Abstract
The mechanisms regulating memory CD8+ T cell function and homeostasis during ageing are unclear. CD8+ effector memory T cells that re-express CD45RA (EMRA T cells) increase considerably in older humans and both ageing and persistent cytomegalovirus (CMV) infection are independent factors in this process. We used MHC class I tetrameric complexes that were mutated in the CD8 binding domain to identify CMV-specific CD8+ T cells with high antigen binding avidity. In individuals who were HLA-A*0201, CD8+ T cells that expressed CD45RA and were specific for the pp65 protein (NLV epitope) had lower avidity than those that expressed CD45RO and demonstrated decreased cytokine secretion and cytolytic potential after specific activation. Furthermore, low avidity NLV-specific CD8+ T cells were significantly increased in older individuals. The stimulation of blood leukocytes with CMV lysate induced high levels of IFNα that in turn induced IL-15 production. Moreover, the addition of IL-15 to CD45RA−CD45RO+ CMV-specific CD8+ T cells induced CD45RA expression while antigen activated cells remained CD45RO+. This raises the possibility that non-specific cytokine driven accumulation of CMV-specific CD8+ CD45RA+ T cells with lower antigen binding avidity may exacerbate the effects of viral re-activation on skewing the T cell repertoire in CMV infected individuals during ageing.
INTRODUCTION
The reduction in thymic output during ageing suggests that T cell memory has to be maintained by periodic proliferation of the pre-existing T cell pool in older individuals (1). However, the repeated episodes of T cell activation throughout life leads to phenotypic and functional differentiation towards an end-stage T cell that is associated with the loss of proliferative capacity (2). This process, known as replicative senescence, may arise from telomere erosion, oxidative damage to DNA as well as stress induced responses (1). However, despite their proliferative dysfunction, highly differentiated end-stage-like CD8+ T cells are increased in older individuals (3) possibly attributable to their relative resistance to apoptosis in vivo (4).
Multiple lines of evidence indicate that the presence of expanded populations of highly differentiated CD8+ T cells is detrimental to immunity. For example, mice that have large T cell expansions have greater disease severity after herpes simplex virus challenge in vivo (5). Also, aged rhesus monkeys harbour large expanded populations of T cells that are associated with poor responses to vaccinia vaccination (6). In humans, infection with CMV and the concurrent accumulation of CMV-specific T cells is detrimental to immunity for co-resident Epstein Barr Virus infection (7). In addition, the accumulation of large numbers of effector memory CD8+ T cells in CMV positive older humans is predictive of earlier mortality (8). Therefore, clarification of how expanded populations of highly differentiated T cells are generated and maintained in older humans and whether they are functionally competent is essential.
Highly differentiated T cells in both CD4+ and CD8+ compartments in humans can be identified by loss of the surface chemokine receptor CCR7 and/or the co-stimulatory molecules CD27 and CD28, and reduction of their telomere length (3, 9, 10). In addition, a highly differentiated (CCR7−, CD28−, CD27−) subset of effector memory T cells that are considered to be close to an end stage (3) can re-express the CD45RA molecule (EMRA) (11). This particular subset of T cells is considerably expanded during ageing and has characteristics of senescent T cells (2, 3, 12). Previous studies and also data included in the current report show that the increase in EMRA CD4+ and CD8+ T cells may also result from persistent CMV infection independent of age (12-14). However, the reason why CMV induces substantially greater numbers of EMRA T cells compared to other persistent viruses, such as Epstein-Barr virus and varicella zoster virus, is not clear, and the functional properties of this population in older humans are not known (15).
In this study we show that the expanded CMV-specific CD8+ T cell population specific for a HLA-A*0201 restricted epitope (NLV) of the immunodominant pp65CMV protein can show either high or low avidity, as identified by tetramers that have been mutated in their MHC binding domain for CD8 (16-18). This low avidity population accumulates in older subjects, preferentially expresses CD45RA and have reduced functional responses to antigen specific stimulation compared to their high avidity CD45RO expressing counterparts. Furthermore we found that CD45RA re-expression could be re-induced in CMV-specific CD8+CD45RO+ T cells by IL-15, but not TCR activation, suggesting that cytokine mediated homeostatic proliferation may be in part, a mechanism for the generation of EMRA T cells in vivo.
MATERIALS AND METHODS
Blood sample collection and PBMC isolation
Written informed consent was obtained and whole blood was collected in standard heparinised tubes from healthy volunteers. Young donors were between 18-35 years and old donors were between 65-95 years of age. The study was approved by the Local Research Ethics Committee of the Royal Free and University College Medical School. Donors did not have any co-morbidity, were not on any immunosuppressive drugs, and retained physical mobility and lifestyle independence. Peripheral Blood Mononuclear Cells (PBMCs) were isolated using Ficoll hypaque (Amersham Biosciences) and either analysed immediately or cyropreserved in 10% DMSO/FCS.
Determination of donor CMV status
The CMV status of donors was obtained by the overnight stimulation of fresh PBMCs with CMV viral lysate and identification of IFNγ production by CD4+ T cells as previously described (9). There was total concordance between IFNγ+ responses and seropositivity obtained from IgG serology obtained from the diagnostic laboratory of UCLH (9).
Flow Cytometric Analysis
PBMCs were analysed by flow cytometry using a combination of the following antibodies: CCR7 PE, CD3 APC, CD3 FITC, CD8 FITC, CD8 PerCP, CD8 APC-Cy7, CD11a PE, CD14 BD Horizon V500, CD16 FITC, CD19 PerCP, CD27 FITC, CD27 PE, CD28 PE-Cy7, CD45RA APC, CD45RO PE, CD56 ECD (Beckman Coulter, UK), HLA-DR eFluro 450 (eBioscience) and, IL-15 PE (all from BD biosciences, Oxford, UK unless stated). Occasionally cells were also stained with either Live/Dead Fixable Blue Dead Cell Stain (Invitrogen, Paisley, UK) or BD Via-Probe (BD Biosciences). Tetramer staining was conducted on HLA matched donor PBMCs for 20 mins at 37°C with either HLA-A*0201 (NLVPMVATV) or HLA-B*0701 (TPRVTGGGAM) CMVpp65-specific conventional tetramers or “null” tetramers that have been mutated in the α3 domain in the conserved binding site for CD8 as previously described (18). Conventional tetramers identify all specific CD8+ T cells that are able to bind specific peptide whilst the null tetramers identify CD8+ T cells that have high avidity for specific peptide without the need for CD8 binding. Therefore using conventional and null tetramers to identify CMVpp65-specific T cells in the same individuals, we can determine the proportion of these cells that have high avidity (19). All samples were fixed with 2% paraformaldehyde then acquired on either the BD FACS Calibur or BD LSR/LSRII using cell quest software or BD FACS Diva software respectively.
Functional activity
Multi-functional responses of CMVpp65-specific CD8+ T cells was performed by staining whole PBMCs initially with either conventional or high avidity HLA-A*0201 (NLVPMVATV) CMVpp65-specific tetramers and CD107a FITC (BD Biosciences) for 2hrs at 37°C. Titrated concentrations of CMVpp65 peptides (Proimmune, Oxford, UK) were added in the presence of monensin and brefeldin A for a further 4 hours at 37°C. Subsequently, PBMCs were stained intracellularly with IFNγ Pe-Cy7 or BD Horizon V450 and TNFα APC (BD Biosciences, Oxford, UK) via Caltag Fix and Perm kit in accordance with manufacturers guidelines.
