Abstract
Background and Aims
Despite recent recognition that (1) plant–herbivore interactions during the establishment phase, (2) ontogenetic shifts in resource allocation and (3) herbivore response to plant volatile release are each pivotal to a comprehensive understanding of plant defence, no study has examined how herbivore olfactory response varies during seedling ontogeny.
Methods
Using a Y-tube olfactometer we examined snail (Helix aspersa) olfactory response to pellets derived from macerated Plantago lanceolata plants harvested at 1, 2, 3, 4, 5, 6 and 8 weeks of age to test the hypothesis that olfactory selection of plants by a generalist herbivore varies with plant age. Plant volatiles were collected for 10 min using solid-phase microextraction technique on 1- and 8-week-old P. lanceolata pellets and analysed by gas chromatography coupled with a mass spectrometer.
Key Results
Selection of P. lanceolata was strongly negatively correlated with increasing age; pellets derived from 1-week-old seedlings were three times more likely to be selected as those from 8-week-old plants. Comparison of plant selection experiments with plant volatile profiles from GC/MS suggests that patterns of olfactory selection may be linked to ontogenetic shifts in concentrations of green leaf volatiles and ethanol (and its hydrolysis derivatives).
Conclusions
Although confirmatory of predictions made by contemporary plant defence theory, this is the first study to elucidate a link between seedling age and olfactory selection by herbivores. As a consequence, this study provides a new perspective on the ontogenetic expression of seedling defence, and the role of seedling herbivores, particularly terrestrial molluscs, as selective agents in temperate plant communities.
Keywords: Green leaf volatiles, seedling herbivory, olfactory response, snails, Helix aspersa, ontogeny, plant defence, Plantago lanceolata, seedling establishment, volatile organic compounds
INTRODUCTION
Susceptibility to herbivore attack varies markedly during the lifetime of an individual plant. During the early stages of establishment, seedlings are particularly vulnerable to herbivory and the loss of even relatively small amounts of tissue can prove fatal or at best impose a severe cost in terms of later competitive or reproductive fitness (Hanley and May, 2006; Hanley and Sykes, 2009). As the plant ages, defensive capability generally increases (Goodger et al., 2004; Barton and Koricheva, 2010) although plants often switch resistance and tolerance mechanisms to match ontogenetic shifts in herbivore identity and preference (Hanley et al., 2007; Gruntman and Novoplansky, 2011). Moreover, the impacts of herbivory on mature plants are often much reduced in comparison with those incurred during younger ontogenetic stages, due to a large extent on the increased ability of the plant to resist herbivory via the expression of chemical or physical defences (Boege and Marquis, 2005; Barton and Koricheva, 2010).
The most distinct ontogenetic changes in constitutive plant defences are both expected (Boege and Marquis, 2005) and observed (Elger et al., 2009; Quintero and Bowers, 2012) to occur during the transition from seedling to juvenile stages. A general increase in allocation to plant defence with seedling age is also predicted by both the growth-differentiation balance hypothesis (Herms and Mattson, 1992) and the optimal defence theory (McKey, 1974). More recently, however, Boege and Marquis (2005) suggested that due to resource allocation and structural constraints, cotyledon-stage seedlings should exhibit higher levels of chemical defences than older seedlings, before constitutive defence levels again increase as the plant passes from the seedling to pre-reproductive maturity. Given that the impact and fitness costs of herbivory also appear to be greater for younger seedlings (Hanley et al., 1995a; Hanley and Fegan, 2007), there is good reason to expect that young seedlings should be better defended than their older conspecifics.
There have been remarkably few tests of changes in the expression of early plant chemical defence, however, and these studies describe conflicting trends. On the one hand, Elger et al. (2009) reported increased concentrations of various secondary metabolites with age in six European grassland forbs (see also Boege, 2005; Neilson et al., 2006), Quintero and Bowers (2012) described positive, but non-linear ontogenetic increases in iridoid glycoside concentrations in Plantago lanceolata, while Wallace and Eigenbrode (2002) found the highest glucosinolate concentrations in the youngest Brassica juncea seedlings (see also Collantes et al., 1999). This apparent inconsistency across the seedling phase is probably symptomatic of the wider variation in how different anti-herbivore resistance and tolerance traits are expressed by different plant species and growth forms throughout plant ontogeny (Barton and Koricheva, 2010; Orians et al., 2010).
