Abstract
Background: Targeted cancer therapy is a new approach for the treatment of cancer. It involves a specific molecular target, mainly a receptor that serves as a target for monoclonal antibodies or tyrosine kinase inhibitors. Side-effects of these new regimens are described to be mild, compared to those of classical chemotherapy. There is a lack in the documentation and understanding of oral complications related to molecularly targeted drugs.
Methods: In this review, we tried to make a systematic review of the databases Pubmed and Scopus, using "targeted cancer therapy" and "oral", or "mucositis", or "stomatitis", or "bleeding", or "hemorrhage" as search terms. Specific drug name searches were not conducted. The search yielded 97 results. Only articles related to EGFR and VEGFR inhibition were selected. Finally 13 articles met the criteria. Results are discussed and possible pathogenetic mechanisms for the complications of targeted cancer therapy regimens are presented.
Results: It appears that the most serious side-effect is mucositis/stomatitis that may affect the whole gastrointestinal tract. It rarely results in treatment discontinuation. Reduced saliva secretion, xerostomia and dysphagia can be severe with some regimens and interfere with food uptake. Osteonecrosis, wound healing impairment, spontaneous gingival bleeding and dysgeusia were also reported.
Conclusions: Considering these data it is obvious that symptoms related to cancer treatment should be considered in the context of the holistic management of patients. Oral complications should not be ignored but recorded during physical examination, because they may significantly impair daily activities and patients' quality of life.
Keywords: bevacizumab, cetuximab, oral complications, molecularly targeted drugs, EGFR, VEGFR
Introduction
The first to describe a concept of selective uptake of molecules by tissues was Ehrlich, in the 19th century. He described the side-chain theory, that formed the basis for the understanding of the effects of serum and the coupling between an antigen and an antibody, that built the basis for the discovery of monoclonal antibodies and targeted cancer therapy.
Molecularly targeted drugs interact with a specific target, mostly a protein, in a selective way. This protein is a growth factor, a growth factor receptor, a signaling molecule, a cell cycle protein, an apoptosis mediator, a molecule implicated in cancer cell dispersal and angiogenesis1.
Side effects of molecularly targeted drugs differ on the severity of reported symptoms compared to classical chemotherapy agents, because they rarely cause alopecia, nausea and vomiting. Reports regarding oral complications are sparse and the most frequently reported sign in clinical control trials is mucositis/stomatitis. The aim of this review of the literature is twofold: 1. to present the oral complications of targeted cancer therapy, in particular those that are the result of therapies that target EGFR and VEGFR, 2. to analyze the possible pathogenetical mechanisms.
Molecularly targeted drugs This general term includes two main categories of molecules, monoclonal antibodies and tyrosine kinase inhibitors. Tyrosine kinase inhibitors connect to the cytoplasmic side of membrane receptors. They are small molecules, administered per os, once daily. Because of their small size they provide enhanced bioavailability.
On the contrary, monoclonal antibodies act on the extracellular domain side. They are large molecules, given by intravenous route once a week and show decreased bioavailability in certain compartments, like the CNS. Because of their size they do not normally pass the basal membrane, thus they are rarely related to symptoms from the gastrointestinal tract. They connect to a certain epitope of an antigen/protein.
Receptors: action and side-effects
1. EGFR EGF receptors are membrane receptors with tyrosine kinase activity. Like all receptors of this family, they need ATP for the phosphorylation of their cytoplasmic domain, that possesses enzymatic activity. They play a major role in cancer progression, because they inhibit apoptosis, enhance cell cycle progression, angiogenesis, cancer cell motility and metastasis, malignant transformation and lead to cancer phenotype2,3.
The overexpression of EGFR in various cancer types, especially in the head and neck cancer, in which an overexpression is present in 42%-98% of the cases, is related to an increased transcriptional activity and anticipates a bad outcome2,3.
EGFR inhibition and oral complications Two different ways of EGFR-molecularly targeted drug interaction offer a more effective inhibition. The first involves the connection of the drug to the extracellular domain of the receptor that inhibits the connection of the ligand. The second targets the intracellular portion that has tyrosine kinase activity and exerts its action by restricting ATP binding or binding to the active site of the enzyme3,4. Thus both monoclonal antibodies and tyrosine kinase inhibitors can effectively inactivate EGFR.
