Abstract
Alzheimer disease (AD) brain is characterized by extracellular plaques of amyloid β (Aβ) peptide with reactive microglia. This study aimed to determine whether a dietary intervention could attenuate microgliosis. Memory was assessed in 12-mo-old male amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mice via Barnes maze testing followed by division into either a control-fed group provided free access to normal chow and water or a treatment group provided free access to normal chow and drinking water supplemented with pomegranate extract (6.25 mL/L) for 3 mo followed by repeat Barnes maze testing for both groups. Three months of pomegranate feeding decreased the path length to escape of mice compared with their initial 12-mo values (P < 0.05) and their control-fed counterparts (P < 0.05). Brains of the 3-mo study pomegranate-fed mice had lower tumor necrosis factor α (TNF-α) concentrations (P < 0.05) and lower nuclear factor of activated T-cell (NFAT) transcriptional activity (P < 0.05) compared with controls. Brains of the 3-mo pomegranate or control mice were also compared with an additional control group of 12-mo-old mice for histologic analysis. Immunocytochemistry showed that pomegranate- but not control-fed mice had attenuated microgliosis (P < 0.05) and Aβ plaque deposition (P < 0.05) compared with 12-mo-old mice. An additional behavioral study again used 12-mo-old male APP/PS1 mice tested by T-maze followed by division into a control group provided with free access to normal chow and sugar supplemented drinking water or a treatment group provided with normal chow and pomegranate extract–supplemented drinking water (6.25 mL/L) for 1 mo followed by repeat T-maze testing in both groups. One month of pomegranate feeding increased spontaneous alternations versus control-fed mice (P < 0.05). Cell culture experiments verified that 2 polyphenol components of pomegranate extract, punicalagin and ellagic acid, attenuated NFAT activity in a reporter cell line (P < 0.05) and decreased Aβ-stimulated TNF-α secretion by murine microglia (P < 0.05). These data indicate that dietary pomegranate produces brain antiinflammatory effects that may attenuate AD progression.
Introduction
One of the defining histopathology of the Alzheimer disease (AD)4 brain is the age-associated progressive accumulation of amyloid β (Aβ) peptide–containing plaques (1, 2). However, abundant reactive microglia are also invested within the plaques (3, 4), and Aβ serves as a potent activating stimulus for these cells (5–8). This is of particular interest because retrospective epidemiologic studies have varyingly reported that the select antiinflammatory therapies are efficacious at decreasing the risk of AD at least during the early or asymptomatic stages of disease (9–12). This suggests that limiting microglial activation during particular times with select reagents may be a promising therapeutic strategy for AD.
Increased numbers of morphologically reactive microglia are a well-characterized histologic observation from AD brains compared with control brains without dementia (13, 14). A common approach for modulation of immune cell phenotype is to attenuate the activity of the transcription factor, nuclear factor of activated T cells (NFAT). NFAT is as a member of the REL family of transcription factors crucial to the regulation of select proinflammatory genes, such as interleukin (IL)-2 and tumor necrosis factor α (TNF-α) (15). Two groups of differently regulated NFAT transcription factor isoforms have been so far identified: 1) Ca2+/calcineurin-activated isoforms and 2) a tonicity-controlled isoform (16). Although various NFAT isoforms are expressed in immune and nonimmune cells, the Ca2+/calcineurin-activated NFAT isoforms play key roles in regulating immune cell phenotype (17, 18). NFAT expression has also been documented in neurons and astrocytes (19–23). Neuronal NFAT can regulate axonal growth (19), survival (21), or apoptosis (20) during development. On the other hand, astrocytic NFAT is reportedly involved in initiation and maintenance of injury-, disease-, and aging-mediated neuroinflammatory processes (22–24). Surprisingly, very little is known about NFAT expression and function in microglia despite the fact that it is the resident immune cell type in the brain. We (25), as well as others (26, 27), demonstrated that microglia also express NFAT, and it behaves as an immunomodulatory transcription factor similar to its role in other immune cells. Specifically, stimuli of microglia or other immune cells that elevate intracellular Ca2+ concentrations lead to activation of calcineurin, which dephosphorylates inactive cytosolic NFAT, allowing it to translocate to the nucleus where it forms a complex with the activator protein 1 (AP-1) family of transcription factors (Fos/Jun) to regulate pro- and antiinflammatory gene transcription (28). Therefore, calcineurin inhibitory agents such as cyclosporine or FK506 have gained clinical utility as immunomodulators via their indirect ability to inhibit NFAT (29, 30). However, the adverse side effects of these agents during prolonged treatment regimens, including severe renal toxicity, decrease their attractiveness for treating the millions of elderly patients suffering from AD (31). On the other hand, several small-molecule agents have been identified that directly prevent NFAT activity by binding calcineurin in or near the NFAT binding site to attenuate NFAT-calcineurin interaction (32–34).
To identify novel NFAT inhibitory agents for use as potential antiinflammatory agents in treating AD, we have relied on the recent demonstration that punicalagin, a polyphenol derived from pomegranate extract, attenuates NFAT activity in vitro (35). In addition, both punicalagin and ellagic acid, another polyphenol component of pomegranate extract, attenuate BACE activity (36), and BACE expression is reportedly NFAT-dependent (37). Based upon the fact that punicalagin and ellagic acid are available in rodent plasma after oral ingestion (38), we hypothesized that dietary pomegranate extract or its purified polyphenol components will not only inhibit NFAT activity and microglial activation in vitro but also demonstrate inhibitory, antiinflammatory activity in vivo using an amyloid precursor protein/presenilin 1 (APP/PS1) transgenic mouse model of AD.
Materials and Methods
Materials.
The anti-NFATc2, anti-phosphorylated (p-) NFATc2, and anti-α-tubulin antibodies were purchased from Santa Cruz Biotechnology. The anti-cluster of differentiation 68 (anti-CD68) antibody was obtained from AbD Serotec. The anti-synaptophysin and anti-βIII-tubulin antibodies were purchased from Chemicon. The anti-glial fibrillary acidic protein (anti-GFAP) antibody was purchased from Sigma. The anti-postsynaptic density protein 95 (anti-PSD95), anti- nuclear factor of κ light polypeptide gene enhancer in B-cell inhibitor (anti-IκB), anti-p-IκB, and anti-β site APP cleavage enzyme (anti-BACE) antibodies were obtained from Cell Signaling. The anti-nitrotyrosine antibody was purchased from Enzo Life Science. The anti-Aβ (4G8) antibody was obtained from Covance. The anti-APP antibody was purchased from Invitrogen, Life Technologies. The anti-4-hydroxy-2-nonenal (anti-HNE) Michael adduct antibody was purchased from EMD Millipore.
Pomegranate extract.
Pomegranate extract (POMx) was obtained from POM Wonderful LLC. A single serving of 240 mL (8 oz) of pomegranate juice reportedly contains 34 g of sugars, 35 g of other carbohydrates, 30 mg of sodium, 430 mg of potassium, 0% vitamin C or A, 4% calcium, 2% iron, anthocyanins (387 mg/L), punicalagins (1561 mg/L), ellagic acid (121 mg/L), and other tannins (417 mg/L) (39). The POMx liquid extract used in this study is reportedly prepared as described (40). Briefly, after juice is pressed from the whole fruit, the seeds are removed from the remaining portions, which are then screw pressed and enzymatically treated to solubilize polyphenol compounds. The material is concentrated and filtered to produce the extract that is further concentrated and pasteurized. Two hundred forty milliliters of the POMx is reported to contain 776 mg of gallic acid equivalents, which include hydrolysable tannins such as punicalagin, punicalin, ellagic acid, and anthocyanins (41). Human studies indicate that ellagic acid but not punicalagin is bioavailable in plasma (39, 42, 43). The ingestion of pomegranate juice containing 25 mg of ellagic acid and 318 mg of punicalagin results in a maximum plasma concentration (Cmax) of 31–33 ng/mL at 1 h postingestion (42, 43). However, rodent studies indicate that both ellagic acid and punicalagin can be absorbed into plasma after oral delivery (44, 45).
Animals.
