Abstract
Objective To do a systematic review and meta-analysis of studies comparing sequential therapy for eradication of Helicobacter pylori with pre-existing and new therapies, thus providing a glimpse of eradication success worldwide.
Design Systematic review and meta-analysis.
Data sources Medline, Embase, and Cochrane Central Register of Controlled Trials up to May 2013; abstract books of major European, American, and Asian gastroenterological meetings.
Study selection Randomised controlled trials in previously untreated adults, in which sequential therapy was compared with a pre-existing or new therapy.
Results 46 randomised controlled trials were reviewed and analysed. 5666 patients were randomised to sequential therapy and 7866 to other (established and new) treatments. The overall eradication rate of sequential therapy was 84.3% (95% confidence interval 82.1% to 86.4%). Sequential therapy was superior to seven day triple therapy (relative risk 1.21, 95% confidence interval 1.17 to 1.25; I2=29.3%; number needed to treat 6 , 95% confidence interval 5% to 7%), marginally superior to 10 day triple therapy (1.11, 1.04 to 1.19; I2= 67.2%; NNT 10, 7 to 15), but not superior to 14 day triple therapy (1.00, 0.94 to 1.06; I2=54.3%), bismuth based therapy (1.01, 0.95 to 1.06; I2=21.1%), and non-bismuth based therapy (0.99, 0.94 to 1.05; I2=52.3%). Data on eradication according to pre-treatment antimicrobial susceptibility testing were available in eight studies, and sequential therapy was able to eradicate 72.8% (61.6% to 82.8%) of the strains resistant to clarithromycin.
Conclusions Eradication rates with pre-existing and new therapies for H pylori are suboptimal. Regional monitoring of resistance rates should help to guide treatment, and new agents for treatment need to be developed.
Introduction
Helicobacter pylori infection causes peptic ulcers, gastric mucosa associated lymphoid tissue lymphoma, and gastric cancer.1 Standard treatments for H pylori infection that have been endorsed by US as well as European scientific societies and by regulatory authorities rely on clarithromycin, metronidazole, or amoxicillin in conjunction with gastric acid inhibitors.2 3 The prevalence of resistance to clarithromycin and metronidazole has increased substantially in recent years, and a corresponding decrease has occurred in the eradication rate for H pylori infection,4 which has declined to unacceptable levels in most Western countries.5 A new treatment regimen that would achieve the eradication rates of 90% or greater seen at the advent of H pylori treatment is urgently needed.5 Such a regimen would need to have high efficacy against clarithromycin resistant and metronidazole resistant strains of H pylori, as these strains are increasingly encountered in routine clinical practice. As the response to eradication therapy is significantly related to the prevalence of primary resistance in the population, the choice of a treatment regimen should be based on the knowledge of the underlying prevalence of resistant strains in the community, which needs to be monitored.
Sequential therapy, a new regimen administering antimicrobials in a given sequence rather than all simultaneously, has generated worldwide interest. This kind of treatment is not actually new, as it uses established drugs, all approved for eradication of H pylori. However, the administration strategy is innovative. The sequential regimen is a simple dual therapy including a proton pump inhibitor plus amoxicillin 1 g (both twice daily) given for the first five days, followed by a triple therapy including a proton pump inhibitor, clarithromycin 500 mg, and a nitroimidazole antimicrobial (all twice daily) for the remaining five days. Initial studies of sequential therapy suggested that its superiority over standard triple therapy might be due to improved eradication of clarithromycin resistant strains.6 7
Recently, several randomised controlled trials have compared sequential therapy with other established and new therapies. These provide a glimpse into eradication rates for H pylori in the countries where those studies were conducted. The aim of this study was to assess the efficacy of sequential therapy compared with other eradication regimens, by doing a systematic review and meta-analysis of randomised controlled trials.
Methods
Search strategy and study selection
This meta-analysis was developed according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement guidelines.8 We searched the medical literature by using Medline (1950 to May 2013), Embase (1980 to May 2013), and the Cochrane Central Register of Controlled Trials (May 2013). Randomised controlled trials examining the eradication rate of sequential therapy compared with other treatments were eligible for inclusion (box). We identified eligible studies with the terms “Helicobacter pylori”, “H. pylori”, “H pylori”, “Campylobacter pylori”, “C. pylori”, “C pylori”, “infection”, “dyspepsia”, “sequential”, “triple”, “concomitant”, “quadruple”, “treatment”, “therapy”, “omeprazole”, “lansoprazole”, “rabeprazole”, “pantoprazole”, “esomeprazole”, “bismuth”, “clarithromycin”, “metronidazole”, “tinidazole”, “amoxicillin”. We imposed no language restrictions. Two investigators (LG and NV) evaluated abstracts of the papers identified by the initial search for appropriateness, independently and in a blinded manner. Foreign language papers were translated where necessary. We also searched the abstract books from the British Society of Gastroenterology (2001-12), American Gastroenterological Association (2000-13), American College of Gastroenterology (2004-12), United European Gastroenterology Week (2000-12), European Helicobacter pylori Study Group (2000-12), and Asian Pacific Digestive Week (2003-12). We used bibliographies of all relevant studies identified to do a recursive search. In addition, we contacted authors to obtain unpublished data from their studies, whenever we deemed it necessary.
Eligibility criteria
Randomised controlled trials*
Patients aged ≥18 years
Patients never treated before for Helicobacter pylori infection
Patients without significant comorbidity (for example, renal failure, hepatic failure, cancer)
Helicobacter pylori infection diagnosed (before and after treatment) using at least one of histology, rapid urease test, 13/14C urea breath test, stool test, culture†
Randomised controlled trials comparing sequential treatment‡ with other eradication regimens
Eradication rate according to intention to treat analysis
Eradication assessed at least four weeks after end of treatment
*Articles and/or abstracts reporting only interim analysis of randomised controlled trials were not included
†Articles and/or abstracts not reporting test used to diagnose infection and/or to follow-up infection were not included
‡Sequential treatment defined as proton pump inhibitors twice daily + amoxicillin 1 g twice daily for five days followed by proton pump inhibitors twice daily + clarithromycin 500 mg twice daily + nitroimidazole derivatives twice daily for next five days
Outcome assessment
The primary outcome was the efficacy of sequential therapy compared with established and new therapies in eradicating H pylori infection. Secondary outcomes included safety and efficacy according to the antimicrobial resistance pattern, where reported.
Data extraction
Two investigators (LG and NV) assessed articles independently, using pre-designed data extraction forms. Disagreement between investigators was resolved by discussion with the other two investigators (DV and CS). Data on eradication were based on intention to treat analysis. In addition, the following clinical data were extracted for each trial: country of origin, type of publication (article, abstract), proton pump inhibitor used, use of tinidazole (versus other nitroimidazole derivatives), duration of comparative eradication treatment, and adverse event rate.
Evaluation of risk of bias
We assessed risk of bias as described in the Cochrane handbook,9 by evaluating the random sequence generation, concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. We considered randomised controlled trials as being at low risk of bias if all the domains except blinding of participants or personnel were properly assured. As the outcome (that is, eradication) was almost always assessed by objective means, we did not consider blinding to be crucial.
