Abstract
The liver is at the nexus of the regulation of lipoprotein uptake, synthesis, and secretion, and it is the site of xenobiotic detoxification by cytochrome P450 oxidation systems (phase I), conjugation systems (phase II), and transporters (phase III). These two major liver systems control vitamin E status. The mechanisms for the preference for α-tocopherol relative to the eight naturally occurring vitamin E forms largely depend upon the liver and include both a preferential secretion of α-tocopherol from the liver into the plasma for its transport in circulating lipoproteins for subsequent uptake by tissues, as well as the preferential hepatic metabolism of non-α-tocopherol forms. These mechanisms are the focus of this review.
Keywords: alpha-tocopherol, xenobiotic metabolism, lipoprotein metabolism
Unlike vitamins A and D, α-tocopherol (which is also a fat-soluble vitamin) does not accumulate to “toxic” levels in the liver or extrahepatic tissues (1). Indeed, when toxicologists searched for evidence of adverse effects of excess α-tocopherol, the only consistent finding was the observation that vitamin E caused increased bleeding tendencies, likely as a result of interference with vitamin K status (1). Thus, it was quite surprising that meta-analyses reported that consumption of vitamin E supplements (400 IU or more) by humans was associated with increased risk of dying (2–4), although the accuracy of these statistical analyses remain in dispute (5, 6). Nonetheless, the relationship between vitamin E and the adverse effects observed in some intervention studies (7–9) have largely gone unidentified.
Vitamin E does not have a known role as a cofactor, nuclear receptor ligand, or essential component of any enzymatic system. Rather, vitamin E protects long-chain polyunsaturated fatty acids (PUFA) from lipid peroxidation (10). Thus, vitamin E is delivered to the same sites as PUFA as a result of the nonspecific trafficking of vitamin E by virtually every lipoprotein receptor and lipid delivery and transport system, as discussed below. Remarkably, the body does regulate the amounts and form of vitamin E by a variety of mechanisms controlled in the liver.
The liver is at the nexus of the regulation of lipoprotein uptake, synthesis, and secretion; and the liver is the site of xenobiotic detoxification by cytochrome P450 oxidation systems (phase I), conjugation systems (phase II), and transporters (phase III) (11). These two major liver systems impact vitamin E status. The purpose of this review is to delineate the mechanisms for vitamin E absorption, disposition, metabolism, and excretion and to relate these to the establishment and regulation of tissue α-tocopherol concentrations in an effort to understand how dysregulation of these mechanisms might result in adverse consequences.
STRUCTURE-FUNCTION RELATIONSHIPS
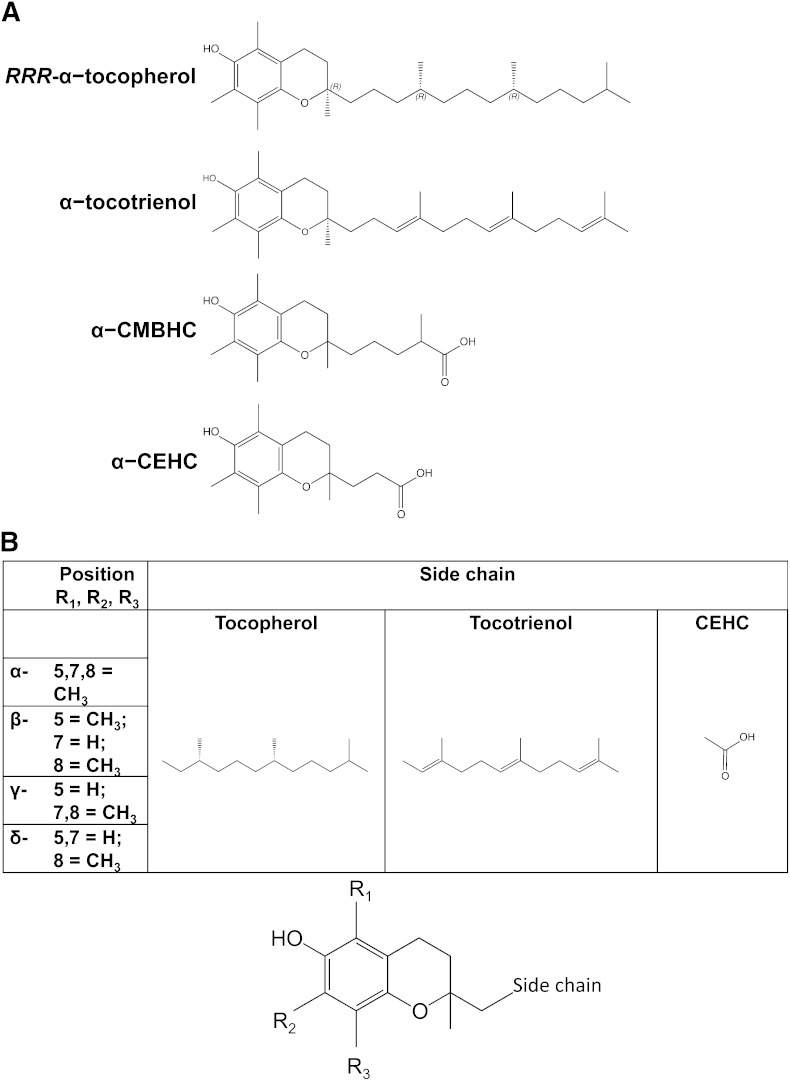
Plants synthesize eight different forms of vitamin E: tocopherols and tocotrienols, which include α, β, γ, and δ forms that differ in the number of methyl groups on the chromanol ring (Fig. 1) Almost immediately upon vitamin E's discovery in 1922 (12), these different forms of vitamin E were recognized to have differing biologic activities in rodents (13). Biologic activities were assessed by the various vitamin E forms to reverse deficiency symptoms, specifically fetal resorption in vitamin E-deficient pregnant rats (14). The different vitamin E forms are interconvertable by plants (15), but there is no convincing evidence that the same is true for animals.
Fig. 1.
Vitamin E structures. (A) α-Tocopherol-related structures. From top to bottom: RRR-α-tocopherol with indicated stereochemistry on the side chain and at position 2, (R)-2,5,7,8-tetramethyl-2-((4R,8R)-4,8,12-trimethyltridecyl)chroman-6-ol; α-tocotrienol, R-2,5,7,8-tetramethyl-2-((3E,7E)-4,8,12-trimethyltrideca-3,7,11-trien-1-yl)chroman-6-ol; α-CMBHC: 2,5,7,8-tetramethyl-2(2’-carboxymethylbutyl)-6-hydroxychroman, or 5-(6-hydroxy-2,5,7,8-tetramethyl-chroman-2-yl)-2-methyl-pentanoic acid; α-CEHC: 2,5,7,8-tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman, or 3-(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)propanoic acid. (B) Various forms of vitamin E and its metabolites. The chromanol ring illustrated at the bottom indicates the position of the substituents at positions 5, 7, and 8 and the side-chain location. The table indicates the various substituents for each α, β, γ, and δ, as well as the side chains for tocopherols, tocotrienols, and CEHCs.
Today, only α-tocopherol is recognized by the Food and Nutrition Board of the Institute of Medicine to meet human vitamin E requirements (1). Importantly, the differences in antioxidant activities of the various vitamin E forms are relatively minor, whereas the differences in biologic activities are quite striking (10). Indeed, the chemically synthesized, all racemic α-tocopherol contains eight stereoisomers, which have identical antioxidant activities but differ by a factor of two in biologic activity, as a result of the stereochemistry of the 2 position (e.g., 2S-α- compared with 2R-α-tocopherol activity) (1). The mechanisms for the preference for α-tocopherol largely depend upon the liver and include both a preferential secretion of α-tocopherol from the liver into the plasma for its transport in circulating lipoproteins for subsequent uptake by tissues, as well as the preferential hepatic metabolism of non-α-tocopherol forms. These mechanisms are the focus of the remainder of this review.
ABSORPTION AND LIPOPROTEIN TRANSPORT
Intestinal vitamin E absorption
The mechanism for vitamin E absorption by the enterocyte has not been entirely clarified. Certainly, absorption is enhanced if vitamin E supplements are consumed with fat (16, 17). Vitamin E absorption is impaired if fat absorption is prevented by a gastrointestinal lipase inhibitor (18), in disorders causing impaired bile secretion, such as in cholestatic liver disease (19), or in cystic fibrosis (20–22). Vitamin E can be absorbed by patients with malabsorption syndromes (23), if the vitamin E is given as a stable micellar form (24). Apparently, cystic fibrosis impedes vitamin E absorption only in those individuals with impaired bile secretion, suggesting that the requirement for gastrointestinal lipase is not as great as that for bile acids (25, 26). The minimum requirements for vitamin E absorption have been reported as its incorporation into micelles containing taurocholate and oleic acid (27). It is unclear whether these components function to solubilize vitamin E for its uptake into enterocytes or whether they function as ligands for specific receptors for internalization of the micelle.
The specific mechanisms for entry of vitamin E into the enterocyte following its solubilization in a micellar form remain controversial. The Niemann-Pick C1-Like 1 (NPC1L1) protein, when tested in vitro in an intestinal model system, does facilitate tocopherol uptake that is ezetimibe-sensitive, demonstrating that the drug, which inhibits cholesterol uptake by NPC1L1, also limits vitamin E absorption (28). Additionally, the scavenger receptor B-1 (SR B-1) has been shown to facilitate both cholesterol and tocopherol uptake into enterocytes and to be inhibited by a specific SR B-1 inhibitor, block lipid transport-1 (BLT-1) (27, 29). However, when evaluated in vivo, cholesterol and tocopherol absorption by mice occurred in quite different locations, with the greatest tocopherol absorption in the distal jejunum, suggesting there may be additional mechanisms/receptors/transporters involved in vitamin E absorption (27).
