Abstract
Chronic obstructive pulmonary disease (COPD) remains a major cause of morbidity and mortality with increasing rates during the last decades. Due to the progressive nature of the disease, underestimation of symptoms by the patients, lack of knowledge and underuse of spirometry by the Primary Care providers the disease remains under-diagnosed in about half of the cases.
Patients with a smoking history of ≥20 pack-years and relevant symptoms (e.g. dyspnea, chronic cough and sputum production) are considered a high risk group. Measurement of spirometric parameters after administration of a short acting bronchodilator confirms the presence of irreversible airflow obstruction and establishes the diagnosis. However in the primary care spirometry is usually not available and differential diagnosis with other obstructive pulmonary diseases (e.g. asthma, bronchiectasis) is not always easy. General Practitioners (GPs) need simple screening tools to decide if a patient belong to a high risk group and pulmonary consultation is necessary. Early and accurate diagnosis of COPD in the primary care setting allowing for a timely and effective management which reduces the rate of decline in lung function improves survival of patients, their quality of life and reduces health-care utilization.
The aim of the present review is to provide the existing information about COPD diagnosis and the related problems in the Primary Care. Also we reviewed numerous simple COPD diagnosis questionnaires as well as the use of hand-held flow meters which could be used as effective screening tools.
Keywords: chronic obstructive pulmonary disease, primary care, diagnosis, questionnaires, spirometry, case-identification, review
Chronic Obstructive Lung Disease (COPD) is a preventable and treatable disease that characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to cigarette smoke and other noxious particles or gases1. Prevalence of clinically significant COPD (GOLD stage II or higher) is estimated to be 10.1% according to the results of an international population-based investigation2. It was estimated that 1 every 4 men and 1 every 6 women without COPD at the age of 55 years will eventually develop COPD at some time during their further life3.
Prevalence rates of COPD are expected to increase in next decades, notably among women and in developing countries populations4. By 2030, COPD is expected to become the third leading cause of death in middle-income countries5. Trends in age-standardized death rates for the 6 leading causes of death in the USA from 1970 through 2002 indicates that, while mortality from several these chronic conditions decline over the period, COPD mortality increased6. In the European Union (EU) the total direct cost of respiratory diseases is estimated to be about 6% of the total health care budget, with COPD accounting for over 50% (38,6 billion Euros)7. The majority of this cost is attributed to hospitalizations for exacerbations8.
Patients with COPD face a significantly increased risk for premature death9. COPD exacerbations influence mortality, pulmonary function, physical activity and quality of life of patients10-12. Physical activity in COPD patients is lower than that observed in healthy subjects of similar age13, and is reduced even in stable GOLD stage II patients14. Patients with more advanced stages are also at increased risk for comorbid conditions (e.g. diabetes, arterial hypertension, cardiovascular disease, osteoporosis, lung cancer, and depression) as well as associated systemic consequences (e.g. weight loss and muscle dysfunction due to inactivity and deconditioning) which play an important prognostic role15-17.
Smoking cessation is the only effective way to change the natural history of the disease and to oppose the deleterious effects of smoking on lung function18.
Underdiagnosis of COPD in the primary care setting
COPD underdiagnosis has been observed in many studies throughout the world. In a large epidemiologic, multicenter, population-based study conducted in Spain, a total of 4,035 men and women (40 to 69 years) who were randomly selected from a target population of 236,412 subjects, had answered a relevant questionnaire and underwent spirometry. The prevalence of COPD was 9.1%, 15% in smokers, 12.8% in ex-smokers, and 4.1% in nonsmokers. There was no previous diagnosis of COPD in 78.2% of cases. Multivariate analysis showed that individuals had a higher probability of having received a previous diagnosis of COPD if they lived in urban areas, were of male gender, were >60 years old, had higher educational levels, had >15 pack-year smoking history, or had symptoms of chronic bronchitis19. During 2000, an estimated 10 million U.S. adults reported physician-diagnosed COPD. However, data from NHANES III estimate that approximately 24 million U.S. adults have evidence of impaired lung function, indicating that COPD is underdiagnosed20.