Statistical analysis
We assessed data for the primary outcome by using a random effects model,10 to give a conservative estimate of the 95% confidence intervals. Results were expressed as relative risk for success of H pylori eradication and as difference in eradication rates among patients assigned to sequential therapy versus other eradication regimens. We also used a random effects model to pool data for safety,10 and expressed them as relative risk for adverse events. We required at least three comparable study groups for every comparison for randomised controlled trials to be included in the meta-analysis. We also calculated prediction intervals at 95% confidence intervals for the primary outcome, as they might be considered a more appropriate future treatment summary.11 12
We assessed heterogeneity between trials with the χ2 test for heterogeneity at a significance level of P<0.1. We also calculated the I2 statistic.13 Its value ranges from 0% to 100%, with 0% representing no observed heterogeneity and larger values indicating increasing heterogeneity. We chose a value below 25% to represent low levels of heterogeneity.13 When the degree of statistical heterogeneity was greater than this cut-off between trial results for the primary outcome, we investigated possible explanations by using subgroup analyses according to country of origin, use of tinidazole as the nitroimidazole derivative in the sequential treatment, type of publication (abstract versus article), proton pump inhibitor used, duration of comparative treatment (when applicable), and trials with a high risk and unclear risk of bias versus a low risk of bias. As exploratory analyses, they may explain some of the observed variability between trials. We used the Cochran Q statistic to compare the relative risks between studies in the analyses.9
We used a random effects model to calculate eradication rates of regimens.10 We calculated proportions, their differences, and 95% confidence intervals by using the method recommended by Newcombe and Altman. We calculated the number needed to treat and 95% confidence intervals from the reciprocal of the risk difference of the meta-analysis. We used Stata version 10.1 to generate forest plots for primary and secondary outcomes with 95% confidence intervals, as well as funnel plots. We assessed funnel plots for evidence of asymmetry and possible publication bias or other small study effects, by using the Egger’s linear regression and regarding a two sided P value of 0.10 or less as significant.14
Results
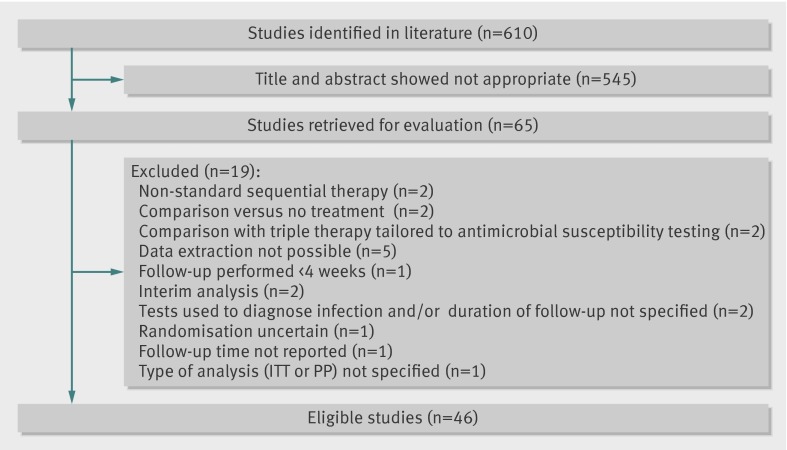
The search strategy we used identified 610 citations, of which we excluded 545 after examining the title and abstract. We retrieved and evaluated 65 articles in more detail. Of these, we excluded 19 for various reasons, leaving 46 randomised controlled trials that were eligible for inclusion,6 7 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 as shown in figure 1, 11 of which were abstracts.15 22 24 36 38 45 46 49 53 56 57 Fifteen studies included more than two arms.16 19 24 27 29 32 33 34 38 39 48 49 50 57 58 Three studies could not be included in the meta-analysis because our criteria required at least three comparable study groups for every comparison.32 47 54 Only four trials were at low risk of bias.7 32 51 55 The table shows detailed characteristics of the studies included in the systematic review and meta-analysis. Supplementary table A shows a complete evaluation of risk of bias.
Fig 1 Flow diagram of systematic review. ITT=intention to treat; PP=per protocol.
Characteristics of studies included in systematic review and meta-analysis
| Author and year | Country | Publication | Comparison* | Test to diagnose† | Follow-up (weeks) | Test to follow-up† | Culture | PPI‡ |
|---|---|---|---|---|---|---|---|---|
| Zullo et al 20036 | Italy | Full paper | TT-7 | H/R/13C-UBT | 6 | H/R/13C-UBT | Yes | R |
| Focareta et al 200315 | Italy | Abstract | TT-7 | R | 6 | 13C-UBT/ST | NA | E |
| De Francesco et al 200416 | Italy | Full paper | TT-7/TT-10 | H/R/13C-UBT | 6-8 | H/R/13C-UBT | NA | R |
| De Francesco e al 200417 | Italy | Full paper | TT-10 | H/R/13C-UBT | 6-8 | 13C-UBT | NA | R |
| Zullo 2005 et al18 | Italy | Full paper | TT-7 | H/R | 4-6 | H/R | NA | R |
| Scaccianoce et al 200619 | Italy | Full paper | TT-7/TT-10 | H/R | 4-6 | 13C-UBT | NA | E |
| Vaira et al 20077 | Italy | Full paper | TT-10 | H/R/C/13C-UBT | 4 and 8 | 13C-UBT | Yes | P |
| Choi et al 200820 | South Korea | Full paper | TT-7 | B | 8 | 13C-UBT/B | NA | O |
| Ma et al 200821 | China | Full paper | TT-7 | 14C-UBT/R | 4 | 14C-UBT/R | NA | O |
| Wu et al 200822 | China | Abstract | TT-7 | H | 4-6 | 13C-UBT | NA | R |
| Hu et al 200923 | China | Full paper | TT-7 | R/H | 4 | 13C-UBT | NA | E |
| Park et al 200924 | South Korea | Abstract | TT-7/TT-10/TT-14 | UBT/R/H | 4 | UBT/R/H | NA | Different |
| Zhao et al 200925 | China | Full paper | TT-7 | H | 4 | 13C-UBT | NA | P |
| Paoluzi et al 201026 | Italy | Full paper | TT-7 | H/R/C-UBT/ST | 8 | H/R/ST/C-UBT | NA | E |
| Aminian et al 201027 | Iran | Full paper | TT-10/BCT | R | 8 | ST | NA | O |
| Liang et al 201028 | China | Full paper | TT-10 | 14C-UBT/R | 4 | 14C-UBT/R | NA | R |
| Molina-Infante et al 201029 | Spain | Full paper | TT-10/ST with levofloxacin 500 bd | C-UBT/R/H | 8 | C-UBT | NA | O |
| Song et al 201030 | China | Full paper | TT-7 | 14C-UBT/H | 4 | 14C-UBT | NA | O |
| Wu et al 201031 | Taiwan | Full paper | NBQT | R/H/C | 6 | 13C-UBT/R/H/C | Yes | E |
| Romano et al 201032 | Italy | Full paper | ST with levofloxacin 250 bd/500 bd | 13C-UBT/H/R | 6 and 10 | 13C-UBT | Yes | O |
| Gao et al 201033 | China | Full paper | TT-7/ BCT | R/H | 4-6 | 13C-UBT | NA | R/O |
| Greenberg et al 201134 | South America | Full paper | TT-14/NBQT | 13C-UBT | 6-8 | 13C-UBT | NA | L |
| Kim et al 201135 | South Korea | Full paper | TT-14 | 13C-UBT/R/H | 4 | 13C-UBT | NA | P |
| Gatta et al 201136 | Italy | Abstract | TT-7 | H/R/C/13C-UBT | 4 | 13C-UBT | Yes | E |
| Wu et al 201137 | China | Full paper | TT-14 | 13C-UBT/R/H | 4 | 13C-UBT/R/H | NA | E |
| Franceschi et al 201238 | Italy | Abstract | TT-7/high dose amoxicillin TT-7 | 13C-UBT | 6 | 13C-UBT | NA | L |
| Choi et al 201239 | South Korea | Full paper | TT-7/TT-10/TT-14 | C-UBT/R/H | 4 | C-UBT/H | NA | R |
| Fakheri et al 201240 | Iran | Full paper | BCT | R/H | 8 | 14C-UBT | NA | P |
| Huang et al 201241 | Taiwan | Full paper | NBQT | C/R/H | 6 | C-UBT/B | Yes | L |
| Oh et al 201242 | South Korea | Full paper | TT-7 | R/H | 4 | 13C-UBT | NA | R |
| Park et al 201243 | South Korea | Full paper | TT-7 | 13C-UBT/R/H | 4 | 13C-UBT | NA | R |
| Chung et al 201244 | South Korea | Full paper | TT-10 | H/R/C | 4 | 13C-UBT | Yes | L |
| Kalapothakos et al 201245 | Greece | Abstract | TT-10 | 13C-UBT/R/H | 8 | 13C-UBT | NA | Different |
| Singh et al 201246 | Malaysia | Abstract | TT-7 | R/H | 6 | C-UBT | NA | R |
| Qian et al 201247 | China | Full paper | ST with levofloxacin 500 od | R/13C-UBT | 4 | 13C-UBT | NA | E |
| Lahbabi et al 201248 | Morocco | Full paper | TT-7 AC/TT-7 AM | H/PCR | 12 | 13C-UBT | NA | NR |
| Harmandar et al 201249 | Turkey | Abstract | TT-14/BCT/ST-14 | B | 4 | B/14C-UBT | NA | P |
| Liou et al 201250 | Taiwan | Full paper | TT-14/ST-14 | 13C-UBT/R/H/C/S | 6 | 13C-UBT | Yes | L |
| Javid et al 201351 | India | Full paper | TT-10 | R/H | 4 | R/H | NA | P |
| Seddik et al 201352 | Morocco | Full paper | TT-7 | H | 4-6 | 13C-UBT | NA | O |
| Yep-Gamarra et al 201353 | Peru | Abstract | TT-10 | B | 4 | B | NA | O |
| Sardarian et al 201354 | Iran | Full paper | Hybrid | H/R | 8 | 14C-UBT | NA | P |
| McNicholl et al 201355 | Spain | Full paper | NBQT | 13C-UBT/R/H/C | 4 | 13C-UBT/H | NR | O |
| Liu et al 201356 | Hong Kong | Abstract | BCT | H/R | 8 | 13C-UBT | NA | E |
| Ang et al 201357 | Singapore | Abstract | TT-10/NBQT | R/H/13C-UBT | 4 | 13C-UBT | NA | NR |
| Zullo et al 201358 | Italy | Full paper | NBQT/hybrid | H/R | 6 | 13C-UBT | NA | O |
bd=twice daily; NA=not assessed; NR=not reported; od=once daily; PPI=proton pump inhibitor.
