Abstract
Blood flow plays crucial roles in vascular development, remodeling and homeostasis, but the molecular pathways required for transducing flow signals are not well understood. In zebrafish embryos, arterial expression of activin receptor-like kinase 1 (alk1), which encodes a TGFβ family type I receptor, is dependent on blood flow, and loss of alk1 mimics lack of blood flow in terms of dysregulation of a subset of flow-responsive arterial genes and increased arterial endothelial cell number. These data suggest that blood flow activates Alk1 signaling to promote a flow-responsive gene expression program that limits nascent arterial caliber. Here, we demonstrate that restoration of endothelial alk1 expression to flow-deprived arteries fails to rescue Alk1 activity or normalize arterial endothelial cell gene expression or number, implying that blood flow may play an additional role in Alk1 signaling independent of alk1 induction. To this end, we define cardiac-derived Bmp10 as the crucial ligand for endothelial Alk1 in embryonic vascular development, and provide evidence that circulating Bmp10 acts through endothelial Alk1 to limit endothelial cell number in and thereby stabilize the caliber of nascent arteries. Thus, blood flow promotes Alk1 activity by concomitantly inducing alk1 expression and distributing Bmp10, thereby reinforcing this signaling pathway, which functions to limit arterial caliber at the onset of flow. Because mutations in ALK1 cause arteriovenous malformations (AVMs), our findings suggest that an impaired flow response initiates AVM development.
Keywords: Bmp10, Alk1/Acvrl1, Hereditary hemorrhagic telangiectasia, Arteriovenous malformation, Zebrafish, Flow response
INTRODUCTION
Blood flow imparts mechanical forces and distributes endocrine factors that influence vascular development and remodeling and allow maintenance of arterial-venous identity, but the molecular pathways that govern these flow-dependent processes are not fully understood (for a review, see Roman and Pekkan, 2012). Among the physical forces imparted by blood flow, shear stress, the frictional force that acts in the direction of blood flow, is the most extensively studied. Laminar shear stress induces expression of the transcription factor Klf2 which coordinates expression of numerous flow-responsive genes that promote cell cycle arrest and vasodilation, thereby favoring vascular quiescence and conferring atheroprotection (Dekker et al., 2002; Dekker et al., 2005; Dekker et al., 2006; Parmar et al., 2006). By contrast, disturbed (low or oscillatory) shear stress activates the transcription factors NF-κB and AP-1, which promote an atherogenic response characterized by inflammation and vasoconstriction (Lan et al., 1994; Khachigian et al., 1995; Bhullar et al., 1998; Hay et al., 2003). However, the repertoire of flow responses that exist in vivo clearly extends well beyond these pathways.
Mounting evidence implicates the TGFβ family type I receptor, Alk1, as a key player in a flow-responsive signaling pathway that functions to promote quiescence in nascent arteries. In zebrafish embryos, alk1 (acvrl1 - Zebrafish Information Network) is expressed predominantly in arteries proximal to the heart, which experience relatively high magnitudes of mechanical forces; preventing heartbeat eliminates alk1 mRNA expression (Corti et al., 2011). Furthermore, either loss of blood flow or loss of alk1 results in increased expression of cxcr4a, which encodes a pro-angiogenic chemokine receptor, and decreased expression of endothelin 1 (edn1), which encodes a vasoconstrictive peptide (Corti et al., 2011). Both of these genes are flow responsive in cultured endothelial cells (Wang et al., 1993; Melchionna et al., 2005), suggesting that Alk1 might lie upstream of cxcr4a and edn1 in a mechanosensitive signaling pathway. In support of this hypothesis, blood flow-mediated repression of cxcr4a correlates with diminished endothelial cell protrusive activity in nascent zebrafish arteries (Bussmann et al., 2011), and zebrafish alk1 mutants, which exhibit abnormally high levels of arterial cxcr4a, develop enlarged arteries containing supernumerary endothelial cells, suggestive of failed flow-induced suppression of endothelial cell migration or proliferation (Roman et al., 2002; Corti et al., 2011). Evidence from mice further supports the idea that Alk1 functions in a flow-responsive pathway to promote quiescence of nascent arteries. In mice, Alk1 is expressed predominantly in embryonic arterial endothelial cells, with weak expression in adults (Seki et al., 2003; Seki et al., 2004). However, Alk1 expression can be induced in adult mice during periods of active angiogenesis in arterial endothelial cells exposed to high shear stress (Seki et al., 2003). Furthermore, recent mouse studies have implicated bone morphogenetic protein (BMP) signaling in general or Alk1 signaling in particular in the maintenance of a quiescent endothelial stalk cell fate (Larrivée et al., 2012; Moya et al., 2012). Together, these data from both mouse and zebrafish support the hypothesis that Alk1 signaling mediates flow-dependent arterial endothelial cell quiescence. Notably, alk1 acts independently of klf2a in zebrafish (Corti et al., 2011), suggesting that multiple flow-dependent pathways coordinate in vivo to control the activation state of the endothelium.
Alk1 signaling is crucial for normal vascular development and homeostasis in mice and zebrafish, with loss of function resulting in embryonic lethality associated with development of direct connections between arteries and veins, or arteriovenous malformations (AVMs) (Oh et al., 2000; Urness et al., 2000; Roman et al., 2002). In humans, ALK1 heterozygosity results in hereditary hemorrhagic telangiectasia type 2 (HHT2), a vascular disorder characterized by predisposition to development of telangiectases and AVMs (Guttmacher et al., 1995; Johnson et al., 1996). However, despite the clear link between ALK1 signaling and AVM prevention, the ALK1 signaling pathway remains poorly defined in vivo. In TGFβ family signaling, ligands bind to a heterotetrameric complex of two type II receptors and two type I receptors, both of which are serine/threonine kinases. The type II receptors phosphorylate and thus activate the type I receptors, and the type I receptors then phosphorylate receptor-specific Smad proteins. Phosphorylated Smads complex with the common partner Smad, Smad4, enter the nucleus and, together with a variety of transcription factors, regulate transcription of target genes (for a review, see Xu et al., 2012). With respect to ALK1, the ligands TGFβ1, TGFβ3, BMP9 and BMP10 can induce ALK1-dependent phosphorylation of Smad1, Smad5 and/or Smad9 (hereafter referred to as Smad1/5/9) and stimulate activity of a phospho-Smad1/5/9 (pSmad1/5/9)-responsive reporter in cultured endothelial cells (ten Dijke et al., 1994; Lux et al., 1999; Oh et al., 2000; Goumans et al., 2002; Goumans et al., 2003; Brown et al., 2005; David et al., 2007; Scharpfenecker et al., 2007; David et al., 2008; Mitchell et al., 2010). However, although TGFβ-mediated activation of ALK1 requires ALK5 (canonical TGFβ1 type I receptor) activity in cultured endothelial cells (Goumans et al., 2003), endothelial cell-specific deletion of Alk5 in mice or Alk5 inhibition in zebrafish embryos does not affect Alk1 activity (Park et al., 2008), suggesting that TGFβ subfamily ligands are not relevant to Alk1 signaling in embryonic development. In fact, BMP9 and BMP10 are the only TGFβ superfamily ligands that bind to ALK1 with high affinity in vitro, and they circulate at physiologically relevant concentrations (Brown et al., 2005; David et al., 2008; Mitchell et al., 2010; Ricard et al., 2012). However, neither Bmp9- (Ricard et al., 2012) nor Bmp10- (Chen et al., 2004) null mice phenocopy Alk1-null mice (Oh et al., 2000; Urness et al., 2000), which present with AVMs. Although the lack of AVMs could reflect ligand redundancy, interference with both ligands via blocking antibodies and/or ligand traps impairs mouse retinal angiogenesis but does not produce retinal AVMs (Larrivée et al., 2012; Ricard et al., 2012). As such, the identity of the activating ALK1 ligand in vivo has remained elusive.
In this work, we use zebrafish embryos to demonstrate that Alk1 kinase activity, in addition to alk1 mRNA expression, requires blood flow, and we provide evidence that this newly defined role for blood flow stems not from mechanical force but from distribution of the cardiac-derived circulating ligand, Bmp10. Taken together, our data define a novel endocrine pathway in which circulating Bmp10 binds to endothelial cell Alk1 to induce phosphorylation of Smad1/5/9, which promotes a program of gene expression that limits endothelial cell number within nascent arteries in response to blood flow. Abrogation of this flow response results in enlarged arteries and ultimately AVMs.