Once vitamin E has been taken up into the enterocyte, the mechanisms for traversing the cell and secretion in lipoproteins are also not well defined. Certainly, humans develop vitamin E-deficiency symptoms (30) if they are unable to synthesize apolipoprotein B (apoB) or have a defect in lipidating apoB due to a defective microsomal triglyceride transfer protein [e.g., humans with homozygous hypobetalipoproteinemia (31, 32) or abetalipoproteinemia (33, 34), respectively]. ApoB is needed for vitamin E transport both in chylomicrons (apoB48) and in very low and low-density lipoproteins (VLDL and LDL, apoB100). Vitamin E deficiency occurs not only as a result of impaired chylomicron assembly but also as a result of impaired hepatic VLDL production and secretion, as observed in normotriglyceridemic hypobetalipoproteinemia (35, 36). The lack of α-tocopherol secretion in VLDL in such a subject resulted in low plasma α-tocopherol concentrations with the preponderance of α-tocopherol transported in high-density lipoproteins (HDL), findings which were in contrast to normal subjects who transported α-tocopherol in both LDL and HDL (37).
The role of HDL α-tocopherol transport from the intestine has been investigated, especially during low-fat intakes (38). The importance of HDL for intestinal α-tocopherol absorption seems unlikely given the severity of vitamin E deficiency in the absence of chylomicron secretion, as described above. However, the finding that HDL is involved in vitamin E absorption provides a mechanism for its absorption in the absence of secretion of apoB-containing lipoproteins. This mechanism explains in part the observed normal adipose α-tocopherol tissue concentrations, which are achieved by abetalipoproteinemic patients when they consume vitamin E supplements (approximately 10 g/day) (39). This role of HDL in α-tocopherol absorption is in addition to minor amounts of apoB lipoproteins these patients secrete that contain α-tocopherol (40, 41).
The process of discrimination between vitamin E forms during intestinal absorption and incorporation into chylomicrons has been studied both in vivo and in vitro. As might be expected from the discussion of mechanisms involved in vitamin E absorption, no discrimination between forms of vitamin E has been observed at this step (42, 43). In studies in thoracic lymph duct-cannulated rats of α-tocopherol absorption, when soybean oil was used as a carrier of the vitamin, α-tocopherol inhibited its own absorption as its concentrations in the oil were increased, while the γ-tocopherol absorption was unaffected (44). Thus, the low γ-tocopherol and high α-tocopherol concentrations did not lead to discrimination between the tocopherols, suggesting that solubility in the intestinal lumen, rather than concentration, is critical for absorption. Indeed, based on their greater fluidity, there may be preference for uptake of tocotrienols into the enterocyte, but following absorption, there is rapid metabolism of the non-α-tocopherol forms (45–48), which is discussed further below.
The estimation of the fractional rate of vitamin E absorption varies widely with no consensus among investigators as to whether the percentage is less than 30% or as much as 90%, because the outcomes are highly dependent upon the analytical techniques used and size of the dose (16, 49). There is evidence for a limitation in α-tocopherol absorption, with high doses being absorbed at lower fractional rates (44, 50–53).
Hepatic secretion of α-tocopherol into plasma
Chylomicrons transport vitamin E from the intestine through the circulation to the liver (43, 54). The body's preference for α-tocopherol occurs as a result of the specific secretion of α-tocopherol from the liver and the hepatic metabolism of non-α-tocopherol forms; this latter topic is discussed further below.
Once the liver takes up chylomicron remnants and begins their degradation, the mechanism by which α-tocopherol is salvaged and secreted from the liver is unknown. The α-tocopherol transfer protein (α-TTP) is required for this process because humans with a defect in the α-TTP gene manifest a vitamin E deficiency disorder called ataxia with vitamin E deficiency (AVED) (55–57). α-TTP's function is to preferentially transfer α-tocopherol compared with other dietary vitamin E forms (58, 59). Hypothetically, this α-tocopherol transfer function is necessary for the observed mechanism that enriches the plasma with α-tocopherol, not with the other non-α-tocopherol (43, 60, 61). Notably, the liver (62), not the intestine, expresses α-TTP; thus, liver, not the intestine, specifically discriminates between forms of vitamin E. The means by which α-TTP facilitates α-tocopherol secretion into plasma is unknown, but it is under investigation (63–70). The current consensus is that α-TTP facilitates α-tocopherol transfer to the hepatic plasma membrane, where the ATP binding cassette transporter A1 (ABCA1) is involved in α-tocopherol transfer to circulating lipoproteins (65), such as VLDL (71) and HDL (72, 73).
Lipoprotein transport
All lipoproteins are involved in α-tocopherol trafficking to the tissues. VLDL apparently leaves the liver preferentially enriched in α-tocopherol (43, 60, 74), despite evidence that the enrichment does not occur inside the hepatocyte (71, 75). The conversion of VLDL to LDL enriches the latter with α-tocopherol. HDL may acquire α-tocopherol during lipolysis of triglyceride-rich lipoproteins, but there may be specific mechanisms that enrich HDL with α-tocopherol (73). VLDL has been estimated to contain approximately 65 α-tocopherol molecules per particle (76), while LDL contains approximately 8–12 α-tocopherol molecules per particle, and HDL contains less than 1 α-tocopherol per particle (77).
VLDL production is also dependent upon adequate hepatic α-tocopherol concentrations to prevent lipid peroxidation (78). Lipid peroxides cause an increase in apoB degradation during its synthesis and lipidation (79).
Circulating HDL concentrations are under a complex regulatory control that includes lipases; transfer proteins; ATP binding cassette (ABC) transporters, including A1, G1, G5, and G8; and SR B-1 (80). The various HDL-cholesterol regulatory controls are also involved in α-tocopherol trafficking.
HDL has a major role in reverse cholesterol transport and cholesterol excretion (81), and it plays a role in plasma vitamin E transport. It is unknown whether HDL specifically traffics vitamin E to the liver for metabolism and excretion. HDL contains approximately 40% of circulating α-tocopherol (82, 83). Supplements, whether they contain α-tocopherol or are tocotrienol-enriched, do not change the percentage of circulating α-tocopherol in the HDL fraction (84, 85). Whether HDL cholesterol concentrations change in humans taking vitamin E supplements remains a controversial topic. Since the late 1970s, vitamin E supplements have been found to increase (86, 87), decrease (88), or leave unchanged (89) the circulating HDL-cholesterol concentrations. The differences in these outcomes may have to do with the various HDL regulators, as discussed below.
ABCA1 mediates the lipidation of apoAI, the major HDL protein (90); however, it has also been found to facilitate the α-tocopherol secretion from cells (73), especially in the intestine (91) and liver (72).
The phospholipid transfer protein (PLTP) and cholesterol ester transfer protein (CETP) are both members of the same protein family, but they have very different functions (92). CETP transfers HDL cholesteryl ester to VLDL and thus decreases HDL cholesterol levels (93), whereas inhibitors of CETP raise HDL cholesterol levels (94). Borel et al. (95) reported in 129 French Caucasian subjects that a genetic CETP variant had increased plasma α-tocopherol concentrations. Hacquebard et al. (96) propose that CETP may facilitate α-tocopherol enrichment of circulating LDL and HDL based on their in vitro studies using lipid emulsions.
PLTP is found in plasma associated with HDL, where it not only transfers phospholipids but also diacylglycerol, α-tocopherol, cerebroside, and lipopolysaccharides (92).
Jiang et al. (97) reported decreased α-tocopherol concentrations in apoB-containing lipoproteins from PLTP-deficient mice, whether the backgrounds were wild-type, apoE-deficient, low-density lipoprotein (LDL) receptor-deficient, or apoB/CETP transgenic. In all four backgrounds, the PLTP deficiency increased oxidative damage (97). PLTP deficiency also decreased α-tocopherol concentrations in both the brain and the vascular wall, decreased hepatic α-tocopherol concentrations, and led to an increase in apoB degradation (98).
Clinical significance
α-Tocopherol is transported in plasma lipoproteins and delivered to tissues by all available mechanisms. This redundancy in α-tocopherol transport systems obviates the need for any specific plasma α-tocopherol transport proteins. The only transport protein for α-tocopherol is the hepatic α-TTP. The α-tocopherol-associated protein is in fact supernatant protein factor (SFP), which binds α-tocopherol poorly in in vitro assays (99). Instead, SFP binds squalene, acting as a squalene transfer protein and promoting cholesterol synthesis, as shown in SPF-knockout mice (100, 101); notably, the mice were not vitamin E-deficient.
α-TTP is critical for intracellular α-tocopherol trafficking to allow the liver to export α-tocopherol to the plasma. Vitamin E deficiency occurs in humans if α-TTP is defective and only dietary sources of α-tocopherol are available (57). Importantly, large supplements of vitamin E obviate the need for α-TTP and ameliorate the deficiency symptoms in AVED humans (102).
Plasma α-tocopherol concentrations are highly dependent upon circulating lipid levels (103). In patients with abetalipoproteinemia, who have extraordinarily low circulating lipids, vitamin E supplements can normalize adipose tissue concentrations despite the apparent impairment in lipoprotein transport (104). Contrariwise, apparently normal circulating α-tocopherol concentrations in patients with cholestatic liver disease and elevated circulating lipids were found to have deficient tissue concentrations (105). Thus, plasma α-tocopherol concentrations can be misleading with regard to assessment of vitamin E status.
METABOLISM OF VITAMIN E
The liver plays a major role in vitamin E metabolism, which is one of the key mechanisms for the α-tocopherol preference, for limiting its accumulation, and for determining the circulating levels of various vitamin E forms. Vitamin E metabolism is nonspecific; the mechanisms involved are promiscuous in that these are general xenobiotic processes. Vitamin E is metabolized by ω-hydroxylation by cytochrome P 450 (CYP), followed by β-oxidation, conjugation, and excretion. All of the possible metabolites from α-, γ-, and δ-tocopherols and tocotrienols have been identified (106). The various steps in metabolism are described more fully below.