Unlike mature plants, where tissue loss can often be fully compensated (Strauss and Agrawal, 1999), seedlings seldom have the opportunity to recover from herbivore attack. Seedlings are often completely defoliated by herbivores (Hulme, 1994; Hanley, 2004) and during early ontogeny lack the resources to re-grow after defoliation (Hanley et al., 2004). Consequently, those plant species that possess constitutive defences during their establishment have a compelling reason to advertise the presence of their defences to would-be herbivores before the herbivores have the opportunity to inflict damage. One way of doing this is to synthesize and release olfactory signals immediately following herbivore attack or even before an attack has commenced.
The role of volatile compounds as olfactory signals of innate anti-herbivore deterrence is well established for mature plants (Unsicker et al., 2009; Yuan et al., 2009; Dicke and Baldwin, 2010). By far the most important groups of volatile organic compounds (VOCs) are terpenes and green leaf volatiles (Dicke and Baldwin, 2010), and while used by some herbivores for plant host location, the most commonly reported response is release of VOCs following herbivore damage and subsequent avoidance of the same plant by other herbivores (Laothawornkitkul et al., 2008; Mooney et al., 2009; Unsicker et al., 2009). However, although there is evidence that invertebrate herbivores detect seedling volatiles and modify their feeding behaviour accordingly, these studies are limited to Carroll et al.'s (2006, 2008) work on armyworm response to two crop species (cowpea and maize seedlings) and a sole investigation of the olfactory selection of wildflower seedlings (Hanley et al., 2011). In this latter study, the olfactory selection of macerated seedling material by the snail Helix aspersa was positively correlated with seedling acceptability of nine grassland plant species, i.e. seedlings of low gustatory acceptability appeared to signal the fact.
The theoretical arguments for, and observed trends in, ontogenetic variation in constitutive seedling defence (Boege and Marquis, 2005; Elger et al., 2009; Quintero and Bowers, 2012), coupled with the likely importance of an ability to signal defensive capability before too much damage is inflicted (Hanley et al., 2011), suggests that any herbivore response to seedling volatiles should vary with plant age. While a handful of studies have examined ontogenetic variation in VOC release in established plants (Shiojiri and Karban, 2006; Bracho-Nunez et al., 2011), no study as yet has analysed ontogenetic variation in this relationship during the seedling stage. Using a plant species known to show considerable early ontogenetic changes in constitutive chemical defences, the aim of this study was to determine whether olfactory selection of food plants by a generalist herbivore varies according to the ontogenetic stage of the target species.
MATERIALS AND METHODS
Study species
Plantago lanceolata L. is a short-lived, perennial herb species native to grasslands and disturbed vegetation of Eurasia, but now globally widespread as an exotic weed (Van der Aart and Vulto, 1992). When compared with other species, P. lanceolata has low acceptability to seedling herbivores (Hulme, 1994; Hanley, 2004), a pattern most likely due to the early ontogenetic development and expression of key constitutive chemical defences: phenolics and iridoid glycosides (Barton, 2007, 2008; Elger et al., 2009; Quintero and Bowers, 2012).
Molluscs are the principal seedling herbivore in temperate ecosystems (Crawley, 1997). Observation of snail (Helix aspersa) feeding on seedlings in experimental arenas suggests that some species are consistently avoided even before physical contact is made (M. E. Hanley, pers. observ.) and the slug, Deroceras reticulatum, is known to exhibit a strong neurophysiological response to plant volatiles (Birkett et al., 2004). Here we used the snail H. aspersa because it is a generalist herbivore frequently employed in experiments comparing seedling acceptability (Hanley and Sykes, 2009; Hanley et al., 2011). Three hundred H. aspersa were collected from around Plymouth, UK, retained in plastic containers kept in incubators (16 °C day/8 °C night temperature and 12-h day/night light cycle) and fed on a mixed diet of lettuce and carrot. The snails were kept under this regime for 6 months before use in the olfactory trials. Four days prior to the start of olfactory trials, a selection of snails were moved to a separate container and starved.