MATERIALS AND METHODS
Zebrafish lines and maintenance
Adult zebrafish (Danio rerio) were maintained according to standard protocols (Westerfield, 1995). When appropriate, embryo medium was supplemented with 0.003% phenylthiourea (PTU) (Sigma, St Louis, MO, USA) at 24 hours post-fertilization (hpf) to prevent melanin synthesis. Mutant line alk1y6 and the alk1y6 genotyping assay have been described previously (Roman et al., 2002). Transgenic lines Tg(fli1a:negfp)y7, Tg(kdrl:gfp)la116, Tg(gata1a:DsRed)sd2 and Tg(fli1a.ep:mrfp-CAAX)pt505 have been described previously (Roman et al., 2002; Traver et al., 2003; Choi et al., 2007; Corti et al., 2011). To drive wild-type alk1 in endothelial cells, we generated ptol-fli1a.ep:alk1-myc by Gateway cloning (Invitrogen, Carlsbad, CA, USA), recombining pDESTtol2pA2 (Kwan et al., 2007) with p5E fli1a.ep (Villefranc et al., 2007), pME-alk1 and p3E-MTpA (Kwan et al., 2007). To drive ligand- and type II receptor-independent, constitutively active alk1 (Roman et al., 2002) in endothelial cells, we generated ptol-fli1a.ebs:alk1CA-mCherry, recombining pDESTtol2pA2, p5E fli1a.ebs (fli1a Ets-binding site; a kind gift from N. Lawson, University of Massachusetts Medical School, Worcester, MA, USA), pME alk1CA and p3E mCherry-pA (Kwan et al., 2007). These constructs were co-injected with transposase mRNA (Kawakami et al., 2004) into one-cell embryos to generate Tg(fli1a.ep:alk1-myc)pt516, hereafter referred to as Tg(fli1a:alk1-myc), and a series of mosaic founders for Tg(fli1a.ebs:alk1CA-mCherry), hereafter referred to as Tg(fli1a:alk1CA-mCh). The constitutively active alk1 transgene causes severe vascular defects and is embryonic lethal in F1 embryos.
Morpholinos
Translation blocking (TB) and splice blocking (SB) morpholino-modified antisense oligonucleotides (GeneTools, Philomath, OR, USA) were as follows: bmp9TB, 5′-GGAGCAAATGTCCTACGCGCCACAT-3′; bmp9SB, 5′-CTCTTTATGTGTACTCACCCTGAAC-3′; bmp10TB, 5′-AAAAGTGATTTCTGCTACCAGCCAT-3′; bmp10SB, 5′-AGGAAAATATGCAGTTACCTTCATT-3′; and bmp10-likeTB, 5′-GCAGCAGAGAATCAGCCATGACTGC-3′. For TB morpholinos, efficacy and specificity were evaluated by injecting into one-cell embryos CMV-driven EGFP DNA-containing morpholino-binding sites upstream of the initiator methionine, with or without cognate or non-cognate morpholino, and assessing EGFP expression at ∼6 hpf. We could not rescue bmp10 or bmp10-like morphant phenotypes by injecting morpholino-resistant mRNA because embryos ventralized (data not shown). The alk1, tnnt2a and control morpholinos have been described previously (Sehnert et al., 2002; Corti et al., 2011).
In situ hybridization and immunostaining
Digoxigenin-labeled riboprobes (Roche, Indianapolis, IN, USA) for cdh5, cxcr4a, edn1 and myl7 (myosin light chain 7, previously known as cardiac myosin light chain) have been described previously (Yelon et al., 1999; Corti et al., 2011). Zebrafish bmp10 was amplified from cDNA using primers 5′-ACCACAGCTGAACTCCGACT-3′ and 5′-TCCACACT TGGCCACTACCATT-3′; and bmp10-like was amplified using primers 5′-CGCAATGAAGCACCAGAGTA-3′ and 5′-CCGTCCACTGTCTCTCATCA-3′. Both fragments were cloned into pCRII-TOPO (Invitrogen). Whole-mount in situ hybridization was performed as described previously (Roman et al., 2002). Immunohistochemistry was performed using primary antibodies MF20 at 1:200 (sarcomeric myosin, Developmental Studies Hybridoma Bank, Iowa City, IA, USA) or 9E10 at 1:200 (myc, Covance, Princeton, NJ, USA), biotinylated horse anti-mouse IgG at 1:200, ABC reagent (Vector Laboratories, Burlingame, CA), and 3,3′-diaminobenzidine (Sigma, St Louis, MO, USA). Embryos were photographed using an MVX-10 MacroView microscope and DP71 camera (Olympus America, Center Valley, PA, USA). For sections, embryos were embedded in JB4 (Polysciences, Warrington, PA, USA), sectioned at 8 μm and imaged using an Olympus BX51 microscope and DP71 camera. Images were compiled with Adobe Photoshop CS2 version 9.0.2 (Adobe Systems, San Jose, CA, USA).
For immunofluorescence, embryos were fixed in 4% paraformaldehyde in PBS overnight at 4°C, embedded in 4% NuSieve GTG agarose (Lonza, Rockland, ME, USA), and sectioned at 50 μm with a VT1000S vibratome (Leica Microsystems, Buffalo Grove, IL, USA). Rabbit anti-phospho-Smad1/5/9 (also known as anti-phospho-Smad1/5/8, 9511, Cell Signaling Technology, Beverly, MA, USA) was used at 1:100 and goat-anti-rabbit Alexa Fluor 647 at 1:200. Sections were mounted with Vectashield HardSet mounting medium (Vector) and imaged with an Olympus Fluoview 1000 confocal microscope outfitted with a UPFLN 40× oil immersion objective, with scan speed 244 Hz. Two-dimensional projections were generated from z-series (1 μm steps) and images processed for presentation and quantitation using MetaMorph 7.7 (Molecular Devices, Sunnyvale, CA, USA). For presentation, images were pseudocolored and colocalization highlighted using the ‘boost colocalization’ function. For quantitation of nuclear phospho-Smad1/5/9, threshold images were created for summed projections of nuclear EGFP panels, alk1-positive arteries were traced with the ‘trace region’ tool, and all nuclei were selected within the traced region using the ‘create regions around objects’ tool. Traced nuclei were transferred to corresponding pSmad1/5/9 summed projections, and average nuclear pixel intensity was measured and normalized to the average pixel intensity for an adjacent non-vascular pSmad1/5/9-positive domain. Results are expressed as percentage of corresponding controls.
Microinjection of rhBMP10 and rhBMP9
Embryos were anesthetized in 160 μg/ml tricaine (Sigma) and embedded in 1% NuSieve GTG agarose in 30% Danieau/PTU. Two nl of Phenol Red/KCl buffer or Qtracker 655 non-targeted quantum dots (Invitrogen) with or without 10 μM rhBMP10 or rhBMP9 protein (R&D Systems, Minneapolis, MN, USA) were injected into the base of one caudal division of the internal carotid artery (CaDI) at 28 hpf. Embryos were either imaged live for cell counts (see below) or fixed at 36 hpf and assayed by immunofluorescence or in situ hybridization as described above.
Live confocal imaging
For live imaging, embryos were anesthetized in 160 μg/ml tricaine and inserted into 500 μm troughs in 2% SeaKem LE agarose (Lonza)/30% Danieau. Z-series (0.5-2 μm steps) were collected using a TCS SP5 multiphoton/confocal microscope (Leica Microsystems, Wetzlar, Germany) outfitted with an APO L 20×/1.00 water immersion objective, non-descanned detectors and spectral detectors. EGFP was excited with a Mai Tai DeepSee Ti:Sapphire laser (Newport/Spectra Physics, Santa Clara, CA, USA) at 900 nm, whereas dsRed and mCherry were excited with a 561 nm diode. Scanning was performed either with a point scanner (400 Hz) with 4× frame averaging or resonant scanner (8000 Hz) with 16× line averaging. Projections were generated using MetaMorph 7.7 and endothelial cell numbers counted as described previously (Corti et al., 2011).