*BCT=bismuth containing therapies; hybrid=PPI twice daily and amoxicillin 1 g twice daily for 14 days plus 500 mg clarithromycin and nitroimidazole derivatives, both twice daily, for last 7 days; NBQT=non-bismuth quadruple therapy; ST-14=sequential therapy lasting 14 days; TT-7=triple therapy lasting 7 days; TT-10=triple therapy lasting 10 days; TT-14=triple therapy lasting 14 days.
†B=biopsy based test; C=culture; C-UBT=urea breath test; H=histology; PCR=polymerase chain reaction; R=rapid urease test; S=serology; ST=stool test.
‡E=esomeprazole; L=lansoprazole; O=omeprazole; P=pantoprazole; R=rabeprazole.
Meta-analysis
Sequential therapy versus triple therapy lasting seven days
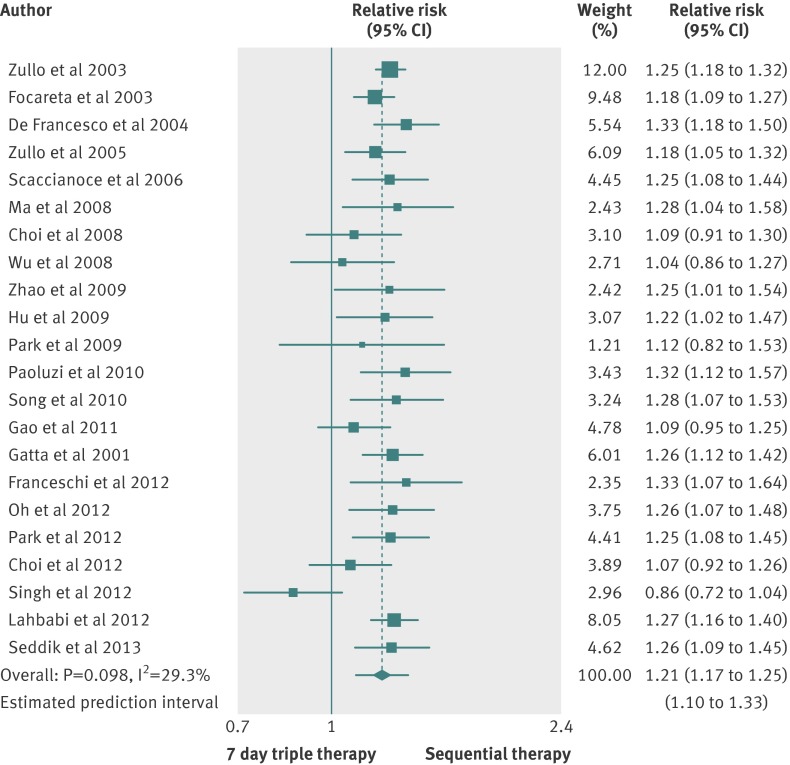
Twenty two studies compared sequential therapy with a triple therapy regimen lasting seven days.6 15 16 18 19 20 21 22 23 24 25 26 30 33 36 38 39 42 43 46 48 52 No trial was at low risk of bias, and two trials did not report full data on the proton pump inhibitor used.24 48 As shown in figure 2, the pooled relative risk was 1.21 (95% confidence interval 1.17 to 1.25), favouring sequential treatment, the number needed to treat was 6 (95% confidence interval 5 to 7), and the 95% prediction intervals ranged from 1.10 to 1.33. We found evidence of heterogeneity (I2=29.3%; P=0.098), without funnel plot asymmetry (Egger’s test coefficient −0.69, 90% confidence interval −1.71 to 0.33; P=0.257).
Fig 2 Forest plot of sequential therapy versus seven day triple therapy
In all, 2449 patients were treated with the sequential therapy compared with 2566 patients treated with triple therapy lasting seven days, and the eradication rate reported was 86.5% (95% confidence interval 82.9% to 89.7%) for the sequential therapy and 71.5% (68.4% to 74.5%) for the triple therapy. The difference in eradication rates was 15% (13% to 18%) favouring sequential treatment, and the 95% prediction intervals ranged from 9% to 22% (supplementary figure A), with evidence of heterogeneity (I2=34.0%; P=0.061).
Because of the heterogeneity, we did subgroup analyses according to country of origin, use of tinidazole in the sequential therapy, type of publication, and proton pump inhibitor used; we did not evaluate risk of bias, as all trials were at high or unclear risk of bias (supplementary table B). We found a slightly statistically significant effect in favour of sequential therapy in trials conducted in China, Italy, Korea, and Morocco.
One of these studies compared sequential therapy with both proton pump inhibitor-amoxicillin-clarithromycin and proton pump inhibitor-amoxicillin-metronidazole.48 The eradication rate of sequential therapy was 24.0% (13.6% to 33.5%) higher than proton pump inhibitor-amoxicillin-clarithromycin and 15.9% (7.1% to 25.1%) higher than proton pump inhibitor-amoxicillin-metronidazole. One trial also compared sequential therapy with a modified triple therapy (and for this reason we did not include this arm in the meta-analysis) consisting of proton pump inhibitor twice daily, clarithromycin 500 mg twice daily, and amoxicillin 1000 mg three times daily for seven days. No significant difference in eradication rate was observed between the two treatments (P=0.750).38
Data on adverse events were available in 18 trials.6 16 18 19 20 21 23 25 26 30 33 38 39 42 43 46 48 52 The pooled relative risk was 1.11 (0.97 to 1.27), indicating no significant difference, with no evidence of heterogeneity (I2=0%; P=0.918).
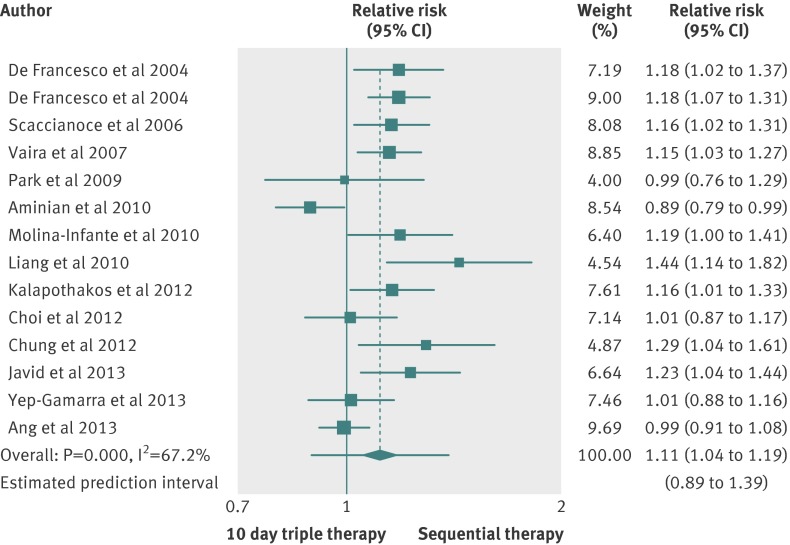
Sequential therapy versus triple therapy lasting 10 days
Fourteen studies compared sequential therapy with a triple therapy regimen lasting 10 days.7 16 17 19 24 27 28 29 39 44 45 51 53 57 Only two trials were at low risk of bias,7 51 and three trials did not report full data on the proton pump inhibitor used.24 45 57 As shown in figure 3, the pooled relative risk was 1.11 (1.04 to 1.19), slightly favouring sequential treatment, the 95% prediction intervals ranged from 0.89 to 1.39, and the number needed to treat was 10 (7 to 15). We found evidence of heterogeneity (I2=67.2%; P=0.000) but no funnel plot asymmetry (Egger’s test coefficient 2.35, −1.96 to 4.90; P=0.126). In all, 1368 patients were treated with the sequential therapy compared with 1378 patients treated with triple therapy lasting 10 days, and the eradication rate reported was 84.3% (79.8% to 88.4%) for the sequential therapy and 75.3% (69.6% to 77.9%) for the triple therapy lasting 10 days. The difference in eradication rates was 9.0% (4% to 14%), favouring sequential treatment, the 95% prediction intervals ranged from −9% to 26% (supplementary figure B), with evidence of heterogeneity (I2=66.4%; P=0.000).