COPD underdiagnosis could be attributed to underestimation of symptoms by the patients. In a large International Survey aimed to quantify morbidity and burden in COPD, 36% of the patients with dyspnea during basic everyday activities described the disease as mild or moderate21.
Chronic obstructive lung diseases (e.g. asthma and COPD) are common among the target-population of a GP. In the Netherlands, for example, an average GP will encounter annually eight new cases of asthma and seven of COPD, while managing 50 patients with asthma and 60 with COPD22. Despite this increased burden of respiratory patients, spirometry remains largely underused in the primary care23,24. This problem has been repeatedly observed even in countries with advanced health care systems. It has been observed in Italy25, but also for diagnosis and treatment of patients in Spain, where only one third of patients with COPD had post-bronchodilator spirometry while about half of them had not undergone spirometry at all26. In the USA a recent epidemiological survey, among more than 1.5 million members of insurance organizations, showed that only 32% of patients with a new COPD diagnosis had undergone spirometry the previous 2 years to 6 months following diagnosis27.
The limited use of spirometry within primary care has been attributed to cost constraints, lack of access and time, low quality of examinations, inaccurate interpretation of results, and inadequately trained staff24,28,29. Furthermore, evidence suggests that drugs are frequently prescribed inappropriately and not according to recommendations based on spirometric disease severity26,30. After publication of the results of a large randomized controlled trial conducted in Italy, which failed to prove a significant advantage of office spirometry in improving the diagnosis of asthma and COPD in general practice31, Enright argued against its use for COPD screening by primary care physicians32. A recent study from Australia33 showed that establishing spirometry into general use is difficult but repeated training courses, review of the results by specialists and feedback regarding the quality of the manoeuvres could improve and maintain competency and minimize error rates.
Taking into account that the cost of COPD treatment is constantly increasing while health-related budget is continuously declining, the need for accurate diagnosis is imperative. All patients who are suspected to have COPD based on history and clinical examination should undergo official spirometry after bronchodilation by respiratory specialists to minimize overdiagnosis and overtreatment, a rather common situation in the primary care setting34.
Evaluating medical history, risk factors, clinical examination and using validated questionnaires
Even though performing high-quality spirometry in the primary care setting and evaluating the results correctly is a matter of debate, taking a detailed medical history, using validated questionnaires and identifying common comorbidities is an effective initial approach for screening subjects who visit a GP. Diagnosis and management of COPD should always be based on post-bronchodilator official spirometry.
The most common respiratory related symptoms of COPD are dyspnea, chronic cough, sputum production, chest tightness and wheezing1. All these symptoms are usually progressive and persistent over time while the adoption of a sedentary way of living may mask breathlessness. Taking into account that symptoms are nonspecific, a GP should always ask about the characteristics of chronic cough in order to reveal other causes (Table 1), other medical conditions that may explain dyspnea (Table 2) or chronic sputum production (e.g. bronchiectasis). Weight loss, reduction in free-fat mass and anxiety are common problems in more advanced stages of the disease and are important prognostic factors35,36. However they might be symptoms of other diseases (e.g. tuberculosis, bronchial cancer) and therefore should always be considered in the differential diagnosis.
Table 1. Causes of chronic cough with normal chest X-ray.
