding is corroborated by results from other studies. For example, the Copenhagen City Heart Study reported that, when included in the same multivariable model, leptin, but not adiponectin, was significantly associated with incident hypertension.31 Concomitantly, that this association appears to be independent of adiposity is consistent with prior results. For example, a study of normotensive males has reported that there is a significant correlation between plasma leptin and blood pressure independent of body mass or abdominal obesity32 and even those of normal body weight but essential hypertension have significantly higher plasma leptin.33 Additionally, leptin-deficient humans show decreased sympathetic tone and normal blood pressure despite severe obesity.34 As such, the existing literature on the association between leptin and blood pressure supports and independent effect of this adipokine.
From a clinical perspective, higher levels of leptin have been associated with CVD risk factors that influence vascular function relevant to blood pressure regulation. These include diabetes mellitus and arterial compliance.35, 36 Given the association between higher leptin and higher blood pressure, interventions that reduce leptin concentrations may have salutary effects on blood pressure and CVD risk. In this regard, previous trials have shown that higher levels of physical activity and/or a diet low in saturated fat are associated with significant reductions in leptin concentrations, independent of changes in fat mass or body mass index.37 Similarly, we have shown that higher levels of sedentary activity are associated with higher leptin concentrations independent of body mass index and physical activity levels.38 Notably, there are no approved therapeutic interventions to lower leptin. As such, recommendations for lifestyle modifications that reduce sedentary behavior, increase physical activity or include a diet with less saturated fat may reduce leptin concentrations and therefore the risk of developing hypertension.
Strengths of this study include a relatively large, well-characterized, multi-ethnic cohort from across the United States and valid and reproducible measures of four different adipokines. Limitations include few subjects at the highest levels of obesity and a cross-sectional study design, which limits inferences on causality. Indeed, it may be possible that there is residual confounding due to an unmeasured cytokine. In this regard, longitudinal studies of those who are not hypertensive at baseline would minimize the potential for errors in interpretation due to reverse causality.
In conclusion, higher concentrations of leptin are associated with higher systolic, diastolic, pulse and mean arterial pressures, as well as being classified as hypertensive or with isolated systolic hypertension, independent of relevant risk factors, measures of adiposity and selected measures of adiposity-associated inflammation, and these associations may to be stronger among women. These findings provide evidence that leptin may be influencing blood pressure independently and by an as yet determined mechanism.
What is known about this topic
Obesity is associated with high blood pressure.
Adipokines are related to higher levels of adiposity.
Leptin has been associated with hypertension.
What this study adds
Leptin is significantly associated with multiple measures of blood pressure (SBP, DBP, pulse pressure and mean arterial pressure) and both hypertension and isolated systolic hypertension.
The associations between leptin and blood pressure are independent of relevant risk factors, several measures of obesity and selected measures of adiposity-associated inflammation.
The association between leptin and blood pressure appear to be different by gender but not race/ethnicity, level of obesity or smoking status.
The association between adiponectin and blood pressure is not independent of adiposity.
Resistin and tumor necrosis factor -α are not significantly associated with blood pressure or hypertension.
Acknowledgments
This research was supported by a grant (R01-HL-088451) and contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
CONFLICTS
The authors have no financial conflicts of interest to report.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM National Center for Health Statistics, editor. Prevalence of obesity in the United States, 2009–2010. Hyattsville, MD: 2012. [Google Scholar]
- 2.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595–600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 3.Leong KS, Wilding JP. Obesity and diabetes. Best Practice & Research Clinical Endocrinology & Metabolism. 1999;13(2):221–237. doi: 10.1053/beem.1999.0017. [DOI] [PubMed] [Google Scholar]
- 4.Jones DW, Kim JS, Andrew ME, Kim SJ, Hong YP. Body mass index and blood pressure in Korean men and women: the Korean National Blood Pressure Survey. Journal of hypertension. 1994;12(12):1433–7. doi: 10.1097/00004872-199412000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Jones DW. Body weight and blood pressure. Effects of weight reduction on hypertension. American journal of hypertension. 1996;9(8):50s–54s. doi: 10.1016/0895-7061(96)00183-5. [DOI] [PubMed] [Google Scholar]
- 6.Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Curr Pharm Des. 2004;10(29):3621–37. doi: 10.2174/1381612043382855. [DOI] [PubMed] [Google Scholar]
- 7.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45(1):9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 8.Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26 (Suppl 2):S28–38. doi: 10.1038/sj.ijo.0802125. [DOI] [PubMed] [Google Scholar]
