Abstract
In the present study, we show that higher levels of lymphocyte GH are expressed in spleen cells from aging animals compared to young animals. Further, leukocytes from primary and secondary immune tissues and splenic T and B cells from aging rats all express higher levels of GHRH receptors compared to younger animals. Bone marrow and splenic T cells express the highest levels of GHRH receptor in aging animals. Spleen cells from aging animals showed no significant change in proliferation or GH induction after treatment with GHRH. Taken together, the data for the first time show alterations in GH synthesis and expression of the GHRH receptor on cells of the immune system that may play a role in the immune response in aging.
Keywords: Growth hormone, GHRH receptors, lymphocytes, aging
1. Introduction
The consequences of immunosenescence show that aged individuals are less able to ward off bacterial, viral and fungal infections, have decreased responses to protective vaccines and higher incidences of autoimmune diseases and cancer [1]. Although the mechanisms are not entirely known, research on aged humans and mice have shown phenotypic and functional alterations in both the innate immune system and the humoral and cellular arms of the acquired immune response [2;3]. Aging is also accompanied by an impairment of the endocrine system. The growth hormone (GH)-axis is no exception where aging is associated with a significant decline in secretion of GH [4]. Old rats show lower pituitary GH mRNA, GH content, and GH-releasing hormone (GHRH) receptor [5]. Age-related reductions in GH secretion in rats and humans appear to result from a decrease in GHRH secretion [4;6–8]. Pituitary GH is a central player in IGF-1 induction and growth, tissue maintenance and repair, and also can improve a variety of immune functions, including B-cell responses and antibody production [9], NK activity [10], macrophage activity [11], and T cell function [10]. Serum GH and IGF-1 decrease with advancing age which, at least in part, may contribute to the age-related decline in immunocompetence [12;13].
It is apparent that non-pituitary sites and cell types also possess the ability to produce GH. The sites include the brain [14], mammary gland [15], placenta [16], skin [17], ovary [18], and cells of the immune system [19]. Our results in rodent spleen cells analyzing GH by mass spectrometry and Western analysis have shown that different molecular weight isoforms of GH can be detected in primary mouse spleen T and B cells [20]. In the mouse, we showed that GH isoforms could be induced by oxidative stress and that the larger molecular weight isoform appeared to reside primarily in the cytoplasm whereas the lower molecular weight isoform was primarily detected in the nucleus [20]. Most recently, in the rat we have shown that stressful cellular conditions likely to occur at sites of inflammation or tumor growth such as hypoxia and alterations in pH also induce the synthesis of lymphocyte GH [21]. The potential function of lymphocyte-derived GH in immunoregulation has been suggested for lymphocyte growth, survival, and cytokine production [22–26].
The evidence also supports the existence of the GHRH receptor (GHRH-R) in extrapituitary tissues including brain, spleen, thymus, ovary and renal medulla suggesting a physiological role(s) beyond the regulation of GH synthesis and secretion [27–32]. Additional human GHRH-R splice variants have been reported in several different cancers [33;34]. The major splice variant of the GHRH-R, named SV1, differs at a short portion of the extracellular portion and is fully functional [35]. Our studies in rats with thymus cell membranes showed two major bands for binding sites of GHRH at 43- and 27 kDa [31] compared to 65-, 47- and 28 kDa complexes in the rat pituitary [33]. In rat pituitary, two distinct classes of GHRH binding sites have been described. The first was of high affinity and low capacity while the second was of lower affinity and higher capacity [36]. There appears to be an age-related decrease in the number of high-affinity GHRH binding sites and an increase in the number of low affinity sites in pituitaries from 14-month old rats [37]. No significant change of GHRH binding affinity and capacity has been detected in aging renal medulla homogenates [38] and nothing is known about aging and GHRH binding sites on cells of the immune system.
Although much is known about the pituitary production of GH and the GHRH-R in the rat neuroendocrine system during aging, nothing is known, however, about the effects of aging on the abilities of cells of the murine immune system to produce GH. Previous studies with human peripheral blood lymphocytes (PBL) suggested no significant differences in lymphocyte GH production between young and elderly subjects [39], whereas aging was associated with a decline in lymphocyte GHRH expression [40]. In the present study, we found surprisingly that the levels of lymphocyte GH increased and the binding of GHRH also increased in aged animals compared to young controls. The alterations in lymphocyte GH synthesis and GHRH receptor expression on cells of the immune system during aging support the idea that GH may be important in the development and/or maintenance of a local immune response in an older animal.
2. Materials and methods
2.1 Cell preparations
Sprague-Dawley female rats were obtained from Harlan Laboratories (Prattville, AL) and B6 female mice from Jackson Laboratories. Animals were aged on site and sacrificed by cervical dislocation and their spleens, thymuses and bone marrow lymphocytes were placed into sterile plastic dishes containing RPMI 1640 medium and 20 mM Hepes (pH 7.4). Cell suspensions were prepared by teasing the tissues and washing two times in RPMI 1640 followed by centrifugation on a Ficoll-Hypaque density gradient as described to remove red blood cells [41]. Rat spleen cells were separated into T and B cell types by nylon wool chromatography [42]. The leukocytes (2 × 106 cells/ml) were suspended in RPMI 1640 supplemented with 10% fetal calf serum. Unless otherwise indicated, cell cultures were treated with hormones and/or chemicals for binding and incubated as described in the figure legends.
2.2 Binding of 125I-labeled GHRH
Binding assays were carried out in the presence of fixed 125I-labeled hGHRH (30,000 cpm, 15 nM) and cold GHRH1-29 (10−6 M–10−11 M) in a total volume of 0.2 ml. The incubations were carried out in microfuge tubes treated with Sigmacote in RPMI 1640 containing 1 × 106 cells in 25 mM Hepes (pH 7.4) and 0.1% BSA at 4°C for 60 min. At the end of the incubations, the cells were centrifuged in a Beckman 12 microfuge, pellets were washed three times in ice-cold buffer, and the tip of the tube was cut off and counted in a gamma-counter. Radioactivity not removed in the presence of an excess of unlabeled hGHRH (1–29) at 10 μM was considered nonspecific. Nonspecific binding was 12–17% of total cell binding.
