Abstract
Study Objectives:
To evaluate the effect of respiratory scoring criteria on diagnosis and classification of sleep disordered breathing (SDB) in chronic heart failure (CHF).
Design:
Cross-sectional observational study.
Setting:
Heart failure and general cardiology clinics at two London hospitals.
Patients or Participants:
One hundred eighty stable patients with CHF and a median age of 69.6 y, 86% male.
Interventions:
SDB was diagnosed by polysomnography. The apnea-hypopnea index (AHI) was initially scored using a conservative hypopnea definition of a ≥ 50% decrease in nasal airflow with a ≥ 4% oxygen desaturation. The AHI was rescored with hypopnea defined according to the American Academy of Sleep Medicine (AASM) alternative scoring rule, requiring an associated ≥ 3% oxygen desaturation or arousal. SDB was defined as AHI ≥ 15/h. Diagnosis and classification of SDB as obstructive sleep apnea (OSA) or central sleep apnea (CSA) with each rule were compared. The effect of mixed apneas on classification of SDB as CSA or OSA was also investigated.
Measurements and Results:
Median AHI increased from 9.3/h to 13.8/h (median difference 4.6/h) when the AASM alternative rule was used to score hypopneas. SDB prevalence increased from 29% to 46% with the alternative scoring rule (P < 0.001). Classification of SDB as OSA or CSA was not significantly altered by hypopnea scoring rules or the categorization of mixed apneas.
Conclusion:
Hypopnea scoring rules can significantly influence the apnea-hypopnea index and diagnosis of sleep disordered breathing in chronic heart failure but do not alter the classification as obstructive sleep apnea or central sleep apnea. Standardization of hypopnea scoring rules is important to ensure consistency in diagnosis of sleep disordered breathing in chronic heart failure patients.
Citation:
Ward NR; Roldao V; Cowie MR; Rosen SD; McDonagh TA; Simonds AK; Morrell MJ. The effect of respiratory scoring on the diagnosis and classification of sleep disordered breathing in chronic heart failure. SLEEP 2013;36(9):1341-1348.
Keywords: Chronic heart failure, diagnosis, hypopnea, mixed apnea, scoring criteria, sleep disordered breathing
INTRODUCTION
Sleep disordered breathing (SDB) is prevalent in patients with chronic heart failure (CHF).1–3 However, the absence of classic symptoms such as daytime sleepiness4,5 makes diagnosis and treatment difficult in patients with CHF. An apnea-hypopnea index (AHI) ≥ 15 events per hour is typically used as the cutoff for diagnosis and treatment of SDB in CHF6–9 but this index is dependent on the criteria used to identify respiratory events.
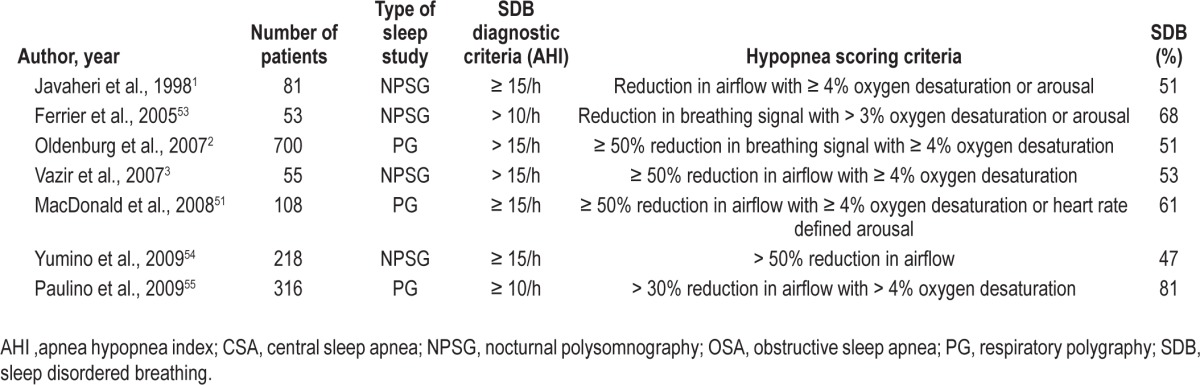
Respiratory scoring rules are known to influence diagnosis of obstructive sleep apnea (OSA)10–12 and an important source of variation is the definition of hypopnea.11,13–15 Although a number of criteria have been used to score hypopnea in CHF (Table 1), the influence of respiratory scoring rules on diagnosis of SDB in patients with CHF is unknown. The first goal of this study was to investigate the effect of two hypopnea scoring rules on the AHI and prevalence of SDB in CHF. We tested the hypothesis that the diagnosis of SDB would be made in a significantly greater number of patients with CHF when hypopneas were scored using the American Academy of Sleep Medicine (AASM) “alternative” rule requiring an associated ≥ 3% oxygen desaturation or electroencephalographic (EEG) arousal,16 compared with a more conservative hypopnea definition requiring a corroborative ≥ 4% oxygen desaturation.17
Table 1.
Hypopnea scoring criteria used in studies reporting the prevalence of sleep disordered breathing in chronic heart failure
The classification of SDB as OSA or central sleep apnea (CSA) is important when determining treatment options in CHF.18 Scoring criteria may also influence the classification of SDB if they result in preferential scoring of central or obstructive respiratory events. Central apneas are reported to be associated with less marked oxygen desaturation than obstructive and mixed apneas.19–21 Therefore, a hypopnea definition requiring a lesser corroborative oxygen desaturation may favor the scoring of central hypopneas, which would systematically bias the classification of SDB toward CSA. The second goal of this study was to evaluate whether the use of the two different hypopnea scoring criteria would result in a shift in classification of SDB from OSA to CSA. We tested the hypothesis that classification of SDB as OSA or CSA would not be changed by the use of two different hypopnea scoring rules.