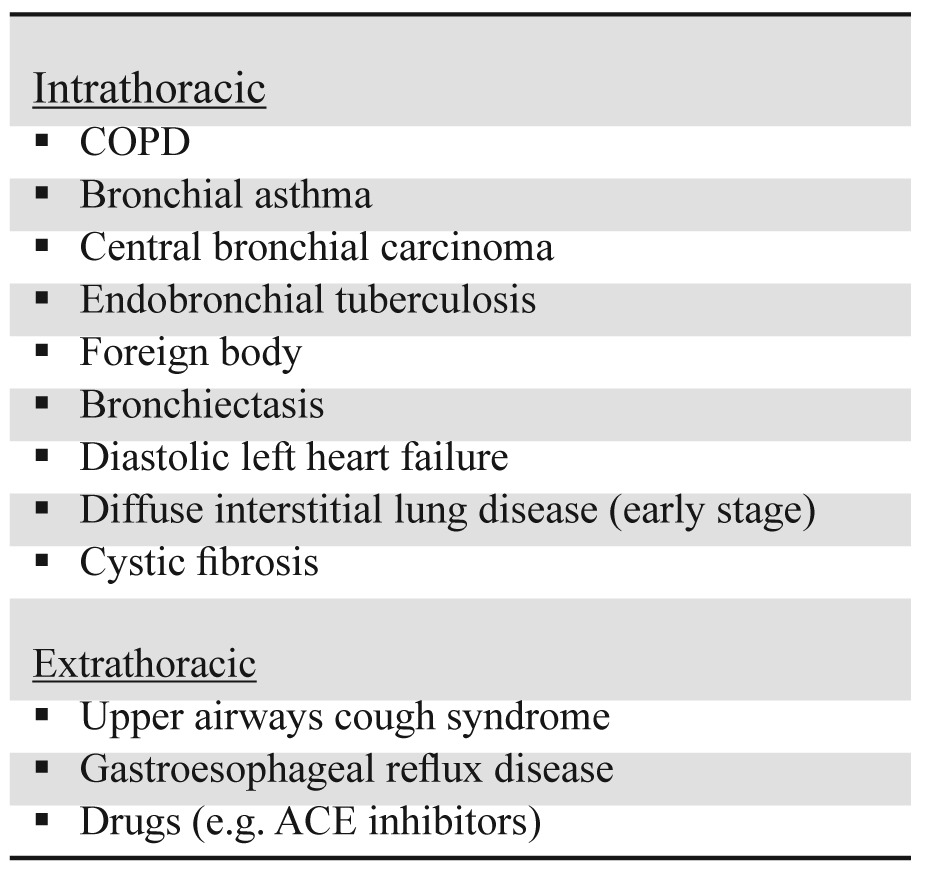
Table 2. Common causes of chronic dyspnea.
We should emphasize that the presence of symptoms is important. Even in the mild stage of the disease, symptomatic patients had a faster decline in lung function, increased respiratory care utilization and lower quality of life than asymptomatic subjects37. Additionally there are no prospective studies or guidelines recommendations that asymptomatic subjects with mild to moderate airflow obstruction would experience additional health benefits if labelled and treated as having COPD38,39. Even though smoking cessation is strongly recommended for every smoker40, incorporating spirometry into an anti-smoking programme as a motivational tool is not always increase cessation rates41,42.
GPs should ask in details and quantify smoking habits (pack-years= cigarettes consumed per day X years with regular smoking / 20) as history of cigarette smoking is considered the most important causative factor for the development of COPD1. Long-term cohort studies proved that current smokers had a greater annual rate of decline in FEV1 compared to never smokers18,43. In a recent study among 3,955 subjects screened for a work-related medical evaluation, quantitative smoking history (≥ 20 pack-years) showed the highest odds ratio for association with COPD and was significantly greater than those for any one of the four respiratory symptoms evaluated44. It was also proved the low positive predicted value of respiratory symptoms for airways obstruction.
Although cigarette smoking is widely acknowledged as the single most important risk factor for COPD, it is now recognized that never smokers may account for between one-fourth and one-third of all COPD cases45. Even though never smokers were less likely to have COPD and had less severe COPD than ever smokers, they comprised 23.3% of those classified with GOLD stage ≥II COPD in the BOLD study46. Predictors of COPD in never smokers include advanced age, low educational level, occupational exposure, prior physician-diagnosed asthma, childhood respiratory diseases, BMI alterations and exposure to biomass smoke (use for cooking/heating)46-48.