Plant propagation and snail culture
Plantago lanceolata and lettuce (Lactuca sativa L. ‘Thom Thumb’) seeds were obtained from a commercial supplier (Herbiseed Ltd, Twyford, UK). During May 2011, sequential sowings of P. lanceolata seeds were made into plastic seed trays (350 × 215 × 70 mm) filled with John Innes No. 2 potting compost maintained in a naturally lit greenhouse. Plant material from individual trays was harvested at 1, 2, 3, 4, 5, 6 and 8 weeks after germination by removing all above-ground material, before it was cleaned of adhering compost and macerated using a mortar and pestle. The macerated material was then divided into Eppendorf tubes, each containing 1·5 g of macerated plant material, which was then frozen for later use. A previous study comparing snail olfactory selection of frozen and fresh pellets showed no discrimination between treatments (Hanley et al., 2011). Lettuce was harvested 3 weeks after germination and processed and stored as above.
Snail olfaction trials
Our method followed that used by Hanley et al. (2011). A glass Y-tube olfactometer of internal diameter 55 mm, with stem and arms 80 mm long (Soham Scientific, Ely, UK), was used to observe snail olfactory response to macerated plant material. Each end of the Y-tube was connected to 10-litre plastic containers containing the test materials. Air flow-meters (Caché Instrumentation, Wakefield, UK) were fitted into holes drilled into each container and connected to an air pump. Airflow was set to 500 cm3 min−1 and was sourced, unfiltered from the room which was a sealed, temperature-controlled laboratory to reduce confounding olfactory stimuli. Illumination was a single fluorescent strip-light positioned directly above the experimental apparatus.
Pellets of plant material were randomly selected from the seven age treatments of the ‘test’ species (P. lanceolata), defrosted and placed in one of the plastic containers. A pellet of the ‘control’ (L. sativa) was placed in the other container to act as a standard against which selection of the ‘test’ pellet was compared. The ‘test’ and ‘control’ pellets were randomly assigned to either the right- or the left-hand container before each trial. For each test one snail was randomly selected from a pool of 20 starved individuals and introduced to the base of the Y-tube apparatus. Each snail was observed for a maximum of 10 min or until it contacted the end of a Y-tube arm. We noted the time taken for each snail to reach the split in the Y-tube (‘Time to Choose’), the direction chosen on reaching the split (‘Proportion of Choice’) and the time taken to reach the end of the Y-tube arms (‘Time to Sample’). Trials where snails made a choice within the specified time frame were conducted 15 times for each test age; trials where the subject snail failed to make a choice within 10 min were discarded. All equipment was thoroughly washed with distilled water between trials to remove mucus.
Volatile collection and analysis
One and 8-week-old P. lanceolata pellets (derived from plants cultivated in separate trays as above) were fully defrosted at room temperature and each pellet placed into a 4-mL screw-topped vial (Chromacol Ltd, London, UK) with a screw cap containing a red Teflon-coated silicon septum. VOCs were collected by introducing a solid-phase microextraction fibre (SPME, blue fibre 65 µm PDMS-DVB; Supelco, Bellefonte, PA, USA) through the septa into the vial. Volatiles were collected for 10 min and analysed immediately after the collection ceased. In total, the VOCs emitted by four 1·5-g standard mass pellets of each of the two plant age groups were analysed.
Samples were analysed by thermal desorption on an Agilent 6890 gas chromatograph coupled to a Hewlett-Packard 5972 Quadrupole Mass Selective Detector, which ionized by electron ionization. The carrier gas was helium (7·51 psi constant) and the injector was operated in a splitless mode. The capillary column was an HP-INNOWAX (30 m, 0·25 mm i.d., 0·25 mm film; Agilent Technologies, Palo Alto, CA, USA). Injection temperature was 250 °C and the oven temperature was held at 50 °C for 2 min, and then programmed at 5 °C min−1 to 70 °C, then at 10 °C min−1 to 240 °C. The mass spectrometer (250 °C) scanned from mass 350 to 40 at a rate of 2·43 times s−1 and data were captured and analysed by Enhanced Chemstation software (v. B.01·00; Agilent Technologies).
VOCs were initially tentatively identified by comparing spectra with those in Wiley 275 and NIST 98 analytical databases, and their peak areas recorded. Confirmation was achieved for a number of the VOCs by comparison of spectra and retention times with those of authenticated standards. For some of the compounds for which standards were unavailable, tentative identifications were determined from Kovat's indices and database matches. VOCs were included for further analysis only if they were found to occur in at least three of the four samples of one of the pellet ages. Peak areas of VOCs identified in both 1- and 8-week-old samples were compared by a series of t-tests. Statistical analyses were conducted using SPSS v.19 (SPSS Inc. 2010).