Activation of CD8+ CD45RA+ and CD8+ CD45RO+ T cells
CD8 fraction of PBMCs was collected by magnetic bead separation (MACS; Miltenyi, Surrey, UK). The non-CD8 fraction was retained for use as antigen presenting cells (APCs) which were pulsed with either medium containing NLVPMVATV (NLV) peptide (Promimmune) or with medium only for 1hr at 37°C prior to irradiation (40-Gy gamma radiation). The CD8+ population was stained with CD45RA APC (Caltag, Buckingham, UK) and CD45RO FITC (Dako, Stockport, UK) and sorted to isolate CD45RA and CD45RO populations of >95% purity using a BD FACS Aria. 200,000 cells of each population were then placed in sterile FACs tubes with different ratios of irradiated peptide pulsed APCs. After 2hr incubation at 37°C, Brefeldin A was added and cells further incubated for 4hr. Subsequently PBMCs were stained for intracellular expression of IFNγ Pe-Cy7 or BD Horizon V450 (BD Biosciences, Oxford, UK).
Polyclonal activation of CD8+ T cells
Total PBMCs, or sorted CD45RA+ or CD45RO+ CD8+ T cells which were added to irradiated APCs (1:1 ratio), were activated with immobilised anti-CD3 (0.5μg/ml) or PMA/ ionomycin (both 0.5μg/ml). After 2hr incubation at 37°C, Brefeldin A was added and cells further incubated for 16hr at room temperature. PBMCs were then stained for CD8, CD27, CD45RA, and intracellular expression of IFNγ (BD Horizon V450, BD Biosciences) via Caltag Fix and Perm in accordance with manufacturers guidelines.
Measurement of IL-15 mRNA production following CMV lysate or cytokine stimulation
The mRNA levels of IL-6, IL-15, TNFα, and IFNα were measured in total PBMCs directly ex vivo and after 4hrs and 24hrs of culture in the presence CMV lysate from infected cells (1:10 of stock solution of 0.64mg/ml). Additionally, IL-15 mRNA levels were measured in total PBMCs before and after 24 hours of culture in the presence of either IFNα (500U/ml: Alpha 2a, 11100-1 from PBL Interferon source), IL-6 or TNFα (both 50ng/ml: R&D systems, USA). Cytokine mRNA was measured in different leukocyte subsets from PBMCs that were stimulated with CMV lysate (24 hours). T cell populations were isolated by CD3 MAC separation and the negative fraction (non-CD3+ cells) were then incubated with a cytokine cocktail (CD14 FITC, CD3 APC, CD56 ECD (Beckman Coulter, UK), HLA-DR e450 (ebiosciences), CD19 PE-Cy7 (eBioscience, UK), CD16 PE and UV live dead (Invitrogen, UK), all BD Biosciences unless stated) prior to sorting. Leukocyte populations were identified by: T cells (CD3+), NK cells (CD3−CD56+), B cells (HLA-DR+CD3−CD56−CD19+), monocytes (HLA-DR+CD3−CD56−CD19−CD16+/−CD14+/−) and DCs (HLA-DR+CD3−CD56− CD19−CD16−CD14−). Total RNA was purified with RNeasy columns (Qiagen, UK). Reverse transcription was performed with random primers using the MuLVRT reverse transcriptase (Invitrogen). The mRNA level of genes was determined by real-time quantitative PCR (RT-qPCRApplied Biosystems) with the TaqMan primers for IL-15 (Hs99999039_m1), IFNA2 (Hs02621172_s1), IL-6 (Hs00985639_m1) and TNFα (Hs01113624_g1). The housekeeping 18S mRNA was amplified from the same cDNA reaction mixture using the TaqMan primers (Hs03928985_g1*). Each sample was run in duplicate and target mRNA level was expressed as a ratio to the level of 18S to control for differing levels of cDNA in each sample.
Induction of CD45RA re-expression by IL-15
A CD8 positive fraction was collected from PBMCs by MACS then stained with: 1) anti-CD45RA APC (Caltag, Buckingham, UK) or; 2) anti-CD45RA APC and anti-CD27 FITC, and sorted using a BD FACS Aria. Cells were individually cultured at 100,000 cells per well with a single dose of IL-15 (10U; R&D Systems, Abingdon, UK) for up to 21 days. At different times, post IL-15 addition, cells were harvested, counted then stained with: 1) NLV/HLA-A*0201 tetramer followed by surface staining with CD45RA APC and CD45RO FITC or; 2) CD45RA APC and CD27 FITC for 30 minutes at 4°C, respectively. We incubated CD45RA−CD45RO+ cells with IL-2 instead of IL-15 as a control.
Statistical Analysis
Graph Pad Prism v5 was used to construct all graphs, dot plots and bar charts. The D’Agostino-Pearson omnibus K2 normality test was used to determine if data fitted a Gaussian (normal) distribution. Statistical significance was evaluated using the Student’s t test if data followed a normal distribution and if data was also paired it was assessed using a Student’s paired t test. Unpaired non-parametric data was evaluated using a Mann-Whitney U test, with a Wilcoxon matched paired test used if data points represented paired observations. All t-tests were two-tailed unless otherwise stated. Linear regression analysis was performed using GraphPad Prism to generate lines of best fit and correlations were assessed by Pearson and Spearman rank. All p-values ≤0.05 were regarded as significant.
RESULTS
CMV-specific EMRA CD8+ T cells increase during ageing
CMV infection and aging are associated with decreases in the naïve CD8+ T cell compartment and concomitant increases in the highly differentiated T cell pool (14, 20). CMV infection is also associated with large oligoclonal expansions of CD8+ T cells in older individuals (7, 20). Using MHC class I tetrameric complexes (tetramers) that are directed to epitopes of the immunodominant CMVpp65 protein in HLA-A*0201 (NLV) or HLA-B*0701 (TPR) positive individuals (representative results for NLV in one young and one old individual are shown in Fig. 1A) we observed an increase in CMVpp65 specific CD8+ T cells during ageing (Fig. 1B, p<0.01, R2=0.157) confirming previous reports (7, 14). When separated further on the basis of HLA-A*0201 (NLV) and HLA-B*0701 (TPR) specific responses the increase in CMV-specific CD8+ T cells during ageing was still observed (n=36, p<0.05, R2=0.134, and n=25, p<0.05, R2=0.227 respectively, Supplementary Fig. 1A).
Figure 1. CMV-specific CD8+ T EMRA cells accumulate during ageing.