Fig 3 Forest plot of sequential therapy versus 10 day triple therapy
Because of the heterogeneity, we did subgroup analyses according to country of origin, use of tinidazole (instead of metronidazole) in the sequential therapy, risk of bias, type of publication, and proton pump inhibitor used (supplementary table C). We found a slightly statistically significant effect in favour of sequential therapy in trials conducted in China, Greece, India, Italy, and Spain.
Data on adverse events were available for 11 trials.7 16 19 27 28 29 39 44 45 51 53 The pooled relative risk was 0.94 (0.79 to 1.13), indicating no significant difference, with no evidence of heterogeneity (I2=0%; P=0.642).
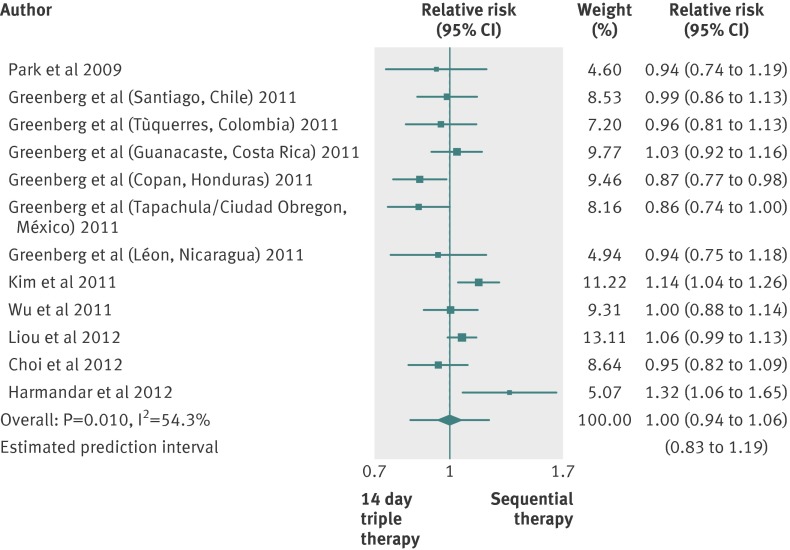
Sequential therapy versus triple therapy lasting 14 days
Seven studies compared sequential therapy with a triple therapy regimen lasting 14 days.24 34 35 37 39 49 50 No trial was at low risk of bias, and one trial did not report full data on the proton pump inhibitor used.24 As shown in figure 4, the pooled relative risk was 1.00 (0.94 to 1.06), and the 95% prediction intervals ranged from 0.83 to 1.19. We found evidence of heterogeneity (I2=54.3%; P=0.010) but no funnel plot asymmetry (Egger’s test coefficient -1.564, −3.46 to 0.33; P=0.167). In all, 1224 patients were treated with the sequential therapy compared with 1227 patients treated with triple therapy lasting 14 days, and the eradication rate reported was 80.8% (76% to 85.1%) for the sequential therapy and 81.3% (79.5% to 84.7%) for the triple therapy. The difference in eradication rates was −0.5% (−5% to 5%), and the 95% prediction intervals ranged from −16% to 15% (supplementary figure C), with evidence of heterogeneity (I2=61.1%; P=0.003).
Fig 4 Forest plot of sequential therapy versus 14 day triple therapy
Because of the heterogeneity, we did subgroup analyses according to country of origin, use of tinidazole in the sequential therapy, type of publication, and proton pump inhibitor used; we did not evaluate risk of bias, as all trials were at high or unclear risk of bias (supplementary table D). We found no statistically significant differences.
Data on adverse events were available for four trials.35 37 39 50 The pooled relative risk was 0.98 (0.73 to 1.33), indicating no significant difference, with evidence of heterogeneity (I2=47.8%; P=0.125).
Effect of duration of triple therapies on eradication rate
The analysis of the studies comparing triple therapies of different durations with sequential therapy allowed us to evaluate the effect of length of the triple regimens on the eradication rate. We found a significant trend (χ2 for trend: P<0.001) between the duration of therapy and the success of treatment, even if the clinical gain was modest. Therapy lasting 14 days eradicated 9.8% (6.3% to 11.9%) and 6% (2.9% to 9.3%) more infections than therapy lasting seven and 10 days. We found no significant difference in eradication rate between therapy lasting seven and 10 days (3.8%, −0.1% to 5.8%).
Sequential therapy versus bismuth containing therapies
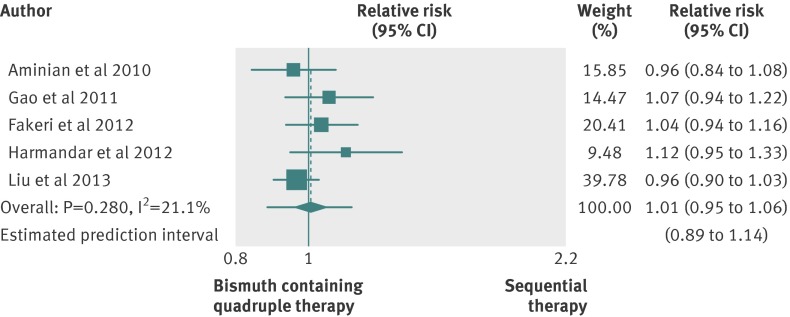
In three studies bismuth containing therapies lasted 14 days,27 40 49 whereas in two trials they had a 10 day duration.33 56 Different bismuth formulations were used, including colloid bismuth subcitrate, bismuth pectin, and other unspecified bismuth salts. In one of the studies lasting two weeks, furazolidone was given during the first week of treatment.40 No trial was at low risk of bias.
The pooled relative risk was 1.01 (0.95 to 1.06) (fig 5), and the 95% prediction intervals ranged from 0.89 to 1.14. We found evidence of low heterogeneity (I2=21.1%; P=0.280) and no evidence of funnel plot asymmetry (Egger’s test coefficient 2.32, −0.32 to 4.9; P=0.131). The eradication rate with bismuth containing therapies, compared with the efficacy of sequential therapy, was not affected by the duration (P=0.592).
Fig 5 Forest plot of sequential therapy versus bismuth containing therapies
In all, 546 patients were treated with the sequential therapy compared with 545 patients treated with bismuth containing quadruple therapy, and the eradication rate reported was 86.2% (82.1% to 89.8%) for the sequential therapy and 84.9% (78.8% to 90.1%) for the bismuth containing quadruple therapy. The difference in eradication rates was 1.3% (−4% to 5%), and the 95% prediction intervals ranged from −11% to 12% (supplementary figure D), with low heterogeneity (I2=22.3%; P=0.272).
Data on adverse events were available for four studies.27 33 40 56 The pooled relative risk was 1.08 (0.71 to 1.63), indicating no significant difference, with evidence of heterogeneity (I2=25.5%; P=0.258).