C2C12 transfection and luciferase assays
On the day prior to transfection, C2C12 cells were seeded at 4×104 cells/well in 24-well plates. Cells were washed once with Optimem I (Invitrogen) and incubated in Optimem I for 2 hours. All wells were transfected with Lipofectamine (2 μl/well; Invitrogen) in complex with BRE-luciferase (400 ng/well) and pRL-TK (40 ng/well). In plasmid test wells, 400 ng/well of pcDNA3-hALK1, pcDNA3-hALK1(R411Q) (a kinase-dead mutant), pcDNA3-hALK3-HA or pCS2-zALK1 were also added. After 4 hours, lipoplexes were removed and DMEM containing 10% FBS and antibiotic-antimycotic solution (Invitrogen) added for 24 hours. Cells were washed twice with serum-free DMEM+antibiotic and incubated for 21 hours, then treated for 21 hour with 0.1-3000 pg/ml rhBMP9 or rhBMP10 (R&D Systems, Abingdon, UK). Cells were then lysed and assayed for Firefly and Renilla luciferase activities using the Dual-Glo Luciferase Assay kit (Promega, Southampton, UK) according to the manufacturer’s instructions. Firefly luciferase activities were normalized to the Renilla control. Data were normalized as percentage responses using GraphPad Prism (San Diego, CA, USA) and EC50 values calculated. Plasmids were obtained as follows: BRE-luciferase from P. ten Dijke (Leiden University Medical Center, Leiden, The Netherlands); pcDNA3-hALK1 and pcDNA3-hALK1(R411Q) from R. Trembath (King’s College London, London, UK); and pcDNA3-hALK3-HA from K. Miyazono (University of Tokyo, Tokyo, Japan).
Statistics
Endothelial cell numbers and pSmad1/5/9 intensities were compared by two-tailed, unpaired Student’s t-test with significance set at P<0.05.
RESULTS
Blood flow is required not only for alk1 expression but also for Alk1 activity
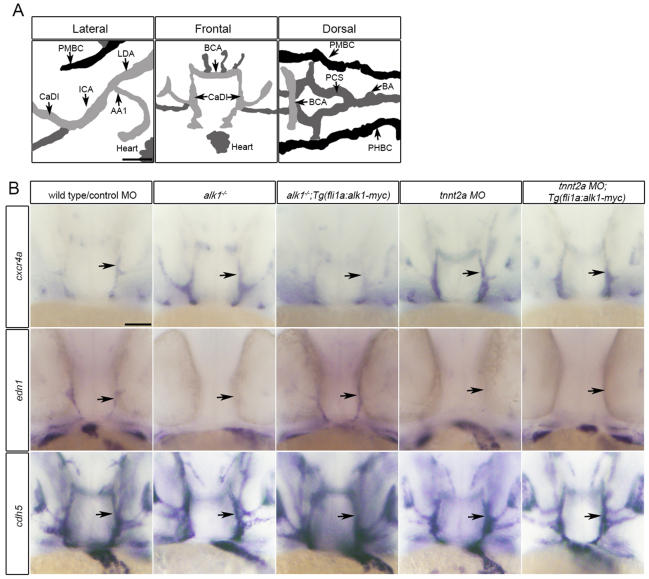
In 36 hpf zebrafish embryos, alk1 is expressed predominantly in endothelial cells within arteries proximal to the heart: the first aortic arch; the cranialward internal carotid artery, caudal division of the internal carotid artery (CaDI) and basal communicating artery (BCA); and the caudalward lateral dorsal aortae (Fig. 1A). We have previously demonstrated that alk1 expression requires blood flow, and that loss of alk1 mimics loss of blood flow in terms of changes in expression of cxcr4a and edn1: both alk1 mutants, which have high flow through assayed vessels, and cardiac troponin-t2a (tnnt2a) morphants, which lack heartbeat and blood flow, exhibit increased cxcr4a expression and decreased edn1 expression in alk1-expressing cranial arteries at 36 hpf compared with corresponding controls (Fig. 1B) (Corti et al., 2011). Given that mammalian orthologs CXCR4 and EDN1 are flow responsive in cultured human endothelial cells (Wang et al., 1993; Melchionna et al., 2005), these data suggest that Alk1 might act downstream of blood flow to control expression of these mechanoresponsive genes. Accordingly, if Alk1 signaling is sufficient downstream of blood flow, then restoration of alk1 in the absence of flow might be expected to rescue expression of cxcr4a and edn1. To test this hypothesis, we generated a stable transgenic line, Tg(fli1a:alk1-myc), that expresses Alk1-myc in all endothelial cells regardless of the presence of blood flow (supplementary material Fig. S1). This transgene restores normal expression of cxcr4a and edn1 in alk1 mutants (Fig. 1B), rescues alk1 mutants to adulthood [n=23 alk1-/- of 130 adults from alk1+/-;Tg(fli1a:alk1-myc) incrosses; 71% rate of rescue], and has no untoward effects on growth and development. However, flow-independent expression of endothelial cell alk1 fails to normalize expression of cxcr4a or edn1 in 36 hpf tnnt2a morphants (Fig. 1B). There are two plausible explanations for this observation: Alk1 signaling may not be sufficient downstream of blood flow to control expression of these genes, or flow may be required for some aspect of Alk1 signaling in addition to alk1 expression.
Fig. 1.
Restoration of alk1 expression does not rescue cxcr4a or edn1 expression in the absence of blood flow. (A) Lateral (anterior left), frontal (anterior upwards) and dorsal (anterior left) views of the zebrafish cranial vasculature at 36 hpf. alk1-positive arteries are light gray, alk1-negative arteries are dark gray and veins are black. alk1-positive arteries are closest to the heart. AA1, aortic arch 1; BA, basilar artery; BCA, basal communicating artery; CaDI, caudal division of the internal carotid artery; ICA, internal carotid artery; LDA, lateral dorsal aorta; PCS, posterior communicating segments; PHBC, primordial hindbrain channel; PMBC, primordial midbrain channel. Scale bar: 50 μm. (B) Whole-mount in situ hybridization for cxcr4a, edn1 and cadherin 5 (cdh5, pan-endothelial control) at 36 hpf. cxcr4a and edn1 are upregulated and downregulated, respectively, in alk1 mutants (column 2) and tnnt2a morphants (column 4) compared with controls (column 1), and expression can be normalized to wild-type/control morphant levels by transgene-mediated, flow-independent alk1 expression in alk1 mutants (column 3) but not in tnnt2a morphants (column 5). cdh5 expression is not altered by alk1 or flow conditions. n≥50 for all groups. Frontal views, dorsal upwards. Arrows indicate left CaDI. Scale bar: 50 μm.
To determine more directly whether restoration of endothelial alk1 expression is sufficient to restore Alk1 signaling in the absence of flow, we assessed a proximal read-out of Alk1 activity: nuclear pSmad1/5/9. pSmad1/5/9 is present in endothelial cell nuclei within the alk1-positive CaDIs and BCA in 36 hpf wild-type embryos, is decreased in these arteries in alk1-/- and is restored in alk1-/-;Tg(fli1a:alk1-myc) (supplementary material Fig. S2 and Table S1), demonstrating that Alk1 signaling is necessary for Smad1/5/9 phosphorylation in these arterial endothelial cells. Because alk1 expression is dependent on blood flow (Corti et al., 2011) and Smad1/5/9 phosphorylation is dependent on alk1 expression, pSmad1/5/9 in these arterial endothelial cells should be dependent on blood flow. Indeed, nuclear pSmad1/5/9 is nearly undetectable in the CaDIs at 24 hpf, prior to the onset of flow through and alk1 expression in these vessels (Fig. 2A; supplementary material Table S1), and is decreased at 36 hpf in tnnt2a morphants (Fig. 2B; supplementary material Table S1) compared with sibling controls (Fig. 2C; supplementary material Table S1). However, restoration of alk1 expression via a fli1a:alk1-myc transgene in flow-deprived tnnt2a morphants fails to rescue nuclear pSmad1/5/9 (Fig. 2D; supplementary material Table S1), whereas endothelial-specific expression of a constitutively active (ligand- and type II receptor-independent) form of alk1 via a fli1a:alk1CA-mCh transgene restores nuclear pSmad1/5/9 in the absence of blood flow (Fig. 2E; supplementary material Table S1). Taken together, these data support the idea that blood flow is required not only for alk1 expression but also for some additional aspect of Alk1 activity, such as type II receptor expression and/or expression or distribution of Alk1 ligand.
Fig. 2.