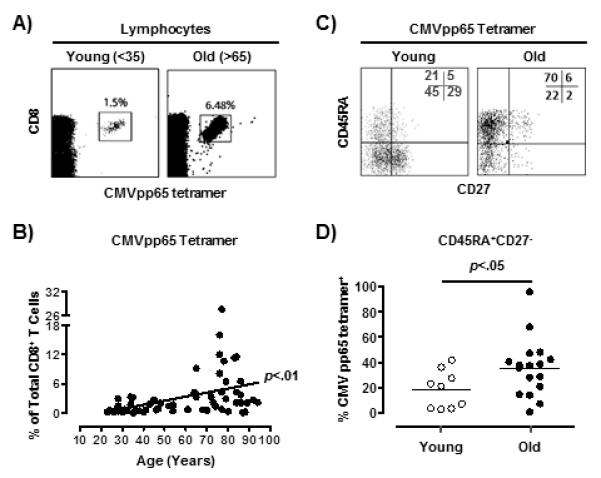
Phenotypic analysis of CMVpp65-specific CD8+ T cells. Representative dot plots showing the size of the CMVpp65-specific population as a percentage of total CD8+ T cells for one young and one old individual (A). Pooled data (n=61) showing the size of the CMVpp65-specific population as a percentage of total CD8+ T cells as stratified by age (B). Line of best fit was generated by linear regression and the correlation assessed by Pearson and Spearman rank (GraphPad Prism). Representative dot plots (one young and one old donor) of CMVpp65-specific CD8+ T cell CD45RA/CD27 subset distribution (C). Cumulative data comparing the percentage of CMVpp65-specific CD8+ T EMRA cells (CD45RA+CD27−) within the total CMV-specific compartment in different age groups (D). One dot represents one donor. Results from two-tailed paired t Test are shown.
We used the relative expression of the surface markers CD45RA and CD27 to define naïve (CD45RA+CD27+), central memory (CD45RA+CD27−), effector memory (CD45RA−CD27−) and CD45RA re-expressing effector memory populations (EMRA; CD45RA+CD27−) (21). We confirmed that there was an increase in CMVpp65-specific CD8+ EMRA T cells (NLV/HLA-A*0201 and TPR/HLA-B*0701) during ageing (representative staining shown in Fig. 1C , 1D), regardless of whether these specific T cells were defined on the basis of being CD27−CD45RA+ or CD45RA+CD45RO−, CD45RA+CCR7− or CD45RA+CD11ahi (Supplementary Fig. 1B) (21) . In addition we found that within the NLV/HLA-A*0201 specific CD8+ T cell population, <5% of EMRA cells populations expressed CD28 (data not shown), confirming that these cells exhibit a late differentiated phenotype (8, 22, 23).
CMV-specific CD8+ T cells with low antigen binding avidity accumulate in old HLA-A*0201+ individuals
Two tetramers directed towards the pp65 peptides NLV (HLA-A*0201) and TPR (HLA-B*0701) were generated using standard recombinant MHC molecules. In addition, NLV and TPR tetramers were generated using HLA-A*0201 or HLA-B*0701, respectively, with an altered α3 domain in the conserved binding site for CD8 (17-19). These mutations have been extensively investigated across a range of human and mouse MHC Class I molecules and consistently impact on CD8 binding but not TCR interactions (24-27). The alteration weakens CD8 co-receptor binding that normally stabilizes the interaction between peptide-MHC and the TCR. Binding to this mutated tetramer is therefore more reliant upon the ability of the TCR to bind peptide and hence requires high avidity of interaction (CD8-independence). Using the two types of tetramers (conventional and null), the size of the conventional (total) and high avidity CMVpp65-specific populations were compared in the same donor (Fig. 2A).
Figure 2. NLV/HLA-A*0201-CMV-specific T cells which accumulate during ageing have reduced avidity.
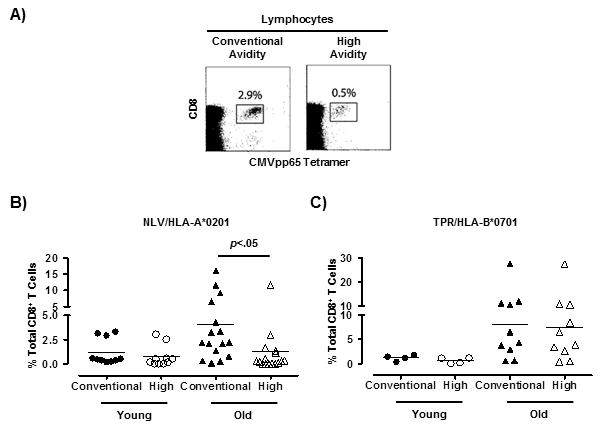
Relative quantification of conventional and high avidity CMVpp65-specific CD8+ T cells in young and old individuals using conventional and null tetramers. Representative example of one donor stained with either the conventional or high avidity CMVpp65-specific tetramers (A). Collective data on NLV/HLA-A*0201-specific (B) and TPR/HLA-B*0701-specific (C) conventional and high avidity populations as a percentage of total CD8+ T cells separated by age group (NLV; 10 young and 15 old donors: TPR; 4 young and 10 old donors). Results from two-tailed paired t Test are shown.
In the young subjects, the size of the conventional and high avidity NLV/HLA-A*0201 or TPR/HLA-B*0701 specific T cell populations were similar suggesting that most of the tetramer defined cells were of high avidity in this age group (Fig. 2B, 2C). However in older humans, there were significantly fewer high avidity (null tetramer positive) compared to conventional avidity CMV-specific CD8+ T cells in NLV/HLA-A*0201 in the same individuals but this was not observed in TPR/HLA-B*0701 subjects (Fig. 2B, 2C). This suggests that the NLV-specific cells that accumulate in older HLA-A*0201 positive individuals have reduced TCR binding avidity. In contrast, all TPR-specific cells that are identified by the conventional tetramer in HLA-B*0701 positive individuals appear to have high TCR binding avidity. However, in some individuals using just the conventional tetramer, we found TPR/HLA-B*0701 binding cells which had a high or low tetramer staining pattern (Supplementary Figure 2A, top 3 panels). Previous work on tumour antigens showed “low” avidity cells to be identified by lower tetramer staining intensity indicating that TPR/HLA-B*0701 specific CD8+ T cells with weak tetramer binding may be a population with low avidity (28). Therefore, cells with low avidity can also be identified in some HLA-B7*0101 positive individuals. Collectively, our results indicate that CMVpp65-specific CD8+ T cells with low avidity accumulate in some individuals during ageing and that this may be associated with HLA-type.
High avidity NLV/HLA-A*0201-specific CD8+ T cells have superior functional responses to challenge with specific antigen
The functional response profiles of the conventional and high avidity NLV/HLA-A*0201-specific CD8+ T cell populations were compared by measuring IFNγ, CD107a expression and TNFα production in the tetramer defined population after activation of total PBMCs with increasing doses of a CMVpp65 peptide pool (Fig. 3 and Supplementary Fig 3). As shown in Fig 2, some individuals showed equal binding of the conventional and null tetramers, indicating that all the cells were of high avidity, while others showed lower null compared to conventional tetramer binding, indicating that cells of low avidity were found in these subjects. When we investigated individuals that showed lower null vs conventional tetramer binding, we found that the high avidity (null tetramer binding) population expressed higher levels of IFNγ, CD107a and TNFα following activation compared with the conventional tetramer binding population that contains a mixture of high and low avidity cells (Fig. 3A representative staining, Fig. 3B dose response to peptide). This was confirmed in a second experiment (Supplementary Fig. 3A). In contrast, when we investigated participants that had equal proportions of null and conventional tetramer binding, the expression of IFNγ, CD107a and TNFα following peptide activation was similar in both null and conventional tetramer positive cells, (Fig 3C). This was confirmed in two further subjects who showed equal null and conventional tetramer binding (Supplementary Fig. 3B). These results collectively indicate that the presence of lower avidity NLV-specific CD8+ T cells is associated with decreased functionality of this population.