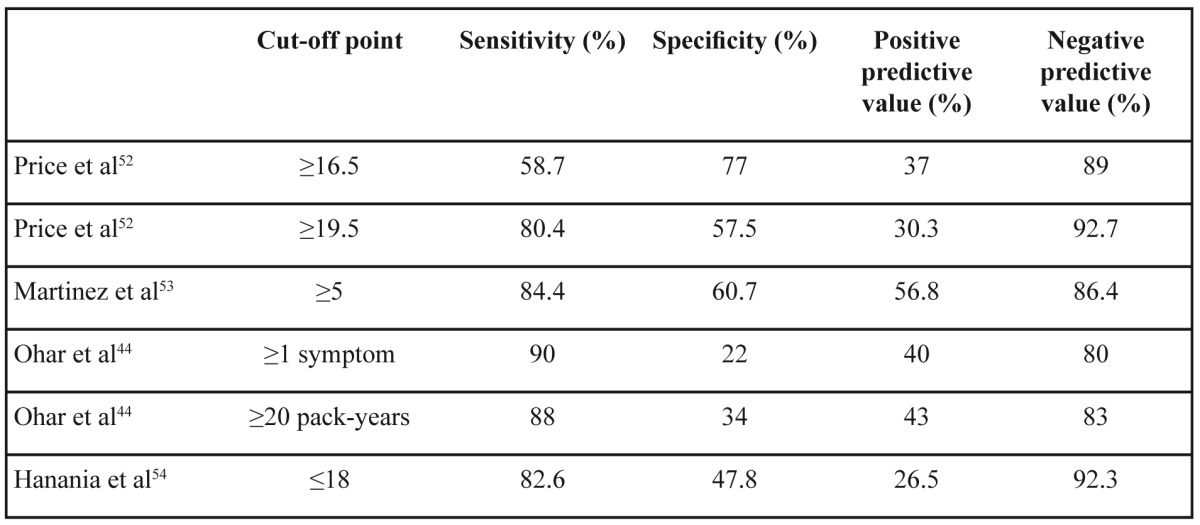
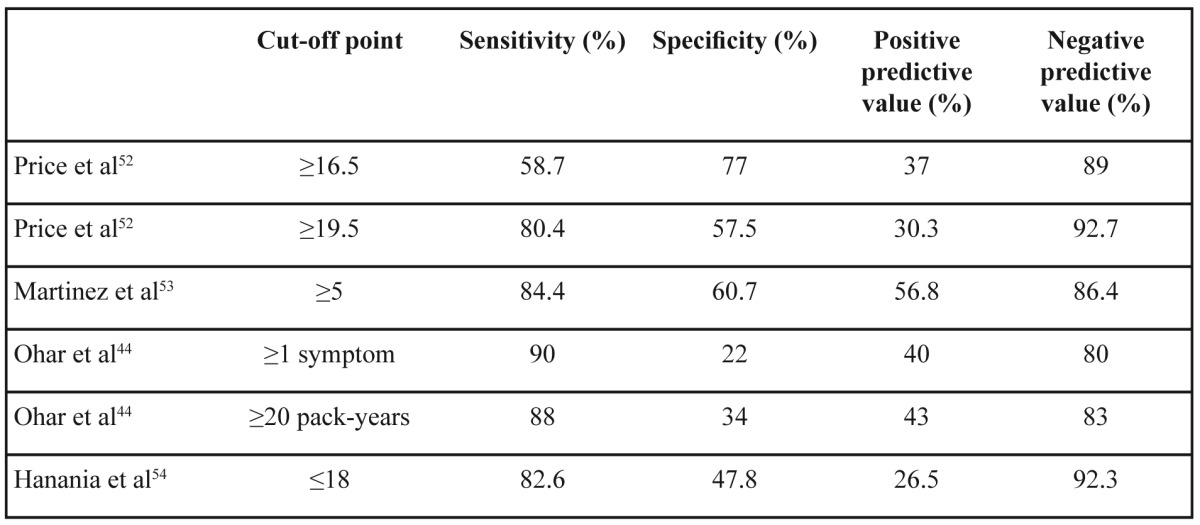
There are well-validated questionnaires for following-up patients with established diagnosis of COPD49-51 which are related to severity of airway obstruction and prognosis. On the other hand there is no widespread use of screening questionnaires in the primary care. There are some simple, self-scored, symptom-based questionnaires which could identify high risk subjects for COPD in a general practice setting52-54. A common characteristic of these questionnaires are their high negative predictive value while positive predictive value reaches 50% because the majority of current smokers without airflow obstruction share the same symptoms with COPD patients (Table 3). This means that a subject with negative score in such a questionnaire probably does not suffer from COPD and an alternative diagnosis should be considered to explain his/her symptoms. If someone had a positive score in a COPD screening questionnaire then official spirometry after bronchodilator and pulmonary consultation are necessary. We should be very careful when we choose a screening questionnaire because external validation may not confirm the results of the original one54.