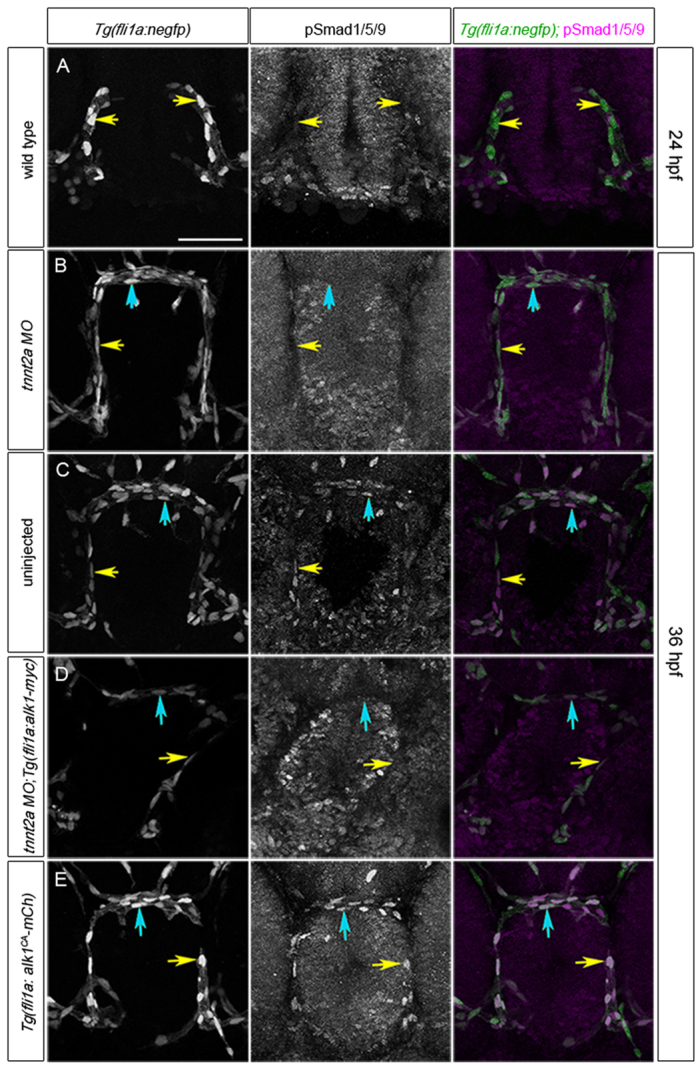
Alk1 activity is dependent on blood flow. (A-E) pSmad1/5/9 (middle column) in endothelial cell nuclei (marked by fli1a:negfp transgene, left column); in merge (right column), EGFP-expressing endothelial cell nuclei are green and pSmad1/5/9 immunofluorescence is magenta. (A) 24 hpf wild type, prior to blood flow; (B) 36 hpf tnnt2a morphant (no flow); (C) 36 hpf wild type; (D) 36 hpf tnnt2a morphant harboring a fli1a:alk1-myc transgene; (E) 36 hpf tnnt2a morphant harboring a fli1a:alk1CA-mCh transgene. Tg(fli1a:alk1CA-mCh) embryos do not have lumenized vessels. In merge (right column), EGFP-expressing endothelial cell nuclei are green, pSmad1/5/9 immunofluorescence is magenta. Yellow and blue arrows indicate endothelial cells in the caudal division of the internal carotid artery (CaDI) and basal communicating artery (BCA), respectively. 2D confocal projections of 50 μm frontal sections, dorsal upwards. Scale bar: 50 μm. See supplementary material Table S1 for fluorescence quantitation.
bmp10 knockdown phenocopies alk1 mutants
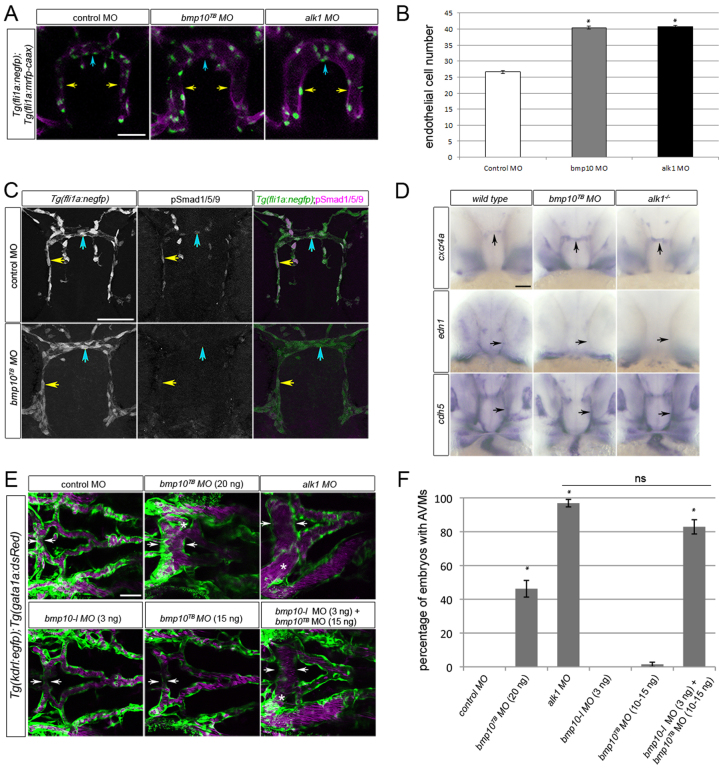
To investigate the effect of blood flow on Alk1 ligand availability, we first needed to determine the physiologically relevant ligand during zebrafish embryonic vascular development. Therefore, we assessed vascular gene expression and architecture between 36 and 48 hpf in bmp9 (ENSDARG00000059173) and bmp10 (ENSDARG00000061769) morphants, and compared results with alk1 mutants/morphants. Translation blocking (TB) morpholinos were validated in vivo as described in the Materials and methods (supplementary material Fig. S3A). Injection of bmp9TB morpholino had no effect on nuclear pSmad1/5/9 in the 36 hpf CaDI/BCA (supplementary material Fig. S3B) nor any effect on cranial vascular architecture at 48 hpf (supplementary material Fig. S3C), but reproducibly generated a venous remodeling defect in the tail that was phenocopied by injection of bmp9 splice blocking (SB) morpholino (data to be presented in a separate manuscript). By contrast, at 36 hpf, bmp10TB morphant CaDIs/BCAs were enlarged and contained supernumerary endothelial cells, a phenotype indistinguishable from alk1 morphants (Fig. 3A,B). Furthermore, in the 36 hpf CaDI/BCA, bmp10TB morphants exhibited decreased nuclear pSmad1/5/9 (Fig. 3C; supplementary material Table S1), increased expression of cxcr4a (50/53, 94% Fig. 3D), and decreased expression of edn1 (15/15, 100%; Fig. 3D). These effects were similar to effects observed in alk1 mutants (supplementary material Fig. S2; Fig. 3D). In addition, by 48 hpf, bmp10TB morphants developed AVMs connecting the arterial system underlying the midbrain and hindbrain to adjacent veins, strongly resembling alk1 mutants/morphants (Fig. 3E). However, the bmp10 morpholino-induced AVM phenotype was observed with relatively weak penetrance (21/43, 49%; Fig. 3F) and low expressivity at a maximum tolerated dose (20 ng), suggesting that an additional ligand might be compensating for bmp10 at later time points. Examination of the zebrafish genome uncovered bmp10-like (ENSDARG00000045632), which is most closely related to bmp10 (64% identity in the active peptide) and likely represents a bmp10 paralog that arose during a teleost whole-genome duplication (Woods et al., 2005). Therefore, we knocked down both bmp10-like and bmp10, and assessed the zebrafish vasculature at 48 hpf. Injection of bmp10-like morpholino (3 ng) or bmp10TB morpholino (10-15 ng) alone had almost no effect on cranial vascular architecture (n=61 and 47, respectively), whereas co-injection of these two morpholinos at these same doses robustly phenocopied alk1 morphants in terms of AVM development (122/146, 84%; Fig. 3E,F), suggesting a strong genetic interaction. Similar results were observed with a bmp10SB morpholino, with no AVMs at 15 ng (n=48) but AVMs upon co-injection with 3 ng bmp10-like morpholino (46/57, 81%). By contrast, co-injection of bmp9TB morpholino (7 ng) with bmp10TB morpholino (15 ng) failed to elicit AVMs (0/29), and co-injection of bmp9TB morpholino (7 ng), bmp10TB morpholino (15 ng) and bmp10-like morpholino (3 ng) did not increase the percentage phenotype (20/31, 65%) beyond combined injection of bmp10TB morpholino and bmp10-like morpholino. These results demonstrate that bmp10-like acts redundantly with bmp10 and that Bmp10 but not Bmp9 is the crucial in vivo Alk1 ligand required for arterial quiescence and AVM prevention during zebrafish embryonic vascular development.
Fig. 3.