α-Tocopherol and non-α-tocopherol metabolism
The α-tocopherol metabolite α-carboxyethyl hydroxychroman (CEHC) is tail-shortened but has an unoxidized head group (Fig. 1) (107). Nearly 60 years ago, Simon et al. (108) described vitamin E metabolism and its oxidized product “Simon metabolites” or α-tocopheronolactone (107). Simon metabolites were thought to occur as a result of in vitro oxidation (109); they are decreased if an antioxidant is present during sample preparation (110). However, they have been found in increased concentrations in urine from diabetic subjects (111). Thus, both CEHCs and tocopheronolactones may be biologically relevant vitamin E metabolites.
Non-α-tocopherols, especially tocotrienols, appear to be metabolized and excreted, while the body retains α-tocopherol. All racemic α-tocopherol (only half is 2R-α-tocopherol, the form recognized by α-TTP) was also converted by humans to α-CEHC at greater rates than RRR-α-tocopherol (112). γ-CEHC was proposed as the major route for γ-tocopherol excretion (113). In cultured hepatocytes, γ-CEHC production is 100 times greater than is α-CEHC from similar amounts of tocopherols added to the medium (114). Moreover, based on pharmacokinetic analyses in humans, γ-CEHC production determined the rate of disappearance of γ-tocopherol (115). Fraiser and Jiang (116) reported that γ-CEHC production was greater from γ-tocotrienol than from γ-tocopherol. In rats, accumulation of α-tocotrienol in various organs could be demonstrated when large quantities of α-tocotrienol were administered by gavage for more than two years; when α-tocotrienol administration was stopped and the diet was replaced with a vitamin E-deficient diet, α-tocotrienol depletion took less than two months, while loss of α-tocopherol was negligible (117). Uchida et al. (118, 119) showed that α-tocopherol stimulated the disappearance of non-α-tocopherol and increased CEHC production. It is likely that metabolism of the tocotrienols observed in rats led to its rapid disappearance, as faster turnover of tocotrienols has also been seen in humans (120). Thus, the consensus of reports provides strong evidence that the body recognizes α-tocopherol as a vitamin, whereas even low concentrations of the other dietary tocols are recognized as xenobiotics and are metabolized and excreted.
Phase I, CYP
CYP4F2 was identified to ω-hydroxylate γ-tocopherol (121) by analysis of individual human CYPs expressed in insect cells. Moreover, CYP4F2 activity toward α-tocopherol was limited relative to other forms of vitamin E (122). An inhibitor of CYP4F, an omega imidazole-containing compound 1,[(R)-2-(9-(1H-imidazol-1-yl)nonyl)-2,5,7,8-tetramethylchroman-6-ol], decreased CEHC production from γ-tocopherol in HepG2 cells in culture and increased plasma δ-tocopherol concentrations when δ-tocopherol and the inhibitor were simultaneously given to mice (123). Additionally, human CYP4F2 variants demonstrated varying ω-hydroxylation in vitro (124), but in a genome-wide association study, they were not found to dramatically change tocopherol concentrations or be associated with plasma γ-tocopherol concentrations (125). The CYP4F2 variant Rs2108622 was associated with increased circulating α-tocopherol in subjects from the ATBC trial (126), suggesting that this variant has reduced ω-hydroxylation activity.
The studies by Sontag et al. (121, 122) would seemingly define CYP4F2 as the CYP involved in vitamin E metabolism. Contrariwise, when cyp4f14 (the mouse equivalent of CYP4F2) was disrupted, the accumulation of tissue γ and δ-tocopherols was observed, but the production of CEHCs was not completely obliterated (127, 128), suggesting that there are other CYP enzymes that may participate in vitamin E metabolism. In early studies of vitamin E metabolism, CYP3A was proposed to be involved in vitamin E metabolism based on the observation that CYP3A inhibitors and stimulators altered CEHC production (114, 129–131). Additionally, studies in mice demonstrated that feeding α-tocopherol increased cyp3a mRNA (132). However, studies in rats injected with vitamin E suggested that excess hepatic α-tocopherol did not upregulate CYP4F or CYP3a (133). When rats were given a CYP3A inducer pregnenolone-16α-carbonitrile (PCN) or inhibitor ketoconazole (KCZ), Li and Shaw (134) concluded there was little impact of CYP3A activity on CEHC excretion. Vitamin E metabolism may be differently regulated in mice and rats because mice have been repeatedly reported to have increased cyp3a11 mRNA in response of excess vitamin E (132, 135–137). Vitamin E does not appear to have an effect on CYP3A activity in humans (88, 138, 139). Thus, in vivo CYP4F2 most likely is the CYP involved in initiating vitamin E metabolism in humans.
Note that CYP4F2’s function is not specific for vitamin E. CYP4 family members are major fatty acid ω-hydroxylases (reviewed in Ref. 140). CYP4F2 ω-hydroxylates vitamin K1 (phylloquinone) (141), and variants in the human population have been found to have altered responses to the vitamin K antagonist warfarin (142). CYP4F2 also converts arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) (143, 144) and participates in leukotriene metabolism (145). Additionally, the CYP4 family modulates eicosanoids during inflammation and metabolizes some clinically significant pharmaceutical agents (146). Human variants in the CYP4F2 gene have been associated with hypertension and increased stroke risk (147–152). These clinical effects are thought to be a result of altered leukotriene metabolism. CYP4A and CYP4F genes are regulated in the opposite direction by peroxisome proliferators, starvation, and high-fat diets (144).
Once the vitamin E tail has been ω-hydroxylated, there is consensus that β-oxidation takes place (107, 109, 114, 116, 129, 135, 153–155). The process of β-oxidation may involve both peroxisomes and mitochondria, but the mitochondria are apparently a significant site for CEHC production (155).
Phase II, conjugation
Most investigators use a combination glucuronidase and sulfatase to prepare their samples and thus report unconjugated metabolite concentrations, because several different conjugates in urine and in plasma have been described. In addition to glucuronide conjugates of CEHC (156), CEHC sulfate (116, 153, 154) and CEHC glycoside (157) have been reported. Johnson et al. (135), using a metabolomics approach, reported novel α-CEHC conjugates in both mouse and human urine, including α-CEHC glycine, α-CEHC glycine glucuronide, and α-CEHC taurine.
The mechanism for glucuronidation has not been investigated, but Hashiguchi et al. (158) have demonstrated in vitro that sulfotransferases (SULT), specifically members of the SULT1 family, displayed sulfating activities toward both tocopherols and their metabolites by studying of all 14 known human cytosolic SULTs. These findings support the hypothesis of Freiser and Jiang (116, 154) that sulfated intermediates may be important for cellular trafficking during vitamin E metabolism.
Phase III, transporters
There are no reports of transporters specifically involved in the transport of CEHCs or their conjugates. One of the hepatic responses to “excess” α-tocopherol is to upregulate α-tocopherol and metabolite biliary secretion. Both the mouse multidrug resistance (mdr1, p-glycoprotein) gene (136) and the Slc22a5 gene (Solute carrier family 22, organic anion transporter, member 5) were upregulated in mice fed high vitamin E diets (136). The rat hepatic genes and proteins MDR (ABCB4) (133, 159) and breast cancer resistance (BCRP) (133) are upregulated in response to increasing tissue α-tocopherol concentrations, whereas the organic anion transporter protein (OATP) (133) was decreased. Previously, mouse mdr2, another ABC transport protein, was shown to be involved in the efflux of α-tocopherol into bile (160). These various transporters are possible candidates for the mechanism of metabolite efflux from the liver, but more research is needed to define the mechanisms for export of vitamin E forms and their metabolites.
CLINICAL ASPECTS OF VITAMIN E METABOLISM
CEHC as a biomarker of vitamin E status
Circulating α-tocopherol concentrations are not reliable for assessment of vitamin E status, especially in subjects with abnormally high or low lipid concentrations. When urinary α-CEHC was initially suggested as a biomarker of adequate vitamin E status, the methodology at that time was not sufficiently sensitive to detect low urinary levels of α-CEHC excreted during times of vitamin E intake only from diet, although plasma α-CEHC increases were reported with supplemental vitamin E intake (109, 161). Refinements in methodology have shown that low levels of α-CEHC are continuously excreted in urine and do increase with higher α-tocopherol intake (162–164). There appears to be a threshold in α-CEHC excretion that corresponds to α-tocopherol intake; this increase in α-CEHC excretion has been proposed as an indicator to α-tocopherol adequacy (165). It is important to note that α-CEHC excretion does increase to a greater extent if supplements or foods fortified with all racemic α-tocopherol are consumed (162), because α-CEHC levels increase to a greater extent in response to 2S-α-tocopherols (112). Additionally, biliary α-CEHC excretion may play an important role when supplemental vitamin E intake becomes excessive. Excretion via the bile may explain why the urinary α-CEHC concentration peaked prior to cessation of supplemental vitamin E intake in a study using deuterium-labeled vitamin E to study metabolism in smokers and nonsmokers (162).
Vitamin K status
Vitamin E supplementation significantly decreased venous thromboembolism by 21% in the Women's Health Study (166), an effect that was attributed to interactions between vitamins E and K. These vitamins appear to share the same metabolic pathway for catabolism; the side chain of both vitamin E and K are ω-hydroxylated, then β-oxidized (167).
Plant-derived phylloquinone (vitamin K1) has a 20 carbon phytyl side chain, while menaquinones (MK) have multiple prenyl units, as indicated by their suffix number (e.g., MK-n) (167). The liver converts phylloquinone to menadione, which is then converted to MK-4 synthesis by UbiA prenyltransferase containing 1 (UbiA1) (168, 169). Importantly, it appears that vitamin E interferes with this process, as extrahepatic tissue vitamin K1 and MK-4 concentrations were lower in rats fed a high vitamin E diet (170) or injected with vitamin E (171). Additionally, high-dose vitamin E supplementation (1,000 IU) in humans increased the degree of under-γ-carboxylation of prothrombin (proteins induced by vitamin K absence-factor II, PIVKA-II) (172). The mechanism by which vitamin E interferes with vitamin K status is unknown.