RESULTS
When harvested at 1 week old, seedlings were at the cotyledon stage. The first true leaf appeared in 2- to 3-week-old seedlings, and by 4 weeks old plants had developed at least two true leaves and had probably achieved independence from their cotyledons to signal the end of the seedling phase (Hanley et al., 2004). By 8 weeks old plants were sufficiently grown to be considered as mature, pre-reproductive plants.
Following arc-sine transformation of the ‘Proportion of Choice’ data, we found an extremely strong negative relationship (r2 = 0·925, F1,5 = 61·74, P < 0·0001) between snail preference and seedling age (Fig. 1). Pellets derived from 1-week-old seedlings were almost three times more likely to be selected (eight of 15 trials, or 53 %) than pellets derived from 8-week-old material (three of 16 trials, or 19 %). However, when we compared mean ‘Time to Choose’ and ‘Time to Sample’ with seedling age, we found no relationships (‘Time to Choose’ r2 = 0·1, F1,5 = 0·01, P = 0·978: ‘Time to Sample’ r2 = 0·1, F1,5 = 0·01, P = 0·976) (data not shown).
Fig. 1.
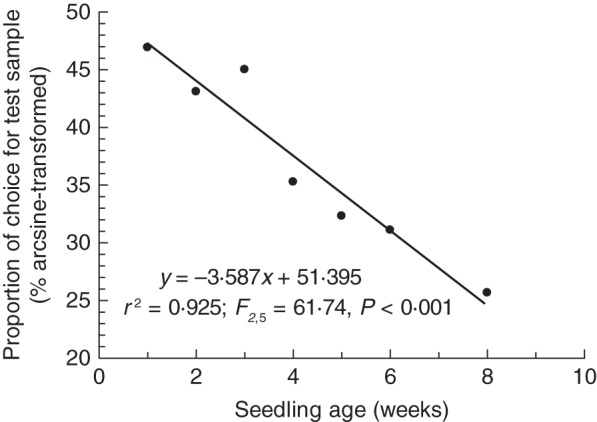
The relationship between seedling age and the proportion of snails (Helix aspersa) moving towards a pellet of macerated material derived from Plantago lanceolata plants aged 1, 2, 3, 4, 5, 6 and 8 weeks. Experiments were conducted using a Y-tube olfactometer and snails were presented with a choice between the test species (P. lanceolata) and a lettuce (Lactuca sativa) control.
In total, 23 compounds were identified from the P. lanceolata leaf pellets. There were both quantitative and qualitative differences in VOC emissions between the 1- and 8-week-old pellets. The major component of both VOC blends was (E)-2-hexenal (Supplementary Data Fig. S1), but the emission of this compound did not differ significantly between pellet ages. Quantitative differences occurred in ten of the compounds, at least half of which were leaf alcohols (Table 1). Eight of the compounds were emitted in significantly higher quantities from the 8-week-old plant pellets and two from the 1-week-old pellets. In addition, 1-week-old plants produced four compounds that were not produced by 8-week-old plants, including ethanol, ethyl acetate, acetic acid and one unidentified compound. In contrast, 8-week-old plants consistently produced two compounds not found in 1-week-old plants: one tentatively identified as (E)-2-hexen-1-ol acetate and the other unidentified.
Table 1.