Table 3. Several screening questionnaires for COPD and their properties.
Official spirometry and portable hand-held spirometers
Spirometry is the best standardized, most reproducible and most objective measurement of airflow limitation1. It is needed to make a confident diagnosis of irreversible airways obstruction (postbronchodilator FEV1/FVC<0.7 according to GOLD guidelines1 or postbronchodilator FEV1/VC<lower limit of normal according to ATS/ERS guidelines for spirometry56) and exclude other diagnoses that may present with similar spirometric patterns (e.g. severe asthma, bronchiectasis, obliterative bronchiolitis). Spirometric severity of the disease is defined according to postbronchodilator FEV1 % pred. (stage I: FEV1≥80%, stage II: 50≤FEV1<80%, stage III: 30≤FEV1<50% and stage IV: FEV1<30%). The two main problems of establishing spirometry in the primary care setting, excluding cost and lack of time during every-day clinical practice, are spirometry performance and evaluation of the results.
Achieving ATS/ERS quality standards for spirometry tests57 depends mainly on training and experience of the examiner (e.g. physician, pulmonary function technologist, staff of primary care practitioners) as well as the cooperation with the patient. Many international organizations have developed educational courses and certification for health care professionals who perform spirometry58,59. As only about half of the spirometry tests in primary care meet the quality goals60, a programme of continuous review and feedback regarding the quality and interpretation of tests, in cooperation with pulmonary specialists, is necessary to improve and maintain competency61.
GOLD guidelines proposed the fixed 0.7 post-bronchodilator FEV1/FVC cut-off point for the diagnosis of the disease and four stages according to FEV1 % predicted, a definition that leads to overdiagnosis of the disease especially among the elderly62 as there is an age-related decline in FEV1/FVC ratio63. In a study among 14,056 symptomatic adults referred for spirometry by their general practitioner, the percentage of false positive diagnoses using the fixed cut-off point definition were 33.2% for the subgroup 61–70 years and 38.7% for those aged 71–80 years compared to definition according to lower limit of normal. Moreover in the subgroup of current or ex-smokers aged ≥50 years positive predictive value of pre-bronchodilator airflow obstruction was 84.2% compared to the post- bronchodilator definition64. A practical approach is using only GOLD stages ≥II for establishing COPD diagnosis among the elderly65.
Another option for the primary care physicians with no direct approach to official spirometry is to use simple, hand-held, expiratory flow-meters that measure FEV1/FEV6 ratio as FEV6 could be used as a good alternative for FVC66. In a study among 204 undiagnosed current and former smokers >50 year old, the pre-bronchodilator FEV1/FEV6 cut-off point of 0.75 showed a positive predictive value of 52% and negative predictive value of 91% for COPD case-finding67. In another study among 1,078 subjects >40 year old who visited a GP (current smokers: 48.4%), the combination of positive IPAG questionnaire (≥17 points) plus post-bronchodilation FEV1/FEV6 <0.7 showed a positive predictive value of 71% and negative predictive value of 97% for COPD case-finding68.
A simplified diagnostic algorithm of COPD in the primary care setting is proposed in Figure 1 taking into account that we are far from establishing a spirometer in every GD site and spirometry has not been proven to be cost-effective as a screening tool for every asymptomatic smoker. Based on the high negative predictive value of both COPD screening questionnaires and FEV1/FEV6 measurements52-54,67,68 we believe that a negative combination could be used to exclude COPD diagnosis. On the other hand their positive predictive value is >50% in most studies so we proposed that if either of them is positive then official spirometry is recommended. We propose using pre- or post-bronchodilator FEV1/FEV667,68.
Figure 1. A suggested diagnostic algorithm for COPD in the Primary Care Setting.