REGULATION OF VITAMIN E CONCENTRATIONS BY TRAFFICKING AND METABOLISM
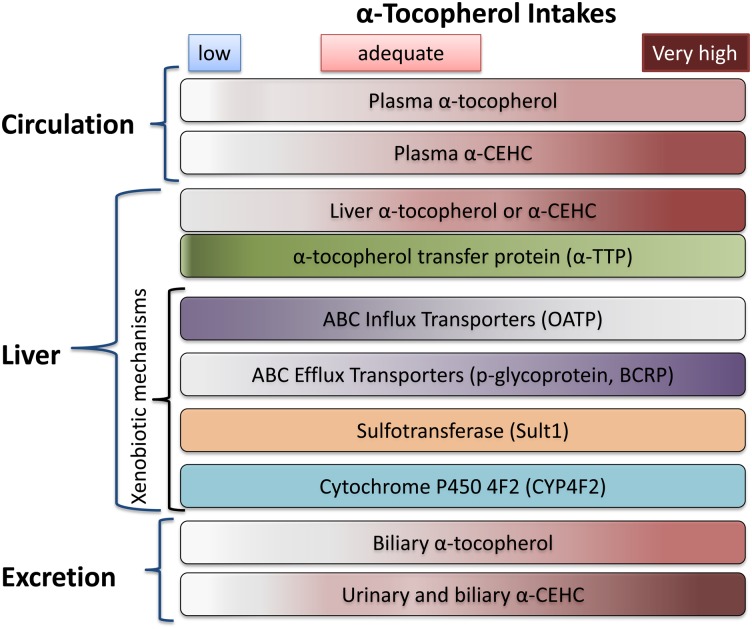
Vitamin E supplied in the diet is in relatively low concentrations, especially compared with amounts in supplements, and dietary vitamin E from plants is usually present as multiple vitamin E forms. When these low dietary amounts are absorbed and reach the liver, α-TTP facilitates α-tocopherol resecretion into plasma, while non-α-tocopherols are metabolized to CEHCs and excreted. Thus, as indicated in Scheme 1, prolonged, low intake of α-tocopherol can be associated with apparently adequate plasma α-tocopherol concentrations, as a result of the α-TTP salvage mechanism. Moreover, oxidative stress can increase the α-TTP gene expression (173), suggesting that hepatic α-TTP may increase with deficiency. Importantly, with high α-tocopherol administration [e.g., 400 IU supplements (174)], the liver secretion of α-tocopherol becomes limiting, plasma concentrations do not increase more than 2-4-fold (175), and xenobiotic metabolism is observed with high levels of circulating α-CEHC and high urinary α-CEHC excretion (162–164). Studies in rats administered vitamin E by subcutaneous injection with 40-fold increases in hepatic α-tocopherol show that hepatic α-TTP (162–164), CYP4F2, and sult1 gene are unchanged, while xenobiotic efflux transporters are upregulated and influx transporters downregulated (133). It should be noted that the vitamin E-related substrates for the sulfotransferase and the transporters are unknown; the transporters are not involved in lipoprotein uptake. The net effect of these processes is, in the face of a high influx of α-tocopherol into the liver, to limit circulating α-tocopherol to a 2- to 4-fold increase (174), to increase α-CEHC excretion in urine (109, 176), and potentially to increase both α-tocopherol and α-CEHC excretion in bile and thereby limit the delivery of α-tocopherol to extrahepatic tissues (177).
Scheme 1.
Relationship between vitamin E in diet, circulation, liver, and excretion. The α-tocopherol and α-CEHC are shown in a rose color, with higher concentrations indicated by darker colors. For all panels, as the vitamin E intake moves from low (<5 mg/day), to adequate (10–50 mg/day), to very high (>1,000 mg/day), the intensity of the color increases. Components with no color change (e.g., α-TTP) do not vary with vitamin E intake. Note that OATP varies inversely with vitamin E intake.
WHY IS α-TOCOPHEROL A VITAMIN?
Although there are plenty of antioxidants present in the diet, only α-tocopherol is a vitamin. α-Tocopherol's special role is that of a fat-soluble antioxidant that prevents the propagation of lipid peroxidation. It is likely that there are specific lipids, probably derived from PUFAs (such as docosahexaenoic acid) that are essential for life and that vitamin E protects (10). The α-tocopherol is the most efficient and safest of the vitamin E forms. The α-tocopheroxyl radical is relatively long-lived (178), and it can be reduced to α-tocopherol by water-soluble antioxidants, such as ascorbic acid (179). Other forms of vitamin E, when they become radicals, are more reactive and can readily form adducts that are potentially cytotoxic (180).
The safety of α-tocopherol can also be inferred from the relative lack of specific mechanisms for its metabolism. The other non-α-tocopherol forms are readily metabolized by xenobiotic pathways, likely because these forms are not effective as antioxidants and therefore should be removed promptly from the body. The tocotrienols may be a special case, because the unsaturated tail potentially could interfere with MK-4’s role in carboxylating vitamin K-dependent proteins in tissues.
Footnotes
Abbreviations: α-TTP, α-tocopherol transfer protein; CYP, cytochrome P 450.
- AVED
- ataxia with vitamin E deficiency
- CEHC
- carboxyethyl hydroxychroman
- MK
- menaquinone
- SR B-1
- scavenger receptor B-1
This work was supported in part by National Institutes of Health Grant R01-DK-081761.
REFERENCES
- 1.Institute of Medicine. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press, Washington, DC. [Google Scholar]
- 2.Vivekananthan D. P., Penn M. S., Sapp S. K., Hsu A., Topol E. J. 2003. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 361: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. 2007. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 297: 842–857. [DOI] [PubMed] [Google Scholar]
- 4.Miller E. R., 3rd, Paston-Barriuso R., Dalal D., Riemersma R. A., Appel L. J., Guallar E. 2005. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 142: 37–46. [DOI] [PubMed] [Google Scholar]
- 5.Abner E. L., Schmitt F. A., Mendiondo M. S., Marcum J. L., Kryscio R. J. 2011. Vitamin E and all-cause mortality: a meta-analysis. Curr. Aging Sci.4: 158–170. [Google Scholar]
- 6.Berry D., Wathen J. K., Newell M. 2009. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin. Trials. 6: 28–41. [DOI] [PubMed] [Google Scholar]
- 7.Cheung M. C., Zhao X. Q., Chait A., Albers J. J., Brown B. G. 2001. Antioxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler. Thromb. Vasc. Biol. 21: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 8.Brown B. G., Zhao X. Q., Chait A., Fisher L. D., Cheung M. C., Morse J. S., Dowdy A. A., Marino E. K., Bolson E. L., Alaupovic P., et al. 2001. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345: 1583–1592. [DOI] [PubMed] [Google Scholar]
- 9.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J. M., Ross C., Arnold A., Sleight P., Probstfield J., et al. 2005. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 293: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 10.Traber M. G., Atkinson J. 2007. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 43: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dussault I., Yoo H. D., Lin M., Wang E., Fan M., Batta A. K., Salen G., Erickson S. K., Forman B. M. 2003. Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc. Natl. Acad. Sci. USA. 100: 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans H. M., Bishop K. S. 1922. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 56: 650–651. [DOI] [PubMed] [Google Scholar]
- 13.Emerson O. H., Emerson G. A., Mohammad A., Evans H. M. 1937. The chemistry of vitamin E. tocopherols from natural sources. J. Biol. Chem. 22: 99–107. [Google Scholar]
- 14.Leth T., Sondergaard H. 1977. Biological activity of vitamin E compounds and natural materials by the resorption-gestation test, and chemical determination of the vitamin E activity in foods and feeds. J. Nutr. 107: 2236–2243. [DOI] [PubMed] [Google Scholar]
- 15.Mene-Saffrane L., DellaPenna D. 2010. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol. Biochem.48: 301–309. [Google Scholar]
- 16.Bruno R. S., Leonard S. W., Park S-I., Zhao Y., Traber M. G. 2006. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled α-tocopheryl acetate. Am. J. Clin. Nutr. 83: 299–304. [DOI] [PubMed] [Google Scholar]
- 17.Jeanes Y. M., Hall W. L., Ellard S., Lee E., Lodge J. K. 2004. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 92: 575–579. [DOI] [PubMed] [Google Scholar]
- 18.Melia A. T., Koss-Twardy S. G., Zhi J. 1996. The effect of orlistat, an inhibitor of dietary fat absorption, on the absorption of vitamins A and E in healthy volunteers. J. Clin. Pharmacol. 36: 647–653. [DOI] [PubMed] [Google Scholar]
- 19.Sokol R. J., Heubi J. E., Iannaccone S., Bove K. E., Balistreri W. F. 1983. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 85: 1172–1182. [PubMed] [Google Scholar]
- 20.Farrell P. M., Bieri J. G., Fratantoni J. F., Wood R. E., di Sant'Agnese P. A. 1977. The occurrence and effects of human vitamin E deficiency. A study in patients with cystic fibrosis. J. Clin. Invest. 60: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias E., Muller D. P., Scott J. 1981. Association of spinocerebellar disorders with cystic fibrosis or chronic childhood cholestasis and very low serum vitamin E. Lancet. 2: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 22.Sitrin M. D., Lieberman F., Jensen W. E., Noronha A., Milburn C., Addington W. 1987. Vitamin E deficiency and neurologic disease in adults with cystic fibrosis. Ann. Intern. Med. 107: 51–54. [DOI] [PubMed] [Google Scholar]
- 23.Papas K., Kalbfleisch J., Mohon R. 2007. Bioavailability of a novel, water-soluble vitamin E formulation in malabsorbing patients. Dig. Dis. Sci. 52: 347–352. [DOI] [PubMed] [Google Scholar]
- 24.Traber M. G., Thellman C. A., Rindler M. J., Kayden H. J. 1988. Uptake of intact TPGS (d-α- tocopheryl polyethylene glycol 1000 succinate) a water miscible form of vitamin E by human cells in vitro. Am. J. Clin. Nutr. 48: 605–611. [DOI] [PubMed] [Google Scholar]
- 25.Winklhofer-Roob B. M., Tuchschmid P. E., Molinari L., Shmerling D. H. 1996. Response to a single oral dose of all-rac-alpha-tocopheryl acetate in patients with cystic fibrosis and in healthy individuals. Am. J. Clin. Nutr. 63: 717–721. [DOI] [PubMed] [Google Scholar]