A third area of uncertainty in the diagnosis of SDB in CHF relates to the effect of mixed apneas on classification of SDB. Mixed apneas contain features of both obstructive and central apnea in the same event. Although some consider they are part of the OSA syndrome,22 mixed apneas are typically categorized as central apneas in patients with CHF.3,23 It is not known if these differences in categorization influence the classification of SDB. The third goal of this study was to evaluate the effect of mixed apneas on the classification of SDB in CHF. We tested the hypothesis that categorization of mixed apneas as central or obstructive apneas does not significantly change the classification of SDB as CSA or OSA in patients with CHF.
The data analyzed for this study were collected during a research project to investigate the utility of single-channel portable monitors for diagnosis of SDB in CHF.24
METHODS
Stable patients with CHF attending cardiology clinics were invited to participate in this study irrespective of clinical suspicion of SDB. CHF was diagnosed in accordance with European guidelines.25 Criteria for inclusion were age 18-90 y and no hospitalization or change in medication for ≥ 4 weeks. Patients receiving treatment for SDB were excluded. Written informed consent was given by all patients before participation and ethical approval for this research was received from the Brompton, Harefield, and NHLI research ethics committee (COREC 07/ Q0404/32).
SDB was diagnosed by nocturnal polysomnography (SOMNO-screen, SOMNOmedics, Randersacker, Germany). Electroen-cephalogram, electrooculograms, and submental electromyogram (EMG) were recorded for analysis of sleep. Thoracoabdominal movements were monitored using respiratory inductance plethysmography belts (Sleepsense, SLP Inc., St. Charles, Illinois, USA). Nasal pressure measurement was used to detect airflow and oxygen saturation was recorded by finger pulse oximeter. Snoring was detected with a tracheal microphone. Bilateral anterior tibialis EMG was monitored for identification of leg movements and body position was recorded by the SOMNOscreen recording unit. Polysomnography was performed in the patient's own home or in the hospital sleep laboratory according to the patient's preference. There were 132 patients (73%) who opted for polysomnography to be performed in their home.
Anthropometric measurements and clinical interview were completed before the sleep study. Subjective daytime sleepiness was quantified with the Epworth Sleepiness Scale (ESS).26 A single maintenance of wakefulness test (Oxford Sleep Resistance; OSLER test) was performed on the morning after polysomnography.27 Heart failure severity was assessed using the New York Heart Association (NYHA) classification,28 assay of brain natriuretic peptide (BNP) (Triage BNP, Biosite Inc, San Diego, California, USA), and echocardiographic assessment of cardiac size and function.
All polysomnography studies were analyzed by one investigator (VR) who was unaware of the clinical status of the patient or involved with recording of the data. Sleep and arousals were scored according to standard criteria.16 Apnea was scored when nasal airflow reduced to < 10% of baseline for ≥ 10 sec. Obstructive apneas were scored when the thoracoabdominal effort signals showed continuing respiratory excursions and central apnea was scored when respiratory efforts were absent.16 Mixed apnea was scored when respiratory effort was absent during the first half of the apnea but three or more obstructed breaths occurred before resumption of airflow.29
Hypopneas were initially scored when the amplitude of the nasal airflow signal decreased ≥ 50% for ≥ 10 sec in association with a ≥ 4% oxygen desaturation (hereafter referred to as ‘hypopnea rule 1’). Hypopneas were categorized as obstructive when snoring, flattening of the nasal pressure inspiratory signal, or out-of-phase deflections on the thorax and abdomen movement signals occurred during the event29; central hypopnea was scored when all of these features were absent. After calculation of the AHI using hypopnea rule 1, the PSG was reanalyzed using the AASM “alternative” hypopnea scoring rule, which requires a ≥ 50% reduction in airflow with an associated ≥ 3% oxygen desaturation or EEG arousal (hereafter referred to as ‘hypopnea rule 2’).
SDB was defined as an AHI ≥ 15 events/h and classified as OSA or CSA according to the predominant type of respiratory event.
As the quality of the pulse oximeter signal was fundamental to differentiating hypopneas scored by each rule, patients with less than 240 min of technically adequate oximetry recording during polysomnography were excluded from the hypopnea analysis.
However, because apneas did not require an associated oxygen desaturation to be scored, these participants were not excluded from the mixed apnea analysis. Hypopnea scoring rule 2 was used for measurement of the AHI during the mixed apnea analysis.
Statistical Analyses
Because respiratory event indices were not parametrically distributed, results are presented as median and interquartile range (IQR). Where the distribution of the data could not be indicated using the IQR due to occurrence of events in < 25% of patients, values are presented as median and range, or mean and standard deviation (SD).
Comparison of continuous variables between two independent groups was performed with the Mann-Whitney U test. The Wilcoxon signed-rank test was used to compare repeated measurements of the AHI, hypopnea index, and proportion of respiratory events measured with each hypopnea rule. Categorical variables were compared using the chi-square test or Fisher exact test. The McNemar test was used to compare the number of patients with CHF in whom SDB was diagnosed using each hypopnea rule. The proportion of patients with CHF classified as having CSA and OSA was compared using the chi-square test. Statistical analyses were performed using SPSS V18.0 (IBM, Chicago, IL, USA).