Mean peak areas of ten (of a total of 23) volatile organic compounds identified by GC/MS from the headspace of P. lanceolata pellets derived from 1- or 8-week-old leaves found to differ significantly between these two age treatments (n = 4); in addition, four compounds (ethanol, ethyl acetate, acetic acid and one unidentified compound) were present only in 1-week-old plants
| Mean peak area (±s.e.) |
|||
|---|---|---|---|
| Compound | 1-week-old P. lanceolata | 8-week-old P. lanceolata | t-test |
| Alcohols | |||
| 1-Hexanol | 18 579 ± 2687 | 26 470 ± 1202 | t = 2·7, P = 0·04 |
| (Z)-3-Hexen-1-ol | 10 941 ± 1677 | 198 111 ± 12 568 | t = 14·8, P < 0·001 |
| (E)-2-Hexen-1-ol | 59 056 ± 18 330 | 285 210 ± 8366 | t = 11·2, P < 0·001 |
| 3-Octanol | 1460 ± 1460† | 32 193 ± 4173 | t = 7·0, P <0·001 |
| 1-Octen-3-ol | 1148 269 ± 134 191 | 1793 094 ± 188 719 | t = 2·8, P = 0·03 |
| Aldehydes | |||
| (Z)-2-Hexenal* | 37 970 ± 4384 | 63 753 ± 7934 | t = 2·8, P = 0·03 |
| Pentanal* | 7739 ± 672 | 1540 ± 1540† | t = 3·7, P = 0·01 |
| Alkanes | |||
| Hexane | 55 724 ± 8836 | 21 117 ± 7443 | t = 3·0, P = 0·02 |
| Unidentified compounds | |||
| 1 | 52 486 ± 2089 | 168 255 ± 34 055 | t = 3·4, P = 0·02 |
| 2 | 16 633 ± 728 | 84 098 ± 20 052 | t = 3·4, P = 0·02 |
* Tentative identification.
† Three of the four replicate samples contained no compound.
DISCUSSION
The strong negative relationship between olfactory selection by snails and plant age corroborates a number of studies reporting increased concentrations of both phenolics and iridoid glycosides during early P. lanceolata ontogeny (Fuchs and Bowers, 2004; Barton, 2007; Elger et al., 2009; Quintero and Bowers, 2012). Indeed, increased defensive capability through the seedling stage appears to be a general trend for a variety of taxonomically unrelated species (Boege, 2005; Neilson et al., 2006; Elger et al., 2009), one of the main predictions of the growth-differentiation balance hypothesis (Herms and Mattson, 1992) and the optimal defence theory (McKey, 1974). However, when taken in tandem with studies of ontogenetic shifts in plant chemistry in P. lanceolata, the fact that 1-week-old (cotyledon-stage) seedlings were selected by snails much more frequently than any other age group offers no support for Boege and Marquis' (2005) expectation that cotyledon-stage seedlings should exhibit higher levels of chemical defences than older seedlings.
By comparison of the volatile profiles of 1- and 8-week-old P. lanceolata plants, we identified several compounds whose concentrations varied with plant age and which may influence snail foraging behaviour. The so-called common green leaf volatiles (GLVs) we detected [(Z)-2-hexenal and five aliphatic alcohols, 1-hexanol, (Z)-3- and (E)-2-hexen-1-ol, 3-octanol and 1-octen-3-ol] are commonly encountered following herbivore damage (Shiojiri et al., 2006; Furstenau et al., 2012). As these GLVs were detected in older plants, they may act as anti-herbivore deterrents in their own right and/or signal to potential herbivores that the plant contains one or more of a range of constitutive chemical defences identified in P. lanceolata (Barton, 2007; Elger et al., 2009; Quintero and Bowers, 2012). Three other volatile compounds, ethanol, ethyl-acetate and acetic acid, were present in small amounts only in 1-week-old seedlings. Each is known to be attractive to insect herbivores (Giblin-Davis et al., 1996; Casana-Giner et al., 2001) and thus these compounds may have an important role in the olfactory detection of young P. lanceolata seedlings by snails.
Although the olfactory signalling of increased chemical defence seems the most plausible explanation for our results, we recognize that age-related changes in seedling selection by snails may be linked to the detection of attractant, rather than repellent, cues. Nonetheless, VOCs are most commonly associated with repellent or toxic chemicals (Unsicker et al., 2009) and the range of volatiles described for P. lanceolata includes the deterrent GLV (Z)-3-hexenyl acetate and various terpenoids (Fontana et al., 2009). What is unclear is whether age affects the plant's ability to synthesize VOCs or simply that VOCs are released as a consequence of the steady accumulation and mobilization of direct chemical defences as the plant grows older.
While the olfactory responses elicited in this experiment were based on cues emanating from macerated plants, post-damage volatile emission is common following herbivore attack. Volatiles released in this way are thought to have a number of functions including the signalling of a plant's direct defensive capabilities to the herbivore, attraction of natural enemies such as parasitoid wasps, and even the mobilization of chemical defences in neighbouring plants (Unsicker et al., 2009; Dicke and Baldwin, 2010). Any or all of these roles may be operating during the establishment phase. Seedlings seldom germinate in isolation and volatile release may be important in signalling the defensive capability of the cohort, or indeed inducing the synthesis of secondary metabolites in nearby (and presumably genetically related) seedlings; this phenomenon is well established for mature plants (Heil and Silva Bueno, 2007; Dicke and Baldwin, 2010).