Mouse use was approved by the University of North Dakota Institutional Animal Care and Use Committee. Only male C57BL/6 APPswe/PS1dE9 mice were used in this study. The mice were bred and raised at the University of North Dakota. Mice were fed 8640 Teklad 22/5 rodent diet “normal chow” (22% crude protein, 5.5% fat, 3.9% crude fiber, 3.0 kcal/g energy density, 29% calories from protein, 17% calories from fat, 54% calories from carbohydrate) and maintained on a 12-h light/dark cycle. Original founder animals for the colony were purchased from the Jackson Laboratory (005864). We used the mice at 12 mo of age after we observed that significant disease pathology and behavioral deficit had occurred (46). These APP/PS1 transgenic mice express the human APP, Aβ precursor protein (A4) with the Swedish mutations K595N/M596L and the human PSEN1, presenilin 1 with the DeltaE9 mutation under control of the mouse prion promoter.
Treatment groups.
The study was designed to behaviorally address longitudinal changes with age and pomegranate feeding by using the APP/PS1 mice at a starting age of 12 mo followed by 1 or 3 mo of treatment. Although consumption of water, POMx, and weight were not measured during the treatment periods, daily assessments of animal health were monitored. For the 3-mo study, all mice at 12 mo of age were randomized into 2 groups with n = 4 mice [control 12-mo-old (C-12) or pomegranate 12-mo-old (P-12) mice] before any treatments began. All of the mice were then Barnes maze tested. After the testing, the control group was maintained for 3 mo to an age of 15 mo on their normal free-access diet of chow and water [control 15-mo-old (C-15)], whereas the pomegranate group was provided chow plus daily free access to 6.25 mL/L of POMx (POMx treatment) in their drinking water for 3 mo to an age of 15 mo [pomegranate 15-mo-old (P-15)]. The 3-mo period was based on a prior study demonstrating that a similar time course was sufficient to ameliorate atherogenic effects in the apolipoprotein E deficient (apoE−/−) mouse model (40). Upon completion of the 3-mo period, mice were repeat tested on the Barnes maze, mice were sacrificed by carbon dioxide inhalation and exsanguination, then brains and spleens were collected from both groups for performing NFAT activity assays and cytokine ELISA analyses. To examine age-related histologic and biochemical changes in the brains during the disease process from 12 to 15 mo of age, an additional group of control 12-mo-old male APP/PS1 mice (12) from the colony was collected. The mice also had free access to normal chow and drinking water and therefore were comparable to both the C-12 and P-12 groups. They were collected to compare with the C-15 and P-15 groups. This group of mice is designated throughout as “12” to differentiate from C-12. An additional 1-mo study was performed to provide another behavioral analysis. Another group of male APP/PS1 mice were taken at 12 mo of age and randomized into 2 groups [n = 6/group; control 12-mo-old (Co-12) and pomegranate 12-mo-old (Po-12)]. Mice were T-maze tested, then control mice were provided daily free access to normal chow and added sugar in their drinking water (11.9% sucrose, 1.05% glucose, and 1.05% fructose) for 1 mo [control 13-mo-old (Co-13)]. Pomegranate-fed mice were also provided daily free access to normal chow but with added POMx (6.25 mL/L) in their drinking water (POMx treatment) for 1 mo [pomegranate 13-mo-old (Po-13)]. Mice were repeat tested in the T-maze after the 1 mo of treatment. Brains and spleens were not collected for subsequent analysis from these animals because they were only used to determine whether 1 mo of feeding was sufficient to produce behavioral change.
Primary microglia culture.
Microglia were derived from postnatal day 1 to postnatal day 2 C57BL/6 mouse brains as described previously (47). Microglia purity was routinely verified at >98% by CD68 immunoreactivity. Microglia were treated in serum-free media DMEM/F12 (catalog no. 12400-024) from Gibco Life Technologies overnight for 24 h with or without stimulation with 10μmol/L Aβ, 10μmol/L ellagic acid, or 10μmol/L punicalagin; cells or media were then collected for subsequent analyses. Aβ amino acids 1–42 fibril preparations were performed as in our prior work (8).
Barnes maze assessment for 3-mo treatment study.
To assess spatial learning and memory in the transgenic APP/PS1 mice with and without POMx treatment, the 12-mo-old male mice (C-12 and P-12) were used for Barnes maze trials before any dietary modification and then again at 15 mo (C-15 and P-15) after treatment. This behavioral paradigm provides mice with a large circular table with perimeter holes to explore, with only 1 hole leading to an escape box, while a bright light is projected onto the table as a motivational stimulus. Briefly, at the beginning of each testing period (12 mo, 15 mo) mice were given an adaptation period to explore the maze and were gently guided to the escape hole. This was followed by a spatial acquisition period to assess learning in which the mice were given 4 timed trials per day for 4 d to identify the correct escape hole. Assessment of long-term and short-term reference memory was made by testing mice on the maze on d 5 after the last acquisition trial (short-term) and again on d 12 (long-term). Statistical comparisons were made across ages and treatment groups comparing latency to escape, path length to escape, and number of errors to escape. After the final Barnes maze assessment at 15 mo, brains were either perfusion fixed for histology or flash-frozen for biochemical analyses.
T-maze assessment for 1-mo treatment study.
A T-maze was used to assess the working memory in male 12-mo-old transgenic APP/PS1 mice before (Co-12 and Po-12) and after 1 mo of control (Co-13) or POMx (Po-13) feeding. Mice were placed on the T-maze starting platform, and the entry door was then raised to allow the mice to walk down the stem and choose an arm. Once a choice was made, identified by the presence of all 4 feet in an arm, the mice were returned to the starting platform for 30 s before the door was raised to allow exploration again. The arm selection steps were repeated 9 times to allow recording of the number of entries into each arm.
Western blotting of 3-mo study mice.
Western blot analyses of hippocampal regions from treated mice were performed to assess specific changes in AD-associated variables using anti-synaptophysin, PSD95, nitrotyrosine, HNE-adduct, IκB, p-IκB, CD68, GFAP, NFATc2, and p-NFATc2 antibodies. The OD of all signals was normalized to their respective loading controls by using anti-βIII-tubulin or α-tubulin antibodies.
Immunohistochemistry of 3-mo study mice.
The fixed brains were cryoprotected, serially sectioned (40μm), and immunolabeled by using anti-CD68, Aβ (4G8), and GFAP antibodies with Vector VIP (Vector Laboratories, Inc.) as the chromogen. To quantify Aβ immunoreactivity, ODs of serial brain sections containing the entire hippocampus (4 sections/brain, 4 brains/condition) were used (46). All immunostains of brains were performed together to minimize variability in staining processing from day to day to allow accurate comparisons. 1.25× images were taken from 4 consecutive serial sections (960μm apart) throughout the brain containing the CA1, CA3, and dentate gyrus hippocampal regions. ODs of the individual regions from each section were measured by using Adobe Photoshop software (Adobe Systems). Each region was identified via marquee, and the same size marquee was applied to all sections per region and condition (4 sections/brain, 4 brains/condition).
ELISA of 3-mo study mice.
Flash-frozen brains and spleens from treated mice were pulverized and lysed to perform ELISAs from tissue lysate. Alternatively, media were collected from 24-h stimulated microglia for ELISAs. Commercial ELISAs were used to quantitate concentrations of TNF-α, IL-1β, or IL-6. All mouse cytokine ELISA kits, IL-1β (catalog no. 88-7910-PL), IL-6 (catalog no. 88-7064-CP2), and TNF-α (catalog no. SMTA00) were purchased from R&D Systems (for TNF-α) and eBioscience (for IL-1β and IL-6). Cytokine values were normalized to wet weight of tissue for tissue analysis.
MTT reduction assay.
The 3-(4,5-dimethylthiazol-2-yl (MTT) assay was used to determine changes in microglial viability with the use of 3[4,5-dimethylthiazol-2-y1]-2,5-diphenyltetrazolium bromide (100μg/mL; catalog no. M-2128) purchased from Sigma, which we dissolved in DMEM/F12. After the 24-h stimulations, media were removed for ELISA and the cells were incubated with MTT for 4 h. The media were aspirated and the reduced formazan precipitate was dissolved in isopropanol. Absorbance values were read at 560/650 nm via plate reader.
HeLa/NFAT-luc stable cell line culture and luciferase assay.