2.3 Western blot analysis
Spleen cells were pelleted by centrifugation and resuspended in Tris/Triton-X lysis buffer, incubated on ice for 45 min and the supernatant harvested by centrifugation. Protein concentration was determined with the Bio-Rad protein assay reagent. The lysate was snap frozen and stored at −70°C until analyzed by Western blotting procedures as previously described [20]. Immunoreactive proteins were visualized using the ECL Western blotting analysis system. Film was scanned and analyzed using Scion Image Software (Scion Corp., Frederick, MD). Blotted membranes were stripped and reprobed with specific antibodies to actin. Densitometric analysis is represented graphically as the triplicate mean ratio of GH/actin with error bars representing the standard error of the mean (p<0.05).
2.4 RNA isolation, blotting and GH cDNA hybridization
Total RNA was prepared by homogenizing lymphocytes in 5 M guanidine thiocyanate with subsequent protease K digestion and extraction with phenol/chloroform. After ethanol precipitation, the RNA pellet was dried under vacuum and dissolved in sterile water. An aliquot was removed to determine the yield and purity by optical density measurements at 260 and 280 nm. Total RNA (10 μg) was blotted onto Gene Screen hybridization membranes with the manifold II slot blotter (Schleicher and Schuell, Inc., Keene, NH) as described by the manufacturers.
Plasmids containing specific rat [43] GH cDNA were kindly provided by Dr. John Baxter and Dr. Fran Denoto (Neurochemistry Laboratories, V.A. Medical Center, Sepulveda, CA). Plasmid DNA was prepared essentially as previously described, and 800 base-pair Hind III inserts were labeled with 32P-dCTP by nick translation to a specific activity of 1-2 × 108 cpm/μg as previously described [44;45]. Hybridization was performed as previously described [46]. After hybridization, the membranes were washed, and the nitrocellulose paper exposed to X-ray film at −70°C with Dupont Cronex Lightning-Plus intensifying screens for 2 to 3 days.
2.5 Proliferation studies
Spleen and thymus suspensions (2 × 106/ml) from 3-month and 12-month old female rats were prepared in RPMI 1640 supplemented with 1% Nutridoma-SP containing 100 U of penicillin and streptomycin per milliliter. These suspensions were incubated in the presence of [3H]thymidine (18h; 1 μC/ml, New England Nuclear) in plastic tissue culture dishes with and without GHRH for 18h at 37°C. The cultures were harvested using a Whittaker Mini-Mash Harvester and the glass fiber filters counted in a Tracor Analytic Model 6892 liquid scintillation system.
2.6 Chemicals and reagents
GHRH1-29 (G-0519) was obtained from Sigma and [125]I]-labeled hGHRH was purchased from Amersham. 32P-dCTP was purchased from New England Nuclear (Boston, MA). Goat GH antiserum (T-20, sc-10365) for detection of rat and mouse GH was purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Monoclonal anti-β-actin Ab (A5441), and all other chemicals were obtained at the highest grade from Sigma-Aldrich Corporation (St. Louis, MO).
2.7 Data analysis
Each experiment was repeated at least three times, and data are reported as mean ± standard error of the mean (SEM). Significant differences between various experimental treatment groups were determined by analysis of variance (ANOVA) and Student’s t-test. Densitometric analysis of the scanned images of Western blots was done using Scion Image Software (Scion Corporation, Frederick, MD). The results of binding studies were analyzed with GraphPad software to determine the binding parameters of the cells. Use of * in figures designates p ≤ 0.05.
3. Results
3.1 Effect of aging on GH expression in the spleen of aging mice and rats
Aging is associated with a significant decline in growth hormone secretion by the pituitary gland [4;47]; however, it is not known whether GH secretion also declines with increasing age in cells of the immune system. A representative experiment showing the expression of a single-sized band of GH by Western blot analysis of cell extracts from spleen cells of mice of different ages is shown in Figure 1. Unexpectedly, the level of GH increased significantly in 7- and 14-month old mice compared to the control of one month old animals. The influence of age on the expression of splenocyte GH was also investigated in 1- and 21-month old rats. Representative blots for three animals from a total of 30 rats per age group are shown in Figure 2. Interestingly, two GH bands are present at approximately 48- and 44 kDa in the rat spleen preparations. The data show that the levels of both bands of GH were also significantly higher (p<.01) in the 21-month old rats (Fig. 2). The influence of age on the expression of GH mRNA in rat spleens is shown in Figure 3. Since cells of the immune system do not contain a sufficient concentration of GH mRNA to routinely enable its detection by Northern blotting (data not shown), this experiment was performed by slot blot analysis. The data show that GH mRNA was significantly higher (p <0.05) in 14-month-old rats than that observed in one month old animals. Thus, spleen cells from aging mice and rats appear to produce greater GH mRNA and GH protein than young animals.
Fig. 1.
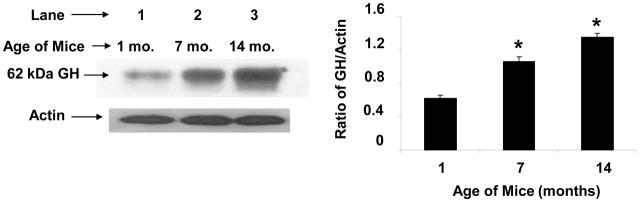
GH protein expression in spleen cells from B6 aging female mice. Cells were harvested from mice and cultured for 18 h after which whole cell extracts were prepared as described in the Methods. After SDS-PAGE (8%) and transfer to PVDF membranes, Western blot analysis was performed using commercial Ab to GH (Santa Cruz) and bands visualized using a chemiluminescence substrate for HRP. Blots were stripped and reprobed with specific Abs to actin. Asterisks (*) denote a significant difference (p<0.001) from the 1-month control. The approximate molecular weight for GH is shown with an arrow on the left. The results shown are typical of an experiment repeated three times. Key: lane 1 (1 month); lane 2 (7 month); lane 3 (14 month) age of mice as source of spleen cells.
Fig. 2.