RESULTS
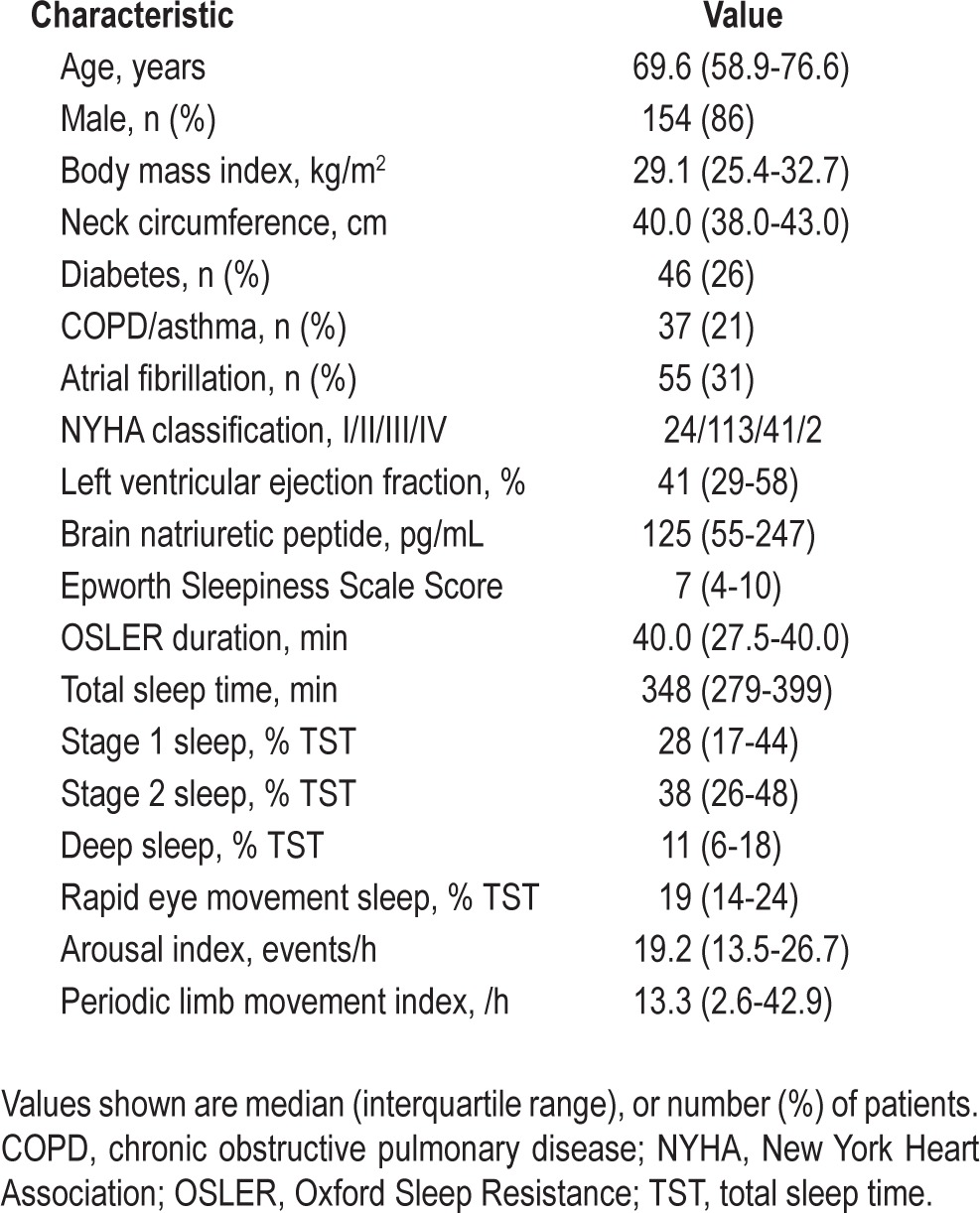
There were 354 patients with CHF identified who fulfilled the recruitment criteria, 180 of whom consented to participate in the study and underwent nocturnal polysomnography. Median age was 69.6 y (58.9-76.6 y), body mass index 29.1 (25.4-32.7) kg/m2, and 86% were male (Table 2). One hundred fifty-four patients (86%) had mild to moderate symptoms of CHF (New York Heart Association class II and III) with median left ventricular ejection fraction 41% (29-58%) and BNP concentration 125 (55-247) pg/mL. Subjective and objective measurements of daytime sleepiness were normal in most patients with CHF, with median ESS of 7 (4-10) and OSLER duration of 40 min (27.5-40 min). The median total sleep time during polysomnography was 348 min (279-399 min) with predominantly light sleep and a median arousal index of 19.2/h (13.5-26.7/h) (Table 2). Periodic limb movements during sleep occurred frequently with an index of 13.3/h (2.6-42.9/h).
Table 2.
Clinical characteristics and polysomnographic measurements of sleep in patients with chronic heart failure (n = 180)
Diagnosis of SDB in CHF Using Two Different Hypopnea Scoring Rules
One hundred seventy patients with CHF had technically adequate oximetry data and were included in the hypopnea analysis.
The median AHI scored with hypopnea rule 1 was 9.3 events/h (4.0-19.3 events/h), compared with 13.8 events/h (7.5-26.3 events/h) with hypopnea rule 2 (P < 0.001; Table 3). The mean difference between the AHI scored with each hypopnea rule was 4.6 events/h, with 95% limits of agreement of -1.9 to 11.2 events/h (Figure 1).
Table 3.