It is possible that seedlings also signal their defensive capability before the herbivore makes contact. Observation of snail feeding behaviour in experimental arenas shows that snails have a remarkable ability to discriminate between seedlings of varying defensive capability even before gustatory contact is made (M. E. Hanley, pers. observ.). However, no study has yet quantified volatile release in intact seedlings, presumably due to the difficulty in entraining volatile samples from small samples. Moreover, while terrestrial molluscs are known to detect volatiles in established plants (Dodds et al., 1999; Birkett et al., 2004), how VOCs influence mollusc olfaction and feeding behaviour remains poorly explored, especially at the critical seedling stage.
Seedling herbivory is a potent selective filter in natural ecosystems, with a remarkable influence over plant community composition and species coexistence (Hanley et al., 1995b; Howe et al., 2002; Asquith and Mejia-Chang, 2005; Hanley and Sykes, 2009). However, despite its importance in our understanding of plant community dynamics, the mechanisms underpinning seedling selection by herbivores such as snails are not readily apparent. The elucidation of terrestrial mollusc response to indirect (VOCs) as well as direct (constitutive and induced chemicals) defences could contribute greatly to our understanding of their role as selective agents in temperate grasslands and how this role varies with plant ontogeny. Moreover, terrestrial molluscs are one of the most important economic pests in arable cropping systems (Simms et al., 2006; Nash et al., 2007) and a better understanding of the processes that underpin mollusc olfaction and feeding behaviour may increase our ability to protect crops during the vulnerable establishment phase. This study highlights a previously unreported relationship between herbivore olfactory selection and early plant ontogeny, and signposts a need for ecologists to focus future research on the relationship between direct and indirect plant defences and herbivore feeding behaviour from the very youngest stages of plant life history.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Figure S1: representative GC/MS traces of volatile organic compounds released from 1- and 8-week old Plantago lanceolata pellets, collected on SPME fibres.
ACKNOWLEDGEMENTS
We thank Jane Ackerman and Peter Russell for technical assistance and two anonymous referees and Handling Editor Kasey Barton for their comments on an earlier version of the manuscript. This work was supported by the Leverhulme Trust (grant no. RPG-083 to M.E.H., P.L.N. and G.M.P.).
LITERATURE CITED
- Asquith NM, Mejia-Chang M. Mammals. edge effects, and the loss of tropical forest diversity. Ecology. 2005;86:379–390. [Google Scholar]
- Barton KE. Early ontogenetic patterns in chemical defense in Plantago (Plantaginaceae): genetic variation and tradeoffs. American Journal of Botany. 2007;94:56–66. doi: 10.3732/ajb.94.1.56. [DOI] [PubMed] [Google Scholar]
- Barton KE. Phenotypic plasticity in seedling defense strategies: compensatory growth and chemical induction. Oikos. 2008;117:917–925. [Google Scholar]
- Barton KE, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. American Naturalist. 2010;175:481–493. doi: 10.1086/650722. [DOI] [PubMed] [Google Scholar]
- Birkett MA, Dodds CJ, Henderson IF, et al. Antifeedant compounds from three species of Apiaceae active against the field slug, Deroceras reticulatum (Muller) Journal of Chemical Ecology. 2004;30:563–576. doi: 10.1023/b:joec.0000018629.58425.18. [DOI] [PubMed] [Google Scholar]
- Boege K. Herbivore attack in Casearia nitida influenced by plant ontogenetic variation in foliage quality and plant architecture. Oecologia. 2005;143:117–125. doi: 10.1007/s00442-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Boege K, Marquis RJ. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution. 2005;20:441–448. doi: 10.1016/j.tree.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bracho-Nunez A, Welter S, Staudt M, Kesselmeier J. Plant-specific volatile organic compound emission rates from young and mature leaves of Mediterranean vegetation. Journal of Geophysical Research-Atmospheres. 2011;116 D16304. http://dx.doi.org/10.1029/2010JD015521 . [Google Scholar]