EGFR inhibition is related to cancer cell apoptosis and cell cycle arrest via p27 activation, a cyclin dependent inhibitor, as well as with anti-angiogenic effects. The last is an interesting aspect of EGFR inhibition and can be explained through the interaction of this receptor with the VEGF, the main factor in angiogenesis. In particular, inhibition of EGFR is related to decreased expression of VEGF and decreased VEGF expression correlates with decreased EGFR levels5,6. Nonetheless, an anti-EGFR medication cannot completely eliminate VEGF plasma levels.
Additionally, EGFRs are present on the endothelium of cancer vascular cells and are related to the vascular density of mammary carcinomas7,8.
Mucositis
It is interesting that most of the phase I-III clinical trials, do not thoroughly report oral complications for cetuximab, a monoclonal antibody that acts as an EGFR inhibitor9. Stomatitis is a frequent (Table 1)10-19, dose independent, symptom and it is characterized by generalized erythema and sensitivity and rarely by ulcerous lesions as in case of classical chemotherapy20. It mostly affects the non-keratinized labial and buccal mucosa, the mucosa of the tongue, of the floor of the mouth and the soft palate and appears 9-16 days after treatment initiation, as this is the epithelial cell turnover time.
EGF plays a major role in the maintenance of mucosal integrity, as well as in its rehabilitation, by acting as a mitogen and by inducing mucus and prostaglandin synthesis1. This action is harnessed in patients with small bowel resection21. Inhibition of squamous epithelium maturation in the gastrointestinal tract is an additional explanation for ulcer formation1,22.
Dysphagia, hyposalivation and dysgeusia
Cetuximab does not increase the risk of dysphagia, reduced saliva secretion and dysgeusia11.
2. VEGFR
There are three categories of tyrosine kinase receptors in this family, VEGFR-1, VEGFR-2, and VEGFR-3 and five possible ligands VEGFA, VEGFB, VEFGC, VEGFD, and placental growth factor (PGF). VEGFA is mainly related to carcinogenesis because it is involved in angiogenesis.
VEGF is the main contributor to the process of angiogenesis23. Angiogenesis in tumor environment is elicited as a result of tissue hypoxia and is essential for the increase of tumor size over 1-2 mm24,25.
VEGF exerts a protective effect on the vasculature, mainly through a two-way action: 1. by reducing smooth muscle cell hyperplasia in the inner layer of the vessels25. 2. by promoting NO and PGI2 synthesis. a. this action leads to vasodilation and inhibition of leukocyte rolling and platelet aggregation26,27.
Crucial for the process of angiogenesis, stimulated by VEGF are: 1. the increased vascular permeability that contributes to the release of plasma proteins28 and 2. the expression of tissue metalloproteinases and activators of plasminogen that support endothelial cell migration. Thus, extracellular proteolysis is essential for angiogenesis29.
Bevacizumab is a monoclonal antibody than binds to VEGF and blocks its binding to the receptor. Other agents that possess anti-angiogenic activity inhibit tyrosine kinase activity (sunitinib) or molecules involved in VEGFR signaling (mTOR inhibitors).
Oral side effects of VEGFR inhibition
Oral side effects of this category of molecularly targeted drugs can commence as a result of anti-angiogenic activity and impaired healing. Hemorrhage is the main side effect that may appear after interventions in the oral cavity or even spontaneously. Other general complications related to the vasculature include hypertension, proteinuria and thrombosis.
Hemorrhage
Hemorrhage may be manifested by intracranial bleeding (1,3%), bleeding of the gastrointestinal tract (16%) and epistaxis (53%)13.
The impact of anti-angiogenic drugs on oral bleeding has not been investigated yet. Only sparse reports of gingival hemorrhage, severity 1, have been reported in the literature for patients receiving bevacizumab30. Drug (bevacizumab) discontinuation is suggested for at least 28 days before and 28 after the surgical intervention in the mouth.
Possible mechanisms of bleeding include31: 1. Uptake of VEGF-bevacizumab by platelets. Within hours after the first administration, almost 97% of VEGF plasma concentrations are bound to the monoclonal antibody and stored in platelets' alpha granules32,33. Thus, platelets serve as antibody reservoirs and are involved in the pharmacodynamics of the drug. 2. Interaction with platelet adhesion to the endothelium. VEGF-platelet interactions are important for platelet migration. In particular, VEGF binds to receptors on activated platelets with the mediation of thrombin and enhances non-activated platelet migration and adhesion through tissue factor activation and thrombin synthesis34. 3. Impaired endothelial cell turnover. VEGF-antibody junction results in endothelial cell apoptosis, through inhibition of molecular pathways35. Subendothelial connective tissue exposure destabilizes the endothelium and leads to thrombosis36. 4. Impaired expression of von Willenbrand factor, tissue plasminogen activator and plasminogen activator inhibitor13,29,37,38.