Comparison of respiratory event frequency and diagnosis of sleep disordered breathing in patients with chronic heart failure using different hypopnea scoring criteria (n = 170)
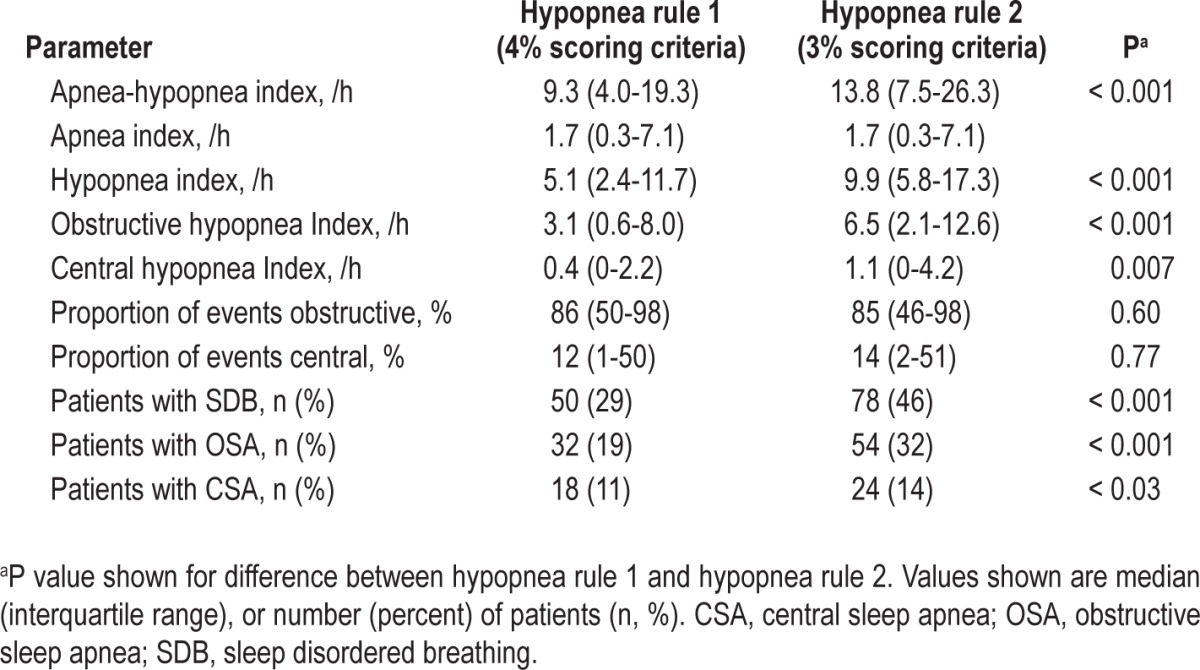
Figure 1.
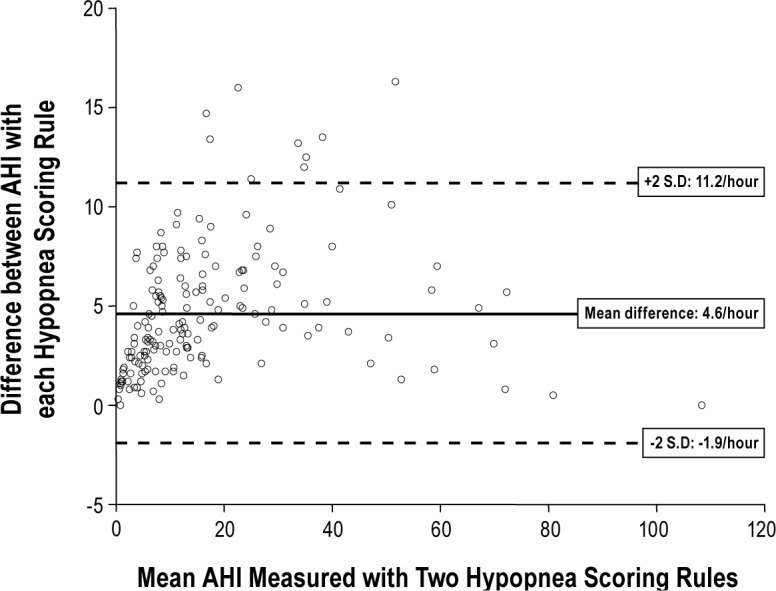
Difference versus average plot to show difference between apnea-hypopnea index (AHI) scored with the two hypopnea rules as a function of mean AHI. SD, standard deviation.
SDB was diagnosed in 50 patients with CHF (29%) when the AHI was scored with hypopnea rule 1 (Table 3). The number of patients with CHF in whom SDB was diagnosed increased significantly to 78 (46%) when hypopnea rule 2 was applied (P < 0.001 by McNemar test; Figure 2).
Figure 2.
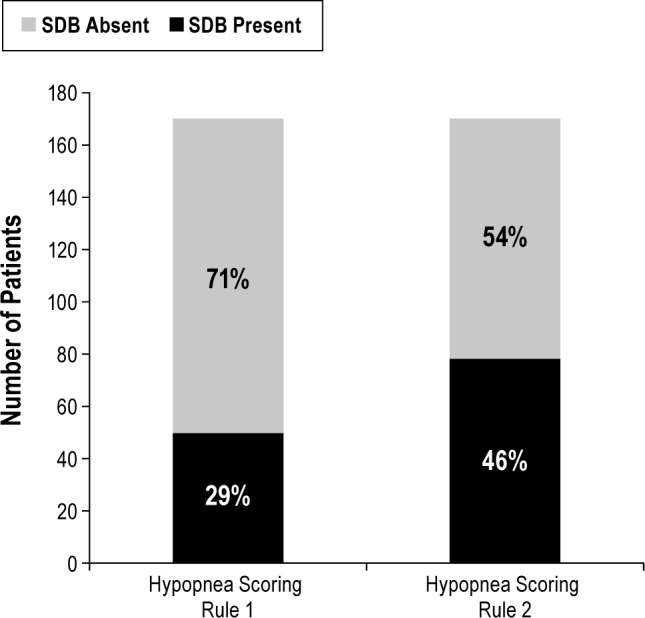
Number of patients with chronic heart failure in whom sleep disordered breathing was diagnosed using different hypopnea scoring criteria (n = 170). SDB, sleep disordered breathing
Using hypopnea scoring rule 1, the median 4% hypopnea index was significantly lower at 5.1 events/h (2.4-11.7 events/h), compared with 9.9 events/h (5.8-17.3 events/h) when hypopnea scoring rule 2 was applied (P < 0.001) (Table 3). Most SDB events were hypopneas and median apnea index was 1.7/h.