HeLa/NFAT-luc cells were purchased from Panomics to measure NFAT response element–directed luciferase expression. Cells were grown in DMEM supplemented with 10% FBS, 100 units/mL penicillin, 100μg/mL streptomycin, and 100μg/mL hygromycin B. Cells were pretreated with 0.1 μmol/L–100 μmol/L punicalagin, 0.1 μmol/L–100 μmol/L ellagic acid, or 0.01–10% POMx for 30 min and then stimulated with or without 500 nmol/L 12-O-tetradecanoylphorbol-13-acetate (TPA) + ionomycin for 6 h. Stimulated cells were then harvested in reporter lysis buffer, and the lysate luciferase activity was measured by using a commercial luciferase reporter assay system (catalog no. E4550) according to the manufacturer’s protocol (Promega Corp.). Values were normalized to their respective protein concentrations.
NFATc1 transcription factor activity assay of 3-mo study mice.
NFATc1 transcription factor activity assays were performed by using the 96-well plate-based TransAm NFATc1 Transcription Factor Assay kit (Active Motif) according to the manufacturer’s protocol. Mouse hippocampal or spleen nuclear lysates were prepared from treated animal tissue for use in activity assay analyses. The activity of NFATc1 was measured by using a microplate reader at 450 nm.
Statistical analysis.
Behavioral data (Barnes maze and T-maze) were analyzed by using a 2-way repeated-measures ANOVA (1-factor repetition) with a Holm-Sidak post hoc test. When comparing only between groups C-15 and P-15, data were analyzed by using a t test. To compare differences in group 12 vs. C-15 vs. P-15, the data were analyzed via a 1-way ANOVA with a Tukey post hoc test or a Kruskal-Wallis 1-way ANOVA when variances were unequal. A 2-way ANOVA with the Holm-Sidak post hoc test was used to compare luciferase activities between TPA and pomegranate polyphenol treatments. Data are expressed as means ± SDs, and significance was considered at P < 0.05. All statistical analyses were performed by using Sigma Plot 12.0 statistical software.
Results
POMx feeding was sufficient to improve Barnes maze behavioral performance in a 3-mo study of APP/PS1 mice.
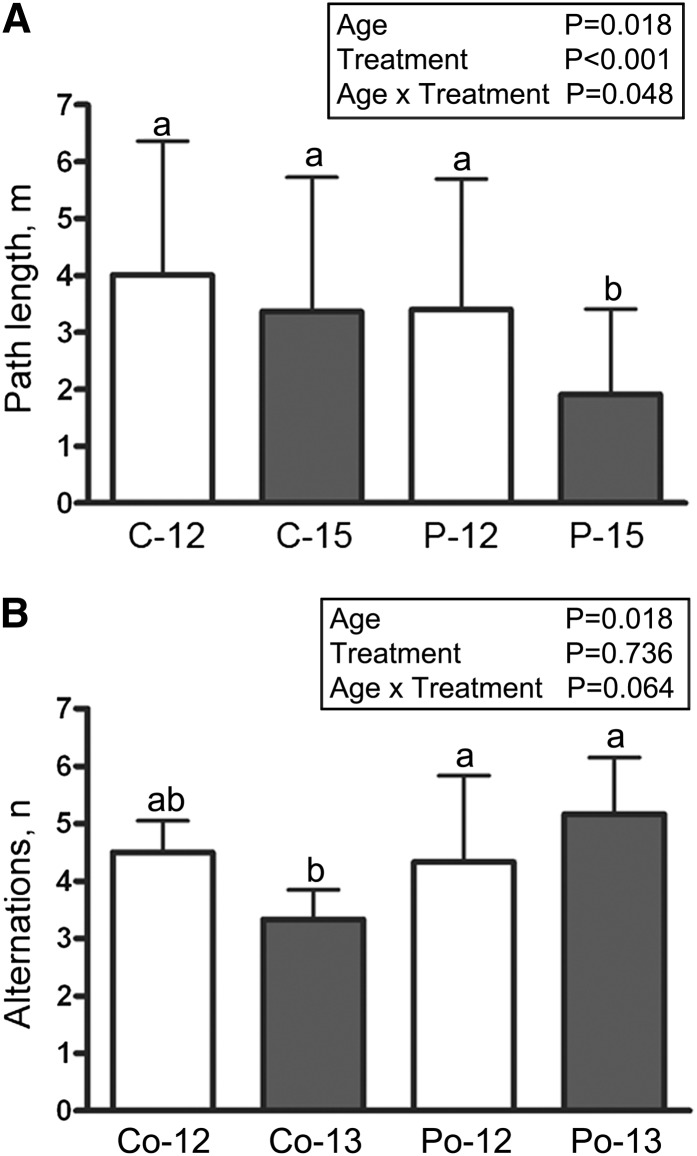
The 3-mo treatment study demonstrated that POMx-treated mice (P-15) had a significantly shorter path length to escape on the Barnes maze compared with both their 12-mo performance (P-12) as well as the 3-mo control group (C-15) (Fig. 1A). However, P-15 mice had no difference in their number of errors to escape compared with any group (Supplemental Fig. 1).
FIGURE 1.
Learning ability in male APP/PS1 transgenic mice supplied with or without 0.625% pomegranate in their normal drinking water for 3 mo of treatment (A) or supplied with 11.9% sucrose, 1.05% glucose, and 1.05% fructose or 0.625% pomegranate in their normal drinking water for 1 mo of treatment (B). For the 3-mo study (A), path lengths to the escape hole are graphed for each time point and each condition (C-12, P-12, C-15, and P-15). For the 1-mo study (B), T-maze analyses of numbers of alternations are graphed for each group (Co-12, Po-12, Co-13, and Po-13). Values are means ± SDs, n = 4 (A) or 6 (B). Means within a group without a common letter differ, P < 0.05. APP/PS1, amyloid precursor protein/presenilin 1; C-12, 3-month study, 12-month-old control mice; C-15, 3-month study, 15-month-old control mice; Co-12, 1-month study, 12-month-old control mice; Co-13, 1-month study, 13-month-old control mice; P-12, 3-month study, pomegranate-fed 12-month-old mice; P-15, 3-month study, pomegranate-fed 15-month-old mice; Po-12, 1-month study, pomegranate-fed 12-month-old mice; Po-13, 1-month study, pomegranate-fed 13-month-old mice.
POMx feeding was sufficient to improve T-maze behavioral performance in 1-mo study APP/PS1 mice.
As further validation of behavioral change, a separate 1-mo, shorter feeding paradigm of 30 d of POMx treatment in the APP/PS1 mice was performed. One month of pomegranate feeding (Po-13) was sufficient to improve their behavioral performance compared with control sugar-water–fed mice (Co-13) as assessed by the number of alternations on the T-maze (Fig. 1B).
Three months of POMx feeding inhibited NFATc1 activation in the APP/PS1 mouse model of AD.
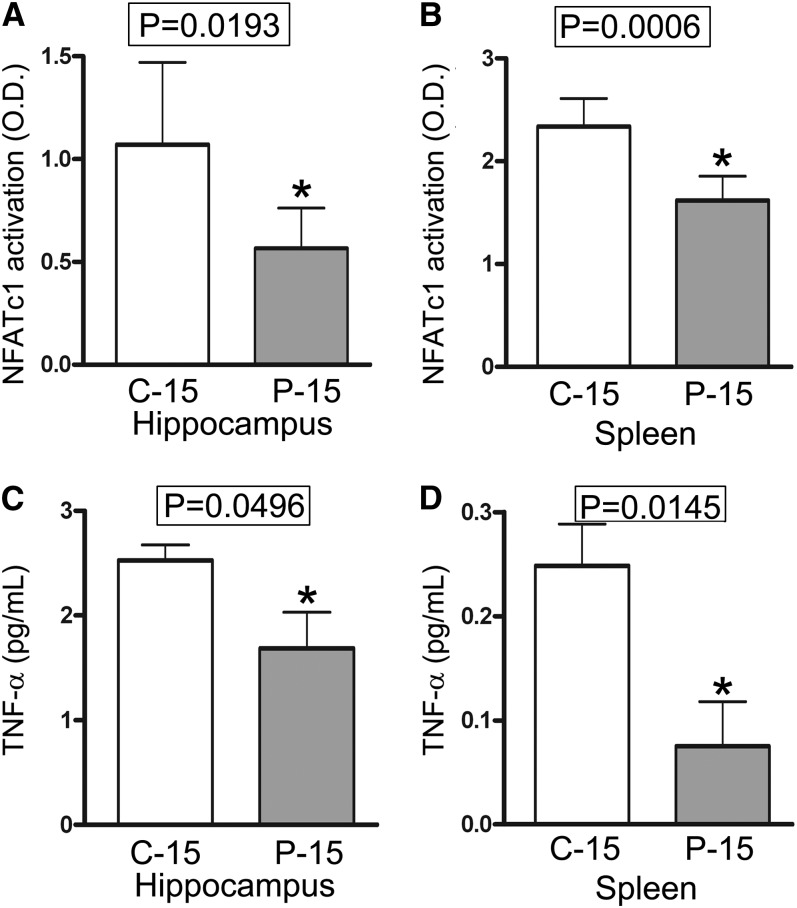
As expected, POMx treatment significantly decreased NFATc1 activity in both the hippocampus and spleen in P-15 compared with C-15 mice (Fig. 2A, B). This demonstrated that 3 mo of dietary supplementation with POMx was sufficient to attenuate not only NFAT activity in peripheral organs but also in the brain.