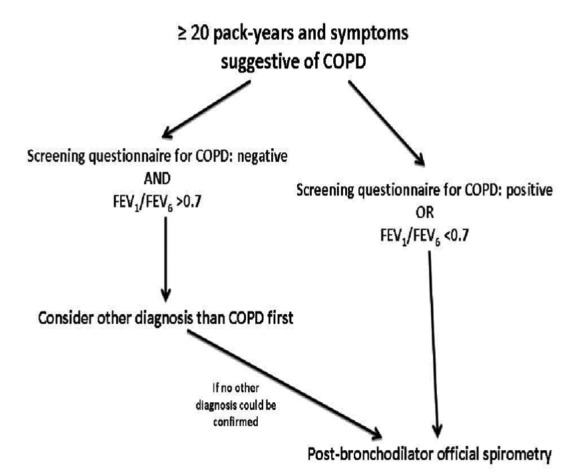
It is important for a GP, who is involved in COPD management, to understand that COPD diagnosis is the result of a holistic decision-making strategy that takes into account medical history, risk factors, physical examination, spirometry, radiographic examinations and long-term response to inhaled bronchodilators and/or corticosteroids. A normal spirometry a few weeks/months after treatment confirms the diagnosis of bronchial asthma meanwhile some patients demonstrate characteristics of both diseases and spirometry alone, even with reversibility test, is not enough to establish a clear diagnosis.
All authors have no conflicts of interest to declare.
References
- 1.Global strategy for the diagnosis, management and prevention of COPD, Updated 2010. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Accessed 2011 July 29;Available from: www.goldcopd.com [Google Scholar]
- 2.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.van Durme YM, Verhamme KM, Verhamme T, van Rooij FJ, Van Pottelberge GR, Hofman A, et al. Prevalence, incidence and lifetime risk for the development of COPD in the elderly: The Rotterdam Study. Chest. 2009;135:368–377. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 4.Mannino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 5.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 7.European Lung White Book: Huddersfield European Respiratory Society Journals Ltd. European Respiratory Society. 2003 [Google Scholar]
- 8.Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119:344–352. doi: 10.1378/chest.119.2.344. [DOI] [PubMed] [Google Scholar]
- 9.Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: Findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–148. doi: 10.2147/copd.s5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunen H, Hacievliyagil SS, Kosar F, Mutlu LC, Gulbas G, Pehlivan E, et al. Factors affecting survival of hospitalized patients with COPD. Eur Respir J. 2005;26:234–241. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 11.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23:689–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Aymerich J, Serra I, Gómez FP, Farrero E, Balcells E, Rodríguez DA, et al. Physical Activity and Clinical and Functional Status in COPD. Chest. 2009;136:62–70. doi: 10.1378/chest.08-2532. [DOI] [PubMed] [Google Scholar]
- 14.Troosters T, Sciurba F, Battaglia S, Langer D, Valluri SR, Martino L, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104:1005–1011. doi: 10.1016/j.rmed.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ, Celli B. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 17.Celli BR. Predictors of mortality in COPD. Respir Med. 2010;104:773–779. doi: 10.1016/j.rmed.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited. An analysis of the Framingham Offspring Cohort. Am J Respir Crit Care Med. 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 19.Peña VS, Miravitlles M, Gabriel R, Jiménez-Ruiz CA, Villasante C, Masa JF. Geographic Variations in Prevalence and Underdiagnosis of COPD. Results of the IBERPOC Multicentre Epidemiological Study. Chest. 2000;118:981–989. doi: 10.1378/chest.118.4.981. [DOI] [PubMed] [Google Scholar]
- 20.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic Obstructive Pulmonary Disease surveillance-United States, 1971-2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 21.Rennard S, Decramer M, Calverley PM, Pride NB, Soriano JB, Vermeire PA, Vestbo J. Impact of COPD in North America and Europe in 2000: subjects perspective of Confronting COPD International Survey. Eur Respir J. 2002;20:799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 22.Derom E, van Weel C, Liistro G, et al. Primary care spirometry. Eur Respir J. 2008;31:197–203. doi: 10.1183/09031936.00066607. [DOI] [PubMed] [Google Scholar]
- 23.Poels PJ, Shermer TR, van Weel C. Underuse of spirometry in the diagnosis of COPD. Monaldi Arch Chest Dis. 2005;63:234–235. doi: 10.4081/monaldi.2005.626. [DOI] [PubMed] [Google Scholar]
- 24.Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3:311–317. doi: 10.2147/copd.s2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caramori G, Bettoncelli G, Tosatto R, Arpinelli F, Visonà G, Invernizzi G, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6–12. doi: 10.4081/monaldi.2005.651. [DOI] [PubMed] [Google Scholar]