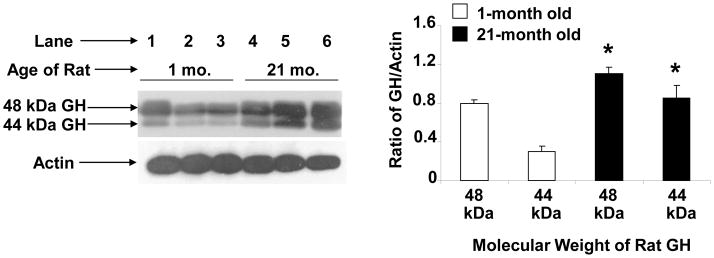
GH protein expression in spleen cells from aging female rats. Cells were harvested from three rats per age group and cultured for 18 h after which whole cell extracts were prepared and Western blot analysis performed as described in the Methods. Asterisks (*) denote a significant difference (p<0.01) between control (1 month) and 21-month-old rats for each molecular weight species. The approximate molecular weight for GH is shown with an arrow on the left. The results shown are typical of an experiment repeated three times. Key: lanes 1–3 (1 month); lanes 4–6 (21 month) old rats as the source of spleen cells.
Fig. 3.
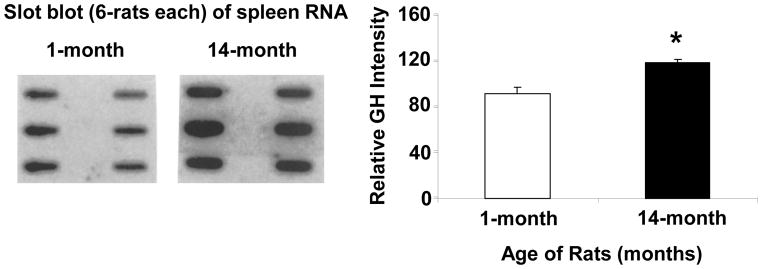
Cytoplasmic GH RNA expression in 1-month and 14-month old female rats. Spleen cells from six 1-month and six 14-month old individual rats were cultured (2 × 106/ml) for 24 h and harvested by centrifugation. Total cytoplasmic RNA isolated, slotted onto Gene Screen hybridization membranes, and probed as described under Materials and Methods. After a 48 h exposure, the autoradiograph was densitometrically scanned and graphed as percentage above the background as shown above. The results shown are typical of an experiment repeated three times. Asterisk (*) denotes a significant difference (p=0.0001) between 1- and 14-month old rats.
3.2 Characteristics of the GHRH binding in spleen and thymus cells of the aging rat
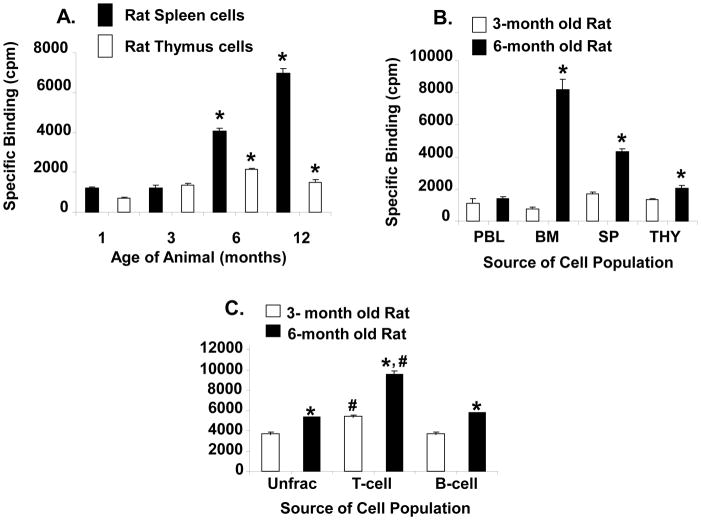
Since alterations of anterior pituitary GHRH binding site parameters were reported in aging rats [37], in the present study we decided to determine whether or not changes in the GHRH receptor were also taking place in lymphoid tissue. Previously, we showed that the binding of GHRH to spleen and thymic cells was saturable with a single high affinity binding site [31]. Homologous competitive binding assays were performed on spleen and thymus cells from 3- and 14-month old rats (Fig. 4). The data indicate no change of apparent binding affinity between spleen and thymus cells of 3- and 14-month old rats (Kd=2.0 nM spleen, Kd=1.0 nM thymus); however, the total number of binding sites was three times higher in the spleen of 14-month old rats (Fig. 4a) and 1.3 times higher in the thymus of 14-month old rats compared to 3-month old rats (Fig. 4b). The increase in total binding sites was significant by 6- and 12-months of age in the spleen (p=0.0001) (Fig. 5a), a finding that closely mirrors when alterations in binding parameters are first noticeable in rat pituitary homogenates [37]. Although the difference in GHRH binding was not as great in thymic tissues between young and old rats, a significant increase in the binding of GHRH in thymic tissue was also observed after 6 months of age (p=0.006) (Fig. 5a). Thus, both spleen and thymus cells from aging rats express more GHRH binding sites than young animals.
Fig. 4.
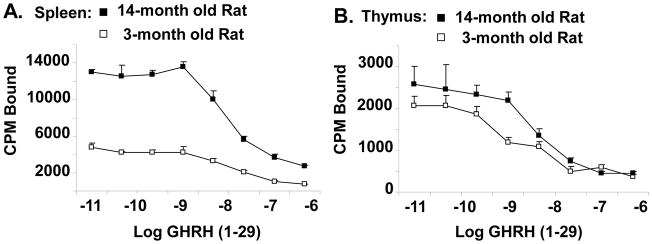
Homologous competitive binding curves for GHRH in 3- and 14-month old female rat spleen (A) and thymus cells (B). Leukocytes were obtained from the tissues by standard methods and washed in RPMI-1640 (pH 7.3). One x 106 cells in RPMI-1640 (pH 7.3) with 0.1% bovine serum albumin, 25 mM HEPES (binding buffer) were incubated (60 min at 4°C) in the presence of a fixed amount of 125I-labeled GHRH (34,461 cpm/tube, 1800 Ci/mmol) with and without various amounts (10−6 M to 10−11 M) of unlabeled GHRH (1–29). Incubated, labeled cells were washed with binding buffer (3 times) and free and bound ligand were separated by centrifugation. Cell-associated radioactivity was determined by a TM Analytical Gamma Counter. Each point represents the mean of three determinations ± SE of the mean.