Knockdown of bmp10 phenocopies zebrafish alk1 mutants. (A) CaDIs (caudal divisions of the internal carotid artery; yellow arrows) and BCA (basal communicating artery; blue arrows) in 36 hpf Tg(fli1a:mrfp-caax);Tg(fli1a:negfp) embryos injected with 20 ng control, 20 ng bmp10TB or 2.5 ng alk1 morpholino. Endothelial cell membranes are magenta; nuclei are green. 2D projections of 10 optical sections (Z-step, 2 μm), frontal views, dorsal upwards. Scale bar: 50 μm. (B) Endothelial cell number in the CaDI/BCA in control, bmp10TB and alk1 morphants. n=7-10 in three independent experiments. Values are mean±s.e.m. Student’s t-test: *P<0.001. (C) pSmad1/5/9 (middle column) in endothelial cells (nuclei marked by fli1a:negfp transgene, left column) in 36 hpf control and bmp10TB morphants. In merge (right column), EGFP-expressing endothelial cell nuclei are green, pSmad1/5/9 immunofluorescence is magenta. Yellow and blue arrows indicate endothelial cells in the CaDI and BCA, respectively. 2D confocal projections of 50 μm frontal sections, dorsal upwards. Scale bar: 50 μm. See supplementary material Table S1 for fluorescence quantitation. (D) Whole-mount in situ hybridization for cxcr4a, edn1 and cdh5 (pan-endothelial control) in wild type/control morphant, bmp10TB morphant and alk1-/- at 36 hpf. Frontal views, dorsal upwards. Arrows indicate BCA (vertical) or CaDI (horizontal). Scale bar: 50 μm. (E) Cranial vasculature in 48 hpf Tg(kdrl:gfp);Tg(gata1a:dsRed) embryos injected with bmp10TB morpholino (15-20 ng, as indicated), alk1 morpholino (2.5 ng) and/or bmp10-like morpholino (3 ng). Arrows highlight width of BCA; asterisk indicates AVM. Endothelial cells are green, red blood cells magenta. 2D confocal projections, dorsal views, anterior leftwards. Scale bar: 50 μm. (F) Quantification of AVM development in 48 hpf morpholino-injected embryos. n=43-146. Values are mean±s.e.m. Student’s t-test: *P<0.001; ns, not significant.
bmp10 paralogs are expressed exclusively in the heart
We have demonstrated that concomitant knockdown of bmp10 and bmp10-like phenocopies alk1 mutants. bmp10 is undetectable at 22 hpf but expressed in the developing heart as early as 24 hpf, with predominant expression in the ventricle by 36 hpf (Fig. 4A; supplementary material Fig. S4A). By 48 hpf, bmp10 is expressed in both heart chambers but appears to be absent from the atrioventricular canal (Fig. 4A). Double staining for bmp10 mRNA and sarcomeric myosin protein revealed that bmp10 is expressed strongly in endocardium but is largely absent from myocardium at 36 hpf (Fig. 4B). bmp10-like is undetectable in the heart at 28 hpf but is expressed at 36 and 48 hpf in distal ventricular myocardium (Fig. 4A,B). Both bmp10 and bmp10-like are expressed independently of heartbeat (supplementary material Fig. S4B), and no discrete extracardiac expression domains of either bmp10 or bmp10-like are observed (data not shown). The temporal difference in ligand expression suggests that Bmp10-like might compensate for Bmp10 after 36 hpf, providing a plausible explanation for the observation that knockdown of bmp10 alone robustly phenocopies alk1 mutants at 36 hpf, but double knockdown of bmp10 and bmp10-like is required for robust phenocopy at 48 hpf (Fig. 3). Furthermore, these data demonstrate that the heart is the most likely source of both Bmp10 and Bmp10-like, and suggest that cardiac-derived Bmp10 ligands might be carried by blood flow to arterial endothelial Alk1.
Fig. 4.
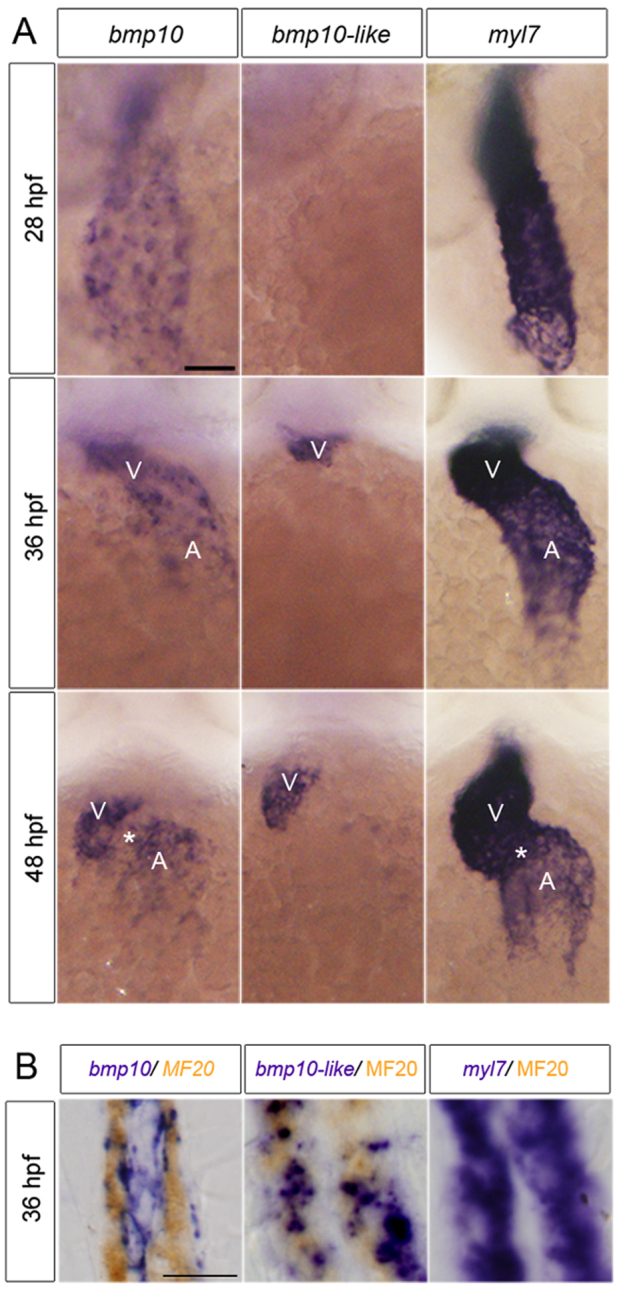
bmp10 and bmp10-like are expressed in the heart. (A) Whole-mount in situ hybridization for bmp10, bmp10-like and myl7 at 28, 36 and 48 hpf. Ventral views, anterior upwards. Scale bar: 50 μm. V, ventricle; A, atrium. Asterisk indicates atrioventricular canal. (B) Sagittal sections (8 μm) through the heart of 36 hpf embryos co-stained with bmp10, bmp10-like or myl7 (purple) and MF20 (sarcomeric myosin; brown). Scale bar: 10 μm.