- 26.Winklhofer-Roob B. M., van't Hof M. A., Shmerling D. H. 1996. Long-term oral vitamin E supplementation in cystic fibrosis patients: RRR-alpha-tocopherol compared with all-rac-alpha-tocopheryl acetate preparations. Am. J. Clin. Nutr. 63: 722–728. [DOI] [PubMed] [Google Scholar]
- 27.Reboul E., Soayfane Z., Goncalves A., Cantiello M., Bott R., Nauze M., Terce F., Collet X., Comera C. 2012. Respective contributions of intestinal Niemann-Pick C1-like 1 and scavenger receptor class B type I to cholesterol and tocopherol uptake: in vivo v. in vitro studies. Br. J. Nutr. 107: 1296–1304. [DOI] [PubMed] [Google Scholar]
- 28.Narushima K., Takada T., Yamanashi Y., Suzuki H. 2008. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol. Pharmacol. 74: 42–49. [DOI] [PubMed] [Google Scholar]
- 29.Reboul E., Klein A., Bietrix F., Gleize B., Malezet-Desmoulins C., Schneider M., Margotat A., Lagrost L., Collet X., Borel P. 2006. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem. 28: 4739–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rader D. J., Brewer H. B., Jr 1993. Abetalipoproteinemia. New insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease. JAMA. 270: 865–869. [DOI] [PubMed] [Google Scholar]
- 31.Mars H., Lewis L. A., Robertson A. L., Butkus A., Williams G. H. 1969. Familial hypobetalipoproteinemia. A genetic disorder of lipid metabolism with nervous system involvement. Am. J. Med. 46: 886–900. [DOI] [PubMed] [Google Scholar]
- 32.Linton M. F., Farese R. V., Jr, Young S. G. 1993. Familial hypobetalipoproteinemia. J. Lipid Res. 34: 521–541. [PubMed] [Google Scholar]
- 33.Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. [DOI] [PubMed] [Google Scholar]
- 34.Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., Wetterau J. R. 2000. The role of the microsomal triglyceride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 20: 663–697. [DOI] [PubMed] [Google Scholar]
- 35.Homer V. M., George P. M., du Toit S., Davidson J. S., Wilson C. J. 2005. Mental retardation and ataxia due to normotriglyceridemic hypobetalipoproteinemia. Ann. Neurol. 58: 160–163. [DOI] [PubMed] [Google Scholar]
- 36.Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. 1981. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J. Clin. Invest. 67: 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traber M. G., Burton G. W., Hughes L., Ingold K. U., Hidaka H., Malloy M., Kane J., Hyams J., Kayden H. J. 1992. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. J. Lipid Res. 33: 1171–1182. [PubMed] [Google Scholar]
- 38.Anwar K., Iqbal J., Hussain M. M. 2007. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 48: 2028–2038. [DOI] [PubMed] [Google Scholar]
- 39.Kayden H. J., Hatam L. J., Traber M. G. 1983. The measurement of nanograms of tocopherol from needle aspiration biopsies of adipose tissue: normal and abetalipoproteinemic subjects. J. Lipid Res. 24: 652–656. [PubMed] [Google Scholar]
- 40.Traber M. G., Rader D., Acuff R., Brewer H. B., Kayden H. J. 1994. Discrimination between RRR- and all rac-α-tocopherols labeled with deuterium by patients with abetalipoproteinemia. Atherosclerosis. 108: 27–37. [DOI] [PubMed] [Google Scholar]
- 41.Aguie G. A., Rader D. J., Clavery V., Traber M. G., Torpier G., Kayden H. J., Fruchart J. C., Brewer H. B., Castro G. 1995. Lipoproteins containing apolipoprotein B in abetalipoproteinemia and homozygous hypobetalipoproteinemia. Identification and characterization. Atherosclerosis. 118: 183–191. [DOI] [PubMed] [Google Scholar]
- 42.Traber M. G., Kayden H. J. 1989. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am. J. Clin. Nutr. 49: 517–526. [DOI] [PubMed] [Google Scholar]
- 43.Traber M. G., Burton G. W., Ingold K. U., Kayden H. J. 1990. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J. Lipid Res. 31: 675–685. [PubMed] [Google Scholar]
- 44.Traber M. G., Kayden H. J., Green J. B., Green M. H. 1986. Absorption of water-miscible forms of vitamin E in a patient with cholestasis and in thoracic duct-cannulated rats. Am. J. Clin. Nutr. 44: 914–923. [DOI] [PubMed] [Google Scholar]
- 45.Tsuzuki W., Yunoki R., Yoshimura H. 2007. Intestinal epithelial cells absorb gamma-tocotrienol faster than alpha-tocopherol. Lipids. 42: 163–170. [DOI] [PubMed] [Google Scholar]
- 46.Abuasal B., Sylvester P. W., Kaddoumi A. 2010. Intestinal absorption of gamma-tocotrienol is mediated by Niemann-Pick C1-like 1: in situ rat intestinal perfusion studies. Drug Metab. Dispos. 38: 939–945. [DOI] [PubMed] [Google Scholar]
- 47.Abuasal B. S., Qosa H., Sylvester P. W., Kaddoumi A. 2012. Comparison of the intestinal absorption and bioavailability of gamma-tocotrienol and alpha-tocopherol: in vitro, in situ and in vivo studies. Biopharm. Drug Dispos. 33: 246–256. [DOI] [PubMed] [Google Scholar]
- 48.Abuasal B. S., Lucas C., Peyton B., Alayoubi A., Nazzal S., Sylvester P. W., Kaddoumi A. 2012. Enhancement of intestinal permeability utilizing solid lipid nanoparticles increases gamma-tocotrienol oral bioavailability. Lipids. 47: 461–469. [DOI] [PubMed] [Google Scholar]
- 49.Novotny J. A., Fadel J. G., Holstege D. M., Furr H. C., Clifford A. J. 2012. This kinetic, bioavailability, and metabolism study of RRR-alpha-tocopherol in healthy adults suggests lower intake requirements than previous estimates. J. Nutr. 142: 2105–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porsgaard T., Hoy C. E. 2000. Absorption by rats of tocopherols present in edible vegetable oils. Lipids. 35: 1073–1078. [DOI] [PubMed] [Google Scholar]
- 51.Kelleher J., Losowsky M. S. 1968. The absorption of vitamin E in man. Biochem. J. 110: 20P–21P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clifford A. J., de Moura F. F., Ho C. C., Chuang J. C., Follett J., Fadel J. G., Novotny J. A. 2006. A feasibility study quantifying in vivo human alpha-tocopherol metabolism. Am. J. Clin. Nutr. 84: 1430–1441. [DOI] [PubMed] [Google Scholar]
- 53.Chuang J. C., Matel H. D., Nambiar K. P., Kim S. H., Fadel J. G., Holstege D. M., Clifford A. J. 2011. Quantitation of [5–14CH3]-(2R, 4’R, 8’R)-alpha-tocopherol in humans. J. Nutr. 141: 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borel P., Mekki N., Boirie Y., Partier A., Grolier P., Alexandre-Gouabau M. C., Beaufrere B., Armand M., Lairon D., Azais-Braesco V. 1997. Postprandial chylomicron and plasma vitamin E responses in healthy older subjects compared with younger ones. Eur. J. Clin. Invest. 27: 812–821. [DOI] [PubMed] [Google Scholar]
- 55.Ouahchi K., Arita M., Kayden H., Hentati F., Ben Hamida M., Sokol R., Arai H., Inoue K., Mandel J. L., Koenig M. 1995. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat. Genet. 9: 141–145. [DOI] [PubMed] [Google Scholar]
- 56.Ben Hamida C., Doerflinger N., Belal S., Linder C., Reutenauer L., Dib C., Gyapay G., Bignal A., Le Paslier D., Cohen D., et al. 1993. Localization of Friedreich ataxia phenotype with selective vitamin E deficiency to chromosome 8q by homozygosity mapping. Nat. Genet. 5: 195–200. [DOI] [PubMed] [Google Scholar]
- 57.Di Donato I., Bianchi S., Federico A. 2010. Ataxia with vitamin E deficiency: update of molecular diagnosis. Neurol. Sci. 31: 511–515. [DOI] [PubMed] [Google Scholar]
- 58.Sato Y., Hagiwara K., Arai H., Inoue K. 1991. Purification and characterization of the alpha-tocopherol transfer protein from rat liver. FEBS Lett. 288: 41–45. [DOI] [PubMed] [Google Scholar]
- 59.Panagabko C., Morley S., Neely S., Lei H., Manor D., Atkinson J. 2002. Expression and refolding of recombinant human alpha-tocopherol transfer protein capable of specific alpha-tocopherol binding. Protein Expr. Purif. 24: 395–403. [DOI] [PubMed] [Google Scholar]
- 60.Traber M. G., Sokol R. J., Burton G. W., Ingold K. U., Papas A. M., Huffaker J. E., Kayden H. J. 1990. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J. Clin. Invest. 85: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traber M. G., Sokol R. J., Kohlschütter A., Yokota T., Muller D. P. R., Dufour R., Kayden H. J. 1993. Impaired discrimination between stereoisomers of a-tocopherol in patients with familial isolated vitamin E deficiency. J. Lipid Res. 34: 201–210. [PubMed] [Google Scholar]
- 62.Sato Y., Arai H., Miyata A., Tokita S., Yamamoto K., Tanabe T., Inoue K. 1993. Primary structure of alpha-tocopherol transfer protein from rat liver. Homology with cellular retinaldehyde-binding protein. J. Biol. Chem. 268: 17705–17710. [PubMed] [Google Scholar]
- 63.Qian J., Atkinson J., Manor D. 2006. Biochemical consequences of heritable mutations in the alpha-tocopherol transfer protein. Biochemistry. 45: 8236–8242. [DOI] [PubMed] [Google Scholar]
- 64.Morley S., Cross V., Cecchini M., Nava P., Atkinson J., Manor D. 2006. Utility of a fluorescent vitamin E analogue as a probe for tocopherol transfer protein activity. Biochemistry. 45: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian J., Morley S., Wilson K., Nava P., Atkinson J., Manor D. 2005. Intracellular trafficking of vitamin E in hepatocytes: role of tocopherol transfer protein. J. Lipid Res. 46: 2072–2082. [DOI] [PubMed] [Google Scholar]