Fig. 5.
Effect of age, source of immune tissue and T cell and B cell populations on GHRH binding in the spleen and thymus of female rats. The binding study was performed as described in the legend to figure 4. In (A), the cells were obtained from spleen and thymus tissues of 1-, 3-, 6- and 12-month old female rats. Values represent the mean ± SEM of specific binding from triplicate individual rats for each cell population at the various ages. In (B), the cells were obtained from peripheral blood lymphocytes (PBL), bone marrow (BM), spleen (SP), and thymus (Thy) tissues of young and old female rats. Values represent the mean ± SEM of triplicate individual rats for each specific cell population at 3- and 6-months of age. *p <0.0001 compared to binding levels of each specific cell population at 3-months of age. In (C), the cells for binding were obtained from the spleen of unfractionated (con) and nylon wool adherent (B-cell) and nylon wool nonadherent (T-cell) enriched cell populations of old female rats. Values represent the mean ± SEM of triplicate individual samples. T-cell and B-cell enriched cell populations show significantly (*p=0.002) increased GHRH receptor expression in 6-month old rats compared to control 3-month old rats. #, significantly (p<0.05) higher levels of GHRH receptors in T-cell enriched cell populations compared to B-cell enriched cell populations in both young and old rat spleen cells.
3.3 Distribution of GHRH receptors in different tissues and cell types in the immune system of aging rats
The expression of the GHRH receptor has been previously documented in various rat tissues, including the spleen and thymus [31;46], but has not been studied in other immune tissues or in aging rats. We, therefore, examined for the presence of GHRH receptors in both primary and secondary lymphoid tissues of aging rats. Thus, peripheral blood lymphocytes (PBL), bone marrow cells, splenocytes and thymic cells of 3- and 6-month old rats were isolated and compared for the presence of the GHRH receptor as described above. The data (Fig. 5b) show that the cells from each of the tissues contained detectable GHRH receptor which in each case, except for the PBL, contained significantly higher numbers of binding sites in the older animal (p=0.0001). In young animals, the presence of the GHRH receptors was most predominant on the spleen followed by the thymus, PBL, and bone marrow, whereas in the aged rat, the GHRH receptor appeared to be most abundant on bone marrow-derived cells followed by the spleen, thymus, and PBL.
In previous studies examining lymphocyte GH production by subpopulations of lymphocytes, we have shown that basal levels of lymphocyte GH mRNA and protein in primary B cells appeared to be significantly higher than the levels seen in primary T cells [46]. This observation was seen both in rats (unpublished observation) and mice [20]. We have also shown that greater levels of GH are induced in T cell-enriched populations compared to B cell-enriched populations after culture of cells under hypoxic conditions (unpublished observation). In order to determine if both T and B cell subpopulations of cells contain GHRH receptors and if they are altered in aging, nylon column enriched T and B cells were tested for GHRH receptors as described above (Fig. 5c). After separation, the T and B cells were judged to be approximately 87% and 63% pure, respectively, by mitogen stimulation and immunofluorescence [48]. The data show that T-cell enriched cell populations and B cell-enriched cell populations show significantly (p=0.0002) increased GHRH receptor expression (T-cell, 1.75-fold; B-cell, 1.6-fold) in 6-month old rats compared to control 3-month old rats. In addition, the data show higher levels of GHRH receptors in T-cell enriched cell populations compared to B-cell enriched cell populations in both young and old rat spleen cells (p<0.05).
3.4 Actions of GHRH on rat spleen and thymus cells in 3- and 12-month old rats
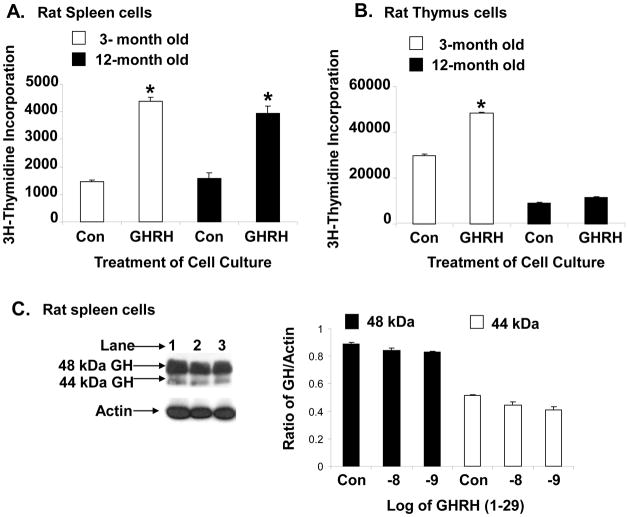
In vitro and in vivo studies have indicated that GHRH is predominately mitogenic in neoplasias and normal cells including lymphocytes [31;49]. Therefore, we investigated whether GHRH would stimulate proliferation in spleen cells obtained from aging rats compared to spleen cells from young rats. Spleen and thymic cells from 3- and 12-month old rats were cultured with GHRH (10−8 M) and 3H-thymidine and harvested 18h later to measure the extent of thymidine incorporation. The data in Figure 6a show that GHRH significantly stimulated thymidine incorporation in 3- and 12-month old spleen cells. Although the proliferation in aged (12-month) spleen cells treated with GHRH is reduced below the control (3-month) spleen cells treated with GHRH (3.0 to 2.1-fold), the data were not statistically significant. Thymic cell proliferation is significantly reduced in 12-month old rats in both control and GHRH-treated cell cultures. In thymic cells, the data were only significant in GHRH-treated 3-month old thymic cells compared to untreated cultures (Fig. 6b). In a separate study, we measured the levels of 44 and 48 kDa lymphocyte GH isoforms by Western blot analysis in cultured 12-month old spleen cells, and found no significant difference between untreated and GHRH-treated cell cultures (Fig. 6c).