Figure 3. High avidity NLV/HLA-A*0201 T cells showed superior antigen-specific functional avidity.
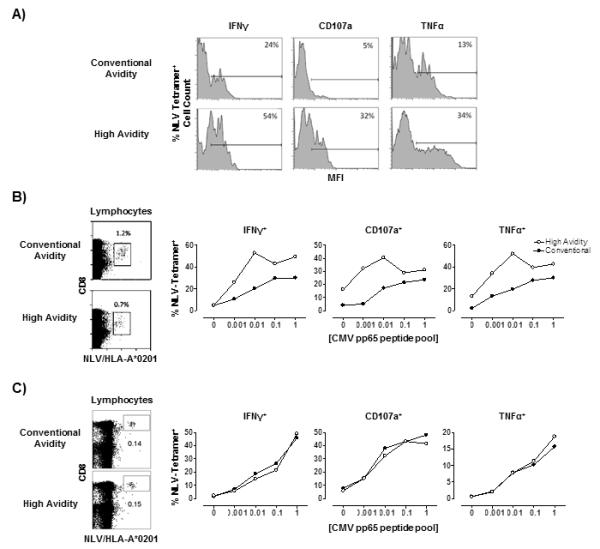
Representative histograms of relative levels of functional markers IFNγ, CD107a and TNFα following stimulation with 0.001μg CMVpp65 peptide are shown for the conventional avidity (top) or high avidity (bottom) NLV/HLA-A*0201-specific populations (A). Numbers shown represent per cent positivity by gating on isotype control. Representative data showing per cent of conventional (top) and null (bottom) tetramer binding cells within the total CD8 pool from an individual with a mixed NLV-specific population (not all tetramer binding cells are high avidity: B left panels; n=2). The functional readouts (IFNγ, CD107a and TNFα) following the stimulation of conventional (black dots) and high (white dots) avidity populations with CMVpp65 peptide concentrations at 0μg, 0.001μg, 0.01μg, 0.1, and 1μg/ml are shown (B; right 3 panels). Similar results were obtained in a second individual also with a mixed high and low avidity NLV-specific population (Supplementary Fig. 3A). Representative data from an individual that has all high avidity cells (same percentage of cells binding both null and conventional tetramer: C left panels). The functional readouts (IFNγ, CD107a and TNFα) following the stimulation of conventional (black dots) and high (white dots) avidity populations with different CMVpp65 peptide concentrations from the same individual is shown (Fig. 3 C, 3 left panels). Similar results were obtained in 2 additional subjects who had similar numbers of conventional and null (all high avidity) NLV-specific T cells (Supplementary Fig. 3B).
The phenotypic characteristics of high avidity CMVpp65-specific CD8+ T cells in HLA-A*0201 (NLV) individuals
We next investigated whether the high avidity CMV (NLV)-specific CD8+ T cells expressed CD45RA or CD45RO (Fig. 4). In a representative example, using the conventional tetramer, the characteristic CD45RA/CD45RO spread is observed in the CMV (NLV)-specific population and the specific cells predominantly express CD45RA (Fig. 4A, top and bottom left). However the high avidity CMV (NLV)-specific cells that were identified with the null tetramer in the same individual predominately expressed CD45RO (Fig. 4A, top and bottom right panels).
Figure 4. Low avidity NLV/HLA-A*0201-specific CD8+ T cells were mainly CD45RA+.
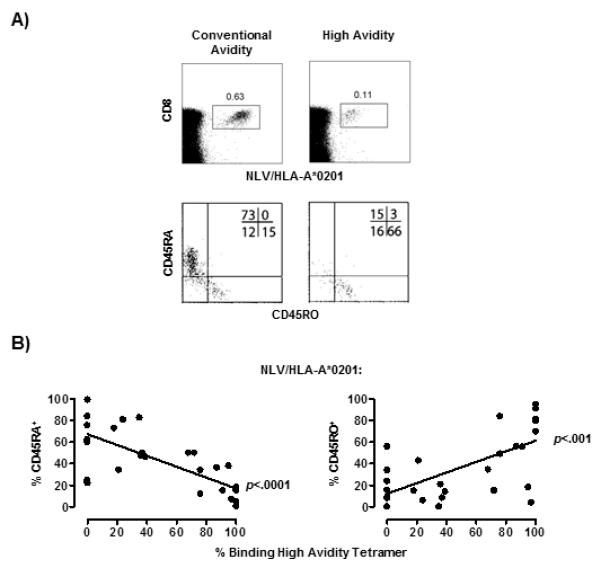
Representative example of one donor stained with either the conventional or high avidity NLV/HLA-A*0201 tetramer together with CD45RA and CD45RO antibodies (A). Collective data (n=29) showing correlation between per cent binding high avidity tetramer within the total NLV/HLA-A*0201 specific populations, with the percentage of cells expressing CD45RA (B, left) or CD45RO (B, right). Lines of best fit were generated by linear regression and the correlation assessed by Pearson and Spearman rank (GraphPad Prism).
We confirmed the observation that CD45RA expression was associated with lower avidity by staining PBMCs from the same individuals with either the conventional tetramer (to quantify CD45RA expression by both high and low avidity cells) or the null tetramer (to determine the percentage of high avidity cells within the NLV-specific population). We found that greater percentages of CD45RA+ tetramer+ positive T cells in the total tetramer pool was associated with a lower proportion of high avidity tetramer+ cells within the same pool (Fig. 4B, left panel; p<0.0001). Conversely, increased expression of CD45RO by the NLV-specific CD8+ T cells was associated with increased proportion of high avidity cells (Fig. 4B right panel).
When we investigated pp65 specific-CD8+ T cells in HLA-B*0701 (TPR) individuals using the conventional and null tetramer, the correlation between avidity and CD45RA/RO phenotype was not observed (both p>0.3, R2<0.09, Supplementary Fig. 2B). As described above, some HLA-B*0701 positive individuals had both high and low tetramer binding cells and we found that that high tetramer binding cells in these individuals were mainly CD45RO+ while the cells with low tetramer binding were CD45RA+ (Supplementary Fig. 2A, bottom 3 panels). Therefore, the association between CD45RA expression and lower avidity also applies to CMVpp65-specific (TPR) CD8+ T cells in some subjects who are HLA-B*0701.
NLV/HLA-A*0201-specific CD8+EMRA T cells have low functional responses to antigen
We investigated whether the lower avidity CD45RA+ NLV/HLA-A*0201-specific T cells had altered functional activity by comparing their response to stimulation with NLV peptide to their CD45RO counterparts in the same individuals (n=4). IFNγ production was used to determine the percentage of reacting cells, as this has been shown to be a better single marker of activation to CMV than TNFα or IL-2 (29). CD8+ T cells that had a mixed CD45RA and CD45RO phenotype were sorted into CD45RA and CD45RA populations (Fig. 5A top row). This individual also has CMV-specific (NLV) CD8+ T cells that were found before sorting and after sorting in the CD45RA or CD45RO populations (Fig. 5A bottom row).