- 26.Miravitlles M, de la Roza C, Naberan K, Lamban M, Gobartt E, Martin A. Use of spirometry and patterns of prescribing in COPD in primary care. Respir Med. 2007;101:1753–1760. doi: 10.1016/j.rmed.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Han MK, Kim MG, Mardon R, Renner P, Sullivan S, Diette GB, et al. Spirometry utilization for COPD. How do we measure up? . Chest. 2007;132:403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 28.Walters JA, Hansen EC, Johns DP, Blizzard EL, Walters EH, Wood-Baker R. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of COPD. Thorax. 2008;63:408–414. doi: 10.1136/thx.2007.082859. [DOI] [PubMed] [Google Scholar]
- 29.Halpin DM, O'Reilly JF, Connellan S, Connellan M. BTS COPD Consortium. Confidence and understanding among general practitioners and practice nurses in the UK about diagnosis and management of COPD. Respir Med. 2007;101:2378–2385. doi: 10.1016/j.rmed.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Jones RC, Dickson-Spillmann M, Mather MJ, Marks D, Shackell BS. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: The Devon primary care audit. Respir Res. 2008;9:62. doi: 10.1186/1465-9921-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lusuardi M, De Benedetto F, Paggiaro P, Sanguinetti CM, Brazzola G, Ferri P, et al. A Randomized Controlled Trial on Office Spirometry in Asthma and COPD in Standard General Practice. Chest. 2006;129:844–852. doi: 10.1378/chest.129.4.844. [DOI] [PubMed] [Google Scholar]
- 32.Enright P. Does screening for COPD by primary care physicians have the potential to cause more harm than good? Chest. 2006;129:833–835. doi: 10.1378/chest.129.4.833. [DOI] [PubMed] [Google Scholar]
- 33.Borg BM, Hartley MF, Fisher MT, Thompson BR. Spirometry training does not guarantee valid results. Respir Care. 2010;55:689–694. [PubMed] [Google Scholar]
- 34.Sichletidis L, Chloros D, Spyratos D, Chatzidimitriou N, Chatziiliadis P, Protopappas N, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82–88. doi: 10.3132/pcrj.2007.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisner MD, Blanc PD, Yelin EH, Katz PP, Sanchez G, Iribarren C, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65:229–234. doi: 10.1136/thx.2009.126201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T, et al. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax. 2008;63:768–774. doi: 10.1136/thx.2007.093724. [DOI] [PubMed] [Google Scholar]
- 38.Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. U.S. Preventive Services Task Force. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;148:535–543. doi: 10.7326/0003-4819-148-7-200804010-00213. [DOI] [PubMed] [Google Scholar]
- 39.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 40.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. Lung Health Study Research Group. The effects of smoking cessation intervention on 14.5 year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 41.Wilt TJ, Niewoehner D, Kim CB, Kane RL, Linabery A, Tacklind J, et al. Use of spirometry for case finding, diagnosis and management of chronic obstructive pulmonary disease (COPD). Agency for Healthcare Research and Quality. Evidence Report/ Technology Assessment No. 121. Prepared by the Minnesota Evidence-based Practice Center. Rockville. AHRQ. 2005:1–169. doi: 10.1037/e439492005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bednarek M, Gorecka D, Wielgomas J, Czajkowska-Malinowska M, Regula J, Mieszko-Filipczyk G, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869–873. doi: 10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respi Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 44.Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137:1345–1353. doi: 10.1378/chest.09-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005;118:1364–1372. doi: 10.1016/j.amjmed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 46.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers. Results from the population-based Burden of Obstructive Lung Disease study. Chest. 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Wang C, Yao W, Chen P, Kang J, Huang S, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33:509–518. doi: 10.1183/09031936.00084408. [DOI] [PubMed] [Google Scholar]