Effect of Hypopnea Scoring Rules on Classification of SDB in CHF
A significantly greater number of both obstructive (P < 0.001) and central hypopneas (P < 0.001) were scored using hypopnea rule 2 compared with hypopnea rule 1 (Table 3). The median increase in the obstructive hypopnea index was 2.5 events/h (0.8-4.6 events/h) and the central hypopnea index increased by 0.4 events/h (0 to 2.0 events/h). In accordance with the greater number of patients in whom SDB was diagnosed using hypopnea rule 2, there was a significant increase in the absolute number of patients with CHF in whom OSA (P < 0.001) and CSA (P < 0.03) were diagnosed using this rule. However, the proportion of patients with SDB classified as having OSA or CSA did not differ significantly with either hypopnea scoring rule; 64% of patients with CHF with SDB were classified as having OSA with rule 1 and 69% were classified as OSA with rule 2 (χ2 = 0.18, P = 0.67). Moreover, in the 50 patients with CHF in whom SDB was diagnosed using hypopnea rule 1, the classification of SDB as OSA or CSA did not change in any patient when hypopneas were scored using rule 2.
Effect of Mixed Apnea Categorization on Classification of SDB in CHF
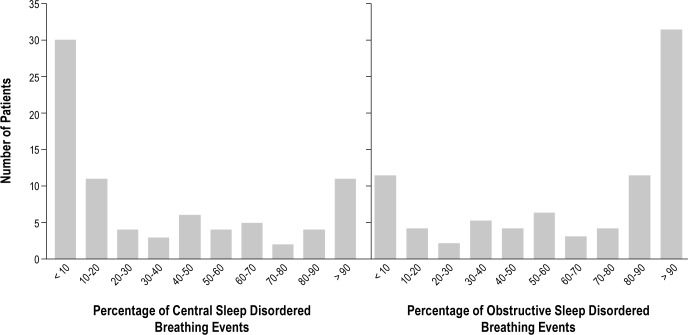
One hundred eighty patients with CHF were included in the mixed apnea analysis with AHI 13.4/h (7.2-25.8/h). SDB was diagnosed in 80 patients (44%) and classified as OSA in 55 patients (31%) and CSA in 25 patients (14%). In most patients with SDB, there was a predominance of either obstructive or central SDB events and a clear distinction between OSA and CSA (Figure 3).
Figure 3.
Classification of sleep disordered breathing in patients with chronic heart failure (n = 80). Data from each patient are displayed twice to show the percentage of central and obstructive sleep disordered breathing events. There was clear predominance of central or obstructive sleep disordered breathing events in most patients.
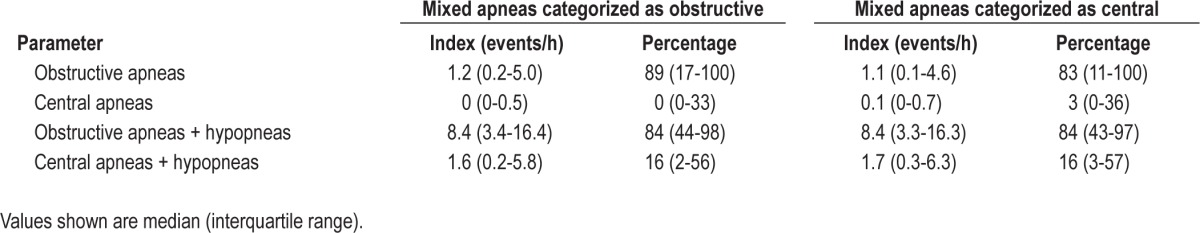
The median apnea index in patients with CHF was 1.9/h (0.3-7.2/h) with predominantly obstructive apneas (83%). Mixed apneas occurred in 40 patients (22%) but the frequency of these events was low, with mean mixed apnea index 0.3 [SD 1.1]/h, range 0-10.6/h. They accounted for a minority of all apneas and the mean percentage of apneas classified as mixed was 1% [SD 2.7] (range 0-15%). Therefore, categorization of mixed apneas as obstructive or central apnea caused minimal change in respiratory indices (Table 4). Moreover, the classification of SDB as OSA or CSA was not changed in any patient when mixed apneas were categorized as obstructive apneas. However, when mixed apneas were categorized as central apneas, SDB classification was changed from OSA to CSA in one patient; this individual had an AHI of 59.8/h and was initially classified as having OSA with 47% obstructive, 44% central, and 9% mixed respiratory events.
Table 4.
Effect of mixed apnea categorization on respiratory event frequency in patients with chronic heart failure (n = 180)
The frequency of mixed apneas was analyzed in the 80 patients with CHF in whom SDB was diagnosed; the mixed apnea index remained low with a mean of 0.6 [1.5]/h (range 0-10.6/h) and mixed apneas comprised a mean of 1% [2.5] (range 0-15%) of all respiratory events. Mixed apneas occurred in a significantly greater proportion of patients with CHF and CSA (56%) compared with patients with OSA (27%; P = 0.03). Median mixed apnea index was 0.2/h (0-0.6/h) in patients with CSA compared with 0/h (0-0.2/h) in patients with OSA (P = 0.04).