Fig. 6.
Effect of age on GHRH-stimulated proliferation of spleen (A) and thymus cells (B) and spleen lymphocyte GH production (C) in female rats. For (A) and (B), cells (2 × 106/ml) were incubated with or without rat GHRH (10−8 M) for 18 h in RPMI containing 1% nutridoma with [3H] thymidine (1 μCi/ml), harvested by mash filtration, and counted as described. Each point represents the mean ± SE of six individual samples. *p <0.05 compared to thymidine incorporation without GHRH treatment at the same age. For (C), spleen cells were harvested from 12-month old rats and cultured (2 × 106/ml) for 18h with and without GHRH as shown in the figure. Whole cell extracts were prepared and Western blot analysis performed as described in the Methods. The results shown are typical of an experiment repeated three times. Kay: Lane 1 (control non-treated); lane 2, 10−8 M GHRH: lane 3, 10−9 M GHRH.
4. Discussion
Alterations in the pituitary gene expression of GH and the GHRH-receptor along with a decrease in hypothalamic GHRH are thought to be the major causes of age-related changes in GH secretion [4–8;47;50;51]. The main goal of the present study was to elucidate whether the production of GH and expression of the GHRH receptor decline similarly in cells of the immune system in the aging animal. We demonstrate here for the first time that rat and mouse GH protein increased in spleen cells from aged rats (Figs. 1–3). Our aging studies in rodents suggest the increase in lymphocyte GH in female rats may be occurring independent of dynamic changes in the GHRH receptor since treatment of cells with GHRH appeared to have no additional effect on the levels of GH (Fig. 6c). In the rat, isoforms of approximately 37, 44, and 48 kDa of lymphocyte GH can be detected where the predominant form is 48 kDa (unpublished observation) whereas in mice, the predominant GH isoform is approximately 62 kDa [20]. The increase in the level of a lower molecular weight GH isoform observed in old rats compared to younger animals could result from proteolysis while higher molecular weight isoforms may represent oligomers or the presence of different glycosylated forms. Additional studies are required to elucidate the exact nature and function of GH isoforms on immune function as well as determine whether similar GH isoforms exist in human lymphocytes. A previous study with human PBL suggested that the secretion of GH by both stimulated lymphocytes and monocytes was not significantly affected in the elderly [39]. Our findings of increased lymphocyte GH synthesis in rodents appear to differ from the studies in humans and suggest there may be significant species differences in lymphocyte GH synthesis in aging.
In this study, we also show for the first time GHRH receptor expression on different immune tissues (Fig. 5b) and on spleen T and B cells in the aging rodent immune system (Fig. 5c). Although no changes in Kd were observed, aging did result in almost a 10-fold increase in GHRH binding to bone marrow cells compared to spleen (2.5-fold), and thymus (1.5-fold). The higher increase in GHRH binding to the bone marrow cells of older animals may suggest a specific proliferative and/or developmental role for GHRH during the aging of bone marrow cells. The proliferation of aging spleen cells after treatment with GHRH was not significantly different from younger animals (Fig. 6a). Both T and B lymphocytes expressed GHRH receptor with relatively more T cells expressing the GHRH receptor in both young and older animals. It is known that T cell interactions with B cells in secondary lymphoid tissues, such as the spleen, provides help to B cells that influence the cytokine milieu and facilitate B cell survival, differentiation, and antibody production [52]. The results suggest the existence of distinct regulatory mechanism(s) of GHRH receptor expression in various immune tissues and between T- and B-cell types. Alterations in GHRH receptor protein production by cells of the aging murine immune system remain to be determined by Western blot analysis to confirm the increase in GHRH receptor expression in the various cell types and tissues.
In a recent work, we showed that GH production by cells of the immune system increases under conditions of hypoxia perhaps via a HIF-1 sensitive response element located in the GH promoter [21]. Aging, which is associated with declining tissue oxygenation, may then be associated with an increase in lymphocyte GH and regulation of the immune response. There is also strong evidence that oxygen limitation and/or activation of HIF-1 plays an important role in mammalian cellular senescence, possibly via a connection to ROS and mitochondrial respiration [53;54]. In this context, the substantial increase in GH production by T cells during hypoxia [21] may specifically increase T cell survival and/or enhance the ability of T cells to provide optimal help to B cells during a normal aging immune response. Thus, T cell overproduction or lack of GH production may play a role in certain autoimmune diseases or immunodeficiencies, respectively.
Taken together, the data suggest that the sensitivity of cells of the immune system to GHRH and their ability to increase basal levels of GH does not diminish with age. With aging and loss of the central supply of GH, the immune system may compensate by increasing the local production of GH and expression of receptors for GHRH to maintain a vigorous immune response. For example, it is known that enhanced local synthesis of estrogen in splenic T cells from animals after trauma enhances immunity [55]. Although compensation by lymphocyte GH for the dwindling supplies of peripheral GH appears possible, it may not be that simple. Dwarf mice which lack Pit-1 and pituitary GH show no compensation in GH production by cells of the immune system and in fact show a modest loss in the production of GH [56]. It appears other mechanisms may be in play in the remodeling of the immune system during aging that facilitate the increase in GH production by cells of the immune system. Also, the majority, if not all, of the lymphocyte GH produced by cells of the immune system normally appears to be retained within the cell. Although this secretion pattern may change in aging, the data suggest that the effect of the intracrine pathway action of lymphocyte GH in aging may be producer cell specific. In this regard, lymphocyte production of IGF-1 may also increase in aging since we showed in rat spleen cells the production of GH and IGF-1 by the same subpopulation of mononuclear leukocytes [57;58].