- 66.Horiguchi M., Arita M., Kaempf-Rotzoll D. E., Tsujimoto M., Inoue K., Arai H. 2003. pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes. Genes Cells. 8: 789–800. [DOI] [PubMed] [Google Scholar]
- 67.Thakur V., Morley S., Manor D. 2010. Hepatic alpha-tocopherol transfer protein: ligand-induced protection from proteasomal degradation. Biochemistry. 49: 9339–9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morley S., Cecchini M., Zhang W., Virgulti A., Noy N., Atkinson J., Manor D. 2008. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J. Biol. Chem. 283: 17797–17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang W. X., Frahm G., Morley S., Manor D., Atkinson J. 2009. Effect of bilayer phospholipid composition and curvature on ligand transfer by the alpha-tocopherol transfer protein. Lipids. 44: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W. X., Thakur V., Lomize A., Pogozheva I., Panagabko C., Cecchini M., Baptist M., Morley S., Manor D., Atkinson J. 2011. The contribution of surface residues to membrane binding and ligand transfer by the alpha-tocopherol transfer protein (alpha-TTP). J. Mol. Biol. 405: 972–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Traber M. G., Burton G. W., Hamilton R. L. 2004. Vitamin E trafficking. Ann. N. Y. Acad. Sci. 1031: 1–12. [DOI] [PubMed] [Google Scholar]
- 72.Shichiri M., Takanezawa Y., Rotzoll D. E., Yoshida Y., Kokubu T., Ueda K., Tamai H., Arai H. 2010. ATP-Binding cassette transporter A1 is involved in hepatic alpha-tocopherol secretion. J. Nutr. Biochem. 21: 451–456. [DOI] [PubMed] [Google Scholar]
- 73.Oram J. F., Vaughan A. M., Stocker R. 2001. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J. Biol. Chem. 276: 39898–39902. [DOI] [PubMed] [Google Scholar]
- 74.Traber M. G., Rudel L. L., Burton G. W., Hughes L., Ingold K. U., Kayden H. J. 1990. Nascent VLDL from liver perfusions of cynomolgus monkeys are preferentially enriched in RRR- compared with SRR-α tocopherol: studies using deuterated tocopherols. J. Lipid Res. 31: 687–694. [PubMed] [Google Scholar]
- 75.Arita M., Nomura K., Arai H., Inoue K. 1997. alpha-Tocopherol transfer protein stimulates the secretion of alpha-tocopherol from a cultured liver cell line through a brefeldin A-insensitive pathway. Proc. Natl. Acad. Sci. USA. 94: 12437–12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parks E. J., Dare D., Frazier K. B., Hellerstein M. K., Neese R. A., Hughes E., Traber M. G. 2000. Dependence of plasma α-tocopherol flux on very low-density triglyceride clearance in humans. Free Radic. Biol. Med. 29: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 77.Bowry V. W., Stanley K. K., Stocker R. 1992. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. USA. 89: 10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan M., Cederbaum A. I., Zhang Y. L., Ginsberg H. N., Williams K. J., Fisher E. A. 2004. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Invest. 113: 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andreo U., Elkind J., Blachford C., Cederbaum A. I., Fisher E. A. 2011. Role of superoxide radical anion in the mechanism of apoB100 degradation induced by DHA in hepatic cells. FASEB J. 25: 3554–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zannis V. I., Chroni A., Krieger M. 2006. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J. Mol. Med. 84: 276–294. [DOI] [PubMed] [Google Scholar]
- 81.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clevidence B. A., Lehmann J. 1989. Alpha- and gamma-tocopherol levels in lipoproteins fractionated by affinity chromatography. Lipids. 24: 137–140. [DOI] [PubMed] [Google Scholar]
- 83.Bjorneboe A., Bjorneboe G. E., Drevon C. A. 1987. Serum half-life, distribution, hepatic uptake and biliary excretion of alpha-tocopherol in rats. Biochim. Biophys. Acta. 921: 175–181. [DOI] [PubMed] [Google Scholar]
- 84.Fairus S., Nor R. M., Cheng H. M., Sundram K. 2006. Postprandial metabolic fate of tocotrienol-rich vitamin E differs significantly from that of alpha-tocopherol. Am. J. Clin. Nutr. 84: 835–842. [DOI] [PubMed] [Google Scholar]
- 85.Fairus S., Nor R. M., Cheng H. M., Sundram K. 2012. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr. J. 11: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hermann W. J., Jr, Ward K., Faucett J. 1979. The effect of tocopherol on high-density lipoprotein cholesterol. A clinical observation. Am. J. Clin. Pathol. 72: 848–852. [DOI] [PubMed] [Google Scholar]
- 87.Aldred S., Sozzi T., Mudway I., Grant M. M., Neubert H., Kelly F. J., Griffiths H. R. 2006. Alpha tocopherol supplementation elevates plasma apolipoprotein A1 isoforms in normal healthy subjects. Proteomics. 6: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 88.Leonard S. W., Joss J. D., Mustacich D. J., Blatt D. H., Lee Y. S., Traber M. G. 2007. Effects of vitamin E on cholesterol levels of hypercholesterolemic patients receiving statins. Am. J. Health Syst. Pharm. 64: 2257–2266. [DOI] [PubMed] [Google Scholar]
- 89.Hatam L. J., Kayden H. J. 1981. The failure of α-tocopherol supplementation to alter the distribution of lipoprotein cholesterol in normal and hyperlipoproteinemic persons. Am. J. Clin. Pathol. 76: 122–126. [DOI] [PubMed] [Google Scholar]
- 90.Oram J. F., Lawn R. M. 2001. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 42: 1173–1179. [PubMed] [Google Scholar]
- 91.Reboul E., Trompier D., Moussa M., Klein A., Landrier J. F., Chimini G., Borel P. 2009. ATP-binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice. Am. J. Clin. Nutr. 89: 177–184. [DOI] [PubMed] [Google Scholar]
- 92.Jiang X. C., Jin W., Hussain M. 2012. The impact of phospholipid transfer protein (PLTP) on lipoprotein metabolism. Nutr. Metab. (Lond.) 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klerkx A. H., El Harchaoui K., van der Steeg W. A., Boekholdt S. M., Stroes E. S., Kastelein J. J., Kuivenhoven J. A. 2006. Cholesteryl ester transfer protein (CETP) inhibition beyond raising high-density lipoprotein cholesterol levels: pathways by which modulation of CETP activity may alter atherogenesis. Arterioscler. Thromb. Vasc. Biol. 26: 706–715. [DOI] [PubMed] [Google Scholar]
- 94.Schaefer E. J., Asztalos B. F. 2006. Cholesteryl ester transfer protein inhibition, high-density lipoprotein metabolism and heart disease risk reduction. Curr. Opin. Lipidol. 17: 394–398. [DOI] [PubMed] [Google Scholar]
- 95.Borel P., Moussa M., Reboul E., Lyan B., Defoort C., Vincent-Baudry S., Maillot M., Gastaldi M., Darmon M., Portugal H., et al. 2009. Human fasting plasma concentrations of vitamin E and carotenoids, and their association with genetic variants in apo C-III, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. Br. J. Nutr. 101: 680–687. [DOI] [PubMed] [Google Scholar]
- 96.Hacquebard M., Vandenbranden M., Malaisse W. J., Ruysschaert J. M., Deckelbaum R. J., Carpentier Y. A. 2008. Vitamin E transfer from lipid emulsions to plasma lipoproteins: mediation by multiple mechanisms. Lipids. 43: 663–671. [DOI] [PubMed] [Google Scholar]
- 97.Jiang X. C., Tall A. R., Qin S., Lin M., Schneider M., Lalanne F., Deckert V., Desrumaux C., Athias A., Witztum J. L., et al. 2002. Phospholipid transfer protein deficiency protects circulating lipoproteins from oxidation due to the enhanced accumulation of vitamin E. J. Biol. Chem. 277: 31850–31856. [DOI] [PubMed] [Google Scholar]
- 98.Desrumaux C., Deckert V., Lemaire-Ewing S., Mossiat C., Athias A., Vandroux D., Dumont L., Monier S., Pais de Barros J. P., Klein A., et al. 2010. Plasma phospholipid transfer protein deficiency in mice is associated with a reduced thrombotic response to acute intravascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 30: 2452–2457. [DOI] [PubMed] [Google Scholar]
- 99.Panagabko C., Morley S., Hernandez M., Cassolato P., Gordon H., Parsons R., Manor D., Atkinson J. 2003. Ligand specificity in the CRAL-TRIO protein family. Biochemistry. 42: 6467–6474. [DOI] [PubMed] [Google Scholar]
- 100.Shibata N., Arita M., Misaki Y., Dohmae N., Takio K., Ono T., Inoue K., Arai H. 2001. Supernatant protein factor, which stimulates the conversion of squalene to lanosterol, is a cytosolic squalene transfer protein and enhances cholesterol biosynthesis. Proc. Natl. Acad. Sci. USA. 98: 2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shibata N., Jishage K., Arita M., Watanabe M., Kawase Y., Nishikawa K., Natori Y., Inoue H., Shimano H., Yamada N., et al. 2006. Regulation of hepatic cholesterol synthesis by a novel protein (SPF) that accelerates cholesterol biosynthesis. FASEB J. 20: 2642–2644. [DOI] [PubMed] [Google Scholar]
- 102.Mariotti C., Gellera C., Rimoldi M., Mineri R., Uziel G., Zorzi G., Pareyson D., Piccolo G., Gambi D., Piacentini S., et al. 2004. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow-up and novel mutations in TTPA gene in Italian families. Neurol. Sci. 25: 130–137. [DOI] [PubMed] [Google Scholar]
- 103.Thurnham D. I., Davies J. A., Crump B. J., Situnayake R. D., Davis M. 1986. The use of different lipids to express serum tocopherol: lipid ratios for the measurement of vitamin E status. Ann. Clin. Biochem. 23: 514–520. [DOI] [PubMed] [Google Scholar]