Figure 5. NLV/HLA-A*0201-specific CD8+ CD45RA+ T cells showed poor response to antigen activation.
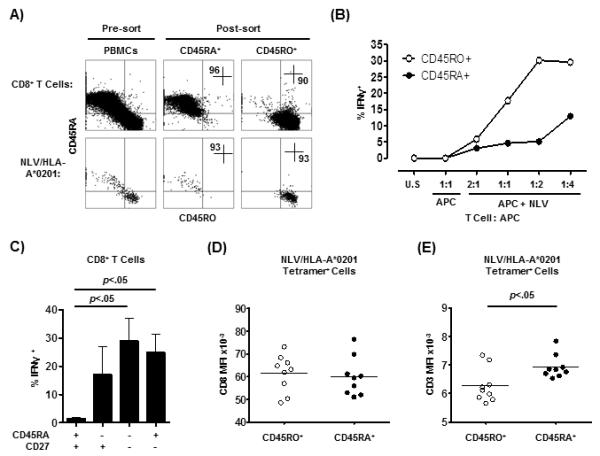
Isolated CD8+ T cells were further sorted into CD45RA+ and CD45RO+ populations (A). NLV/HLA-A*0201-specific cell were identified within the unfractionated or sorted CD8+ T cells using conventional tetramers (A, top row). Numbers represent percentages within the quadrants. The CD45RA+ and CD45RO+ CD8+ T cell populations were stimulated separately with un-pulsed APCs or increasing ratios of NLV peptide-pulsed APCs and the IFNγ response recorded (B). U.S. represents the un-stimulated control (containing no APCs). Three additional experiments showing similar results are shown in Supplementary Fig. 3C. PBMCs (n=7) were stimulated with anti-CD3 and the IFNγ responses of CD8+ T cell populations defined by relative expression of CD45RA v CD27 were measured (C). The levels of CD8 (D) and CD3 (E) co-receptor expression on the different NLV/CMVpp65-specific CD8+ T cell subsets was measured by flow cytometry (n=9). Results from two-tailed paired t Test are shown.
Stimulation with increasing numbers of APCs pulsed with NLV peptide induced a dose-dependent increase of IFNγ positive cells within the CD45RO population however the response in the NLV-stimulated CD45RA population was considerably lower (Fig. 5B and Supplementary Fig. 3C). Therefore CMV (NLV) T cells that express CD45RA have lower TCR avidity and also lower functional responsiveness to specific TCR stimulation by peptide compared to those that express CD45RO.
To determine whether the EMRA CD8+ T cell population was functionally deficient in general or only defective when responding to the CMV-specific peptide we activated PBMCs with a polyclonal stimulus (anti-CD3) and examined the capacity of CD8+ T cell subsets, identified by relative CD45RA and CD27 expression, to secrete IFNγ (Fig. 5C). We found that the EMRA population was very functional. We also ruled out the possibility that reduced avidity of CD45RA expressing CMV-specific T cells was due to lower expression of CD3 or CD8 by showing that the CD45RA+ NLV/pp65-specific CD8+ T cell population expressed similar levels of CD8 and greater level of CD3 (p<.05) compared to the CD45RO+ NLV/pp65-specific populations in the same individuals (Fig. 5D and E).
We also investigated whether NLV-specific CD45RA expressing T cells that have poor responses to specific peptide can respond to polyclonal stimulation. We stimulated CD8+ T cell populations that were sorted into CD45RA and CD45RO subsets with NLV peptide or either anti-CD3 or PMA/ionomycin and assessed IFNγ production by the NLV-specific cells (Supplementary Fig. 3D). We found that although the CD8+ EMRA T cells did not respond to NLV peptide compared to the CD45RO population (Supplementary Fig. 3C) the same cells responded to a similar extent to anti-CD3 or PMA/ionomycin stimulation as their CD45RO counterparts (Supplementary Fig. 3D) indicating that the CD45RA re-expressing T cells are not inherently functionally defective. Therefore the reduced response of the CMV-specific CD45RA expressing CD8+ T cells to peptide stimulation (NLV) is likely to be related to their reduced avidity for antigen.
CMV can induce IL-15 expression in monocytes indirectly via induction of IFNα secretion by DCs
Previous studies have shown that CMV induces the secretion of high levels of IFNα (9, 30) that in turn induces IL-15 (31) that leads to considerable bystander activation during certain viral infections (32). This together with the observation that the addition of IL-15 to cultured primed/memory CD45RO+ T cells without any antigenic stimulus can induce their re-expression of CD45RA (33-36), prompted us to test the hypothesis that IL-15 driven homeostatic proliferation could be a mechanism for the CMV infection induced increase in EMRA CD8+ T cells.
We confirmed that CMV lysate stimulated PBMC induced a high level of mRNA to IFNα that was also found previously at the protein level (Fig. 6A) (9). In addition, we also showed that IL-15 was induced in these cultures after 24 hours (Fig. 6A). We next showed that the addition of IFNα but not IL-6 or TNFα could induce the potent (>6-10 fold increase) production of IL-15 by PBMCs (Fig. 6B). In addition, when we isolated leucocyte subsets, we found that while IFNα production after CMV lysate stimulation was mainly produced by the dendritic cell population, IL-15 was mainly produced by monocytes (Mono; Fig 6C). Finally we showed a link between the secretion of IFNα and IL-15 by PBMCs after CMV lysate stimulation because the addition of an anti- type 1 IFNR Ab to CMV stimulated PBMCs partially blocked the increase in IL-15 mRNA by these cells (Fig. 6D).
Figure 6. CMV can induce IL-15 expression in monocytes indirectly via induction of IFNα secretion by DCs.
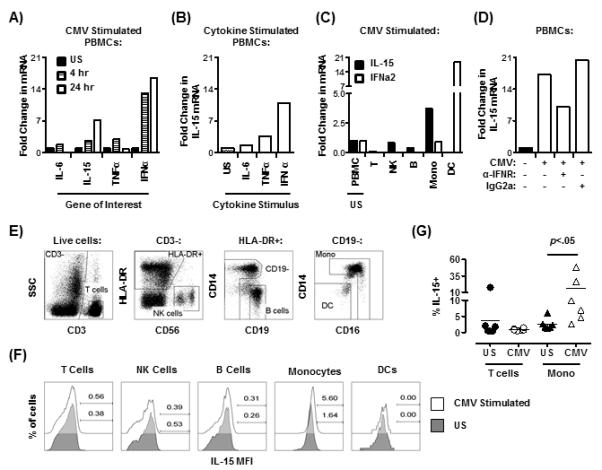
The production of IL-6, IL-15, TNFα and IFNα mRNA was determined by RT-PCR following treatment of total PBMCs with CMV lysate for the times indicated (A). IL-15 mRNA production in total PBMCs was determined following stimulation with either IL-6, TNFα (both 50ng/ml), or IFNα (500U/ml) (B). IL-15 and IFNα mRNA expression was measured in isolated CD3+ T cells (T), NK cells (NK), B cells (B), monocytes (Mono), and dendritic cells (DC) (C). IL-15 mRNA production in total PBMCs was determined following stimulation with CMV lysate (24 hours) with or without anti-IFN-1 receptor (α-IFNR) or isotype control (IgG2a) (D; representative of 2 experiments performed). Fold change is compared to unstimulated controls (US). All data is representative of n >3 unless stated. Representative flow cytometric analysis of one donor demonstrating gating to identify different leukocyte populations (E). Surface IL-15 expression by the different leukocyte populations with or without stimulation with CMV lysate for 24 hours (F). Numbers shown represent per cent positivity by gating relative to the isotype control. Cumulative data showing percentage of IL-15 expressing T cells and monocytes that were either unstimulated (US) or stimulated with CMV lysate for 24 hours (G). Results from Wilcoxon paired signed rank test are shown.