It is well known that the immune system and neuroendocrine system are linked and regulate each other (i.e., bidirectional communication) [59]. Efferent nerves of the sympathetic nervous system (SNS) innervate primary and secondary lymphoid organs and release noradrenaline (NA) and other neuropeptides into the lymphoid microenvironment [60]. Sympathetic NA innervation may be altered in the primary and secondary lymphoid organs of rats at early middle age concomitantly with age-related immunosuppression [61]. Evidence has also been provided that human lymphocyte GH may be involved in antigen-driven TH1 IFN-γ production and that physiologic levels of the stress-related hormones cortisol and NA inhibit lymphocyte GH production [24]. We have found in vitro that treatment of mouse spleen cells with propranolol increased the expression of lymphocyte GH (data not shown). In another study, the administration of GH has been shown to improve conduction velocities and nerve regeneration in a sectioned ulnar nerve model of the rat [62]. Thus, lymphocyte GH may be part of a mechanism responsible for slowing the decline of sympathetic innervation of lymphoid organs during aging at least in the spleen of rodents. On the other hand, it is possible that our data indicate a loss of childhood capacity in the control of lymphocyte GH rather than a compensation by cells of the immune system in aging. The inability to limit the expression of lymphocyte GH may contribute to undesirable consequences that promote a tumor phenotype [63]. It is also a well-established concept that, under physiological conditions, GH also may function to counteract the effects of stress [64;65]. On the one hand, the increase in GH may protect aging immune cells from apoptosis induced during inflammation and/or from oxidative stress. The increase in the levels of the GHRH receptor may promote differentiation, proliferation, wound healing as well as selected immune functions [27;34;35;66;67]. On the other hand, in malignant tissues, knowledge about the GH-axis in lymphocytes may provide valuable insights about how tumor cells may evade destruction. A more thorough understanding of this pathway may yield an important therapeutic strategy to manage immune responses to disease, injury, stress, and tumor formation in an aging adult.
Highlights.
Higher levels of lymphocyte GH are expressed in spleen cells from aging rats and mice than young animals.
Cells from immune tissues of old rats express higher levels of GHRH receptor than young rats.
Bone marrow and splenic T cells express the highest levels of GHRH receptor in aging animals.
Spleen cells from old rats showed no change in growth of GH induction after treatment with GHRH.
Acknowledgments
This work was supported by grants from the National Institute of Health of Neurology and Communicative Disorders (RO1 NS24636) and the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases (RO1 DK38024). The author is grateful for the support of Dr. J. Edwin Blalock, to the laboratory of Dr. Lori McMahon as the source of rat tissue and the laboratory of Dr. Hui Chen Hsu as the source of mouse tissue, to Dr. Carmel McNicholas for reading the manuscript, and Diane Weigent for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000;21:515–521. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 2.Solana R, Pawelec G. Molecular and cellular basis of immunosenescence. Mech Ageing Dev. 1998;102:115–129. doi: 10.1016/s0047-6374(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 3.Ponnappan S, Ponnappan U. Aging and immune function: Molecular mechanisms to interventions. Antioxidants Redox Signaling. 2010;14:1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocrin Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 5.San Frutos MG, Cacicedo L, Mendez CF, Vicent D, Gonzalez M, Sanchez-Franco F. Pituitary alterations involved in the decline of growth hormone gene expression in the pituitary of aging rats. J Gerontology. 2007;62A:585–597. doi: 10.1093/gerona/62.6.585. [DOI] [PubMed] [Google Scholar]
- 6.Sonntag WE, Meites J. Decline in GH secretion in aging animals and man. In: Everett AV, Walton JR, editors. Regulation of neuroendocrine aging. Karger; Basel: 1988. pp. 111–124. [Google Scholar]
- 7.Uberti ECD, Ambrosio MR, Cella SG, Margutti AR, Trasforini G, Rigamonti AE, Petrone E, Muller EE. Defective hypothalamic growth hormone (GH)-releasing hormone activity may contribute to declining GH secretion with age in man. J Clin Endocinol Metab. 1997;82:2885–2888. doi: 10.1210/jcem.82.9.4216. [DOI] [PubMed] [Google Scholar]
- 8.Russell-Aulet M, Jaffe CA, Demott-Friberg R, Barkan AL. In vivo semiquantification of hypothalamic growth hormone-releasing hormone (GHRH) output in humans: Evidence for relative GHRH deficiency in aging. J Clin Endocinol Metab. 1999;84:3490–3497. doi: 10.1210/jcem.84.10.6063. [DOI] [PubMed] [Google Scholar]
- 9.Kimata H, Yoshida A. Differential effect of growth hormone, insulin like growth facator 1, insulin-like growth factor 2 and insulin on Ig production and growth in human plasma cells. Blood. 1994;83:1569–1574. [PubMed] [Google Scholar]
- 10.Stephenson JR, Lee JM, Bailey N, Shepherd AG, Melling J. Adjuvant effect of human growth hormone with an inactivated flavivirus vaccine. J Infect Dis. 1991;164:188–191. doi: 10.1093/infdis/164.1.188. [DOI] [PubMed] [Google Scholar]
- 11.Edwards CK, Lorence RM, Dunham DM, Arkins S, Yunger LM, Greager JA, Walter RJ, Dantzer R, Kelley KW. Hypophysectomy inhibits the synthesis of tumor necrosis factor alpha by rat macrophages: partial restoration by exogenous growth hormone or interferon gamma. Endocrinology. 1991;128:989–986. doi: 10.1210/endo-128-2-989. [DOI] [PubMed] [Google Scholar]
- 12.Gelato MC. Aging and immune function: a possible role for growth hormone. Horm Res. 1996;45:38–45. doi: 10.1159/000184758. [DOI] [PubMed] [Google Scholar]