DISCUSSION
The principal finding of this study is that the criteria used to score hypopnea can significantly influence the measured AHI and at a cutoff of ≥ 15/h, the diagnosis of SDB in CHF. When hypopneas were defined using the AASM “alternative” rule (a ≥ 50% reduction in nasal airflow with a ≥ 3% oxygen desaturation or EEG arousal), the AHI was significantly higher compared with the more conservative hypopnea definition requiring an associated ≥ 4% oxygen desaturation. However, the criteria used to score hypopnea had no effect on the classification of SDB as OSA or CSA. Because patients with CHF with clinically significant SDB may have minimal symptoms,5 understanding the effect of hypopnea scoring criteria is important if diagnosis of SDB and the need for treatment are determined by the measured AHI. This contrasts with treatment of OSA in adults without CHF, where both symptoms and the metric of SDB severity are important when determining the need for therapy.30,31
The importance of a uniform definition of hypopnea is emphasized by reports of significant differences in the AHI and prevalence of OSA with the use of different scoring criteria.11–13,15 The definition of hypopnea used for diagnosis of SDB in CHF is inconsistent, as shown in Table 1. Historically, hypopneas were defined as a reduction in oronasal airflow32 or thoracoabdominal movement,33,34 but this definition was refined to require the reduction in breathing to be associated with a corroborative oxygen desaturation or arousal from sleep.22,35,36 Current guidelines from the AASM to standardize scoring of respiratory events during sleep require all hypopneas to be associated with an oxygen desaturation. However, these guide-lines list two different rules by which hypopnea can be scored.16 The AASM “recommended” rule requires a ≥ 30% reduction in nasal pressure signal amplitude for ≥ 10 sec with a ≥ 4% oxygen desaturation, whereas the “alternative” rule requires a ≥ 50% reduction in nasal pressure signal amplitude with a ≥ 3% oxygen desaturation or arousal from sleep.
The effect of hypopnea scoring criteria has only been studied in populations with OSA. As CSA events may be associated with lesser oxygen desaturation,19–21 the effect of hypopnea scoring criteria could differ in patients with CHF in whom CSA is prevalent. Our finding that the diagnosis of SDB changed in 28 patients with CHF (16%) using different hypopnea scoring rules is similar to reports investigating the influence of scoring rules in adults without CHF, in whom the prevalence of OSA increased by up to 19% when a more lenient rule was used to score hypopnea.13,15 Moreover, the two hypopnea scoring rules used in the current study were almost identical to those evaluated by Ruehland et al.,12 who reported a similar change in the median AHI of 3.5/h and 12% change in OSA prevalence, in adults without CHF.
Although the current study has shown the choice of respiratory scoring criteria can significantly alter the diagnosis of SDB in CHF, the effect of the different hypopnea scoring rules may be of a similar magnitude to the normal night-to-night variation in SDB. In patients with CHF monitored on consecutive nights, 18% of patients in whom SDB was diagnosed on 1 night did not fulfill criteria for diagnosis of SDB on the second night.37 In addition, the severity of SDB changed from moderate (AHI 15-30/h) to severe (AHI ≥ 30/h) on consecutive nights in 37% of patients with CHF.38 Therefore, taking into account the effects of both respiratory scoring criteria and night-to- night variability on the measured AHI, use of a strict AHI cutoff of ≥ 15/h in patients with CHF may be too restrictive, leading to treatment for SDB not being offered to some who may benefit.
The current study is unable to identify the optimum definition for hypopnea in patients with CHF as this would require data on clinically relevant outcomes. In a community-based cohort of adults without CHF, Punjabi et al.39 reported that hypopneas associated with a ≥ 4% oxygen desaturation were associated with prevalent cardiovascular disease, whereas hypopneas associated with a lesser oxygen desaturation or arousal were not. However, some authors argue that the requirement for a corroborative oxygen desaturation to score hypopnea results in underdetection of clinically relevant events,40 leading to under-estimation of SDB severity in individuals with respiratory events causing arousals without desaturation.41 Clinical characteristics including baseline oxygen saturation, body mass index, sleep stage, and resting lung volume will influence the amount of oxygen desaturation during a hypopnea.32,42 Thus, hypopneas requiring a corroborative oxygen desaturation may be scored more readily in overweight adults than in lean individuals.40 Current definitions of hypopnea requiring a corroborative ≥ 3% or ≥ 4% oxygen desaturation were developed for diagnosis of OSA and may not be suitable for patients with CHF who are less likely to be overweight, or in whom there may be less oxygen desaturation during CSA.
This study has also found that mixed apneas were identified in only 22% of patients with CHF and the frequency of these events was low. Previous studies have also reported a low frequency of mixed apneas in patients with CHF but have not examined the effect of mixed apneas on classification of SDB.1,3 In the current study, classification of SDB as OSA or CSA was unchanged in 99% of patients with CHF, irrespective of whether mixed apneas were considered as obstructive or central events. Mixed apneas comprise features of both obstructive and central apnea within the same event. Conflicting reports about their pathogenesis43–45 may explain the discrepancy in categorization as obstructive22 or central apneas3,23 in different studies. The results from the current study suggest that mixed apneas are infrequent events in most patients with CHF and ultimately their categorization may be unimportant. Classification and treatment of SDB in patients with CHF with mixed apneas should therefore be decided according to the frequency and proportion of the other respiratory events.
Several limitations relating to the hypopnea analysis are recognized. The hypopnea rule 2 used in the current study is the same as the “alternative” hypopnea rule recommended by the AASM guidelines. However, the ≥ 50% reduction in airflow amplitude required for our hypopnea rule 1 differs from the AASM “recommended” rule,16 which stipulates a ≥ 30% reduction in airflow, although the ≥ 4% corroborative oxygen desaturation required is the same. This difference in airflow amplitude criteria may be of limited clinical significance due to the difficulty in determining baseline breathing amplitude in patients without a stable breathing pattern or with frequent respiratory events.22 Moreover, the difference between a ≥ 30% and ≥ 50% reduction in breathing amplitude may be difficult to accurately detect with semiquantitative airflow sensors. Ruehland et al.12 reported that the larger airflow reduction (≥ 50% versus ≥ 30%) required to score hypopneas using the AASM “alternative” rule had minimal effect on the AHI.