Two isoforms of IL-15 exist; a short signal peptide isoform which remains within the cytoplasm and has unknown function, and a long signal peptide isoform which can induce NK and T cell proliferation when transpresented on the cell surface to neighbouring cells, or if secreted (37, 38). The membrane bound form of IL-15 identifies the bioactive cytokine which is mainly involved in CD8 expansion (39). We therefore investigated whether the IL-15 that is induced at the transcriptional level in monocytes after CMV lysate stimulation is also detected at the protein level, on the surface of these cells by flow cytometry (Fig 6E). We stimulated PBMC with CMV lysate and investigated the expression of IL-15 in different leucocyte subsets gated as shown (Fig. 6E). We found that monocytes but not T, B, NK or dendritic cells showed membrane staining with this cytokine (representative experiment shown in Fig. 6E). In addition in 6 separate individuals, we found that monocytes but not T cells expressed surface IL-15 after CMV lysate stimulation (Fig. 6F). Since T cells express IL-15R yet do not show membrane staining for this cytokine, it is unlikely that the IL-15 observed on the surface of monocytes is due to the binding of secreted IL-15. This indicates that the CMV lysate stimulation can induce the expression of IL-15 at both the transcriptional and translational level.
IL-15 induces CMV-specific CD8+CD45RO+ T cells to re-express CD45RA in vitro
Next, CD8+ T cells were isolated from HLA-A*0201 positive donors (Fig. 7A). The cells were stained for both CD45RA and CD45RO, and NLV-specific cells were identified by staining with the CMV (NLV) tetramer (Fig. 7A, bottom left panel). Subsequently, we sorted CD45RA−CD45RO+ cells from the CD8 population and <2% of the total CD8 population and <1% of the CMV (NLV)-specific cells within this remaining population expressed CD45RA. IL-15 induced the expansion of CD8+ T cells but maintenance of the NLV specific population by day 14 (Supplementary Fig 4A) and induced CD45RA re-expression in both the total CD8+ CD45RO+ and CMV (NLV)-specific CD8+ CD45RO+ T cell subset (Fig. 7A). However, the CD45RA re-expressing cells also expressed CD45RO. In a previous study we showed that compared to IL-2 and IL-7, IL-15 induces greater levels of CD45RA re-expression on antigen specific cells (34). In contrast, when CMV (NLV)-specific CD8+ CD45RO+ T cell populations were stimulated with CMV peptide pulsed irradiated autologous APCs and IL-2, they proliferated, retained CD45RO expression but did not express CD45RA (Supplementary Figure 4B, top row). CMV (NLV)-specific CD8+CD45RA+ T cells also expanded and switched expression from CD45RA to CD45RO following TCR stimulation (Supplementary Figure 4B, bottom row).
Figure 7. IL-15 induces CD45RA expression.
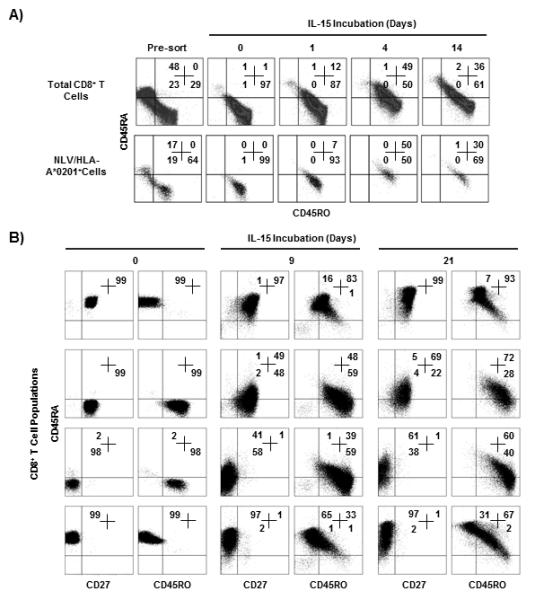
CD45RA−CD45RO+CD8+ T cells were isolated form a CMV seropositive donor and incubated with IL-15 (A). The CD45RA/CD45RO phenotype was determined at different times in the total CD8 population (top panels) or in the NLV-specific CD8+ T cells (bottom panels). Naïve (CD45RA+CD27+), central memory (CD45RA−CD27+), effector memory (CD45RA−CD27−) and EMRA (CD45RA+CD27−) CD8+ T cell subsets were isolated and their CD45RA/CD45RO phenotype was determined before (day 0) and after culture for different times with IL-15 (B). Numbers represent percentages in quadrants. Experiments shown are representative of three performed with very similar results.
IL-15 induces different populations of CD45RA re-expressing CD8+ T cells
The four CD8+ T cell population determined by CD45RA and CD27 expression (21) were cultured with IL-15 for 21 days (Fig 7B, representative example of three experiments.) This cytokine induced the expansion of all four subsets (~3 fold) however the kinetics of expansion was slower in the EMRA subset (Supplementary Fig. 4C). Both the naïve and the EMRA populations remained CD45RA+CD27+ and CD45RA+CD27− phenotype respectively in the presence of IL-15 however a large proportion of both populations now also expressed CD45RO (Fig. 7B). In the presence of IL-15, up to 69% of central memory CD8+ T cells now expressed CD45RA but these cells also expressed CD45RO (Fig 7B). Similarly, approximately 61% of CD45RA−CD27− effector memory T cells re-expressed CD45RA and became CD45RA+CD27− but these cells retained CD45RO expression. The level of CD45RA re-expression with IL-2 was between 2-25% of that induced by IL-15 (data not shown). Collectively this data shows that although IL-15 can induce the expression of CD45RA on CM and EM T cells, these cells retain CD45O expression and therefore are not identical to the CM and EM cells that are freshly isolated from peripheral blood in vivo.
DISCUSSION
Previous studies have identified CMV infection as a risk factor to survival in older humans (8, 13) possibly through the induction of large expanded populations of highly differentiated CD8+ T cells (7, 14). However, it is not clear how these expanded populations of T cells exert their negative influence. The main aims of the present study were to determine how highly differentiated CMV-specific CD8+ CD45RA+CD27− (EMRA) T cell populations are generated and whether they are functionally competent in older humans. These cells accumulate during ageing and are also CD28− and constitute part of the increased effector cell populations defined within the “immune risk phenotype” found in some older individuals in the OCTA/ NONA studies (8, 13).