Sequential therapy versus non-bismuth quadruple therapy
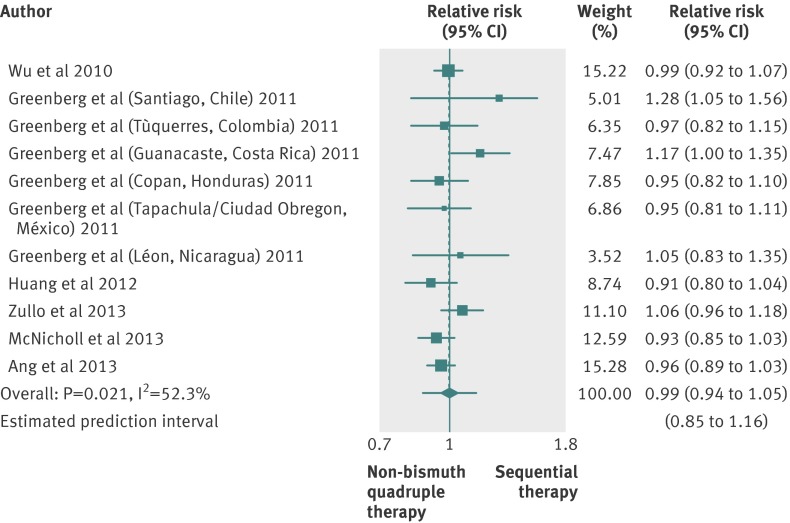
Six studies compared sequential therapy with a non-bismuth containing quadruple therapy regimen (sometimes called concomitant therapy), including a proton pump inhibitor, amoxicillin, clarithromycin, and metronidazole.31 34 41 55 57 58 In four trials, non-bismuth quadruple therapy lasted 10 days,31 41 55 57 and in the remaining two it lasted five days,34 58 and one trial did not report full data on the proton pump inhibitor used.57 Only one trials was at low risk of bias.55
The pooled relative risk was 0.99 (0.94 to 1.05) (fig 6), and the 95% prediction intervals ranged from 0.85 to 1.16. We found evidence of heterogeneity (I2=52.3%; P=0.021) and no evidence of funnel plot asymmetry (Egger’s test coefficient 0.96, −0.72 to 2.65; P=0.324).
Fig 6 Forest plot of sequential therapy versus non-bismuth containing quadruple therapy
In all, 1039 patients were treated with sequential therapy compared with 1031 patients treated with concomitant therapy, and the eradication rate reported was 81.7% (76.1% to 86.7%) for sequential therapy and 81.3% (74.9% to 87%) for the non-bismuth containing quadruple therapy. The difference in eradication rates was 0.4% (−4% to 4%), and the 95% prediction intervals ranged from −14% to 13% (supplementary figure E), with evidence of heterogeneity (I2=55.3%; P=0.014).
Because of the heterogeneity, we did subgroup analyses according to country of origin, risk of bias, proton pump inhibitor used, duration of non-bismuth containing quadruple therapy, type of publication, and use of tinidazole in the sequential therapy (supplementary table E). We found a slightly statistically significant effect in favour of sequential therapy in trials conducted in Chile and Costa Rica.
Data on adverse events were available for four studies.31 41 55 58 The pooled relative risk was 0.92 (0.77 to 1.10), indicating no significant difference, with evidence of heterogeneity (I2=33%; P=0.214).
Data not included in meta-analysis
Sequential therapy versus levofloxacin based sequential therapy
Three studies compared sequential therapy with a modified sequential therapy in which levofloxacin was used instead of the clarithromycin used in the original scheme.29 32 47 Overall, 355 patients were randomised to sequential therapy and 356 patients were randomised to the modified sequential therapy. One study compared sequential therapy with a modified sequential therapy using levofloxacin at dosages of 500 mg daily and 1000 mg daily.32 Of the remaining two, one used levofloxacin at a dosage of 1000 mg daily,29 and the other used it at a dosage of 500 mg daily.47
In the studies using levofloxacin 1000 mg daily, 240 patients were randomised to standard sequential therapy and 240 to the levofloxacin based sequential therapy. The overall eradication rates of sequential therapy and of levofloxacin based sequential therapy were 78.7% (73% to 83.7%) and 90% (85.4% to 93.4%), with a difference of −11.3% (−4.8% to −17.7%) in favour of modified sequential therapy. Adverse event rates were 24.4% (19.1% to 30.4%) with the standard sequential therapy and 24.3% (19.1% to 30.4%) with the levofloxacin based sequential therapy, with a non-significant difference of 0.1% (−7.8% to 7.6%).
In the studies using levofloxacin 500 mg daily, 240 patients were randomised to standard sequential therapy and 241 to the levofloxacin based sequential therapy. The overall eradication rates of sequential therapy and levofloxacin based sequential therapy were 79.5% (73.9% to 84.4%) and 89.6% (85% to 93.1%), with a difference of −10.1% (−3.6% to −16.5%) in favour of modified sequential therapy. Adverse event rates were 14.3% (10.1% to 19.4%) with the standard sequential therapy and 13.8% (9.7% to 18.9%) with the levofloxacin based sequential therapy, with a non-significant difference of 0.5% (−6.8% to 5.9%). The eradication rates of the modified sequential therapy using 1000 mg and 500 mg daily of levofloxacin were not significant different (difference 0.4%, −5.1% to 5.9%).
10 day sequential therapy versus 14 day sequential therapy
Only two studies compared sequential therapy lasting 10 days with sequential therapy lasting 14 days.49 50 In all, 340 patients were randomised to the 10 day therapy and 340 to the 14 day therapy. The overall eradication rates of 10 and 14 day sequential therapy were 87.6% (83.6% to 90.9%) and 89.7% (85.9% to 92.7%), with a non-significant difference of −2.1% (−6.9% to 2.7%). Adverse events were reported in one study only,50 and no significant difference was seen between treatments (difference 5.5%, −13.5% to 2.5%).
Sequential therapy versus hybrid therapy
Hybrid therapy is an evolution of sequential therapy that uses a proton pump inhibitor twice daily and amoxicillin 1 g twice daily for 14 days plus 500 mg clarithromycin and nitroimidazole derivatives, both twice daily, for the last seven days. Two randomised controlled trials compared sequential therapy with hybrid therapy: 300 patients were randomised to each treatment.54 58 The eradication rate was 81% (76% to 85.2%) for the sequential therapy and 86.6% (82.2% to 90%) for the hybrid therapy, with no significant difference (−5.6%, −11.6% to 0.2%).
Adverse event rates were 23% (18.3% to 28.1%) for the sequential therapy and 27% (22% to 32.2%) for the hybrid therapy, with no significant difference between the two therapies (−4%, −10.9% to 2.9%).
Eradication rate of sequential therapy according to antimicrobial resistance
Eight studies provided data on eradication according to pre-treatment antimicrobial susceptibility testing.6 7 31 32 36 41 44 50 Two studies were at low risk of bias.7 32 However, the number of patients with antimicrobial susceptibility testing was small and the 95% confidence intervals for eradication rates were wide, so caution is necessary in interpreting the following results.
Strains resistant to clarithromycin
The overall eradication rate of sequential therapy in patients harbouring strains resistant to clarithromycin was 72.8% (61.6% to 82.8%). In patients harbouring strains resistant to clarithromycin, sequential therapy achieved a significantly higher eradication rate than did triple therapy lasting seven (difference in eradication rate 49.6%, 27.7% to 66.9%) and 10 days (49.8%, 20.3% to 70.5%). However, compared with triple therapy lasting 14 days, non-bismuth containing quadruple therapy, or sequential therapy in which levofloxacin was used instead of clarithromycin, no significant difference was seen (supplementary table F).
Strains resistant to metronidazole
The overall eradication rate of sequential therapy in patients harbouring strains resistant to metronidazole was 86.4% (78% to 93%). In patients harbouring strains resistant to metronidazole, sequential therapy achieved a significantly higher eradication rate than did triple therapy lasting seven (difference in eradication rate 24.1%, 7.2% to 41.3%) and 10 days (17%, 2.1% to 32.7%). Triple therapy lasting 14 days achieved a slightly higher eradication than sequential therapy (difference in eradication rate 16.4%, 0.14% to 32.8%). However, when we compared sequential therapy with non-bismuth containing quadruple therapy or sequential therapy in which levofloxacin was used instead of clarithromycin, we found no significant difference (supplementary table G).
Strains resistant to both clarithromycin and metronidazole
The overall eradication rate of sequential therapy in patients harbouring strains resistant to both clarithromycin and metronidazole was just 37% (16.2% to 60.7%). Only sequential therapy in which levofloxacin was used instead of clarithromycin was able to overcome the resistance to both antimicrobials (supplementary table H).