A further consideration is that the criteria used to differentiate central and obstructive hypopneas in the current study have not been systematically evaluated. Difficulty in identifying the reduction in neural respiratory drive during a central hypopnea may have led to misclassification of some hypopneas. The criteria we used have also been used in previous studies evaluating SDB in CHF29,46 and were selected as the most suitable noninvasive method to classify hypopnea. However, signs of obstruction such as snoring may occur during central hypopnea due to change in upper airway caliber and compliance with the reduction in respiratory drive. The gold standard for distinguishing central from obstructive hypopnea is esophageal manometry,16 but this was not practical in view of the high number of polysomnography studies required.
A potential limitation of our respiratory event analysis is the use of nasal pressure transducer without thermistor for measurement of airflow during polysomnography. Thermal devices and nasal pressure transducers have differing accuracy for detection of apnea and hypopnea.47,48 Nasal pressure monitoring has a high sensitivity for detection of hypopneas,47,49 although it can result in overestimation of the number of apneas due to the nonlinear relationship between nasal pressure and nasal airflow.10 This may have resulted in overestimation of the number of apneas in the current study, whereas some mixed apneas may have been incorrectly diagnosed if low volume breaths or mouth breathing after central apnea were not detected by nasal pressure transducer. However, this would mean the true prevalence and frequency of mixed apneas in patients with CHF may be lower than we have observed and therefore it is unlikely the choice of airflow sensor in the current study has affected the results of our mixed apnea analysis.
The definition of mixed apnea in the current study required respiratory effort to be absent in the first half of the apnea followed by three or more obstructed breaths before resumption of airflow.29 Alternative mixed apnea definitions do not specify the minimum number of obstructed breaths.16 Other authors have used a temporal definition to specify the minimum duration of the central component is > 50% of the apnea3 or that neither the central nor obstructive component can exceed 75% of the duration of the apnea.50 The findings of the current study may not be applicable when these different criteria are used.
The prevalence of CSA in our cohort of patients is lower than reported in many previous studies evaluating SDB in CHF.1–3,51 This may be explained by advances in CHF treatment including the greater use of cardiac resynchronization therapy in our contemporary cohort, which has been shown to reduce the severity of CSA.52 In addition, susceptibility to OSA may have been increased in the current study because most patients were overweight or obese.
CONCLUSION
In patients with CHF, the criteria used to define hypopnea significantly influence the AHI and prevalence of SDB. The number of patients with CHF in whom SDB was diagnosed, using an AHI cutoff of ≥ 15/h, increased by 16% using the AASM “alternative” rule compared with the more conservative hypopnea scoring rule. Because the AHI may be the principal determinant of the need to treat SDB in patients with CHF, it is important that respiratory scoring criteria are standardized to ensure consistency in diagnosis of SDB. Although it is evident that the criteria used to define hypopnea significantly influence the diagnosis of SDB in CHF, the effect of the different definitions on clinically relevant outcomes or response to therapy requires further evaluation.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Cowie has received research grants and honoraria from ResMed. Dr.
Morrell has received research equipment from ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This project was supported by the NIHR respiratory Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, and by a research grant from the British Heart Foundation charity. The salaries of Professors Cowie and Simonds are supported by the National Institute of Health Research Biomedical Research Units at the Royal Brompton Hospital.
Footnotes
A commentary on this article appears in this issue on page 1277.
REFERENCES
- 1.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep-disordered breathing in patients with symptomatic heart failure. A contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Vazir A, Hastings PC, Dayer M, et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail. 2007;9:243–50. doi: 10.1016/j.ejheart.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 5.Hastings PC, Vazir A, O'Driscoll DM, Morrell MJ, Simonds AK. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27:748–55. doi: 10.1183/09031936.06.00063005. [DOI] [PubMed] [Google Scholar]
- 6.Hastings PC, Vazir A, Meadows GE, et al. Adaptive servo-ventilation in heart failure patients with sleep apnea: a real world study. Int J Cardiol. 2010;139:17–24. doi: 10.1016/j.ijcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–6. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 8.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10:581–6. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 10.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 11.Redline S, Kapur VK, Sanders MH, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med. 2000;161:369–74. doi: 10.1164/ajrccm.161.2.9904031. [DOI] [PubMed] [Google Scholar]
- 12.Rueland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manser RL, Rochford P, Pierce RJ, Byrnes GB, Campbell DA. Impact of different criteria for defining hypopneas in the apnea-hypopnea index. Chest. 2001;120:909–14. doi: 10.1378/chest.120.3.909. [DOI] [PubMed] [Google Scholar]
- 14.Tang JP, Rosen CL, Larkin EK, et al. Identification of sleep-disordered breathing in children: variation with event definition. Sleep. 2002;25:72–9. doi: 10.1093/sleep/25.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Tsai WH, Flemons WW, Whitelaw WA, Remmers JE. A comparison of apnea-hypopnea indices derived from different definitions of hypopnea. Am J Respir Crit Care Med. 1999;159:43–8. doi: 10.1164/ajrccm.159.1.9709017. [DOI] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S. Treatment of obstructive and central sleep apnoea in heart failure: practical options. Eur Respir Rev. 2007;16:183–6. [Google Scholar]