- 9.Landsberg L, Krieger DR. Obesity, metabolism, and the sympathetic nervous system. American journal of hypertension. 1989;2(3 Pt 2):125S–132S. doi: 10.1093/ajh/2.3.125s. [DOI] [PubMed] [Google Scholar]
- 10.Hall JE, Louis K. Dahl Memorial Lecture. Renal and cardiovascular mechanisms of hypertension in obesity. Hypertension. 1994;23(3):381–94. doi: 10.1161/01.hyp.23.3.381. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier U, Gressner AM. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin Chem. 2004;50(9):1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Criqui MH, Kamineni A, Allison MA, Ix JH, Carr JJ, Cushman M, et al. Risk Factor Differences for Aortic Versus Coronary Calcified Atherosclerosis: The Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(11):2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.McClelland RLKR, Haessler J, Blumenthal RS, Goff DC. Estimation of risk factor associations when the response is influenced by medication use: an imputation approach. Stat Med. 2008;27(24):5039– 53. doi: 10.1002/sim.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 18.Lilja M, Rolandsson O, Shaw JE, Pauvaday V, Cameron AJ, Tuomilehto J, et al. Higher leptin levels in Asian Indians than Creoles and Europids: a potential explanation for increased metabolic risk. Int J Obes (Lond) 2010;34(5):878–885. doi: 10.1038/ijo.2010.19. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. American journal of hypertension. 2001;14(6, Supplement 1):S103–S115. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 20.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 21.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100(2):270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. Journal of hypertension. 2006;24(5):789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 23.Hausberg M, Morgan DA, Chapleau MA, Sivitz WI, Mark AL, Haynes WG. Differential modulation of leptin-induced sympathoexcitation by baroreflex activation. Journal of hypertension. 2002;20(8):1633–41. doi: 10.1097/00004872-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51(8):2434–40. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- 25.Beltowski J, Wojcicka G, Borkowska E. Human leptin stimulates systemic nitric oxide production in the rat. Obesity research. 2002;10(9):939–46. doi: 10.1038/oby.2002.128. [DOI] [PubMed] [Google Scholar]
- 26.Beltowski JGWj, Gorny D, Marciniak A. Human leptin administered intraperitoneally stimulates natriuresis and decreases renal medullary Na+, K+-ATPase activity in the rat -- impaired effect in dietary-induced obesity. Medical science monitor : international medical journal of experimental and clinical research. 2002;8(6):BR221–9. [PubMed] [Google Scholar]
- 27.Villarreal D, Reams G, Freeman RH, Taraben A. Renal effects of leptin in normotensive, hypertensive, and obese rats. The American journal of physiology. 1998;275(6 Pt 2):R2056–60. doi: 10.1152/ajpregu.1998.275.6.R2056. [DOI] [PubMed] [Google Scholar]
- 28.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31(1 Pt 2):409–14. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 29.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension. 2001;37(2 Part 2):670–6. doi: 10.1161/01.hyp.37.2.670. [DOI] [PubMed] [Google Scholar]
- 30.Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am J Physiol Renal Physiol. 2003;285(4):F619–28. doi: 10.1152/ajprenal.00388.2002. [DOI] [PubMed] [Google Scholar]
- 31.Asferg C, Mogelvang R, Flyvbjerg A, Frystyk J, Jensen JS, Marott JL, et al. Leptin, Not Adiponectin, Predicts Hypertension in the Copenhagen City Heart Study. American journal of hypertension. 2009;23(3):327–333. doi: 10.1038/ajh.2009.244. [DOI] [PubMed] [Google Scholar]
- 32.Barba G, Russo O, Siani A, Iacone R, Farinaro E, Gerardi MC, et al. Plasma leptin and blood pressure in men: graded association independent of body mass and fat pattern. Obesity research. 2003;11(1):160–6. doi: 10.1038/oby.2003.25. [DOI] [PubMed] [Google Scholar]
- 33.Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, et al. High plasma immunoreactive leptin level in essential hypertension. American journal of hypertension. 1997;10(10 Pt 1):1171–4. doi: 10.1016/s0895-7061(97)00310-5. [DOI] [PubMed] [Google Scholar]
- 34.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 35.Atabek ME, Kurtoglu S, Demir F, Baykara M. Relation of serum leptin and insulin-like growth factor-1 levels to intima-media thickness and functions of common carotid artery in children and adolescents with type 1 diabetes. Acta Paediatr. 2004;93(8):1052–7. doi: 10.1111/j.1651-2227.2004.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 36.Lilja M, Rolandsson O, Norberg M, Soderberg S. The impact of leptin and adiponectin on incident type 2 diabetes is modified by sex and insulin resistance. Metabolic syndrome and related disorders. 2012;10(2):143–151. doi: 10.1089/met.2011.0123. [DOI] [PubMed] [Google Scholar]
- 37.Reseland JE, Anderssen SA, Solvoll K, Hjermann I, Urdal P, Holme I, et al. Effect of long-term changes in diet and exercise on plasma leptin concentrations. The American Journal of Clinical Nutrition. 2001;73(2):240–245. doi: 10.1093/ajcn/73.2.240. [DOI] [PubMed] [Google Scholar]
- 38.Allison MA, Jensky NE, Marshall SJ, Bertoni AG, Cushman M. Sedentary behavior and adiposity-associated inflammation the multi-ethnic study of atherosclerosis. Am J Prev Med. 2012;42(1):8–13. doi: 10.1016/j.amepre.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