- 13.Kelley KW, Arkins S, Minshall C, Liu Q, Dantzer R. Growth hormone, growth factors and hematopoiesis. Horm Res. 1996;45:38–45. doi: 10.1159/000184757. [DOI] [PubMed] [Google Scholar]
- 14.Render CL, Hull KL, Harvey S. Neural expression of the pituitary GH gene. J Endocrinology. 1995;147:413–422. doi: 10.1677/joe.0.1470413. [DOI] [PubMed] [Google Scholar]
- 15.Mol JA, VanGardesen E, Seman PJ, Wolswinkel J, Rijinberk A, Rutterman GR. Growth hormone mRNA in mammary gland tumours of dogs and cats. J Clin Invest. 1995;95:2028–2034. doi: 10.1172/JCI117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogilvie S, Buhl WC, Olson JA, Shiverick KT. Identification of a novel family of growth hormone-related proteins secreted by rat placenta. Endocrinology. 1990;126:3271–3273. doi: 10.1210/endo-126-6-3271. [DOI] [PubMed] [Google Scholar]
- 17.Palmetshofer A, Zechner D, Luger TA, Barta A. Splicing variants of the human growth hormone mRNA: detection in pituitary, mononuclear cells and dermal fibroblasts. Mol Cell Endocrinol. 1995;113:225–234. doi: 10.1016/0303-7207(95)03633-i. [DOI] [PubMed] [Google Scholar]
- 18.Izadyar FZJ, Van Tol H, Colenbrander B, Bevers MM. Messenger RNA expression and protein localization of growth hormone in bovine ovarian tissue and in cumulus oocyte complexes (COCs) during in vitro maturation. Mol Reprod Dev. 1999;53:398–406. doi: 10.1002/(SICI)1098-2795(199908)53:4<398::AID-MRD5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Weigent DA, Blalock JE. Production of peptide hormones and neurotransmitters by the immune system. In: Blalock JE, editor. Neuroimmunoendocrinology. Karger; Basel: 1997. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 20.Weigent DA. High molecular weight isoforms of growth hormone in cells of the immune system. Cell Immunol. 2011;271:44–52. doi: 10.1016/j.cellimm.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigent DA. Hypoxia and cytoplasmic alkalinization upregulate growth hormone expression in lymphocytes. Cell Immunol. 2013 doi: 10.1016/j.cellimm.2013.03.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigent DA, Baxter JB, Wear WE, Smith LR, Bost KL, Blalock JE. Production of immunoreactive growth hormone by mononuclear leukocytes. FASEB J. 1988;2:2812–2818. doi: 10.1096/fasebj.2.12.3044906. [DOI] [PubMed] [Google Scholar]
- 23.Arnold RE, Weigent DA. The inhibition of apoptosis in EL4 lymphoma cells overexpressing growth hormone. Neuroimmunomodulation. 2004;11:149–159. doi: 10.1159/000076764. [DOI] [PubMed] [Google Scholar]
- 24.Malarkey WB, Wang J, Cheney C, Glaser R, Nagaraja H. Human lymphocyte growth hormone stimulates interferon gamma production and is inhibited by cortisol and norepinephrine. J Neuroimmunol. 2002;123:180–187. doi: 10.1016/s0165-5728(01)00489-1. [DOI] [PubMed] [Google Scholar]
- 25.Arnold RE, Weigent DA. The inhibition of superoxide production in EL4 lymphoma cells overexpressing growth hormone. Immunopharm Immunotoxicol. 2003;25:159–177. doi: 10.1081/iph-120020467. [DOI] [PubMed] [Google Scholar]
- 26.Weigent DA, Arnold RE. Expression of insulin-like growth factor-1 and insulin-like growth factor-1 receptors in EL4 lymphoma cells overexpressing growth hormone. Cell Immunol. 2005;234:54–66. doi: 10.1016/j.cellimm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ International union of pharmacology. XXXV. The glucagon receptor family. Pharm Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 28.Twery MJ, Moss RL. Sensitivity of the forebrain neurons to growth hormone-releasing hormone. Peptides. 1985;6:609–613. doi: 10.1016/0196-9781(85)90161-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Jr, Obal F, Zheng T, Fang J, Taishi P, Krueger JM. Intrapreoptic microinjection of GHRH or its antagonist alters sleep in rats. J Neurosci. 1999;19:2187–2194. doi: 10.1523/JNEUROSCI.19-06-02187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagnato A, Moretti C, Ornishi J, Frajese G, Catt KJ. Expression of the growth hormone-releasing hormone gene and its peptide product in the rat ovary. Endocrinology. 1992;130:1097–1102. doi: 10.1210/endo.130.3.1537276. [DOI] [PubMed] [Google Scholar]
- 31.Guarcello V, Weigent DA, Blalock JE. Growth hormone releasing hormone receptors on thymocytes and splenocytes from rats. Cell Immunol. 1991;136:291–302. doi: 10.1016/0008-8749(91)90353-d. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J. Differential gene expression of growth hormone (GH)-releasing hormone (GRH) and GRH receptor in various rat tissues. Endocrinology. 1995;136:4147–4150. doi: 10.1210/endo.136.9.7649123. [DOI] [PubMed] [Google Scholar]
- 33.Halmos G, Schally AV, Vargas JL, Plonowski A, Rekasi Z, Czompoly T. Human renal cell carcinoma expresses distinct binding sites for growth hormone-releasing hormone. Proc Natl Acad Sci USA. 2000;97:10555–10560. doi: 10.1073/pnas.180313097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halmos G, Schally AV, Czompoly T, Krupa M, Varga JL, Rekasi Z. Expression of growth hormone-releasing hormone and its receptor splice variants in human prostate cancer. J Clin Endocinol Metab. 2002;87:4707–4714. doi: 10.1210/jc.2002-020347. [DOI] [PubMed] [Google Scholar]
- 35.Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 36.Abribat T, Boulanger L, Gaudreau P. Characterization of [25K-Tyr10]human growth hormone-releasing factor (1–44)amide binding to rat pituitary: evidence for high and low affinity classes of sites. Brain Res. 1990;528:291–299. doi: 10.1016/0006-8993(90)91670-c. [DOI] [PubMed] [Google Scholar]
- 37.Abribat T, Deslauriers N, Brazeau P, Gaudreau P. Alterations of pituitary growth hormone-releasing factors binding sites in aging rats. Endocrinology. 1991;128:633–635. doi: 10.1210/endo-128-1-633. [DOI] [PubMed] [Google Scholar]
- 38.Boulanger L, Girard N, Strecko J, Gaudreau P. Characterization of a growth hormone-releasing hormone binding site in the rat renal medulla. Peptides. 2002;23:1187–1194. doi: 10.1016/s0196-9781(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 39.Luz C, Collaziol D, Preissler T, da Cruz IM, Glock L, Bauer ME. Healthy aging is associated with unaltered production of immunoreactive growth hormone but impaired neuroimmunomodulation. Neuroimmunomodulation. 2006;13:160–169. doi: 10.1159/000097535. [DOI] [PubMed] [Google Scholar]