FIGURE 2.
NFATc1 activation in nuclear extracts and TNF-α concentrations in tissue lysates were inhibited in both the spleen and brain in male APP/PS1 mice supplied with or without 0.625% pomegranate in their normal drinking water for 3 mo of treatment. Values are means ± SDs, n = 4. *P value provided on graph. APP/PS1, amyloid precursor protein/presenilin 1; C-12, 3-month study, 12-month-old control mice; C-15, 3-month study, 15-month-old control mice; NFATc1, nuclear factor of activated T cells cytoplasmic 1; P-12, 3-month study, pomegranate-fed 12-month-old mice; P-15, 3-month study, pomegranate-fed 15-month-old mice.
Three months of POMx feeding attenuated brain cytokine concentrations.
As expected, the concentrations of TNF-α, both in the hippocampus and spleen, were significantly decreased in the P-15 mice compared with the C-15 group (Fig. 2C, D). It is interesting to note that brain exhibited higher concentrations of TNF-α compared with the spleen. This is likely a reflection of the active gliosis and inflammatory changes that characterize these mice in response to the massive plaque Aβ deposition that occurs in the brain. Surprisingly, POMx treatment had no effect on concentrations of IL-1β (Supplemental Fig. 2A, B) or IL-6 (Supplemental Fig. 2C, D) in either tissue type when comparing P-15 with C-15 mice. Therefore, although a clear effect of attenuated cytokine concentrations was detected in brains of POMx-fed P-15 mice, this appeared to be a cytokine-selective response.
Synaptic markers did not change in APP/PS1 mice fed 3 mo with POMx.
We found no significant differences in pre- or postsynaptic changes as indicated by the concentrations of synaptophysin (Supplemental Fig. 3A, B) and PSD95 (Supplemental Fig. 3A, C), respectively, when comparing P-15 with C-15 mice or the 12-mo control group (12).
Three months of POMx feeding did not attenuate concentrations of oxidative damage–related protein markers.
To further investigate the mechanisms of POMx-mediated behavioral improvement, Western blot analyses of the hippocampal lysates from the group of 12-mo-old mice (12) and the C-15 and P-15 groups were next used to examine changes in markers of protein and lipid oxidation. Surprisingly, POMx feeding produced no significant difference in concentrations of 2 different markers of oxidative damage, protein nitrotyrosine or HNE protein Michael adducts between any group (Supplemental Fig. 4). These results suggested that the improved behavioral performance of POMx-fed animals was not related to reduced levels of oxidative change or damage in the brain.
Three months of POMx feeding decreased microgliosis but not astrogliosis in the APP/PS1 mice.
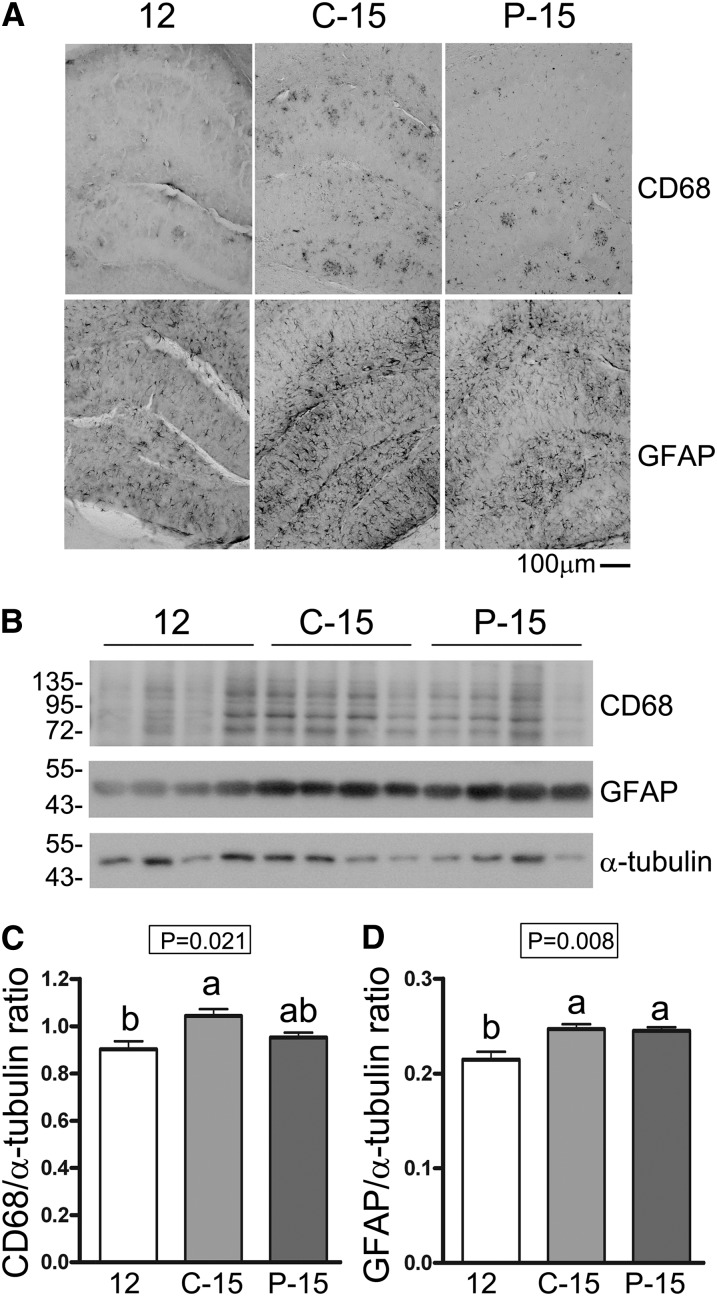
On the basis of the changes in TNF-α observed, we next assessed whether POMx treatment altered the microglial or astroglial activation state in the group of 12-mo-old mice (12) compared with the C-15 and P-15 groups. Immunohistochemistry and Western blots of hippocampal brain sections showed that the age- and disease-associated increase in microglial CD68 concentrations demonstrated in the C-15 group compared with the 12-mo control group (12) was attenuated in the P-15 mice (Fig. 3A–C). However, both P-15 and C-15 groups of mice demonstrated significantly elevated GFAP concentrations compared with the 12-mo control mice (12) (Fig 3A, B, D). This suggested that POMx feeding was able to halt progression of at least the microglial component of gliosis during disease.
FIGURE 3.
Microgliosis but not astrogliosis was attenuated in the brains of male APP/PS1 mice supplied with 0.625% pomegranate in their normal drinking water for 3 mo of treatment compared with 12- and 15-mo-old male control APP/PS1 mice provided normal drinking water. (A) Coronal sections were immunostained by using anti-CD68 and anti-GFAP antibodies and representative images of hippocampi are shown. (B) Western blot analyses of hippocampal lysates and corresponding quantitated ODs of CD68 (C) and GFAP (D) bands are shown. Values are means ± SDs, n = 4. Means without a common letter differ, P < 0.05. APP/PS1, amyloid precursor protein/presenilin 1; C-15, 3-month study, 15-month-old control mice; CD68, cluster of differentiation 68; GFAP, glial fibrillary acidic protein; P-15, 3-month study, pomegranate-fed 15-month-old mice; 12, untreated 12-month-old control mice.