Circulating Bmp10 acts via Alk1 to limit endothelial cell number in nascent arteries
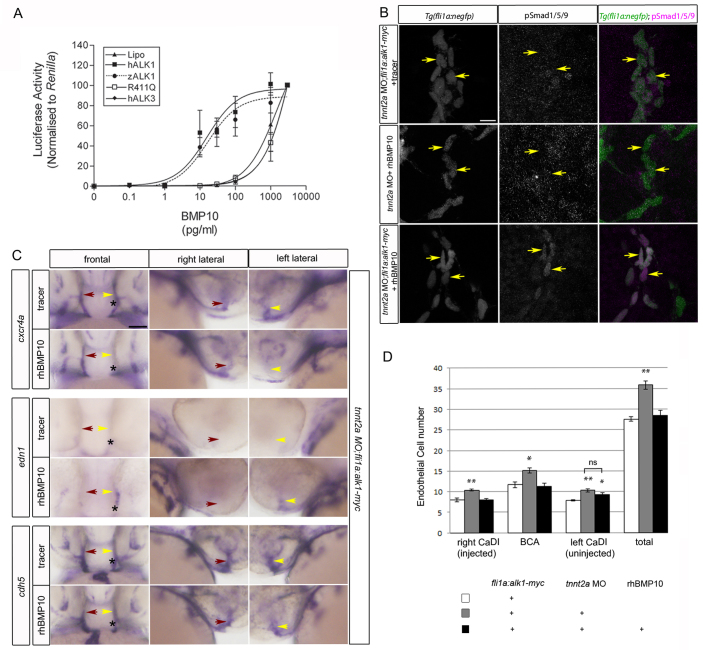
Given that bmp10 paralogs are detectable only in the heart in zebrafish (Fig. 4) and mouse (Neuhaus et al., 1999; Chen et al., 2004), and that Bmp10 circulates in embryonic mouse plasma (Ricard et al., 2012), it stands to reason that blood flow may be required to transport Bmp10 to arterial endothelial cell Alk1, explaining why restoration of alk1 gene expression alone is insufficient to rescue defects in arterial endothelial cell cxcr4a, edn1 and pSmad1/5/9 in the absence of flow (Figs 1, 2). To test this hypothesis, we injected tnnt2a morpholino into Tg(fli1a:alk1-myc) embryos, once again generating embryos that lack heartbeat but nonetheless express endothelial alk1. Non-transgenic siblings injected with tnnt2a morpholino served as blood flow deficient and therefore alk1-deficient controls. At 28 hpf, we injected tracer (phenol red or quantum dots) alone or together with recombinant human (rh) BMP10 directly into the base of one CaDI. rhBMP10 can act through zebrafish Alk1 to activate BMP responsive element (BRE)-driven luciferase activity in transfected C2C12 cells with an EC50 similar to that required for activation of human ALK1 (Fig. 5A). Restoration of either alk1 alone [tnnt2a morphant;Tg(fli1a:alk1-myc)] or rhBMP10 alone (tnnt2a morphant + rhBMP10) failed to rescue nuclear pSmad1/5/9 in the CaDI, whereas restoration of both alk1 and rhBMP10 increased nuclear pSmad1/5/9 ∼3-fold near the site of rhBMP10 injection (Fig. 5B; supplementary material Table S1). Injection of rhBMP10 into alk1 mutants failed to rescue pSmad1/5/9, supporting the idea that rhBMP10 is not acting through other endothelial receptors besides Alk1 (supplementary material Fig. S5; Table S1). Furthermore, injection of rhBMP10 into tnnt2a morphant;Tg(fli1a:alk1-myc) embryos decreased expression of cxcr4a (13/23, 57%) and increased expression of edn1 (14/22, 64%) on the injected side, driving expression of these genes toward levels observed in the presence of normal blood flow (Fig. 5C). In addition, similarly restoring rhBMP10/Alk1 signaling to one CaDI in flow-deprived tnnt2a morphant embryos significantly decreased endothelial cell number within the injected CaDI and BCA but not the uninjected CaDI, normalizing endothelial cell number in the injected CaDI/BCA to that observed in the presence of normal blood flow (Fig. 5D). Because expression of CXCR4 and EDN1 can be repressed or induced, respectively, by mechanical force in cultured endothelial cells (Wang et al., 1993; Melchionna et al., 2005), these results support the idea that Bmp10/Alk1/pSmad1/5/9 signaling may be crucial in transducing a mechanical signal into a biochemical response in arterial endothelial cells in vivo. In total, our results define a novel blood flow responsive signaling pathway - in which blood flow is required for alk1 expression as well as, via circulating Bmp10, Alk1 activity - that is crucial for flow-dependent limitation of endothelial cell number in and therefore caliber of nascent arteries.
Fig. 5.
Bmp10/Alk1 lies downstream of blood flow in regulation of pSmad1/5/9, cxcr4a and edn1. (A) BRE:luciferase activity in C2C12 cells transfected with human (h) ALK1, zebrafish (z) alk1, hALK1 kinase dead mutant (R411Q), hALK3 (which does not bind BMP10) or lipofectamine (lipo), and treated with rhBMP10. Data are normalized to pTK-Renilla luciferase activity. Values are mean±s.e.m., n=3 biological replicates, each representing 3 technical replicates. (B) pSmad1/5/9 (middle column) in endothelial cells (nuclei marked by fli1a:negfp transgene, left column) of the CaDI (caudal division of the internal carotid artery) of 36 hpf tnnt2a morphants in which endothelial cell alk1 expression [Tg(fli1a:alk1-myc)], BMP10 availability (rhBMP10; 2 nl of 10 μM rhBMP10 injected into base of CaDI at 28 hpf), or both, are restored. In merge (right column), EGFP-expressing endothelial cell nuclei are green, pSmad1/5/9 immunofluorescence is magenta. Yellow arrows indicate endothelial cells in the injected CaDI. 2D confocal projections of 50 μm frontal sections, dorsal upwards. Scale bar: 10 μm. See supplementary material Table S1 for fluorescence quantitation. (C) Whole-mount in situ hybridization for cxcr4a, edn1 and cdh5 (pan-endothelial control) in 36 hpf tnnt2a morphant;Tg(fli1a:alk1-myc) embryos injected at 28 hpf into the base of the left CaDI with 2 nl tracer with or without 10 μM rhBMP10. Yellow arrow indicates injected CaDI, red arrow indicates uninjected CaDI. Asterisk indicates injection site. Frontal views, dorsal upwards; lateral views, right (uninjected) side or left (injected) side. Scale bar: 50 μm. (D) Quantitation of CaDI endothelial cell number in 36 hpf Tg(fli1a:alk1-myc) embryos left uninjected (white bars) or injected at the one-cell stage with tnnt2a morpholino (gray, black bars). At 28 hpf, tnnt2a morphants were injected in the right CaDI with 2 nl tracer (gray bars) or 2 nl 10μM rhBMP10 (black bars). tnnt2a morphants injected intravascularly with tracer exhibited increased endothelial cell number throughout the CaDI/BCA, whereas intravascular injection of rhBMP10 normalized endothelial cell number in the injected CaDI and BCA (basal communicating artery). n=10-12 per group in two independent experiments. Values are mean±s.e.m. Student’s t-test: *P<0.05; **P<0.001; ns, not significant.
DISCUSSION
Early embryonic blood vessel development is controlled largely by paracrine signaling interactions that orchestrate endothelial cell migration and proliferation, and coalescence into vascular cords, as well as subsequent angiogenic sprouting that serves to elaborate the primitive vascular network. However, once blood vessels form a lumen, biomechanical forces and endocrine factors also come into play. In this work, we present evidence that blood flow is crucial for limiting endothelial cell number within nascent arteries, and that Alk1 acts downstream of blood flow in mediating this effect. Furthermore, we demonstrate that blood flow is required not only for alk1 expression but also for Alk1 activity, and that the latter requirement is met by provision of circulating ligand, Bmp10. Thus, flow-dependent induction of alk1 and distribution of ligand synergize to enhance Alk1 activity.
It is well established that both BMP9 and BMP10 bind ALK1 with high affinity and can induce Smad1/5/9 phosphorylation and pSmad1/5/9-dependent transcriptional responses in cultured endothelial cells (David et al., 2007; David et al., 2008; Mitchell et al., 2010). However, our work is the first to define Alk1 ligands unequivocally in vivo. We have demonstrated in zebrafish that bmp10 paralogs are required for Alk1 function during embryonic development, whereas bmp9 is dispensable. By contrast, neither Bmp9 nor Bmp10 mouse nulls have been reported to exhibit AVMs. Bmp9-null mice live to adulthood without apparent vascular abnormalities (Ricard et al., 2012), whereas ventricular hypoplasia and impaired trabeculation but not AVMs have been reported in Bmp10 nulls (Chen et al., 2004). There are several possible explanations for this discrepancy between zebrafish and mice. First, Bmp9 and Bmp10 may act redundantly in mouse embryonic vascular development, reflecting a difference in the spatial and/or temporal ligand requirement in mouse versus zebrafish vasculature. Indeed, treatment of Bmp9-null mice with Bmp10 blocking antibodies results in early postnatal retinal vascular defects, hinting at functional redundancy, although AVMs were not noted in these vessels (Ricard et al., 2012). Furthermore, like rhBMP10, rhBMP9 was effective in restoring nuclear pSmad1/5/9 in the CaDI/BCA in tnnt2a morphant;Tg(fli1a:alk1-myc) zebrafish embryos (data not shown), suggesting that Bmp9 and Bmp10 may function redundantly in vivo if available to Alk1 contemporaneously. In zebrafish, bmp9 is first detected in liver around 48 hpf (C.J.M. and B. Shravage, unpublished) and the liver is first vascularized around 55 hpf (Korzh et al., 2008), supporting the idea that Bmp9 is not redundant with Bmp10 between 24 and 48 hpf, the time period during which AVMs develop in alk1 mutants (Roman et al., 2002; Corti et al., 2011). A second possible reason for the discordance between mice and zebrafish in terms of definition of Alk1 ligand stems from the fact that slowed heartbeat and impaired circulation precede death of Bmp10 mouse nulls at embryonic day (E) 9.5-E10.5 (Chen et al., 2004). Thus, it is reasonable to hypothesize that the absence of AVMs in Bmp10 nulls is due to the earlier death of Bmp10 nulls versus Alk1 nulls (E10.5-11.5) and/or insufficient circulation to precipitate AVM formation, which we have demonstrated to be a flow-dependent process (Corti et al., 2011). Because trabeculation in zebrafish does not occur until 60-72 hpf in zebrafish (Liu et al., 2010; Peshkovsky et al., 2011), it is not surprising that we noted no defects in heart development or function through 48 hpf in bmp10 or bmp10-like morphants (data not shown).