Human CD45RA+ T cells that are activated by TCR ligation express CD45RO (40). This also applies to CD8+ EMRA T cells that are re-activated in vitro (4, 35, 41) raising the question of how CMV-specific CD8+ EMRA T cells are generated in vivo. It has been shown previously that IL-15 alone or in combination with IL-7 can induce total CD8+ T central memory cells and EBV-specific CD8+ T cells to re-express CD45RA (34-36). We now show that IL-15 induces the re-expression of CD45RA by CMV-specific CD8+CD45RO+CD45RA− T cells. However these cells retain CD45RO expression also. Therefore, although CD45RA−CD27− T cells re-express CD45RA after IL-15 treatment, they are not exactly the same as the EMRA population that is observed in vivo as they express CD45RO. Similarly the IL-15 induced re-expression of CD45RA by CD45RA−CD27+ populations does not equate them to freshly isolated naïve cells. However, a common observation of freshly isolated peripheral blood T cells is that large populations of CD8+ CD45RA+CD45RO+ T cells exist naturally and it is possible that these cells are in transition between phenotypically defined differentiation states. If indeed homeostatic cytokines like IL-15 can induce the generation of EMRA cells then additional factors that induce the loss of CD45RO while inducing the re-expression of CD45RA remain to be defined. A comparison of the transcriptional profiles between freshly isolated CD45RA+CD27+ cells and the similar cells induced by IL-15 treatment will be required to assess whether they are actually similar populations.
Although CMV-specific CD8+ T cells with lower avidity accumulate in older subjects, we have not tested directly if they also had reduced functionality. However, since CD8+ T cells with lower avidity also have decreased functional capacity and these cells increase in older HLA-A*0201 subjects, it is very likely that these cells in older subjects have decreased functionality. In support of this, Ouyang and Pawelec (42) and Hadrup et al (43) have already shown that CMVpp65 specific CD8+ T cells in older humans are dysfunctional in terms of IFNγ after specific stimulation compared to younger subjects.
An extensive study by Chidrawar and Moss (14) showed that the absolute number of CD8+ EMRA (CD45RA re-expressing memory) T cells increases during ageing and that CMV infection accentuates this increase. In the present study we show an increase in the percentage of CMVpp65 (conventional) tetramer positive cells that are CD45RA+ CD27− during ageing. Since this population contains the lower avidity cells, the data collectively suggests that the absolute number of lower avidity pp65 specific T cells accumulate in older subjects. Still it is likely that there are sufficient numbers of highly avid and functional CMV-specific T cells available to control viral reactivation during ageing since older CMV positive individuals do not experience increased pathology that is attributable to the virus. However it is possible that the association of CMV seropositivity and decreased health during ageing (8) may be due to the increase in low avidity cells that interfere with efficient responses to other pathogens.
A novel point in the present study is that we link IFNα secretion induced by CMV stimulation to IL-15 secretion and CD45RA re-expression by CD8+ T cells. This suggests that lifelong CMV re-activation may have a direct effect on specific T cells, where excessive differentiation through repeated stimulation occurs (9) but also a non-specific effect that may arise from excessive cytokine induced homeostatic proliferation. Bone-marrow derived granulocyte/ monocytes progenitor cells are a major site for CMV latency (44) and compelling evidence suggests that the bone marrow may host virus-specific memory T cells (45). This is particularly evident in older subjects who have increased levels of IL-15 present in the bone marrow (46). Further, T cells may be found in close proximity to IL-15 producing cells in the bone marrow (47). The CD8+ T cell expansion induced by IL-15 may be considerable and it is interesting to note that in macaques, the infusion of IL-15 led to a 100 fold expansion of effector memory CD8+ T cells (48) in vivo.
T cells recognizing the same antigen presented by MHC can vary in their sensitivity by several orders of magnitude (19) and it is the highly avid CD8+ T cells (49), with multi-functional potential (29), that are most efficient at controlling viruses. An important observation in this study was that CMVpp65-specific T cells that expressed CD45RA in individuals who were HLA-A*0201 had lower avidity and functionality than those that expressed CD45RO in the same individuals. This was less robust for individuals who were HLA-B*0701, suggesting that HLA type of the individual plays a role in determining the avidity and functionality of antigen specific CD8+ T cells consistent with previous reports (50, 51). Other HLA-A*0201 restricted CD8+CD45RA+ T cells that are specific for different CMV viral proteins may also have lower avidity and/or functional capacity than their CD45RO counterparts and a recent study in a limited number of participants identified a large population of IE-1 CMV-specific HLA-A*0201 restricted CD45RA+CD8+ T cell population with low TCR binding avidity (52).
Previous studies have shown that highly differentiated EMRA T cells in both the CD4 and CD8 compartments have characteristics of senescence including loss of CD28 expression (1, 2, 12, 20, 53). This suggests the intriguing possibility that homeostatic cytokines may induce senescence signalling pathways in T cells and may also give rise to CD8+ T cells with lower avidity. Despite exhibiting senescence, the EMRA population is not functionally deficient since they can secrete high levels of cytokines and mediate cytotoxic activity very efficiently in response to TCR stimulation (34, 53, 54). However, the CD45RA+ CD8+ T cells that are specific for the pp65 (NLV) epitope in individuals who are HLA-A*0201 positive do not secrete high levels of cytokines in response to antigen specific stimulation, probably reflecting their lower avidity for specific antigen rather than their inherent dysfunction. As high functional avidity is critically important for optimal pathogen clearance (19), the current data suggests that expansions of low avidity NLV/HLA-A*0201-specific CD8+ T cells would provide suboptimal immunity to CMV reactivation in vivo. However it is not clear if immunity to this protein is protective. It is possible that the removal of low avidity CMV-specific CD8+ T cells may increase the efficiency of the immune system in older CMV-infected humans, as suggested by studies in rodents (5). However, in practice this may prove difficult and costly in humans. However it is possible that targeting homeostatic cytokines to prevent non-specific T cell accumulation may counteract the negative effects of large expansions of effector T cells that have been shown to be detrimental in older humans.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Prof. Andy Sewell whose group developed the null tetramers that we used. We are also grateful to the blood donors who participated in this study.
1This work was funded by the British Biotechnology and Biological Sciences Research Council to ANA, the NIHR Biomedical Research Centre, Oxford, the Wellcome Trust and the Oxford Martin School to PK, and a Medical Research Council Capacity Building Studentship to SJG.