POMx feeding for 3 mo decreased Aβ plaque load in the APP/PS1 mice.
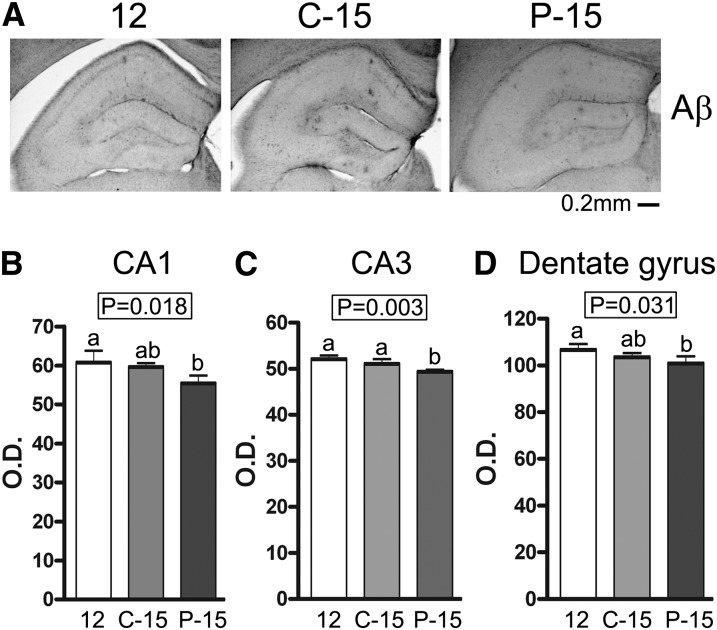
To quantify deposition of Aβ peptide–containing plaques, brain sections from the group of 12-mo-old mice (12) and the C-15 and P-15 groups of mice were immunostained by using anti-Aβ (4G8) antibody. The P-15 group demonstrated a significant decrease in immunoreactivity in the CA1, CA3, and dentate gyrus regions compared with the 12-mo-old mice (12) and a significant decrease in immunoreactivity in CA3 compared with the C-15 group (Fig. 4). We next assessed whether POMx feeding could alter BACE or APP protein concentrations to help explain the decrease in plaque load. Western blot analysis indicated no change in overall protein concentrations of APP or BACE in the hippocampus of any treatment group (Supplemental Fig. 5). These findings demonstrated that POMx was able to decrease plaque load in a fashion apparently independent of APP processing, which might involve increased clearance.
FIGURE 4.
β-Amyloid peptide immunoreactive plaques were decreased in male APP/PS1 mice supplied with 0.625% pomegranate in their normal drinking water for 3 mo of treatment compared with 12-and 15-mo-old male control APP/PS1 mice provided normal drinking water. (A) Images of immunostained anti-Aβ (4G8) were taken from hippocampi. Quantification of Aβ immunoreactivity OD in CA1 (B), CA3 (C), and the dentate gyrus (D) regions of the hippocampi was performed. Values are means ± SDs, n = 4. Means without a common letter differ, P < 0.05. Aβ, amyloid β APP/PS1, amyloid precursor protein/presenilin 1; C-15, 3-month study, 15-month-old control mice; P-15, 3-month study, pomegranate-fed 15-month-old mice; 12, untreated 12-month-old control mice.
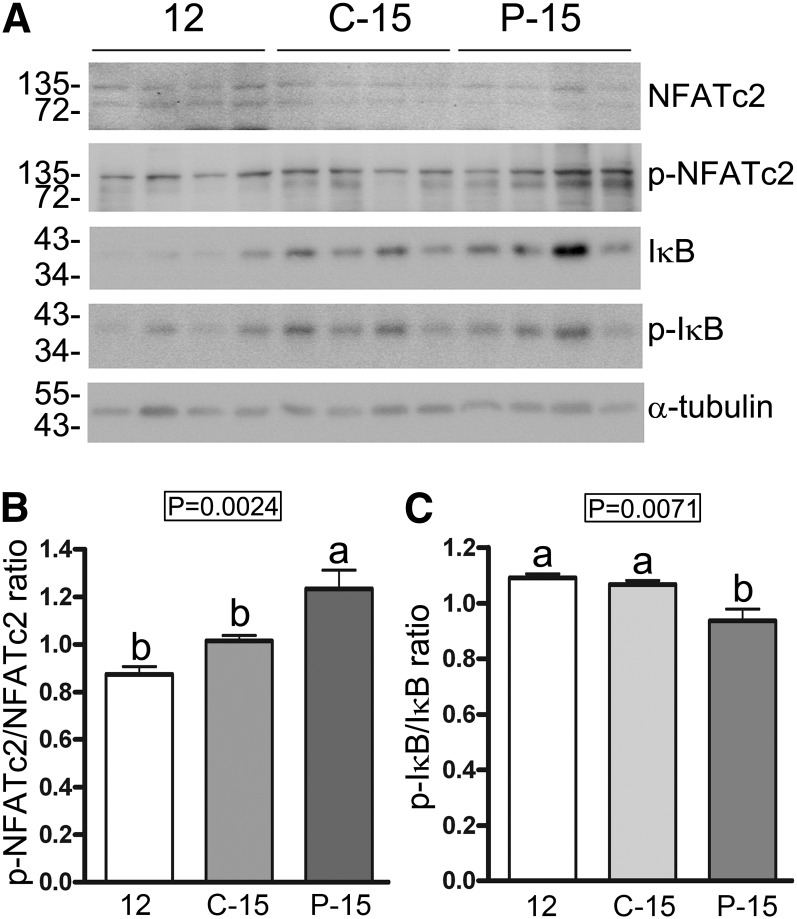
POMx feeding for 3 mo suppressed NFATc2 expression in the APP/PS1 mice.
We assumed that some of the effects observed were a consequence of the attenuated NFAT activity. As an additional means of assessing activity, we quantified active concentrations of another isoform, NFATc2, in the 12-mo-old group (12) compared with the C-15 and P-15 groups of mice. As expected, POMx feeding significantly increased the concentrations of inactive hippocampal NFATc2 (p-NFATc2) in the P-15 group of mice compared with both the 12-mo (12) and C-15 groups (Fig. 5A, B). Because POMx and punicalagin have reported NF-κB inhibitory activity in human intestine Caco-2 cells (48), we also assessed changes in phosphorylated concentrations of the NF-κB inhibitory protein, IκB. POMx feeding decreased the ratio of phosphorylated to nonphosphorylated IκB in the P-15 group compared with the 12-mo-old (12) and C-15 groups, demonstrating a likely condition of decreased IκB degradation and increased inhibition of NF-κB (Fig. 5A, C). These data suggested that dietary POMx is sufficient in these mice to attenuate activity of 2 critical proinflammatory transcription factors in the brain.
FIGURE 5.
Concentrations of active NFATc2 were decreased and concentrations of IκB were increased in brains of male APP/PS1 mice supplied with 0.625% pomegranate in their normal drinking water for 3 mo of treatment compared with 12- and 15-mo-old male control APP/PS1 mice provided normal drinking water. (A) Western blot analyses of hippocampal lysates and corresponding quantitated ODs of p-NFATc2 (B) and p-IκB (C) were normalized against NFATc2 and IκB, respectively. OD values ± SDs were averaged and graphed, n = 4. Means without a common letter differ, P < 0.05. APP/PS1, amyloid precursor protein/presenilin 1; C-15, 3-month study, 15-month-old control mice; IκB, nuclear factor of κ light polypeptide gene enhancer in B-cell inhibitor; NFATc2, nuclear factor of activated T cells cytoplasmic 2; p-, phosphorylated; P-15, 3-month study, pomegranate-fed 15-month-old mice; 12, untreated 12-month-old control mice.