In cranial arterial endothelial cells, both blood flow and Bmp10/Alk1 function are required for phosphorylation of Smad1/5/9, repression of cxcr4a and induction of edn1. Concomitant restoration of endothelial alk1 expression and injection of rhBMP10 normalizes these molecular end points and restores arterial endothelial cell number in the absence of flow. These data strongly suggest that Alk1 acts downstream of blood flow to limit endothelial cell migration, proliferation and/or vascular tone, and thus limit arterial endothelial cell number and vessel caliber. However, changes in expression of cxcr4a and edn1 cannot fully explain defects in vessel architecture resulting from loss of alk1. Although ALK1 signaling can repress CXCR4 or induce EEN1 in cultured endothelial cells (Star et al., 2010; Park et al., 2012; Young et al., 2012), supporting our in vivo data, previous work has demonstrated that increased cxcr4a is not necessary nor is loss of edn1 sufficient for AVM development (Corti et al., 2011), and additional work has demonstrated that concomitant increase in cxcr4a and loss of edn1 is insufficient to generate AVMs (E. Rochon and B.L.R., unpublished). Thus, further work is required to define the molecular mechanisms and cellular behaviors that lead to arterial enlargement in the absence of alk1.
The multifaceted regulation of Alk1 signaling by blood flow is remarkable, with flow required for both alk1 expression and Alk1 activity. Given that mammalian CXCR4 and EDN1 respond to shear stress and/or cyclic strain in cultured endothelial cells (Wang et al., 1993; Melchionna et al., 2005), it seems likely that Alk1 is important in transducing mechanical force into a biochemical signal in vivo. However, the mechanism by which blood flow upregulates alk1 expression is currently unknown, and it remains formally possible that the flow dependence of alk1 expression stems at least in part from a circulating factor. Circulation of ligand clearly contributes to the dependence of Alk1 activation on blood flow: blood flow distributes cardiac-derived circulating Bmp10 to arterial endothelial cell Alk1, thereby explaining the blood flow dependence of Smad1/5/9 phosphorylation in these cells. However, a recent study reported that mechanical force induces Smad1/5/9 phosphorylation in intact mouse endothelium and cultured endothelial cells in a ligand-independent manner (Zhou et al., 2012), contradicting our conclusions. This discrepancy could possibly be explained by the fact that ligand independence in that study was examined via treatment with Noggin, which sequesters most BMP ligands, but not BMP9 or BMP10 (Seemann et al., 2009). Alternatively, oscillatory shear stress applied in that study may have different effects on BMP signaling than pulsatile laminar shear stress, which acts within zebrafish cranial arteries (Chen et al., 2011), or the type I receptor responsible for oscillatory shear-induced pSmad1/5/9 may not be Alk1. Further work is required to better define the roles of and probe interactions between endocrine factors and mechanical force in the regulation of Alk1 signaling.
In summary, our data demonstrate that blood flow induces alk1 expression and provides Bmp10 to arterial endothelial cell Alk1, thereby activating Smad1/5/9 phosphorylation, decreasing cxcr4a expression and inducing edn1 expression. These changes in gene expression, along with changes in expression of yet to be identified genes, serve to dampen angiogenic behavior and to stabilize arterial endothelial cell number and caliber at the onset of blood flow. Taken together with our previous work (Corti et al., 2011), our data suggest that loss of Alk1 function abrogates this important flow response and results in increased nascent arterial caliber, which in turn leads to increased hemodynamic forces within downstream arteries. In an attempt to normalize these hemodynamic forces, downstream vessels mount an Alk1-independent flow response that causes normally transient conduits between this overloaded arterial system and neighboring veins to be retained and enlarged, thereby forming high flow AVMs. Thus, our model suggests that in individuals with HHT, abrogation of one flow response - due to impaired ALK1 signaling - leads to activation of an independent flow response that acts to normalize hemodynamic forces, ultimately leading to AVMs.
Supplementary Material
Acknowledgments
We thank Z. Kupchinsky and D. S. Wright for outstanding fish care, and L. Brilli, P. Corti and B. Shravage for technical contributions. We also thank N. Lawson (University of Massachusetts Medical School, Worcester, MA, USA) for the p5E fli1a.ebs plasmid and for helpful comments on the manuscript, and C. B. Chen (University of Utah, Salt Lake City, UT, USA) and K. Kawakami (National Institute of Genetics, Shizuoka, Japan) for Gateway/tol2 vectors.
Footnotes
Funding
This work was supported by the National Institutes of Health (NIH) [R01 HL079108 to B.L.R.]. D.W.L. was supported by the NIH [T32 HL094295-02]. P.D.U. was supported by a British Heart Foundation Programme Grant [RG/08/002/24718]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
D.W.L. performed the majority of the zebrafish experiments, generated all zebrafish data figures and wrote the paper; S.Y. performed bmp9 morpholino studies, generated the Tg(fli1a:alk1-myc)pt516 line and assisted in most zebrafish experiments; J.P.D. analyzed bmp10 and bmp10-like morphant shunting phenotype; C.J.M. performed preliminary characterization of bmp9 and bmp10 expression patterns and morphant phenotypes; P.D.U. performed luciferase assays and generated the accompanying figure; B.L.R. conceived and directed the study, generated ptol-fli1a.ebs:alk1ca-mCherry, and wrote the paper.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.095307/-/DC1
References
- Bhullar I. S., Li Y. S., Miao H., Zandi E., Kim M., Shyy J. Y., Chien S. (1998). Fluid shear stress activation of IkappaB kinase is integrin-dependent. J. Biol. Chem. 273, 30544–30549 [DOI] [PubMed] [Google Scholar]
- Brown M. A., Zhao Q., Baker K. A., Naik C., Chen C., Pukac L., Singh M., Tsareva T., Parice Y., Mahoney A., et al. (2005). Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J. Biol. Chem. 280, 25111–25118 [DOI] [PubMed] [Google Scholar]
- Bussmann J., Wolfe S. A., Siekmann A. F. (2011). Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development 138, 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K., Weiss D., Suo J., Vega J. D., Giddens D., Taylor W. R., Jo H. (2007). Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 116, 1258–1266 [DOI] [PubMed] [Google Scholar]
- Chen H., Shi S., Acosta L., Li W., Lu J., Bao S., Chen Z., Yang Z., Schneider M. D., Chien K. R., et al. (2004). BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131, 2219–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Patrick M. J., Corti P., Kowalski W., Roman B. L., Pekkan K. (2011). Analysis of early embryonic great-vessel microcirculation in zebrafish using high-speed confocal μPIV. Biorheology 48, 305–321 [DOI] [PubMed] [Google Scholar]
- Choi J., Dong L., Ahn J., Dao D., Hammerschmidt M., Chen J. N. (2007). FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev. Biol. 304, 735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti P., Young S., Chen C. Y., Patrick M. J., Rochon E. R., Pekkan K., Roman B. L. (2011). Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138, 1573–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L., Mallet C., Mazerbourg S., Feige J. J., Bailly S. (2007). Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109, 1953–1961 [DOI] [PubMed] [Google Scholar]
- David L., Mallet C., Keramidas M., Lamandé N., Gasc J. M., Dupuis-Girod S., Plauchu H., Feige J. J., Bailly S. (2008). Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ. Res. 102, 914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R. J., van Soest S., Fontijn R. D., Salamanca S., de Groot P. G., VanBavel E., Pannekoek H., Horrevoets A. J. (2002). Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 100, 1689–1698 [DOI] [PubMed] [Google Scholar]
- Dekker R. J., van Thienen J. V., Rohlena J., de Jager S. C., Elderkamp Y. W., Seppen J., de Vries C. J., Biessen E. A., van Berkel T. J., Pannekoek H., et al. (2005). Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am. J. Pathol. 167, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R. J., Boon R. A., Rondaij M. G., Kragt A., Volger O. L., Elderkamp Y. W., Meijers J. C., Voorberg J., Pannekoek H., Horrevoets A. J. (2006). KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 107, 4354–4363 [DOI] [PubMed] [Google Scholar]