- Carroll MJ, Schmelz EA, Meagher RL, Teal PEA. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. Journal of Chemical Ecology. 2006;32:1911–1924. doi: 10.1007/s10886-006-9117-9. [DOI] [PubMed] [Google Scholar]
- Carroll MJ, Schmelz EA, Teal PEA. The attraction of Spodoptera frugiperda neonates to cowpea seedlings is mediated by volatiles induced by conspecific herbivory and the elicitor inceptin. Journal of Chemical Ecology. 2008;34:291–300. doi: 10.1007/s10886-007-9414-y. [DOI] [PubMed] [Google Scholar]
- Casana-Giner V, Gandia-Balaguer A, Hernandez-Alamos MM, et al. Attractiveness of 79 compounds and mixtures to wild Ceratitis capitata (Diptera: Tephritidae) in field trials. Journal of Economic Entomology. 2001;94:898–904. doi: 10.1603/0022-0493-94.4.898. [DOI] [PubMed] [Google Scholar]
- Collantes HG, Gianoli E, Niemeyer HM. Defoliation affects chemical defences in all plant parts of rye seedlings. Journal of Chemical Ecology. 1999;25:491–499. [Google Scholar]
- Crawley MJ. Plant–herbivore dynamics. In: Crawley MJ, editor. Plant ecology. 2nd edn. Oxford: Blackwell Science; 1997. pp. 401–474. [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dodds CJ, Henderson IF, Watson P, Leake LD. Action of extracts of Apiaceae on feeding behaviour and neurophysiology of the field slug Deroceras reticulatum. Journal of Chemical Ecology. 1999;25:2127–2145. [Google Scholar]
- Elger A, Lemoine DG, Fenner M, Hanley ME. Plant ontogeny and defence: older seedlings are better defended. Oikos. 2009;118:767–773. [Google Scholar]
- Fontana A, Reichelt M, Hempel S, Gershenzon J, Unsicker SB. The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. Journal of Chemical Ecology. 2009;35:833–843. doi: 10.1007/s10886-009-9654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Bowers MD. Patterns of iridoid glycoside production and induction in Plantago lanceolata and the importance of plant age. Journal of Chemical Ecology. 2004;30:1723–1741. doi: 10.1023/b:joec.0000042398.13765.83. [DOI] [PubMed] [Google Scholar]
- Furstenau B, Rosell G, Guerrero A, Quero C. Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. Journal of Chemical Ecology. 2012;38:378–388. doi: 10.1007/s10886-012-0110-1. [DOI] [PubMed] [Google Scholar]
- Giblin-Davis RM, Pena JE, Oehlschlager AC, Perez AL. Optimization of semiochemical-based trapping of Metamasius hemipterus sericeus (Olivier) (Coleoptera: Curculionidae) Journal of Chemical Ecology. 1996;22:1389–1410. doi: 10.1007/BF02027720. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Ades PK, Woodrow IE. Cyanogenesis in Eucalyptus polyanthemos seedlings: heritability, ontogeny and effect of soil nitrogen. Tree Physiology. 2004;24:681–688. doi: 10.1093/treephys/24.6.681. [DOI] [PubMed] [Google Scholar]
- Gruntman M, Novoplansky A. Ontogenetic contingency of tolerance mechanisms in response to apical damage. Annals of Botany. 2011;108:965–973. doi: 10.1093/aob/mcr204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME. Seedling herbivory and the influence of plant species richness in seedling neighbourhoods. Plant Ecology. 2004;170:35–42. [Google Scholar]
- Hanley ME, Fegan EL. Timing of cotyledon damage affects growth and flowering in mature plants. Plant, Cell and Environment. 2007;30:812–819. doi: 10.1111/j.1365-3040.2007.01671.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, May OC. Cotyledon damage at the seedling stage affects growth and flowering potential in mature plants. New Phytologist. 2006;169:243–250. doi: 10.1111/j.1469-8137.2005.01578.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. Impact of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany. 2009;103:1347–1353. doi: 10.1093/aob/mcp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. The effect of seedling age on the likelihood of herbivory by the slug Deroceras reticulatum. Functional Ecology. 1995a;9:754–759. [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. An experimental field study of the effects of mollusc grazing on seedling recruitment and survival in grassland. Journal of Ecology. 1995b;83:621–627. [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvill B. Early plant growth: identifying the end point of the seedling phase. New Phytologist. 2004;164:61–66. doi: 10.1111/j.1469-8137.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics. 2007;8:157–178. [Google Scholar]