- 19.Fletcher EC, Goodnight-White S, Munafo D, Miller CC, 3rd, Luckett R, Qian W. Rate of oxyhemoglobin desaturation in obstructive versus nonobstructive apnea. Am Rev Respir Dis. 1991;143:657–60. doi: 10.1164/ajrccm/143.3.657. [DOI] [PubMed] [Google Scholar]
- 20.Series F, Cormier Y, La Forge J. Influence of apnea type and sleep stage on nocturnal postapneic desaturation. Am Rev Respir Dis. 1990;141:1522–6. doi: 10.1164/ajrccm/141.6.1522. [DOI] [PubMed] [Google Scholar]
- 21.Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position reduces severity of central sleep apnea / Cheyne-Stokes respiration. Sleep. 2006;29:1045–51. doi: 10.1093/sleep/29.8.1045. [DOI] [PubMed] [Google Scholar]
- 22.AASM Task Force Report. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 23.Tkacova R, Niroumand M, Lorenzi-Filho G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure: role of PCO2 and circulatory delay. Circulation. 2001;103:238–43. doi: 10.1161/01.cir.103.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Ward NR, Cowie MR, Rosen SD, et al. Utility of overnight pulse oximetry and heart rate variability analysis to screen for sleep-disordered breathing in chronic heart failure. Thorax. 67:1000–5. doi: 10.1136/thoraxjnl-2012-201684. 201. [DOI] [PubMed] [Google Scholar]
- 25.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 26.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 27.Bennett L, Stradling J, Davies R. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res. 1997;6:142–5. doi: 10.1046/j.1365-2869.1997.00039.x. [DOI] [PubMed] [Google Scholar]
- 28.The Criteria Committee of the New York Heart Association. 9th ed. Boston, MA: Little, Brown & Co.; 1994. Nomenclature and criteria for diagnosis of diseases of the heart and great Vessels; pp. 253–6. [Google Scholar]
- 29.Takasaki Y, Orr D, Popkin J, Rutherford R, Liu P, Bradley TD. Effect of nasal continuous positive airway pressure on sleep apnea in congestive heart failure. Am Rev Respir Dis. 1989;140:1578–84. doi: 10.1164/ajrccm/140.6.1578. [DOI] [PubMed] [Google Scholar]
- 30.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute for Health and Clinical Excellence (NICE) Continuous positive airway pressure for the treatment of obstructive sleep apnoea/ hypopnoea syndrome. London (UK): National Institute for Health and Clinical Excellence (NICE); 2008. (Technology appraisal guidance; no. 139) [Google Scholar]
- 32.Bradley TD, Martinez D, Rutherford R, et al. Physiological determinants of nocturnal arterial oxygenation in patients with obstructive sleep apnea. J Appl Physiol. 1985;59:1364–8. doi: 10.1152/jappl.1985.59.5.1364. [DOI] [PubMed] [Google Scholar]
- 33.Gould GA, Whyte KF, Rhind GB, et al. The sleep hypopnea syndrome. Am Rev Respir Dis. 1988;137:895–8. doi: 10.1164/ajrccm/137.4.895. [DOI] [PubMed] [Google Scholar]
- 34.Whyte KF, Allen MB, Fitzpatrick MF, Douglas NJ. Accuracy and significance of scoring hypopneas. Sleep. 1992;15:257–60. doi: 10.1093/sleep/15.3.257. [DOI] [PubMed] [Google Scholar]
- 35.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 36.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 37.Maestri R, La Rovere M, Robbi E, Pinna G. Night-to-night repeatability of measurements of nocturnal breathing disorders in clinically stable chronic heart failure patients. Sleep Breathing. 2011;15:673–8. doi: 10.1007/s11325-010-0418-4. [DOI] [PubMed] [Google Scholar]
- 38.Vazir A, Hastings PC, Papaioannou I, et al. Variation in severity and type of sleep-disordered breathing throughout 4 nights in patients with heart failure. Respir Med. 2008;102:831–9. doi: 10.1016/j.rmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–5. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guilleminault C, Hagen C, Huynh N. Comparison of hypopnea definitions in lean patients with known obstructive sleep apnea hypopnea syndrome (OSAHS) Sleep Breathing. 2009;13:341–7. doi: 10.1007/s11325-009-0253-7. [DOI] [PubMed] [Google Scholar]
- 41.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104:781–7. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 42.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180:788–93. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alex CG, Onal E, Lopata M. Upper airway occlusion during sleep in patients with Cheyne-Stokes respiration. Am Rev Respir Dis. 1986;133:42–5. doi: 10.1164/arrd.1986.133.1.42. [DOI] [PubMed] [Google Scholar]
- 44.Iber C, Davies SF, Chapman RC, Mahowald MM. A possible mechanism for mixed apnea in obstructive sleep apnea. Chest. 1986;89:800–5. doi: 10.1378/chest.89.6.800. [DOI] [PubMed] [Google Scholar]
- 45.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol. 1986;61:1438–43. doi: 10.1152/jappl.1986.61.4.1438. [DOI] [PubMed] [Google Scholar]
- 46.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–8. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 47.Series F, Marc I. Nasal pressure recording in the diagnosis of sleep apnoea hypopnoea syndrome. Thorax. 1999;54:506–10. doi: 10.1136/thx.54.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ballester E, Badia JR, Hernandez L, Farre R, Navajas D, Montserrat JM. Nasal prongs in the detection of sleep-related disordered breathing in the sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;11:880–3. doi: 10.1183/09031936.98.11040880. [DOI] [PubMed] [Google Scholar]
- 49.Norman RG, Ahmed MM, Walsleben JA, Rapoport DM. Detection of respiratory events during NPSG: nasal cannula/pressure sensor versus thermistor. Sleep. 1997;20:1175–84. [PubMed] [Google Scholar]
- 50.Bliwise D, Bliwise NG, Kraemer HC, Dement W. Measurement error in visually scored electrophysiological data: respiration during sleep. J Neurosci Methods. 1984;12:49–56. doi: 10.1016/0165-0270(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 52.Oldenburg O, Faber L, Vogt J, et al. Influence of cardiac resynchronisation therapy on different types of sleep disordered breathing. Eur J Heart Fail. 2007;9:820–6. doi: 10.1016/j.ejheart.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–22. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 54.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15:279–85. doi: 10.1016/j.cardfail.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 55.Paulino A, Damy T, Margarit L, et al. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102:169–75. doi: 10.1016/j.acvd.2008.12.006. [DOI] [PubMed] [Google Scholar]