Strains resistant to levofloxacin
Only one study tested primary resistance to levofloxacin.32 No significant difference in eradication rate was found between the sequential therapy and the “modified” sequential therapy (in which levofloxacin was used instead of clarithromycin), but the number of patients studied was extremely low (supplementary table I).
Overall eradication rate of sequential therapy
In the 46 randomised controlled trials, 5666 patients were randomised to receive sequential treatment, and the overall eradication rate, pooled with a random model, was 84.3% (82.1% to 86.4%). Supplementary figure F shows the eradication rates of sequential treatment according to the country of origin.
Discussion
The results of this meta-analysis clearly show that, for eradication of Helicobacter pylori infection, sequential therapy is superior to seven day triple therapy and similar to regimens of longer duration and those including more than two antimicrobial agents.
Implications of findings
H pylori infection has been shown to cause peptic ulcer disease, gastric mucosa associated lymphoid tissue lymphoma, and gastric cancer.1 A recent study estimated that the number of new cases of gastric cancer (non-cardia gastric carcinoma and gastric mucosa associated lymphoid tissue lymphoma) attributable to H pylori infection in 2008 was 470 000 in less developed regions and 190 000 in the more developed regions of the world.59 Eradication therapy has been shown to improve outcomes in peptic ulcer disease as well as mucosa associated lymphoid tissue lymphoma, by reducing recurrence.5 Given the importance of eradicating H pylori infection in patients with ulcer disease, the infection should be treated optimally with a combination regimen that has an acceptably high eradication rate.
The triple treatment including a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole to treat H pylori infection, proposed at the first Maastricht conference,60 has become universal since all the consensus conferences and guidelines around the world recommended it. However, the most recent data show that this combination has lost efficacy, with an eradication rate ranging from 71% in the United States to 60% in Western Europe.5 61 62 The eradication rate is uniformly less than the 80% target set at the beginning and well below what should be expected for treatment of an infectious disease.63 64 65
One of the main reasons for this poor performance is the increasing number of strains of H pylori that are resistant to antibiotics. A recent European multicentre survey found that primary resistance (that is, the resistance found in patients infected but never treated) for clarithromycin was 17.5% (an increase in comparison with earlier surveys66 67), resistance to levofloxacin had already reached 14.1%, and the resistance to metronidazole remained stable at 34.9%.4
Investigators in different parts of the world have made several attempts to find new regimens as a more effective alternative to traditional triple therapies. One of these is sequential therapy.68 Analysis of the first studies showed promising results, with eradication rates consistently higher than 90%.69 70 However, the number of studies was small and most of them were done in Italy. Since then, several randomised controlled trials have been carried out in different parts of the world comparing sequential therapy with traditional and novel treatments, providing a glimpse into current eradication rates with new and old treatments.
Our results show that sequential therapy is clearly superior to the triple therapy regimen administered for seven days. But when a comparison was made with triple therapy lasting 10 days, the results were less clear-cut. Although the estimate of the effect size was better for the sequential therapy, prediction intervals were not significant, suggesting that future trials may not be able to observe a superior effect. When the comparison was made with triple therapy lasting 14 days and with bismuth based and non-bismuth based quadruple therapies, the sequential regimen was not significantly superior to any of these treatments.
Pre-treatment susceptibility data were also available in eight studies.6 7 31 32 36 41 44 50 Previous data suggested a high eradication rate with sequential therapy in strains resistant to clarithromycin.6 7 Our data show that sequential therapy was able to eradicate 72.8% of strains resistant to clarithromycin. In the head to head comparisons, sequential therapy achieved significantly better results only when compared with triple therapy lasting seven or 10 days; we found no significant differences in other comparisons. For the strains resistant to metronidazole, the pooled eradication rate was 86.4%; however, as in the previous case, sequential therapy achieved significantly better results only when compared with triple therapy lasting seven or 10 days. For the strains resistant to both clarithromycin and metronidazole, only sequential therapy in which levofloxacin was used instead of clarithromycin was able to significantly overcome the resistance to both antimicrobials. Data on resistance and eradication should, however, be interpreted with caution owing to the small number of patients included in the trials.
If we consider the studies that could not be included in the meta-analysis (our criteria required at least three comparable study groups for every comparison), the modified levofloxacin based sequential therapy showed eradication rates of about 90% and was able to achieve a high eradication rate in strains resistant to clarithromycin, metronidazole, or both. Recently, a new therapy consisting of proton pump inhibitor, amoxicillin 1 g, levofloxacin 500 mg, and tinidazole 500 mg, all given twice daily for five days, has been shown to reach a high eradication rate.71 However, the prevalence of primary resistance to levofloxacin found by the authors in this study was quite low (7.9%) compared with that reported by a recent multicentre survey in southern Europe (13.1%).4 Large well conducted trials that include areas of high resistance are required before this treatment can be considered effective. A hybrid therapy consisting of proton pump inhibitor and amoxicillin 1 g twice daily for 14 days plus clarithromycin 500 mg and tinidazole 500 mg twice daily for the last seven days gave an eradication rate of 86.6%. Additional studies confirming these findings are awaited. Finally, two studies compared sequential therapy lasting 10 and 14 days. The first was a large multicentre, open label, randomised trial, conducted in six centres in Taiwan,50 and the treatment efficacy was similar between the sequential therapy lasting 10 days (87%) and that lasting 14 days (90.7%) (P=0.153). The second study, a randomised controlled trial conducted in Turkey and published only as an abstract, also found no significant difference.49
Recently published data are in line with the poor eradication rates found by our systematic review. In a meta-analysis, bismuth based quadruple therapy failed to show an improvement in eradication rate compared with the triple therapy.72 A large randomised, open label, non-inferiority, phase III trial conducted at 39 sites in Europe compared the efficacy and safety of 10 days’ therapy with omeprazole plus a single three in one capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline (quadruple therapy) with seven days of triple therapy in adults. The eradication rate, according to the intention to treat analysis, was 80% (95% confidence interval 73.9% to 84.9%) for quadruple therapy compared with only 55% (48.6% to 62.1%) for the triple therapy.61 Another meta-analysis, using a fixed effect model, showed that non-bismuth based quadruple therapy was superior to the triple therapy. However, by adopting a more conservative analysis (random effect model), we observed that the prediction interval ranged from 0.92 to 1.40, suggesting that future trials may not be able to detect a superior effect.73 As the response to eradication therapy is related to the prevalence of primary resistance in the population, the choice of a treatment regimen should be based on a knowledge of the underlying prevalence of resistant strains in the community and on the patient’s history (no treatment or previous treatments).74 National monitoring studies such as one started (but later discontinued) in the United States may help clinicians to choose the appropriate therapy.75
One of the studies included in our meta-analysis was a multicentre trial conducted in several South American countries.34 We decided to pool eradication data separately for each country. As recently shown,76 a significant dispersion can be seen across the countries, highlighted by the values of I2. Part of this dispersion could possibly be due to the differing prevalence of resistant organisms in the individual countries. The analysis by country shows that in Colombia, Mexico, and Nicaragua, no significant difference existed between treatments tested. In the other three countries, the results were different: in Chile, the non-bismuth based quadruple therapy was inferior to the triple therapy lasting 14 days and to sequential therapy; in Costa Rica, sequential therapy was superior to the non-bismuth based quadruple therapy; and in Honduras, triple therapy lasting 14 days was superior to sequential therapy.
Limitations of study
When considering the results of this meta-analysis, some limitations should be acknowledged. As with any systematic review and meta-analysis, the results rely on the quality and reporting of the trials. Most of the studies included had problems with concealment of allocation and blinding, which are important safeguards against bias in randomised controlled trials.77 Allocation concealment seeks to prevent selection bias by concealing what treatment the next patient will receive before and until assignment.77 78 In contrast, blinding seeks to prevent performance and detection bias by protecting the sequence after assignment.77 79 Empirical studies show that the effects of experimental interventions measured as odds ratios are exaggerated on average by 21% if allocation concealment is unclear or inadequate and by 18% if trials are not reported as “double blind.”78 80 81 82 83 We also found significant heterogeneity, and subgroup analyses failed to identify plausible explanations. Subgroup analyses are entirely observational in their nature and have the limitations of any observational investigation, including possible bias through confounding by other study level characteristics.9 Therefore, the presence of heterogeneity without plausible explanations, as well as the presence of only a few trials at low risk of bias, affects the quality and the strength of the evidence.84 The applicability of the results should also be viewed with caution, as information regarding the efficacy of the sequential therapy in several Western countries is lacking. No studies have been reported from Canada, and few southern European countries are represented. One open label, randomised controlled trial is ongoing in the United States, comparing triple therapy containing clarithromycin and lasting 10 days with sequential therapy.85 Finally, data on the response of treatments according to pre-treatment sensitivity was available in a minority of the overall patients studied, not allowing a thorough analysis of the results.