Osteonecrosis
An increased risk of osteonecrosis of the mandible has been reported for patients receiving bisphosphonates and anti-angiogenic medications compared to those receiving only bisphosphonates39.
Impaired healing
It is related to an impaired synthesis of extracellular matrix in the granulation tissue as a result of tissue transglutaminase inhibition, an enzyme that activates TGF-ß140.
Dysgeusia
Sunitinib, a tyrosine kinase inhibitor is related to an increased frequency of dysgeusia (42%)15(Table 1).
Table 1. Frequency of mucositis/stomatitis, xerostomia and dysgeusia after cancer treatment with certain molecularly targeted drugs.
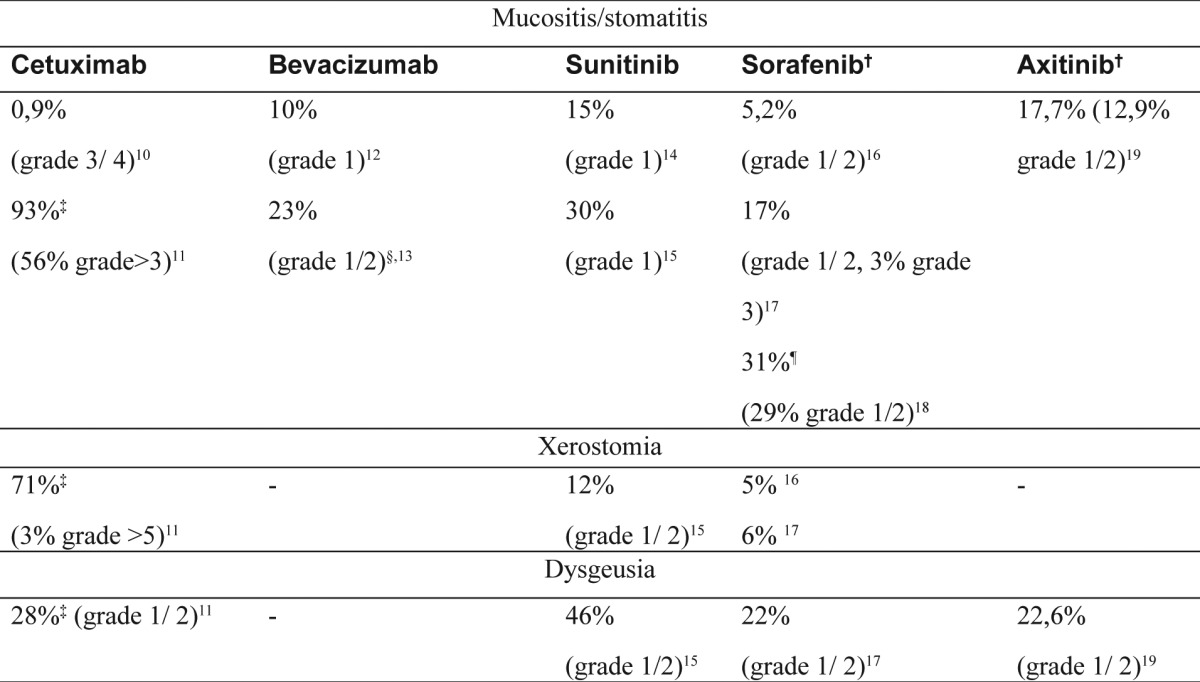
†multiple tyrosine kinase inhibitor, ‡in combination with radiotherapy, §in combination with 5-FU and leucovorin, ¶in patients with previous bevacizumab treatmentSIDS: Frequent oral side-effects of targeted cancer therapy
Conclusion
Although reports and clinical trials that address oral complications of the drugs of targeted cancer therapy are sparse, stomatitis/mucositis is a severe complication that impairs food uptake and causes discomfort. There are many possible mechanisms through which anti-angiogenic medications may cause hemorrhage, however, oral bleeding has not been reported as a frequent side-effect of targeted cancer therapy. Reduced saliva secretion, xerostomia, dysphagia and osteonecrosis have also been reported. It is unclear whether the impa irment of wound healing with these drugs is clinically significant. The consideration of these side-effects as important complications of cancer therapy, will result in better quality of life for cancer patients.
Conflict of interest statement
The authors of this manuscript disclose no financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work.