- Goumans M. J., Valdimarsdottir G., Itoh S., Rosendahl A., Sideras P., ten Dijke P. (2002). Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 21, 1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans M. J., Valdimarsdottir G., Itoh S., Lebrin F., Larsson J., Mummery C., Karlsson S., ten Dijke P. (2003). Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell 12, 817–828 [DOI] [PubMed] [Google Scholar]
- Guttmacher A. E., Marchuk D. A., White R. I., Jr (1995). Hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 333, 918–924 [DOI] [PubMed] [Google Scholar]
- Hay D. C., Beers C., Cameron V., Thomson L., Flitney F. W., Hay R. T. (2003). Activation of NF-kappaB nuclear transcription factor by flow in human endothelial cells. Biochim. Biophys. Acta 1642, 33–44 [DOI] [PubMed] [Google Scholar]
- Johnson D. W., Berg J. N., Baldwin M. A., Gallione C. J., Marondel I., Yoon S. J., Stenzel T. T., Speer M., Pericak-Vance M. A., Diamond A., et al. (1996). Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 13, 189–195 [DOI] [PubMed] [Google Scholar]
- Kawakami K., Takeda H., Kawakami N., Kobayashi M., Matsuda N., Mishina M. (2004). A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 [DOI] [PubMed] [Google Scholar]
- Khachigian L. M., Resnick N., Gimbrone M. A., Jr, Collins T. (1995). Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J. Clin. Invest. 96, 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzh S., Pan X., Garcia-Lecea M., Winata C. L., Pan X., Wohland T., Korzh V., Gong Z. (2008). Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev. Biol. 8, 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- Lan Q., Mercurius K. O., Davies P. F. (1994). Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem. Biophys. Res. Commun. 201, 950–956 [DOI] [PubMed] [Google Scholar]
- Larrivée B., Prahst C., Gordon E., del Toro R., Mathivet T., Duarte A., Simons M., Eichmann A. (2012). ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 22, 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Bressan M., Hassel D., Huisken J., Staudt D., Kikuchi K., Poss K. D., Mikawa T., Stainier D. Y. (2010). A dual role for ErbB2 signaling in cardiac trabeculation. Development 137, 3867–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A., Attisano L., Marchuk D. A. (1999). Assignment of transforming growth factor β1 and β3 and a third new ligand to the type I receptor ALK-1. J. Biol. Chem. 274, 9984–9992 [DOI] [PubMed] [Google Scholar]
- Melchionna R., Porcelli D., Mangoni A., Carlini D., Liuzzo G., Spinetti G., Antonini A., Capogrossi M. C., Napolitano M. (2005). Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J. 19, 629–631 [DOI] [PubMed] [Google Scholar]
- Mitchell D., Pobre E. G., Mulivor A. W., Grinberg A. V., Castonguay R., Monnell T. E., Solban N., Ucran J. A., Pearsall R. S., Underwood K. W., et al. (2010). ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol. Cancer Ther. 9, 379–388 [DOI] [PubMed] [Google Scholar]
- Moya I. M., Umans L., Maas E., Pereira P. N., Beets K., Francis A., Sents W., Robertson E. J., Mummery C. L., Huylebroeck D., et al. (2012). Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev. Cell 22, 501–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus H., Rosen V., Thies R. S. (1999). Heart specific expression of mouse BMP-10 a novel member of the TGF-beta superfamily. Mech. Dev. 80, 181–184 [DOI] [PubMed] [Google Scholar]
- Oh S. P., Seki T., Goss K. A., Imamura T., Yi Y., Donahoe P. K., Li L., Miyazono K., ten Dijke P., Kim S., et al. (2000). Activin receptor-like kinase 1 modulates transforming growth factor-β 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 97, 2626–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. O., Lee Y. J., Seki T., Hong K. H., Fliess N., Jiang Z., Park A., Wu X., Kaartinen V., Roman B. L., et al. (2008). ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111, 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Shao D., Upton P. D., Desouza P., Adcock I. M., Davies R. J., Morrell N. W., Griffiths M. J., Wort S. J. (2012). BMP-9 induced endothelial cell tubule formation and inhibition of migration involves Smad1 driven endothelin-1 production. PLoS ONE 7, e30075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K. M., Larman H. B., Dai G., Zhang Y., Wang E. T., Moorthy S. N., Kratz J. R., Lin Z., Jain M. K., Gimbrone M. A., Jr, et al. (2006). Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshkovsky C., Totong R., Yelon D. (2011). Dependence of cardiac trabeculation on neuregulin signaling and blood flow in zebrafish. Dev. Dyn. 240, 446–456 [DOI] [PubMed] [Google Scholar]
- Ricard N., Ciais D., Levet S., Subileau M., Mallet C., Zimmers T. A., Lee S. J., Bidart M., Feige J. J., Bailly S. (2012). BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 119, 6162–6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman B. L., Pekkan K. (2012). Mechanotransduction in embryonic vascular development. Biomech. Model. Mechanobiol. 11, 1149–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman B. L., Pham V. N., Lawson N. D., Kulik M., Childs S., Lekven A. C., Garrity D. M., Moon R. T., Fishman M. C., Lechleider R. J., et al. (2002). Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 129, 3009–3019 [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M., van Dinther M., Liu Z., van Bezooijen R. L., Zhao Q., Pukac L., Löwik C. W., ten Dijke P. (2007). BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 120, 964–972 [DOI] [PubMed] [Google Scholar]
- Seemann P., Brehm A., König J., Reissner C., Stricker S., Kuss P., Haupt J., Renninger S., Nickel J., Sebald W., et al. (2009). Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet. 5, e1000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert A. J., Huq A., Weinstein B. M., Walker C., Fishman M., Stainier D. Y. (2002). Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 31, 106–110 [DOI] [PubMed] [Google Scholar]
- Seki T., Yun J., Oh S. P. (2003). Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 93, 682–689 [DOI] [PubMed] [Google Scholar]
- Seki T., Hong K. H., Yun J., Kim S. J., Oh S. P. (2004). Isolation of a regulatory region of activin receptor-like kinase 1 gene sufficient for arterial endothelium-specific expression. Circ. Res. 94, e72–e77 [DOI] [PubMed] [Google Scholar]
- Star G. P., Giovinazzo M., Langleben D. (2010). Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: a potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc. Res. 80, 349–354 [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Ichijo H., Franzén P., Laiho M., Miyazono K., Heldin C. H. (1994). Characterization of type I receptors for transforming growth factor-beta and activin. Science 264, 101–104 [DOI] [PubMed] [Google Scholar]
- Topper J. N., Cai J., Qiu Y., Anderson K. R., Xu Y. Y., Deeds J. D., Feeley R., Gimeno C. J., Woolf E. A., Tayber O., et al. (1997). Vascular MADs: two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc. Natl. Acad. Sci. USA 94, 9314–9319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D., Paw B. H., Poss K. D., Penberthy W. T., Lin S., Zon L. I. (2003). Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 [DOI] [PubMed] [Google Scholar]
- Urness L. D., Sorensen L. K., Li D. Y. (2000). Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet. 26, 328–331 [DOI] [PubMed] [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. L., Tang C. C., Wung B. S., Chen H. H., Hung M. S., Wang J. J. (1993). Cyclical strain increases endothelin-1 secretion and gene expression in human endothelial cells. Biochem. Biophys. Res. Commun. 195, 1050–1056 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1995). The Zebrafish Book. Eugene, OR: University of Oregon Press; [Google Scholar]
- Woods I. G., Wilson C., Friedlander B., Chang P., Reyes D. K., Nix R., Kelly P. D., Chu F., Postlethwait J. H., Talbot W. S. (2005). The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 15, 1307–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Liu J., Derynck R. (2012). Post-translational regulation of TGF-β receptor and Smad signaling. FEBS Lett. 586, 1871–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelon D., Horne S. A., Stainier D. Y. (1999). Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 214, 23–37 [DOI] [PubMed] [Google Scholar]
- Young K., Conley B., Romero D., Tweedie E., O’Neill C., Pinz I., Brogan L., Lindner V., Liaw L., Vary C. P. (2012). BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood 120, 4263–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Lee P. L., Tsai C. S., Lee C. I., Yang T. L., Chuang H. S., Lin W. W., Lin T. E., Lim S. H., Wei S. Y., et al. (2012). Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc. Natl. Acad. Sci. USA 109, 7770–7775 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.