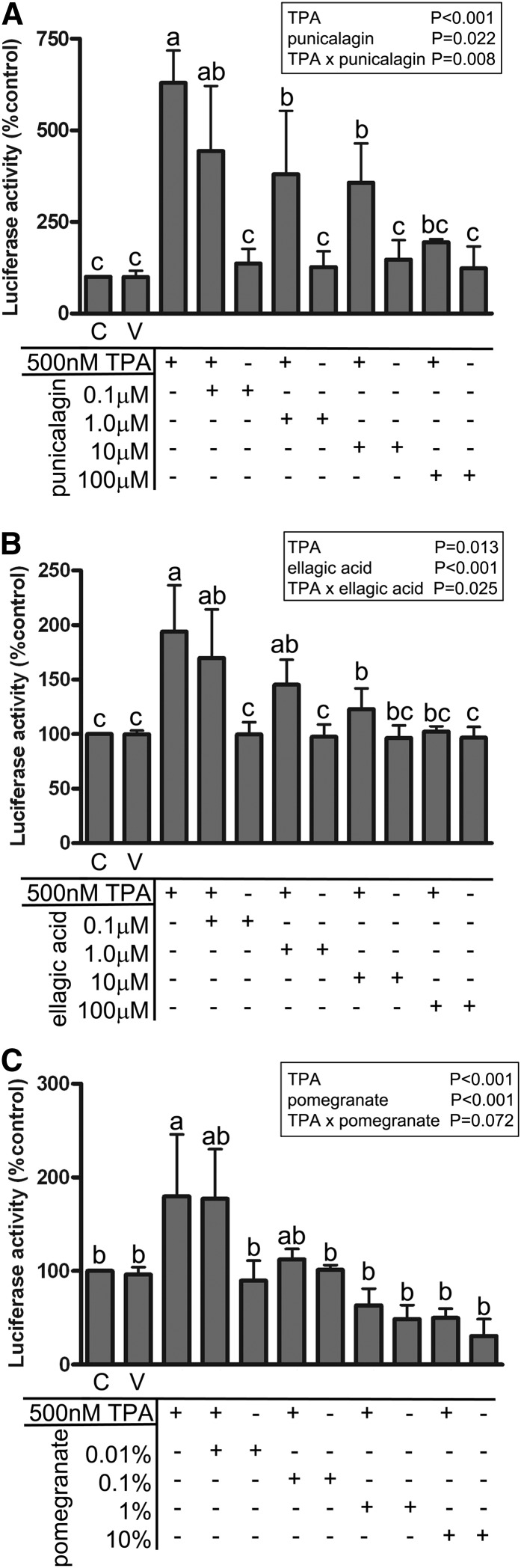
POMx and its components, punicalagin and ellagic acid, inhibited NFAT-dependent luciferase activity in vitro.
Although the purpose of this study was not to isolate or identify a bioactive component of POMx, per se, we were interested in at least validating that particular polyphenols of POMx have true NFAT inhibitory ability. We next compared POMx with punicalagin and another abundant polyphenol component, ellagic acid, for their NFAT inhibitory ability. We used a commercial HeLa/NFAT-luc cell line stably expressing an NFAT promotor–driven luciferase construct stimulated with phorbol ester (TPA) and ionomycin in the presence or absence of POMx, punicalagin, and ellagic acid. All 3 exhibited a dose-dependent ability to attenuate NFAT-driven luciferase activity (Fig. 6). These results verified that POMx as well as at least 2 of its components, punicalagin and ellagic acid, have a bioactive action that involves inhibition of NFAT activity.
FIGURE 6.
NFAT activity in HeLa cells stimulated with or without TPA and ionomycin was inhibited by treatment with pomegranate extract and its components, punicalagin and ellagic acid. Values are means ± SDs, n = 3. Means within a group without a common letter differ, P < 0.05. C, control; NFAT, nuclear factor of activated T cells; TPA, 12-O-tetradecanoylphorbol-13-acetate; V, vehicle.
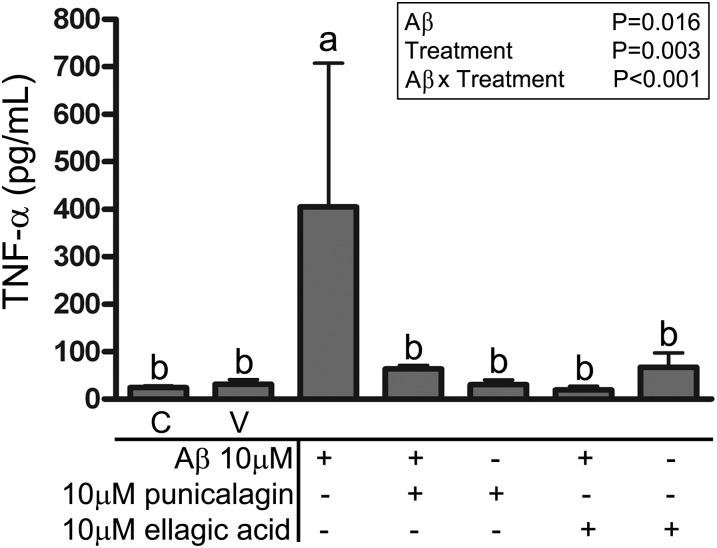
POMx components, ellagic acid and punicalagin, attenuated microglial proinflammatory cytokine release in vitro.
We next assessed whether punicalagin or ellagic acid were able to attenuate TNF-α secretion in microglia. To do this primary murine microglia were cultured and pretreated with or without punicalagin or ellagic acid for 30 min followed by stimulation with Aβ1–42 fibrils for 24 h. ELISA analysis verified that Aβ-stimulated secretion of TNF-α was effectively inhibited by either punicalagin or ellagic acid (Fig. 7). Neither punicalagin nor ellagic acid affected cell viability (Supplemental Fig. 6). These findings confirmed that the potent NFAT inhibitory components of POMx, punicalagin and ellagic acid, were very effective at attenuating microglial TNF-α secretion in response to stimulation with fibrillar amyloid, which is consistent with the known role of NFAT in regulating microglial phenotype.
FIGURE 7.
TNF-α release from primary murine cortical microglia cultures stimulated with or without Aβ was inhibited by treatment with pomegranate extract components, ellagic acid and punicalagin. Values are means ± SDs, n = 3–8. Means within a group without a common letter differ, P < 0.05. Aβ, amyloid β C, control; TNF-α, tumor necrosis factor α V, vehicle.
Discussion
Although we have not suggested a particular bioactive component of the POMx responsible for the effects we observed, we did find a robust antiinflammatory protective effect in the brains of these AD mouse models simply from free-access dietary ingestion of POMx. These results supported similar conclusions from prior studies that oral POMx is sufficient to improve memory-related behavioral performance and decrease plaque load in another AD transgenic mouse model (49) or in response to acute Aβ brain injection (50). Our selection of the particular commercial POMx, POM Wonderful brand, was based on the reported consistency of batch-to-batch preparation and the polyphenol content from this source (51, 52) as well as the fact that this particular liquid concentrate extract has been used in humans to demonstrate increased plasma ellagic acid concentrations after consumption (41, 42).
The antiinflammatory action of dietary POMx has been reported in a variety of studies using a plethora of different model systems (48, 53–55). The mechanism responsible or the precise bioactive component remains unclear. There are numerous studies documenting the ability of POMx or its polyphenols such as punicalagin to attenuate inflammatory conditions through mechanisms as diverse as inhibition of proinflammatory cytokine transcription (35, 48, 56) to antioxidant protection (51, 52, 57–59) to modulation of microbiota (60, 61). We have expanded prior work demonstrating benefits of POMx in 2 different AD mouse paradigms (49, 50) by now providing a mechanistic linkage to antiinflammatory effects in the brain. By using an APP/PS1 line we observed significant behavioral improvement from POMx feeding, which correlated with decreased microgliosis, NFAT activity, and brain TNF-α concentratons. On the basis of our observations of the ability of punicalagin and ellagic acid to inhibit NFAT activity and cytokine secretion in vitro, we predict that some component of the extract, such as punicalagin or ellagic acid, is absorbed and able to enter the brain to inhibit microglial activation via attenuation of, particularly, NFAT activity. Indeed, prior work has validated that ellagic acid can be directly taken up into cells, supporting the notion that it can function intracellularly to directly inhibit NFAT (62). However, we have not ruled out the possibility that these large polyphenol molecules may also act extracellularly to exert their antiinflammatory action. For instance, a recent study indicated that punicalagin can directly interact with cytokines to perhaps behave as a molecular trap to attenuate cytokine stimulation of cells (53).