- 40.Khorram O, Garthwaite M, Golos T. The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system. J Clin Endocinol Metab. 2001;86:3157–3161. doi: 10.1210/jcem.86.7.7652. [DOI] [PubMed] [Google Scholar]
- 41.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 2012;21:77–82. [PubMed] [Google Scholar]
- 42.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 43.Seeburg PH, Shine J, Martial J, Baxter JD, Goodman HM. Nucleotide sequence and amplification in bacteria of a structural gene to rat GH. Nature. 1977;270:486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- 44.Martial JA, Hallewell RA, Baxter JD, Goodman HM. Human growth hormone: Complementary DNA cloning and expression in bacteria. Science. 1979;205:602–607. doi: 10.1126/science.377496. [DOI] [PubMed] [Google Scholar]
- 45.Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1982. [Google Scholar]
- 46.Weigent DA, Blalock JE. The production of growth hormone by subpopulations of rat mononuclear leukocytes. Cell Immunol. 1991;135:55–65. doi: 10.1016/0008-8749(91)90253-8. [DOI] [PubMed] [Google Scholar]
- 47.Rosen CJ. Growth hormone and aging. Endocrine. 2000;12:197–201. doi: 10.1385/ENDO:12:2:197. [DOI] [PubMed] [Google Scholar]
- 48.Weigent DA, Langford MP, Stanton GJ, Blalock JE. Interferon-induced transfer of viral resistance by human B and T lymphocytes. Cell Immunol. 1984;87:678–683. doi: 10.1016/0008-8749(84)90035-2. [DOI] [PubMed] [Google Scholar]
- 49.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: not only a neurohormone. Trends Endocrinol Metab. 2011;8:311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Sonntag WE, Steger RW, Fermen LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980;107:1875–1879. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- 51.Chapman IM, Hartman ML, Pezzoli SS, Jr, Harrell FE, Hintz RL, Alberti KGMM, Thorner MO. Effect of aging on the sensitivity of growth hormone secretion to insulin-like growth factor-I negative feedback. J Clin Endocinol Metab. 1997;82:2996–3004. doi: 10.1210/jcem.82.9.4223. [DOI] [PubMed] [Google Scholar]
- 52.MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 53.Hwang AB, Lee SJ. Regulation of life span by mitochondrial respiration: the HIF-1 and ROS connection. Aging. 2011;3:304–310. doi: 10.18632/aging.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leiser SF, Begun A, Kaeberlein M. HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell. 2011;10:318–326. doi: 10.1111/j.1474-9726.2011.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samy TSA, Zheng R, Matsutani T, Rue ILW, Bland KI, Chaudry IH. Mechanism for normal splenic T lymphocyte functions in proestrus females after trauma: enhanced local synthesis of 17B-estradiol. Am J Physiol Cell Physiol. 2003;285:C139–C149. doi: 10.1152/ajpcell.00058.2003. [DOI] [PubMed] [Google Scholar]
- 56.Weigent DA, Blalock JE. Effect of the administration of growth hormone-producing lymphocytes on weight gain and immune function in dwarf mice. Neuroimmunomodulation. 1994;1:50–58. doi: 10.1159/000097090. [DOI] [PubMed] [Google Scholar]
- 57.Baxter JB, Blalock JE, Weigent DA. Characterization of immunoreactive insulin-like growth factor-I from leukocytes and its regulation by growth hormone. Endocrinology. 1991;129:1727–1734. doi: 10.1210/endo-129-4-1727. [DOI] [PubMed] [Google Scholar]
- 58.Weigent DA, Baxter JB, Blalock JE. The production of growth hormone and insulin-like growth factor-I by the same subpopulation of rat mononuclear leukocytes. Brain Behav Immun. 1992;6:365–376. doi: 10.1016/0889-1591(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 59.Weigent DA, Blalock JE. Associations between the neuroendocrine and immune systems. J Leukocyte Biol. 1995;58:137–150. doi: 10.1002/jlb.58.2.137. [DOI] [PubMed] [Google Scholar]
- 60.Taub DD. Neuroendocrine interactions in the immune system. Cell Immunol. 2011;252:1–6. doi: 10.1016/j.cellimm.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ThyagaRajan S, Madden KS, Teruya B, Stevens SY, Felten DL, Bellinger DL. Age-associated alterations in sympathetic noradrenergic innervation of primary and secondary lymphoid organs in female Fischer 344 rats. J Immunol. 2011;233:54–64. doi: 10.1016/j.jneuroim.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saceda J, Isla A, Santiago S, Morales C, Odene C, Hernandez B, Deniz K. Effect of recombinant human growth hormone on peripheral nerve regeneration: Experimental work on the ulnar nerve of the rat. Neurosci Lett. 2011;504:146–150. doi: 10.1016/j.neulet.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 63.Perry JK, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE. The contribution of growth hormone to mammary neoplasia. J Mammary Gland Biol Neoplasia. 2008;13:131–145. doi: 10.1007/s10911-008-9070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelley KW, Dantzer R. Growth hormone and prolactin as natural antagonists of glucocorticoids in immunoregulation. In: Plotnikoff N, Murgo A, Faith R, Wybran J, editors. Stress and Immunity. CRC Press; Boca Raton: 1991. pp. 433–453. [Google Scholar]
- 65.Kelley KW, Weigent DA, Kooijman R. Protein hormones and immunity. Brain Behav Immun. 2007;21:384–392. doi: 10.1016/j.bbi.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovacs M, Schally AV, Hohla F, Rick FG, Pozsgai E, Szalontay L, Varga JL, Zarandi M. A correlation of endocrine and anticancer effects of some antagonists of GHRH. Peptides. 2010;31:1839–1846. doi: 10.1016/j.peptides.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Weigent DA, Kraneveld AD, Blalock JE. Neuroimmunoendocrinology. In: Nijkamp FP, Parnham MJ, editors. Principles of Immunopharmacology. Springer-Verlag; Basel: 2011. pp. 179–198. [Google Scholar]