- 104.Kayden H. J. 1983. Tocopherol content of adipose tissue from vitamin E deficient humans. In Biology of Vitamin E. R. Porter and J. Whelan, editors. Pittman Books, London. 70–91. [Google Scholar]
- 105.Sokol R. J., Heubi J. E., Iannaccone S. T., Bove K. E., Balistreri W. F. 1984. Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis. N. Engl. J. Med. 310: 1209–1212. [DOI] [PubMed] [Google Scholar]
- 106.Zhao Y., Lee M. J., Cheung C., Ju J. H., Chen Y. K., Liu B., Hu L. Q., Yang C. S. 2010. Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. J. Agric. Food Chem. 58: 4844–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brigelius-Flohé R., Traber M. G. 1999. Vitamin E: function and metabolism. FASEB J. 13: 1145–1155. [PubMed] [Google Scholar]
- 108.Eisengart A., Milhorat A. T., Simon E. J., Sundheim L. 1956. The metabolism of vitamin E. II. Purification and characterization of urinary metabolites of alpha-tocopherol. J. Biol. Chem. 221: 807–817. [PubMed] [Google Scholar]
- 109.Schultz M., Leist M., Petrzika M., Gassmann B., Brigelius-Flohé R. 1995. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 62(suppl.): 1527S–1534S. [DOI] [PubMed] [Google Scholar]
- 110.Li Y. J., Luo S. C., Lee Y. J., Lin F. J., Cheng C. C., Wein Y. S., Kuo Y. H., Huang C. J. 2008. Isolation and identification of alpha-CEHC sulfate in rat urine and an improved method for the determination of conjugated alpha-CEHC. J. Agric. Food Chem. 56: 11105–11113. [DOI] [PubMed] [Google Scholar]
- 111.Sharma G., Muller D., O'Riordan S., Bryan S., Hindmarsh P., Dattani M., Mills K. 2010. A novel method for the direct measurement of urinary conjugated metabolites of alpha-tocopherol and its use in diabetes. Mol. Nutr. Food Res. 54: 599–600. [DOI] [PubMed] [Google Scholar]
- 112.Traber M. G., Elsner A., Brigelius-Flohe R. 1998. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 437: 145–148. [DOI] [PubMed] [Google Scholar]
- 113.Swanson J. E., Ben R. N., Burton G. W., Parker R. S. 1999. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J. Lipid Res. 40: 665–671. [PubMed] [Google Scholar]
- 114.Birringer M., Pfluger P., Kluth D., Landes N., Brigelius-Flohe R. 2002. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 132: 3113–3118. [DOI] [PubMed] [Google Scholar]
- 115.Leonard S. W., Paterson E., Atkinson J. K., Ramakrishnan R., Cross C. E., Traber M. G. 2005. Studies in humans using deuterium-labeled α- and γ-tocopherol demonstrate faster plasma γ-tocopherol disappearance and greater γ-metabolite production. Free Radic. Biol. Med. 38: 857–866. [DOI] [PubMed] [Google Scholar]
- 116.Freiser H., Jiang Q. 2009. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J. Nutr. 139: 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel V., Khanna S., Roy S., Ezziddin O., Sen C. K. 2006. Natural vitamin E alpha-tocotrienol: retention in vital organs in response to long-term oral supplementation and withdrawal. Free Radic. Res. 40: 763–771. [DOI] [PubMed] [Google Scholar]
- 118.Uchida T., Abe C., Nomura S., Ichikawa T., Ikeda S. 2012. Tissue distribution of alpha- and gamma-tocotrienol and gamma-tocopherol in rats and interference with their accumulation by alpha-tocopherol. Lipids. 47: 129–139. [DOI] [PubMed] [Google Scholar]
- 119.Uchida T., Nomura S., Ichikawa T., Abe C., Ikeda S. 2011. Tissue distribution of vitamin E metabolites in rats after oral administration of tocopherol or tocotrienol. J. Nutr. Sci. Vitaminol. (Tokyo). 57: 326–332. [DOI] [PubMed] [Google Scholar]
- 120.Yap S. P., Yuen K. H., Wong J. W. 2001. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J. Pharm. Pharmacol. 53: 67–71. [DOI] [PubMed] [Google Scholar]
- 121.Sontag T. J., Parker R. S. 2002. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism: novel mechanism of regulation of vitamin E status. J. Biol. Chem. 277: 25290–25296. [DOI] [PubMed] [Google Scholar]
- 122.Sontag T. J., Parker R. S. 2007. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J. Lipid Res. 48: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 123.Ohnmacht S., Nava P., West R., Parker R., Atkinson J. 2008. Inhibition of oxidative metabolism of tocopherols with omega-N-heterocyclic derivatives of vitamin E. Bioorg. Med. Chem. 16: 7631–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bardowell S. A., Stec D. E., Parker R. S. 2010. Common variants of cytochrome P450 4F2 exhibit altered vitamin E-{omega}-hydroxylase specific activity. J. Nutr. 140: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Major J. M., Yu K., Wheeler W., Zhang H., Cornelis M. C., Wright M. E., Yeager M., Snyder K., Weinstein S. J., Mondul A., et al. 2011. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 20: 3876–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Major J. M., Yu K., Chung C. C., Weinstein S. J., Yeager M., Wheeler W., Snyder K., Wright M. E., Virtamo J., Chanock S., et al. 2012. Genome-wide association study identifies three common variants associated with serologic response to vitamin E supplementation in men. J. Nutr. 142: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bardowell S. A., Ding X., Parker R. S. 2012. Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism. J Lipid Res. 53: 2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bardowell S. A., Duan F., Manor D., Swanson J. E., Parker R. S. 2012. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. J. Biol. Chem. 287: 26077–26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Birringer M., Drogan D., Brigelius-Flohe R. 2001. Tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic. Biol. Med. 31: 226–232. [DOI] [PubMed] [Google Scholar]
- 130.Parker R. S., Sontag T. J., Swanson J. E. 2000. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 277: 531–534. [DOI] [PubMed] [Google Scholar]
- 131.Ikeda S., Tohyama T., Yamashita K. 2002. Dietary sesame seed and its lignans inhibit 2,7,8-trimethyl-2(2’-carboxyethyl)-6-hydroxychroman excretion into urine of rats fed gamma-tocopherol. J. Nutr. 132: 961–966. [DOI] [PubMed] [Google Scholar]
- 132.Kluth D., Landes N., Pfluger P., Muller-Schmehl K., Weiss K., Bumke-Vogt C., Ristow M., Brigelius-Flohe R. 2005. Modulation of Cyp3a11 mRNA expression by alpha-tocopherol but not gamma-tocotrienol in mice. Free Radic. Biol. Med. 38: 507–514. [DOI] [PubMed] [Google Scholar]
- 133.Traber M. G., Labut E. M., Leonard S. W., Lebold K. M. 2011. alpha-Tocopherol injections in rats up-regulate hepatic ABC transporters, but not cytochrome P450 enzymes. Free Radic. Biol. Med. 51: 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y. J., Shaw H. M. 2007. Pregnenolone and dexamethasone, modulators of cytochrome P450-3A, not increase but reduce urinary alpha-CEHC excretion in rats. Biofactors. 31: 67–76. [DOI] [PubMed] [Google Scholar]
- 135.Johnson C. H., Slanar O., Krausz K. W., Kang D. W., Patterson A. D., Kim J. H., Luecke H., Gonzalez F. J., Idle J. R. 2012. Novel metabolites and roles for alpha-tocopherol in humans and mice discovered by mass spectrometry-based metabolomics. Am. J. Clin. Nutr. 96: 818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mustacich D. J., Gohil K., Bruno R. S., Yan M., Leonard S. W., Ho E., Cross C. E., Traber M. G. 2009. Alpha-tocopherol modulates genes involved in hepatic xenobiotic pathways in mice. J. Nutr. Biochem. 20: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Traber M. G., Siddens L. K., Leonard S. W., Schock B., Gohil K., Krueger S. K., Cross C. E., Williams D. E. 2005. a-Tocopherol modulates Cyp3a expression, increases g-CEHC production and limits tissue g-tocopherol accumulation in mice fed high g-tocopherol diets. Free Radic. Biol. Med. 38: 773–785. [DOI] [PubMed] [Google Scholar]
- 138.Clarke M. W., Burnett J. R., Wu J. H., Hodgson J. M., Ledowski T., Puddey I. B., Croft K. D. 2009. Vitamin E supplementation and hepatic drug metabolism in humans. J. Cardiovasc. Pharmacol. 54: 491–496. [DOI] [PubMed] [Google Scholar]
- 139.Werba J. P., Cavalca V., Veglia F., Massironi P., De Franceschi M., Zingaro L., Tremoli E. 2007. A new compound-specific pleiotropic effect of statins: modification of plasma gamma-tocopherol levels. Atherosclerosis. 193: 229–233. [DOI] [PubMed] [Google Scholar]
- 140.Hsu M. H., Savas U., Griffin K. J., Johnson E. F. 2007. Human cytochrome p450 family 4 enzymes: function, genetic variation and regulation. Drug Metab. Rev. 39: 515–538. [DOI] [PubMed] [Google Scholar]
- 141.McDonald M. G., Rieder M. J., Nakano M., Hsia C. K., Rettie A. E. 2009. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 75: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Caldwell M. D., Awad T., Johnson J. A., Gage B. F., Falkowski M., Gardina P., Hubbard J., Turpaz Y., Langaee T. Y., Eby C., et al. 2008. CYP4F2 genetic variant alters required warfarin dose. Blood. 111: 4106–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lasker J. M., Chen W. B., Wolf I., Bloswick B. P., Wilson P. D., Powell P. K. 2000. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of CYP4F2 and CYP4A11. J. Biol. Chem. 275: 4118–4126. [DOI] [PubMed] [Google Scholar]