Prior work in rodents has demonstrated that both punicalagin and ellagic acid are readily absorbed and found in at least the plasma, suggesting that it is feasible that brain penetration occurred (38). In addition, although plasma punicalagin concentrations do not reportedly increase after POMx consumption (63, 64), several studies have reported an increase in particularly ellagic acid concentrations after POMx ingestion in humans (41, 42). Because a number of microflora-derived metabolites are generated from the ingested polyphenols in POMx, there are actually numerous molecules that likely enter the blood and possibly the brain after ingestion that could have contributed to the effects observed (38, 41, 42, 45, 56). For this reason, our intent with this initial study was not to isolate and validate a particular bioactive extract molecule or metabolite. Instead, we intended to demonstrate that the antiinflammatory action of dietary POMx was not limited to peripheral organs but could extend even to the brain. This was demonstrated via the decrease in microgliosis, NFAT activity, and TNF-α concentrations. Future work will identify what component(s) from ingestion of a standardized extract of pomegranate are detectable in the plasma and brain and are responsible for the effects observed.
Although extract feeding produced behavioral improvement, it is not clear precisely how this occurred. For example, the decrease in TNF-α concentrations might have been responsible for the improvement. Prior studies have demonstrated that attenuation of TNF-α action in the brain results in rapid behavioral improvement in AD subjects (65). On the other hand, the slight but significant decrease in plaque load might also have contributed to the behavioral improvement. On the basis of the current data set it is difficult to explain the mechanism of decreased plaque density as well. Although we were unable to measure activities of β or γ secretases or assess rates of Aβ production and clearance in the current work, a future study assessing these variables will at least shed insight into whether or not the POMx modulated common variables of Aβ production.
It is curious that only TNF-α concentrations were attenuated in the spleen and brain of the pomegranate-fed mice because the in vitro data showed that POMx, punicalagin, or ellagic acid all potently inhibit NFAT activity and cytokine secretion. On the other hand, work from others has also reported cytokine-specific regulatory effects of POMx and punicalagin using human Caco-2 cells (53). Even without a broad cytokine inhibitory action, the therapeutic benefits of POMx for neuroinflammatory conditions are likely to be myriad. Elevated TNF-α concentrations in the brain are implicated in adverse outcomes in conditions ranging from stroke (66) to injury (67) to multiple sclerosis (68) and Parkinson disease (69). This suggests that the therapeutic benefits of dietary POMx can be extended to a range of central nervous system inflammatory conditions.
Although we predict that a component of the ingested extract was absorbed into the plasma and entered the brain to inhibit microglial NFAT or NF-κB activity to attenuate TNF-α secretion, this linear cause-and-effect relationship has not yet been established and requires future work. We focused the study on advanced stages of disease in the mouse model when there was already abundant microgliosis and plaque deposition. It will be interesting for translation to human use to determine whether earlier intervention will delay disease onset. It is not difficult to imagine prolonged use of a standardized, mechanistically validated dietary supplement at early ages to slow the progression of AD.
Supplementary Material
Acknowledgments
L.R. designed, conducted, and analyzed experiments and wrote the initial version of the manuscript; C.K.C. designed and analyzed experiments and prepared the final manuscript version; and K.L.P. analyzed the experiments and helped prepare the final version of the manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AD, Alzheimer disease; Aβ, amyloid β AP-1, activator protein 1; apoE, apolipoprotein E; APP, amyloid precursor protein; BACE, β-site APP cleavage enzyme; C-12, 3-month study, 12-month-old control mice; C-15, 3-month study, 15-month-old control mice; CD68, cluster of differentiation 68; Co-12, 1-month study, 12-month-old control mice; Co-13, 1-month study, 13-month-old control mice; GFAP, glial fibrillary acidic protein; HNE, 4-hydroxy-2-nonenal; IL, interleukin; IκB, nuclear factor of κ light polypeptide gene enhancer in B-cell inhibitor; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; NFAT, nuclear factor of activated T cells; NFATc1, nuclear factor of activated T cells cytoplasmic 1; NFATc2, nuclear factor of activated T cells cytoplasmic 2; p-, phosphorylated; P-12, 3-month study, pomegranate-fed 12-month-old mice; P-15, 3-month study, pomegranate-fed 15-month-old mice; Po-12, 1-month study, pomegranate-fed 12-month-old mice; Po-13, 1-month study, pomegranate-fed 13-month-old mice; POMx, pomegranate extract in drinking water; PS1, presenilin 1; PSD95, postsynaptic density protein 95; TNF-α, tumor necrosis factor α TPA, 12-O-tetradecanoylphorbol-13-acetate; 12, untreated 12-month control mice.
Literature Cited
- 1.Ikeda S, Allsop D, Glenner GG. A study of the morphology and distribution of amyloid beta protein immunoreactive plaque and related lesions in the brains of Alzheimer's disease and adult Down's syndrome. Prog Clin Biol Res. 1989;317:313–23 [PubMed] [Google Scholar]
- 2.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer's disease: a double-labeling immunohistochemical study of senile plaques. Am J Pathol. 1988;132:86–101 [PMC free article] [PubMed] [Google Scholar]
- 4.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–82 [DOI] [PubMed] [Google Scholar]
- 5.Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, et al. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:5296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meda L, Bernasconi S, Bonaiuto C, Sozzani S, Zhou D, Otvos L, Jr, Mantovani A, Rossi F, Cassatella MA. Beta-amyloid (25–35) peptide and IFN-gamma synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes and microglial cells. J Immunol. 1996;157:1213–8 [PubMed] [Google Scholar]
- 7.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40–CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–5 [DOI] [PubMed] [Google Scholar]
- 8.Sondag CM, Dhawan G, Combs CK. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J Neuroinflammation. 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Côté S, Carmichael PH, Verreault R, Lindsay J, Lefebvre J, Laurin D. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2012;8:219–26 [DOI] [PubMed] [Google Scholar]
- 10.Jaturapatporn D, Isaac MG, McCleery J, Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer's disease. Cochrane Database Syst Rev. 2012;2:CD006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, et al. Extended results of the Alzheimer's disease anti-inflammatory prevention trial. Alzheimers Dement. 2011;7:402–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leoutsakos JM, Muthen BO, Breitner JC, Lyketsos CG. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer's Disease Anti-inflammatory Prevention Trial. Int J Geriatr Psychiatry. 2012;27:364–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Styren SD, Civin WH, Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer's disease brain. Exp Neurol. 1990;110:93–104 [DOI] [PubMed] [Google Scholar]
- 14.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200 [DOI] [PubMed] [Google Scholar]
- 15.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47 [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Rodríguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc Natl Acad Sci USA. 1999;96:7214–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84 [DOI] [PubMed] [Google Scholar]
- 18.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 1998;10:599–611 [DOI] [PubMed] [Google Scholar]
- 19.Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–70 [DOI] [PubMed] [Google Scholar]
- 20.Luoma JI, Zirpel L. Deafferentation-induced activation of NFAT (nuclear factor of activated T-cells) in cochlear nucleus neurons during a developmental critical period: a role for NFATc4-dependent apoptosis in the CNS. J Neurosci. 2008;28:3159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedito AB, Lehtinen M, Massol R, Lopes UG, Kirchhausen T, Rao A, Bonni A. The transcription factor NFAT3 mediates neuronal survival. J Biol Chem. 2005;280:2818–25 [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Ortiz JM, Serrano-Pérez MC, Pastor MD, Martín ED, Calvo S, Rincón M, Tranque P. Mechanical lesion activates newly identified NFATc1 in primary astrocytes: implication of ATP and purinergic receptors. Eur J Neurosci. 2008;27:2453–65 [DOI] [PubMed] [Google Scholar]