References
- 1.Widakowich C, de Castro G Jr, de Azambuja E, Dinh P, Awada A. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12:1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- 2.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 3.Harari PM, Huang SM, Herbst RS, Quon H. Molecular Targeting of the Epidermal Growth Factor Receptor. In: Harrison LB, Session RB, Hong WK, editors. Head and Neck Cancer: A Multidisciplinary Approach. 2nd ed. . Philadelphia: Lippincott, Williams and Wilkings; 2003 [Google Scholar]
- 4.Arteaga CL. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol. 2002;29:3–9. doi: 10.1053/sonc.2002.35642. [DOI] [PubMed] [Google Scholar]
- 5.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 6.Ciardiello F, Caputo R, Damiano V, Caputo R, Troiani T, Vitagliano D, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546–1556. [PubMed] [Google Scholar]
- 7.Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9:1200–1210. [PubMed] [Google Scholar]
- 8.de Jong JS, van Diest PJ,, van der Valk P, Baak JP. Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol. 1998;184:53–57. doi: 10.1002/(SICI)1096-9896(199801)184:1<53::AID-PATH6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Watters AL, Epstein JB, Agulnik M. Oral complications of targeted cancer therapies: a narrative literature review. Oral Oncol. 2011;47:441–448. doi: 10.1016/j.oraloncology.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 11.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 12.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 13.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 17.Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia JA, Hutson TE, Elson P, Cowey CL, Gilligan T, Nemec C, et al. Sorafenib in patients with metastatic renal cell carcinoma refractory to either sunitinib or bevacizumab. Cancer. 2010;116:5383–5390. doi: 10.1002/cncr.25327. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 20.Lacouture ME, Maitland ML, Segaert S, Setser A, Baran R, Fox LP, et al. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010;18:509–522. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 21.Stern LE, Erwin CR, O'Brien DP, Huang F, Warner BW. Epidermal growth factor is critical for intestinal adaptation following small bowel resection. Microsc Res Tech. 2000;51:138–148. doi: 10.1002/1097-0029(20001015)51:2<138::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 24.Braun AH, Achterrath W, Wilke H, Vanhoefer U, Harstrick A, Preusser P. New systemic frontline treatment for metastatic colorectal carcinoma. Cancer. 2004;100:1558–1577. doi: 10.1002/cncr.20154. [DOI] [PubMed] [Google Scholar]
- 25.Laitinen M, Zachary I, Breier G, Pakkanen T, Hakkinen T, Luoma J, et al. VEGF gene transfer reduces intimal thickening via increased production of nitric oxide in carotid arteries. Hum Gene Ther. 1997;8:1737–1744. doi: 10.1089/hum.1997.8.15-1737. [DOI] [PubMed] [Google Scholar]
- 26.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280:1375–1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 28.Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557–562. doi: 10.1002/jcp.1041530317. [DOI] [PubMed] [Google Scholar]
- 29.Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902–908. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 30.Chura JC, Van Iseghem K, Downs LS, Carson LF, Judson PL. Bevacizumab plus cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Gynecol Oncol. 2007;107:326–330. doi: 10.1016/j.ygyno.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Learn about Avastin Today. [Internet] [retrieved 2011 Sept 17] 2011;Available from: http://www.avastin.com/avastin/patient [Google Scholar]
- 32.Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187–2190. [PubMed] [Google Scholar]
- 33.Verheul HM, Lolkema MP, Qian DZ, Hilkes YH, Liapi E, Akkerman JW, et al. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res. 2007;13:5341–5347. doi: 10.1158/1078-0432.CCR-07-0847. [DOI] [PubMed] [Google Scholar]
- 34.Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor–stimulated endothelial cells promote adhesion and activation of platelets. Blood. 2000;96:4216–4221. [PubMed] [Google Scholar]
- 35.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 36.Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol. 2003;21:3542 author–reply 3543. doi: 10.1200/JCO.2003.99.046. [DOI] [PubMed] [Google Scholar]
- 37.Brock TA, Dvorak HF, Senger DR. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol. 1991;138:213–221. [PMC free article] [PubMed] [Google Scholar]
- 38.Nemerson Y. Tissue factor and hemostasis. Blood. 1988;71:1–8. [PubMed] [Google Scholar]
- 39.Christodoulou C, Pervena A, Klouvas G, Galani E, Falagas ME, Tsakalos G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 40.Haroon ZA, Amin K, Saito W, Wilson W, Greenberg CS, Dewhirst MW. SU5416 delays wound healing through inhibition of TGF-beta 1 activation. Cancer Biol Ther. 2002;1:121–126. doi: 10.4161/cbt.55. [DOI] [PubMed] [Google Scholar]