- 48.Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, et al. American Thoracic Society Statement: Occupational contribution to the burden of airways disease. Am J Respir Crit Care Med. 2003;167:787–797. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- 49.Miravitlles M, Llor C, de Castellar R, Izquierdo I, Baró E, Donado E. Validation of the COPD severity score for use in primary care: the NEREA study. Eur Respir J. 2009;33:519–527. doi: 10.1183/09031936.00087208. [DOI] [PubMed] [Google Scholar]
- 50.Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38:29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 51.Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Félez M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:680–685. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 52.Price DB, Tinkelman DG, Nordyke RJ, Isonaka S, Halbert RJ. COPD Questionnaire Study Group. Scoring System and Clinical Application of COPD Diagnostic Questionnaires. Chest. 2006;129:1531–1539. doi: 10.1378/chest.129.6.1531. [DOI] [PubMed] [Google Scholar]
- 53.Martinez FJ, Raczek AE, Seifer FD, Conoscenti CS, Curtice TG, D'Eletto T FD, et al. Development and initial validation of a self-scored COPD population screener questionnaire (COPD-PS) COPD. 2008;5:85–95. doi: 10.1080/15412550801940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanania NA, Mannino DM, Yawn BP, Mapel DW, Martinez FJ, Donohue JF, et al. Predicting risk of airflow obstruction in primary care: Validation of the lung function questionnaire (LFQ) Respir Med. 2010;104:1160–1170. doi: 10.1016/j.rmed.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Kotz D, Nelemans P, van Schayck CP, Wesseling GJ. External validation of a COPD diagnostic questionnaire. Eur Respir J. 2008;31:298–303. doi: 10.1183/09031936.00074307. [DOI] [PubMed] [Google Scholar]
- 56.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 57.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 58.Apply for the office spirometry certificate (OSC) American Association for Respiratory Care. http://www.aarc.org/osc.
- 59.HERMES Spirometry: the European Spirometry Driving Licence. Breath. 2011;7:259–264. [Google Scholar]
- 60.Leuppi JD, Miedinger D, Chhajed PN, Buess C, Schafroth S, Bucher HC, Tamm M. Quality of spirometry in primary care for case finding of airway obstruction in smokers. Respiration. 2010;79:469–474. doi: 10.1159/000243162. [DOI] [PubMed] [Google Scholar]
- 61.Borg BM, Hartley MF, Fisher MT, Thompson BR. Spirometry training does not guarantee valid results. Respir Care. 2010;55:689–694. [PubMed] [Google Scholar]
- 62.Vollmer WM, Gíslason T, Burney P, Enright PL, Gulsvik A, Kocabas A, Buist AS. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34:588–597. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Mørkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J. 2002;20:1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 64.Schermer TR, Smeele IJ, Thoonen BP, Lucas AE, Grootens JG, van Boxem TJ, et al. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J. 2008;32:945–952. doi: 10.1183/09031936.00170307. [DOI] [PubMed] [Google Scholar]
- 65.Calverley P. Fulfilling the promise of primary care spirometry (editorial) Eur Respir J. 2008;31:8–10. doi: 10.1183/09031936.00124507. [DOI] [PubMed] [Google Scholar]
- 66.Vandevoorde J, Verbanck S, Schuermans D, Kartounian J, Vincken W. FEV1/FEV6 and FEV6 as an alternative for FEV1/FVC and FVC in the spirometric detection of airway obstruction and restriction. Chest. 2005;127:1560–1564. doi: 10.1378/chest.127.5.1560. [DOI] [PubMed] [Google Scholar]
- 67.Frith P, Crockett A, Beilby J, Marshall D, Attewell R, Ratnanesan A, et al. Simplified COPD screening: validation of the PiKo-6® in primary care. Prim Care Respir J. 2011;20:190–198. doi: 10.4104/pcrj.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sichletidis L, Spyratos D, Papaioannou M, Chloros D, Tsiotsios A, Tsagaraki V, et al. A combination of the IPAG questionnaire and PiKo-6® flow meter is a valuable screening tool for COPD in the primary care setting. Prim Care Respir J. 2011;20:184–189. doi: 10.4104/pcrj.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]