- 23.Sama MA, Mathis DM, Furman JL, Abdul HM, Artiushin IA, Kraner SD, Norris CM. Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem. 2008;283:21953–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H III, et al. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagamoto-Combs K, Combs CK. Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells). J Neurosci. 2010;30:9641–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–10 [DOI] [PubMed] [Google Scholar]
- 27.Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108:115–25 [DOI] [PubMed] [Google Scholar]
- 28.Macián F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–89 [DOI] [PubMed] [Google Scholar]
- 29.Henderson DJ, Naya I, Bundick RV, Smith GM, Schmidt JA. Comparison of the effects of FK-506, cyclosporin A and rapamycin on IL-2 production. Immunology. 1991;73:316–21 [PMC free article] [PubMed] [Google Scholar]
- 30.González-Pinto IM, Rimola A, Margarit C, Cuervas-Mons V, Abradelo M, Alvarez-Laso C, Londono MC, Bilbao I, Sanchez-Turrion V. Five-year follow-up of a trial comparing tacrolimus and cyclosporine microemulsion in liver transplantation. Transplant Proc. 2005;37:1713–5 [DOI] [PubMed] [Google Scholar]
- 31.Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol. 2009;297:F499–509 [DOI] [PubMed] [Google Scholar]
- 32.Roehrl MH, Wang JY, Wagner G. Discovery of small-molecule inhibitors of the NFAT-calcineurin interaction by competitive high-throughput fluorescence polarization screening. Biochemistry. 2004;43:16067–75 [DOI] [PubMed] [Google Scholar]
- 33.Kang S, Li H, Rao A, Hogan PG. Inhibition of the calcineurin-NFAT interaction by small organic molecules reflects binding at an allosteric site. J Biol Chem. 2005;280:37698–706 [DOI] [PubMed] [Google Scholar]
- 34.Sieber M, Karanik M, Brandt C, Blex C, Podtschaske M, Erdmann F, Rost R, Serfling E, Liebscher J, Patzel M, et al. Inhibition of calcineurin-NFAT signaling by the pyrazolopyrimidine compound NCI3. Eur J Immunol. 2007;37:2617–26 [DOI] [PubMed] [Google Scholar]
- 35.Lee SI, Kim BS, Kim KS, Lee S, Shin KS, Lim JS. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem Biophys Res Commun. 2008;371:799–803 [DOI] [PubMed] [Google Scholar]
- 36.Kwak HM, Jeon SY, Sohng BH, Kim JG, Lee JM, Lee KB, Jeong HH, Hur JM, Kang YH, Song KS. beta-Secretase (BACE1) inhibitors from pomegranate (Punica granatum) husk. Arch Pharm Res. 2005;28:1328–32 [DOI] [PubMed] [Google Scholar]
- 37.Cho HJ, Jin SM, Youn HD, Huh K, Mook-Jung I. Disrupted intracellular calcium regulates BACE1 gene expression via nuclear factor of activated T cells 1 (NFAT 1) signaling. Aging Cell. 2008;7:137–47 [DOI] [PubMed] [Google Scholar]
- 38.Cerdá B, Llorach R, Ceron JJ, Espin JC, Tomas-Barberan FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28 [DOI] [PubMed] [Google Scholar]
- 39.Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–5 [DOI] [PubMed] [Google Scholar]
- 40.Aviram M, Volkova N, Coleman R, Dreher M, Reddy MK, Ferreira D, Rosenblat M. Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein E-deficient (E 0) mice and in vitro in cultured macrophages and lipoproteins. J Agric Food Chem. 2008;56:1148–57 [DOI] [PubMed] [Google Scholar]
- 41.Seeram NP, Zhang Y, McKeever R, Henning SM, Lee RP, Suchard MA, Li Z, Chen S, Thames G, Zerlin A, et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food. 2008;11:390–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–8 [DOI] [PubMed] [Google Scholar]
- 43.Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006;54:8956–61 [DOI] [PubMed] [Google Scholar]
- 44.Lei F, Xing DM, Xiang L, Zhao YN, Wang W, Zhang LJ, Du LJ. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:189–94 [DOI] [PubMed] [Google Scholar]
- 45.Cerdá B, Ceron JJ, Tomas-Barberan FA, Espin JC. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J Agric Food Chem. 2003;51:3493–501 [DOI] [PubMed] [Google Scholar]
- 46.Dhawan G, Combs CK. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer's disease. J Neuroinflammation. 2012;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Floden AM, Combs CK. Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. J Neurosci. 2006;26:4644–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–5 [DOI] [PubMed] [Google Scholar]
- 49.Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–15 [DOI] [PubMed] [Google Scholar]
- 50.Choi SJ, Lee JH, Heo HJ, Cho HY, Kim HK, Kim CJ, Kim MO, Suh SH, Shin DH. Punica granatum protects against oxidative stress in PC12 cells and oxidative stress-induced Alzheimer's symptoms in mice. J Med Food. 2011;14:695–701 [DOI] [PubMed] [Google Scholar]
- 51.Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008;56:1415–22 [DOI] [PubMed] [Google Scholar]
- 52.Borges G, Mullen W, Crozier A. Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices. Food Funct. 2010;1:73–83 [DOI] [PubMed] [Google Scholar]
- 53.Hollebeeck S, Winand J, Herent MF, During A, Leclercq J, Larondelle Y, Schneider YJ. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012;3:875–85 [DOI] [PubMed] [Google Scholar]
- 54.Rosenblat M, Aviram M. Pomegranate juice protects macrophages from triglyceride accumulation: inhibitory effect on DGAT1 activity and on triglyceride biosynthesis. Ann Nutr Metab. 2011;58:1–9 [DOI] [PubMed] [Google Scholar]
- 55.Lin CC, Hsu YF, Lin TC. Effects of punicalagin and punicalin on carrageenan-induced inflammation in rats. Am J Chin Med. 1999;27:371–6 [DOI] [PubMed] [Google Scholar]
- 56.González-Sarrías A, Larrosa M, Tomas-Barberan FA, Dolara P, Espin JC. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr. 2010;104:503–12 [DOI] [PubMed] [Google Scholar]
- 57.Heber D, Seeram NP, Wyatt H, Henning SM, Zhang Y, Ogden LG, Dreher M, Hill JO. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J Agric Food Chem. 2007;55:10050–4 [DOI] [PubMed] [Google Scholar]
- 58.Rosenblat M, Volkova N, Attias J, Mahamid R, Aviram M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum's ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010;1:99–109 [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Wang D, Lee RP, Henning SM, Heber D. Absence of pomegranate ellagitannins in the majority of commercial pomegranate extracts: implications for standardization and quality control. J Agric Food Chem. 2009;57:7395–400 [DOI] [PubMed] [Google Scholar]
- 60.Bialonska D, Kasimsetty SG, Schrader KK, Ferreira D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J Agric Food Chem. 2009;57:8344–9 [DOI] [PubMed] [Google Scholar]
- 61.Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int J Food Microbiol. 2010;140:175–82 [DOI] [PubMed] [Google Scholar]
- 62.Lan J, Lei F, Hua L, Wang Y, Xing D, Du L. Transport behavior of ellagic acid of pomegranate leaf tannins and its correlation with total cholesterol alteration in HepG2 cells. Biomed Chromatogr. 2009;23:531–6 [DOI] [PubMed] [Google Scholar]
- 63.Cerdá B, Espin JC, Parra S, Martinez P, Tomas-Barberan FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–20 [DOI] [PubMed] [Google Scholar]
- 64.Cerdá B, Periago P, Espin JC, Tomas-Barberan FA. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53:5571–6 [DOI] [PubMed] [Google Scholar]
- 65.Tobinick EL, Gross H. Rapid cognitive improvement in Alzheimer's disease following perispinal etanercept administration. J Neuroinflammation. 2008;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNFalpha decoy receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell SJ, Jiang Y, Davis AE, Farrands R, Holbrook J, Leppert D, Anthony DC. Immunomodulatory effects of etanercept in a model of brain injury act through attenuation of the acute-phase response. J Neurochem. 2007;103:2245–55 [DOI] [PubMed] [Google Scholar]
- 68.Taoufik E, Tseveleki V, Chu SY, Tselios T, Karin M, Lassmann H, Szymkowski DE, Probert L. Transmembrane tumour necrosis factor is neuroprotective and regulates experimental autoimmune encephalomyelitis via neuronal nuclear factor-kappaB. Brain. 2011;134:2722–35 [DOI] [PubMed] [Google Scholar]
- 69.Zhou QH, Sumbria R, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection with a brain-penetrating biologic tumor necrosis factor inhibitor. J Pharmacol Exp Ther. 2011;339:618–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.