Conclusion
Sequential therapy is superior to seven day triple therapy and similar to regimens of longer duration or including more than two antimicrobial agents. The search for a new agent to treat H pylori is important and should continue, but until such an agent is discovered, any single therapy is unlikely to be effective all over the world.
What is already known on this topic
Triple treatments including a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole have lost efficacy, which is now uniformly less than the 80% target set initially
Several attempts have been made to find new regimens as a more effective alternative to treat H pylori
One such regimen is sequential therapy, originally found to give eradication rates consistently higher than 90%
What this study adds
The overall efficacy of sequential therapy is 84.3% (95% confidence interval 82.1% to 86.4%)
This is superior to seven day triple therapy and similar to regimens of longer duration or including more than two antimicrobial agents
The search for more effective eradication regimens or for new agents to treat H pylori should therefore continue
We are grateful to the following investigators for providing information from their studies: F Franceschi (Rome, Italy), E R Greenberg (Seattle, USA), J Molina-Infante (Caceres, Spain), J M Liou (Taipei, Taiwan), J W Chung (Incheon, Korea), M Singh (Sabah, Malaysia), H Sardarian (Sari, Iran), P N Kalapothakos (Sparti, Greece), M Lahbabi (Fez, Morocco), K Liu (Hong Kong, China), T L Ang (Singapore), A Zullo (Rome, Italy), V Yep-Gamarra (Trujillo, Peru).
Contributors: LG, CS, NV, and DV conceived the study. LG, CS, and DV collected the data. LG and CS analysed and interpreted the data and drafted the manuscript. All authors commented on the drafts and approved the final draft. LG is the guarantor.
Funding: None.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; CS has received consulting fees for advisory committees or review panels from Pfizer, Janssen-Cilag, and Sidem and for speaking and teaching from AstraZeneca; NV has received consulting fees for speaking and teaching from AstraZeneca, Takeda, Ironwood, and Otsuka and has ownership interest (stock shareholder) in Meridian Diagnostics and Orexo; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not needed.
Data sharing: No additional data available.
Cite this as: BMJ 2013;347:f4587
Web Extra. Extra material supplied by the authors
Supplementary tables
Supplementary figures
References
- 1.McColl KE. Clinical practice: Helicobacter pylori infection. N Engl J Med 2010;362:1597-604. [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection-the Maastricht IV/ Florence consensus report. Gut 2012;61:646-64. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808-25. [DOI] [PubMed] [Google Scholar]
- 4.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34-42. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N. Helicobacter pylori treatment: a practical approach. Am J Gastroenterol 2006;101:497-9. [DOI] [PubMed] [Google Scholar]
- 6.Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003;17:719-26. [DOI] [PubMed] [Google Scholar]
- 7.Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med 2007;146:556-63. [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011 (available from www.cochrane-handbook.org).
- 10.DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med 1996;15:1237-48. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham PL, Moran JL. Robust meta-analytic conclusions mandate the provision of prediction intervals in meta-analysis summaries. J Clin Epidemiol 2012;65:503-10. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Focareta R, Forte G, Forte F, Ciarleglio A, Grimaldi E, Ievoli F, et al. Could the 10 days sequrntial therapy be considered a first choice treatment for the eradication of Helicobacter pylori infection? Dig Liver Dis 2003;35(suppl 5):S33. [Google Scholar]
- 16.De Francesco V, Zullo A, Hassan C, Della VN, Pietrini L, et al. The prolongation of triple therapy for Helicobacter pylori does not allow reaching therapeutic outcome of sequential scheme: a prospective, randomised study. Dig Liver Dis 2004;36:322-6. [DOI] [PubMed] [Google Scholar]
- 17.De Francesco V, Zullo A, Margiotta M, Marangi S, Burattini O, Berloco P, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004;19:407-14. [DOI] [PubMed] [Google Scholar]
- 18.Zullo A, Gatta L, De Francesco V, Hassan C, Ricci C, Bernabucci V, et al. High rate of Helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: a prospective controlled study. Aliment Pharmacol Ther 2005;21:1419-24. [DOI] [PubMed] [Google Scholar]
- 19.Scaccianoce G, Hassan C, Panarese A, Piglionica D, Morini S, Zullo A. Helicobacter pylori eradication with either 7-day or 10-day triple therapies, and with a 10-day sequential regimen. Can J Gastroenterol 2006;20:113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol 2008;51:280-4. [PubMed] [Google Scholar]
- 21.Ma CX, Peng GL, Zhao YK, Zhang XY, Zhan LY. Treatment outcomes of functional dyspepsia patients with Helicobacter pylori infection: a comparison between sequential treatment regimen and conventional triple therapy. Acad J Second Mil Med Univ 2008;29:908-11. [Google Scholar]
- 22.Wu HY, Hsi YT, Lam JTW, Lam WM. Ten-day sequential therapy as the first-line eradication of Helicobacter pylori infection in chinese patients: a randomized-controlled trial. Gut 2008;57(suppl 2):A330. [Google Scholar]
- 23.Hu SQ, Zhang M. A 10-day sequential therapy for Helicobacter pylori-infected patients: an analysis of 39 cases. World Chin J Dig 2009;17:1693-5. [Google Scholar]
- 24.Park S, Chun HJ, Kim ES, Park SC, Jung ES, Lee SD, et al. The 10-day sequential therapy for Helicobacter pylori eradication in Korea: less effective than expected. Gastroenterology 2009;136:A339-40. [Google Scholar]
- 25.Zhao QX, Huang DY. Efficacy of tinidazole-containing sequential therapy in the eradication of Helicobacter pylori infection. World Chin J Dig 2009;17:3666-9. [Google Scholar]
- 26.Paoluzi OA, Visconti E, Andrei F, Tosti C, Lionetti R, Grasso E, et al. Ten and eight-day sequential therapy in comparison to standard triple therapy for eradicating Helicobacter pylori infection: a randomized controlled study on efficacy and tolerability. J Clin Gastroenterol 2010;44:261-6. [DOI] [PubMed] [Google Scholar]
- 27.Aminian K, Farsad F, Ghanbari A, Fakhreih S, Hasheminasab SM. A randomized trial comparing four Helicobacter pylori eradication regimens: standard triple therapy, ciprofloxacin based triple therapy, quadruple and sequential therapy. Trop Gastroenterol 2010;31:303-7. [PubMed] [Google Scholar]
- 28.Liang Z, Quan H, Xu G, Yu A. Follow-up study of 10-day sequential therapy on eradication of Helicobacter pylori. Chin J Gastroenterol 2010;15:483-5. [Google Scholar]
- 29.Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M, Hernandez-Alonso M, Vinagre G, Duenas C, et al. Clinical trial: clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther 2010;31:1077-84. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Huo H, Jiang X, Zhou J. Clinical study on effects of different eradication therapies for Helicobacter pylori on functional dyspepsia. Chin J Gastroenterol 2010;15:240-2. [Google Scholar]
- 31.Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, e al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol 2010;8:36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut 2010;59:1465-70. [DOI] [PubMed] [Google Scholar]
- 33.Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World J Gastroenterol 2010;16:4357-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 2011;378:507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YS, Kim SJ, Yoon JH, Suk KT, Kim JB, Kim DJ, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther 2011;34:1098-105. [DOI] [PubMed] [Google Scholar]
- 36.Gatta L, Vaira D, Vakil N, Ricci C, Fiorini G, Castelli V, et al. Sequential therapy vs. standard triple therapy lasting 7 days: results of a prospective study. Gastroenterology 2011;140(suppl 1):S148. [Google Scholar]
- 37.Wu GL, Lan Y, Zhang XJ. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication. World Chin J Dig 2011;19:3100-3. [Google Scholar]