- Hanley ME, Collins SA, Swann C. Advertising acceptability: is mollusc olfaction important in seedling selection? Plant Ecology. 2011;212:727–731. [Google Scholar]
- Heil M, Silva Bueno JC. Within-plant signalling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or to defend. Quarterly Review of Biology. 1992;67:283–335. [Google Scholar]
- Howe HF, Brown JS, Zorn-Arnold B. A rodent plague on prairie diversity. Ecology Letters. 2002;5:30–36. [Google Scholar]
- Hulme PE. Seedling herbivory in grassland: relative impact of vertebrate and invertebrate herbivores. Journal of Ecology. 1994;82:873–880. [Google Scholar]
- Laothawornkitkul J, Paul ND, Vickers CE, et al. Isoprene emissions influence herbivore feeding decisions. Plant, Cell and Environment. 2008;31:1410–1415. doi: 10.1111/j.1365-3040.2008.01849.x. [DOI] [PubMed] [Google Scholar]
- McKey D. Adaptive patterns in alkaloid physiology. American Naturalist. 1974;108:305–320. [Google Scholar]
- Mooney AC, Robertson HM, Wanner KW. Neonate silkworm (Bombyx mori) larvae are attracted to mulberry (Morus alba) leaves with conspecific feeding damage. Journal of Chemical Ecology. 2009;35:552–559. doi: 10.1007/s10886-009-9639-z. [DOI] [PubMed] [Google Scholar]
- Nash MA, Thomson LJ, Hoffmann AA. Slug control in Australian canola: monitoring, molluscicidal baits and economic thresholds. Pest Management Science. 2007;63:851–859. doi: 10.1002/ps.1411. [DOI] [PubMed] [Google Scholar]
- Neilson EH, Goodger JQD, Woodrow IE. Novel aspects of cyanogenesis in Eucalyptus camphora subsp humena. Functional Plant Biology. 2006;33:487–496. doi: 10.1071/FP05293. [DOI] [PubMed] [Google Scholar]
- Orians CM, Hochwender CG, Fritz RS, Snäll T. Growth and chemical defenses in willow seedlings: trade-offs are transient. Oecologia. 2010;163:283–290. doi: 10.1007/s00442-009-1521-8. [DOI] [PubMed] [Google Scholar]
- Quintero C, Bowers MD. Changes in plant chemical defences and nutritional quality as a function of ontogeny in Plantago lanceolata (Plantaginaceae) Oecologia. 2012;168:471–481. doi: 10.1007/s00442-011-2114-x. [DOI] [PubMed] [Google Scholar]
- Shiojiri K, Karban R. Plant age, communication and resistance to herbivores: young sagebrush plants are better emitters and receivers. Oecologia. 2006;149:214–220. doi: 10.1007/s00442-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Shiojiri K, Kishimoto K, Ozawa R, et al. Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences USA. 2006;103:16672–16676. doi: 10.1073/pnas.0607780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms LC, Ester A, Wilson MJ. Control of slug damage to oilseed rape and wheat with imidacloprid seed dressings in laboratory and field experiments. Crop Protection. 2006;25:549–555. [Google Scholar]
- Strauss SY, Agrawal AA. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology and Evolution. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. [DOI] [PubMed] [Google Scholar]
- Unsicker S, Kunert G, Gershenzon J. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Current Opinion in Plant Biology. 2009;12:479–485. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Van der Aart PJM, Vulto JC. Biogeography and human effects. In: Kuiper PJC, Bos M, editors. Plantago: a multidisciplinary study. Berlin: Springer; 1992. pp. 5–6. [Google Scholar]
- Wallace SK, Eigenbrode SD. Changes in the glucosinilate-myrosinase defence system in Brassica juncea cotyledons during seedling development. Journal of Chemical Ecology. 2002;28:243–256. doi: 10.1023/a:1017973005994. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Himanen SJ, Holopainen JK, Chen F, Stewart CN. Smelling global climate change: mitigation of function for plant volatile organic compounds. Trends in Ecology and Evolution. 2009;24:323–331. doi: 10.1016/j.tree.2009.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