- 144.Hardwick J. P. 2008. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem. Pharmacol. 75: 2263–2275. [DOI] [PubMed] [Google Scholar]
- 145.Kikuta Y., Kusunose E., Kusunose M. 2000. Characterization of human liver leukotriene B(4) omega-hydroxylase P450 (CYP4F2). J. Biochem. 127: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 146.Kalsotra A., Strobel H. W. 2006. Cytochrome P450 4F subfamily: at the crossroads of eicosanoid and drug metabolism. Pharmacol. Ther. 112: 589–611. [DOI] [PubMed] [Google Scholar]
- 147.Munshi A., Sharma V., Kaul S., Al-Hazzani A., Alshatwi A. A., Shafi G., Koppula R., Mallemoggala S. B., Jyothy A. 2012. Association of 1347 G/A cytochrome P450 4F2 (CYP4F2) gene variant with hypertension and stroke. Mol. Biol. Rep. 39: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 148.Ding H., Cui G., Zhang L., Xu Y., Bao X., Tu Y., Wu B., Wang Q., Hui R., Wang W., et al. 2010. Association of common variants of CYP4A11 and CYP4F2 with stroke in the Han Chinese population. Pharmacogenet. Genomics. 20: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deng S., Zhu G., Liu F., Zhang H., Qin X., Li L., Zhiyi H. 2010. CYP4F2 gene V433M polymorphism is associated with ischemic stroke in the male Northern Chinese Han population. Prog. Neuropsychopharmacol. Biol. Psychiatry. 34: 664–668. [DOI] [PubMed] [Google Scholar]
- 150.Liu X., Wu J., Liu H., Lai G., Zhao Y. 2012. Disturbed ratio of renal 20-HETE/EETs is involved in androgen-induced hypertension in cytochrome P450 4F2 transgenic mice. Gene. 505: 352–359. [DOI] [PubMed] [Google Scholar]
- 151.Fava C., Montagnana M., Danese E., Sjogren M., Almgren P., Guidi G. C., Hedblad B., Engstrom G., Minuz P., Melander O. 2012. The functional variant V433M of the CYP4F2 and the metabolic syndrome in Swedes. Prostaglandins Other Lipid Mediat. 98: 31–36. [DOI] [PubMed] [Google Scholar]
- 152.Liu H., Zhao Y., Nie D., Shi J., Fu L., Li Y., Yu D., Lu J. 2008. Association of a functional cytochrome P450 4F2 haplotype with urinary 20-HETE and hypertension. J. Am. Soc. Nephrol. 19: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang Q., Freiser H., Wood K. V., Yin X. 2007. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J. Lipid Res. 48: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Freiser H., Jiang Q. 2009. Optimization of the enzymatic hydrolysis and analysis of plasma conjugated gamma-CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E. Anal. Biochem. 388: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mustacich D. J., Leonard S. W., Patel N. K., Traber M. G. 2010. Alpha-tocopherol beta-oxidation localized to rat liver mitochondria. Free Radic. Biol. Med. 48: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pope S. A., Burtin G. E., Clayton P. T., Madge D. J., Muller D. P. 2002. Synthesis and analysis of conjugates of the major vitamin E metabolite, alpha-CEHC. Free Radic. Biol. Med. 33: 807–817. [DOI] [PubMed] [Google Scholar]
- 157.Cho J. Y., Kang D. W., Ma X., Ahn S. H., Krausz K. W., Luecke H., Idle J. R., Gonzalez F. J. 2009. Metabolomics reveals a novel vitamin E metabolite and attenuated vitamin E metabolism upon PXR activation. J. Lipid Res. 50: 924–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hashiguchi T., Kurogi K., Sakakibara Y., Yamasaki M., Nishiyama K., Yasuda S., Liu M. C., Suiko M. 2011. Enzymatic sulfation of tocopherols and tocopherol metabolites by human cytosolic sulfotransferases. Biosci. Biotechnol. Biochem. 75: 1951–1956. [DOI] [PubMed] [Google Scholar]
- 159.Mustacich D. J., Leonard S. W., Devereaux M. W., Sokol R. J., Traber M. G. 2006. Alpha-tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Radic. Biol. Med. 41: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 160.Mustacich D. J., Shields J., Horton R. A., Brown M. K., Reed D. J. 1998. Biliary secretion of alpha-tocopherol and the role of the mdr2 P-glycoprotein in rats and mice. Arch. Biochem. Biophys. 350: 183–192. [DOI] [PubMed] [Google Scholar]
- 161.Radosavac D., Graf P., Polidori M. C., Sies H., Stahl W. 2002. Tocopherol metabolites 2, 5, 7, 8-tetramethyl-2-(2’-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and 2, 7, 8-trimethyl-2-(2’-carboxyethyl)-6-hydroxychroman (gamma-CEHC) in human serum after a single dose of natural vitamin E. Eur. J. Nutr. 41: 119–124. [DOI] [PubMed] [Google Scholar]
- 162.Bruno R. S., Leonard S. W., Li J., Bray T. M., Traber M. G. 2005. Lower plasma alpha-carboxyethyl-hydroxychroman after deuterium-labeled alpha-tocopherol supplementation suggests decreased vitamin E metabolism in smokers. Am. J. Clin. Nutr. 81: 1052–1059. [DOI] [PubMed] [Google Scholar]
- 163.Morinobu T., Yoshikawa S., Hamamura K., Tamai H. 2003. Measurement of vitamin E metabolites by high-performance liquid chromatography during high-dose administration of alpha-tocopherol. Eur. J. Clin. Nutr. 57: 410–414. [DOI] [PubMed] [Google Scholar]
- 164.Imai E., Tsuji T., Sano M., Fukuwatari T., Shibata K. 2011. Association between 24 hour urinary alpha-tocopherol catabolite, 2,5,7,8-tetramethyl-2(2’-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and alpha-tocopherol intake in intervention and cross-sectional studies. Asia Pac. J. Clin. Nutr. 20: 507–513. [PubMed] [Google Scholar]
- 165.Lebold K. M., Ang A., Traber M. G., Arab L. 2012. Urinary alpha-carboxyethyl hydroxychroman can be used as a predictor of alpha-tocopherol adequacy, as demonstrated in the Energetics Study. Am. J. Clin. Nutr. 96: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Glynn R. J., Ridker P. M., Goldhaber S. Z., Zee R. Y., Buring J. E. 2007. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women's Health Study. Circulation. 116: 1497–1503. [DOI] [PubMed] [Google Scholar]
- 167.Harrington D. J., Soper R., Edwards C., Savidge G. F., Hodges S. J., Shearer M. J. 2005. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J. Lipid Res. 46: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 168.Nakagawa K., Hirota Y., Sawada N., Yuge N., Watanabe M., Uchino Y., Okuda N., Shimomura Y., Suhara Y., Okano T. 2010. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 468: 117–121. [DOI] [PubMed] [Google Scholar]
- 169.Okano T., Shimomura Y., Yamane M., Suhara Y., Kamao M., Sugiura M., Nakagawa K. 2008. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 283: 11270–11279. [DOI] [PubMed] [Google Scholar]
- 170.Tovar A., Ameho C. K., Blumberg J. B., Peterson J. W., Smith D., Booth S. L. 2006. Extrahepatic tissue concentrations of vitamin K are lower in rats fed a high vitamin E diet. Nutrition & Metabolism. 3: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Farley S. M., Leonard S. W., Labut E. M., Raines H. F., Card D. J., Harrington D. J., Mustacich D. J., Traber M. G. 2012. Vitamin E decreases extra-hepatic menaquinone-4 concentrations in rats fed menadione or phylloquinone. Mol. Nutr. Food Res. 56: 912–922. [DOI] [PubMed] [Google Scholar]
- 172.Booth S. L., Golly I., Sacheck J. M., Roubenoff R., Dallal G. E., Hamada K., Blumberg J. B. 2004. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am. J. Clin. Nutr. 80: 143–148. [DOI] [PubMed] [Google Scholar]
- 173.Ulatowski L., Dreussi C., Noy N., Barnholtz-Sloan J., Klein E., Manor D. 2012. Expression of the alpha-tocopherol transfer protein gene is regulated by oxidative stress and common single-nucleotide polymorphisms. Free Radic. Biol. Med. 53: 2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dimitrov N. V., Meyer C., Gilliland D., Ruppenthal M., Chenoweth W., Malone W. 1991. Plasma tocopherol concentrations in response to supplemental vitamin E. Am. J. Clin. Nutr. 53: 723–729. [DOI] [PubMed] [Google Scholar]
- 175.Fuller C. J., Chandalia M., Garg A., Grundy S. M., Jialal I. 1996. RRR-alpha-tocopheryl acetate supplementation at pharmacologic doses decreases low-density-lipoprotein oxidative susceptibility but not protein glycation in patients with diabetes mellitus. Am. J. Clin. Nutr. 63: 753–759. [DOI] [PubMed] [Google Scholar]
- 176.Smith K. S., Lee C. L., Ridlington J. W., Leonard S. W., Devaraj S., Traber M. G. 2003. Vitamin E supplementation increases circulating vitamin E metabolites tenfold in end-stage renal disease patients. Lipids. 38: 813–819. [DOI] [PubMed] [Google Scholar]
- 177.Mustacich D. J., Vo A. T., Elias V. D., Payne K., Sullivan L., Leonard S. W., Traber M. G. 2007. Regulatory mechanisms to control tissue alpha-tocopherol. Free Radic. Biol. Med. 43: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Burton G. W., Ingold K. U. 1981. Autoxidation of biological molecules. I. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 103: 6472–6477. [Google Scholar]
- 179.Buettner G. R. 1993. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300: 535–543. [DOI] [PubMed] [Google Scholar]
- 180.Wang X., Thomas B., Sachdeva R., Arterburn L., Frye L., Hatcher P. G., Cornwell D. G., Ma J. 2006. Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc. Natl. Acad. Sci. USA. 103: 3604–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]