- 38.Franceschi F, Campanale M, Finizio R, Barbaro F, Tortora A, Gigante G, et al. High dose amoxicillin-based first line regimen compared to sequential therapy in the eradication of H. pylori infection. Gastroenterology 2012;142:S487. [Google Scholar]
- 39.Choi HS, Chun HJ, Park SH, Keum B, Seo YS, Kim YS, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol 2012;18:2377-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fakheri H, Taghvaei T, Hosseini V, Bari Z. A comparison between sequential therapy and a modified bismuth-based quadruple therapy for Helicobacter pylori eradication in Iran: a randomized clinical trial. Helicobacter 2012;17:43-8. [DOI] [PubMed] [Google Scholar]
- 41.Huang YK, Wu MC, Wang SS, Kuo CH, Lee YC, Chang LL, et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis 2012;13:232-8. [DOI] [PubMed] [Google Scholar]
- 42.Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol 2012;27:504-9. [DOI] [PubMed] [Google Scholar]
- 43.Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, et al. Randomised clinical trial: comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naive patients. Aliment Pharmacol Ther 2012;35:56-65. [DOI] [PubMed] [Google Scholar]
- 44.Chung JW, Jung YK, Kim YJ, Kwon KA, Kim JH, Lee JJ, et al. Ten-day sequential versus triple therapy for H. pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol 2012;27:1675-80. [DOI] [PubMed] [Google Scholar]
- 45.Kalapothakos P, Liantiniotis G, Koulentis M, Koutoufaris G, Georgantas P, Rigas S. Ten days sequential treatment for Helicobacter pylori eradication in clinical practice in Greece. Gut 2012;61(suppl 3):A444. [Google Scholar]
- 46.Singh M, Way LG, Logasamy N, Chettiar R, Na CS, Menon J. Helicobacter pylori eradication in East Malaysia: sequentail vs standard 7 day triple therapy—a prospective randomized trial. Gut 2012;61(suppl 3):A212. [Google Scholar]
- 47.Qian J, Ye F, Zhang J, Yang YM, Tu HM, Jiang Q, et al. Levofloxacin-containing triple and sequential therapy or standard sequential therapy as the first line treatment for Helicobacter pylori eradication in China. Helicobacter 2012;17:478-85. [DOI] [PubMed] [Google Scholar]
- 48.Lahbabi M, Alaoui S, El Rhazi K, El Abkari M, Nejjari C, Amarti A, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori infection: result of the HPFEZ randomised study. Clin Res Hepatol Gastroenterol 2012; published online 15 Nov. [DOI] [PubMed]
- 49.Harmandar FA, Ustundag Y, Buyukuysal C, Ilikhan S, Gun Dogan B. The efficacy of clarithromycin based sequential therapy in eradication of Helicobacter pylori in Turkey. Gut 2012;61(suppl 3):A443. [Google Scholar]
- 50.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomized trial. Lancet 2012;6736:61579-87. [DOI] [PubMed] [Google Scholar]
- 51.Javid G, Zargar SA, Bhat K, Khan BA, Yatoo GN, Gulzar GM, et al. Efficacy and safety of sequential therapy versus standard triple therapy in Helicobacter pylori eradication in Kashmir India: a randomized comparative trial. Indian J Gastroenterol 2013;32:190-4. [DOI] [PubMed] [Google Scholar]
- 52.Seddik H, Ahid S, El AT, El Hamdi FZ, Hassar M, Abouqal R, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a prospective randomized study. Eur J Clin Pharmacol 2013; published online 22 May. [DOI] [PubMed]
- 53.Yep-Gamarra V, Rodriguez-Ulloa C, Diaz-Velez C, Aldave-Herrera A, Donet JA, Rodas JI, et al. sequential versus triple standard therapy for Helicobacter pylori eradication. Gastroenterology 2013;144(suppl 1):S333. [Google Scholar]
- 54.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 2013;18:129-34. [DOI] [PubMed] [Google Scholar]
- 55.McNicholl AG, Marin AC, Molina-Infante J, Castro M, Barrio J, Ducons J, et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2013; published online 11 May. [DOI] [PubMed]
- 56.Liu SHK, Seto WK, Hung IF, Leung WK. 10-day sequential versus 10-day bismuth-containing quadruple therapy as empirical first-line treatment for Helicobacter pylori: an open label randomized crossover trial. Gastroenterology 2013;144(suppl 1):S54. [Google Scholar]
- 57.Ang TL, Fock KM, Ang D. A randomized controlled trial of triple therapy versus sequential therapy versus concomitant therapy as first line treatment for H. pylori Infection. Gastroenterology 2013;144(suppl 1):S53. [DOI] [PubMed] [Google Scholar]
- 58.Zullo A, Scaccianoce G, De Francesco V, Ruggiero V, D’Ambrosio P, Castorani L, et al. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol 2013; published online 5 June. [DOI] [PubMed]
- 59.De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607-15. [DOI] [PubMed] [Google Scholar]
- 60.Malfertheiner P, Megraud F, O’Morain C, Bell D, Bianchi PG, Deltenre M, et al. Current European concepts in the management of Helicobacter pylori infection—the Maastricht consensus report. Eur J Gastroenterol Hepatol 1997;9:1-2. [DOI] [PubMed] [Google Scholar]
- 61.Malfertheiner P, Bazzoli F, Delchier JC, Celinski K, Giguere M, Riviere M, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011;377:905-13. [DOI] [PubMed] [Google Scholar]
- 62.Gisbert JP. Sequential or concomitant therapy for Helicobacter pylori eradication? J Clin Gastroenterol 2010;44:313-25. [DOI] [PubMed] [Google Scholar]
- 63.Megraud F, O’Morain CA, Malfertheiner P, Axon AT, Hunt RH. Guidelines for clinical trials in Helicobacter pylori infection. Gut 1997;41(suppl 2):S1-9. [PubMed] [Google Scholar]
- 64.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59:1143-53. [DOI] [PubMed] [Google Scholar]
- 65.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007;12:275-8. [DOI] [PubMed] [Google Scholar]
- 66.European Study Group on Antibiotic Susceptibility of Helicobacter pylori. Results of a multicentre European survey in 1991 of metronidazole resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis 1992;11:777-81. [PubMed] [Google Scholar]
- 67.Glupczynski Y, Megraud F, Lopez-Brea M, Andersen LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis 2001;20:820-3. [DOI] [PubMed] [Google Scholar]
- 68.Zullo A, Rinaldi V, Winn S, Meddi P, Lionetti R, Hassan C, et al. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther 2000;14:715-8. [DOI] [PubMed] [Google Scholar]
- 69.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009;104:3069-79. [DOI] [PubMed] [Google Scholar]
- 70.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med 2008;148:923-31. [DOI] [PubMed] [Google Scholar]
- 71.Federico A, Nardone G, Gravina AG, Iovene MR, Miranda A, Compare D, et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology 2012;143:55-61. [DOI] [PubMed] [Google Scholar]
- 72.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol 2010;105:65-73. [DOI] [PubMed] [Google Scholar]
- 73.Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol 2012;5:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence based medicine rather than medicine based evidence. Clin Gastroenterol Hepatol 2013; published online 7 June. [DOI] [PMC free article] [PubMed]
- 75.Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis 2004;10:1088-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gatta L, Vakil N, Vaira D. Treatment of Helicobacter pylori in Latin America. Lancet 2012;379:407-8. [DOI] [PubMed] [Google Scholar]
- 77.Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed] [Google Scholar]
- 79.Schulz KF, Chalmers I, Altman DG. The landscape and lexicon of blinding in randomized trials. Ann Intern Med 2002;136:254-9. [DOI] [PubMed] [Google Scholar]
- 80.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
- 81.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-9. [DOI] [PubMed] [Google Scholar]
- 82.Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess 2003;7:1-76. [PubMed] [Google Scholar]
- 83.Balk EM, Bonis PA, Moskowitz H, Schmid CH, Ioannidis JP, Wang C, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA 2002;287:2973-82. [DOI] [PubMed] [Google Scholar]
- 84.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conventional therapy vs sequential therapy in treatment of Helicobacter pylori infection. http://clinicaltrials.gov/ct2/show/NCT01723059.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Supplementary figures