Abbreviations used
- APC
Antigen presenting cell
- CMV
cytomegalovirus
- EMRA
CD45RA re-expressing effector memory cells
- Mono
Monocytes
- NLV
NLVPMVATV
- TPR
TPRVTGGGAM
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 2.Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 3.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunne PJ, Faint JM, Gudgeon NH, Fletcher JM, Plunkett FJ, Soares MV, Hislop AD, Annels NE, Rickinson AB, Salmon M, Akbar AN. Epstein-Barr virus-specific CD8(+) T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–940. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- 5.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 8.Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 10.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, van Lier RA. Evidence that human CD8+CD45RA+CD27- cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 12.Libri V, Azevedo RI, Jackson SE, Di Mitri D, Lachmann R, Fuhrmann S, Vukmanovic-Stejic M, Yong K, Battistini L, Kern F, Soares MV, Akbar AN. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132:326–339. doi: 10.1111/j.1365-2567.2010.03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011 doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 16.Melenhorst JJ, Scheinberg P, Chattopadhyay PK, Lissina A, Gostick E, Cole DK, Wooldridge L, van den Berg HA, Bornstein E, Hensel NF, Douek DC, Roederer M, Sewell AK, Barrett AJ, Price DA. Detection of low avidity CD8(+) T cell populations with coreceptor-enhanced peptide-major histocompatibility complex class I tetramers. J Immunol Methods. 2008;338:31–39. doi: 10.1016/j.jim.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laugel B, van den Berg HA, Gostick E, Cole DK, Wooldridge L, Boulter J, Milicic A, Price DA, Sewell AK. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282:23799–23810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 18.Kasprowicz V, Ward SM, Turner A, Grammatikos A, Nolan BE, Lewis-Ximenez L, Sharp C, Woodruff J, Fleming VM, Sims S, Walker BD, Sewell AK, Lauer GM, Klenerman P. Defining the directionality and quality of influenza virus-specific CD8+ T cell cross-reactivity in individuals infected with hepatitis C virus. J Clin Invest. 2008;118:1143–1153. doi: 10.1172/JCI33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker LJ, Sewell AK, Klenerman P. T cell sensitivity and the outcome of viral infection. Clin Exp Immunol. 2010;159:245–255. doi: 10.1111/j.1365-2249.2009.04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pita-Lopez ML, Gayoso I, DelaRosa O, Casado JG, Alonso C, Munoz-Gomariz E, Tarazona R, Solana R. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun Ageing. 2009;6:11. doi: 10.1186/1742-4933-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 22.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantisan S, Torre-Cisneros J, Lara R, Zarraga S, Montejo M, Solana R. Impact of cytomegalovirus on early immunosenescence of CD8+ T lymphocytes after solid organ transplantation. J Gerontol A Biol Sci Med Sci. 2012;68:1–5. doi: 10.1093/gerona/gls130. [DOI] [PubMed] [Google Scholar]
- 24.Wooldridge L, van den Berg HA, Glick M, Gostick E, Laugel B, Hutchinson SL, Milicic A, Brenchley JM, Douek DC, Price DA, Sewell AK. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–27501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson SL, Wooldridge L, Tafuro S, Laugel B, Glick M, Boulter JM, Jakobsen BK, Price DA, Sewell AK. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–24293. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias MC, Almeida JR, Fastenackels S, van Bockel DJ, Hashimoto M, Venturi V, Gostick E, Urrutia A, Wooldridge L, Clement M, Gras S, Wilmann PG, Autran B, Moris A, Rossjohn J, Davenport MP, Takiguchi M, Brander C, Douek DC, Kelleher AD, Price DA, Appay V. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood. 2011;118:2138–2149. doi: 10.1182/blood-2011-01-328781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potter TA, Rajan TV, Dick RF, 2nd, Bluestone JA. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989;337:73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- 28.Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, Rimoldi D, Lienard D, Speiser D, Guillaume P, Batard P, Cerottini JC, Romero P, Valmori D. Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. [PubMed] [Google Scholar]
- 29.Kirchner A, Hoffmeister B, Cherepnev GG, Fuhrmann S, Streitz M, Lachmann R, Bunde T, Meij P, Schonemann C, Hetzer R, Lehmkuhl HB, Volkmer-Engert R, Volk HD, Gratama JW, Kern F. Dissection of the CMV specific T-cell response is required for optimized cardiac transplant monitoring. J Med Virol. 2008;80:1604–1614. doi: 10.1002/jmv.21229. [DOI] [PubMed] [Google Scholar]
- 30.Feldman SB, Ferraro M, Zheng HM, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral induction of low frequency interferon-alpha producing cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XH, Sun SQ, Hwang IK, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8(+) T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 32.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco J, Godelaine D, Van Pel A, Boon T, van der Bruggen P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood. 2006;108:2897–2905. doi: 10.1182/blood-2005-11-007237. [DOI] [PubMed] [Google Scholar]
- 34.Dunne PJ, Belaramani L, Fletcher JM, Fernandez de Mattos S, Lawrenz M, Soares MV, Rustin MH, Lam EW, Salmon M, Akbar AN. Quiescence and functional reprogramming of Epstein-Barr virus (EBV)-specific CD8+ T cells during persistent infection. Blood. 2005;106:558–565. doi: 10.1182/blood-2004-11-4469. [DOI] [PubMed] [Google Scholar]
- 35.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 36.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 37.Tagaya Y, Kurys G, Thies TA, Losi JM, Azimi N, Hanover JA, Bamford RN, Waldmann TA. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci U S A. 1997;94:14444–14449. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, Malavasi F, Ponzi AN, Paus R, Bulfone-Paus S. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- 39.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59:479–490. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 41.Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, Keyserling HL, Ploss A, Rice CM, Orenstein WA, Mulligan MJ, Ahmed R. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol. 2004;39:607–613. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 44.Prosch S, Docke WD, Reinke P, Volk HD, Kruger DH. Human cytomegalovirus reactivation in bone-marrow-derived granulocyte/monocyte progenitor cells and mature monocytes. Intervirology. 1999;42:308–313. doi: 10.1159/000053965. [DOI] [PubMed] [Google Scholar]
- 45.Palendira U, Chinn R, Raza W, Piper K, Pratt G, Machado L, Bell A, Khan N, Hislop AD, Steyn R, Rickinson AB, Buckley CD, Moss P. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112:3293–3302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- 46.Herndler-Brandstetter D, Landgraf K, Tzankov A, Jenewein B, Brunauer R, Laschober GT, Parson W, Kloss F, Gassner R, Lepperdinger G, Grubeck-Loebenstein B. The impact of aging on memory T cell phenotype and function in the human bone marrow. J Leukoc Biol. doi: 10.1189/jlb.0611299. [DOI] [PubMed] [Google Scholar]
- 47.Herndler-Brandstetter D, Landgraf K, Jenewein B, Tzankov A, Brunauer R, Brunner S, Parson W, Kloss F, Gassner R, Lepperdinger G, Grubeck-Loebenstein B. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol. 186:6965–6971. doi: 10.4049/jimmunol.1100243. [DOI] [PubMed] [Google Scholar]
- 48.Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, Lane HC. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. 2011;118:6845–6848. doi: 10.1182/blood-2011-09-377804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, Costagliola D, Rouzioux C, Agut H, Marcelin AG, Douek D, Autran B, Appay V. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacey SF, Villacres MC, La Rosa C, Wang Z, Longmate J, Martinez J, Brewer JC, Mekhoubad S, Maas R, Leedom JM, Forman SJ, Zaia JA, Diamond DJ. Relative dominance of HLA-B*07 restricted CD8+ T-lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum Immunol. 2003;64:440–452. doi: 10.1016/s0198-8859(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 51.Harari A, Cellerai C, Enders FB, Kostler J, Codarri L, Tapia G, Boyman O, Castro E, Gaudieri S, James I, John M, Wagner R, Mallal S, Pantaleo G. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc Natl Acad Sci U S A. 2007;104:16233–16238. doi: 10.1073/pnas.0707570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan N, Cobbold M, Cummerson J, Moss PA. Persistent viral infection in humans can drive high frequency low-affinity T-cell expansions. Immunology. 2010;131:537–548. doi: 10.1111/j.1365-2567.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lachmann R, Bajwa M, Vita S, Smith H, Cheek E, Akbar A, Kern F. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol. 2012;86:1001–1009. doi: 10.1128/JVI.00873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


