Abstract
Schwannomas are generally benign, slow growing tumors, which can originate from any nerve that has a Schwann cell sheath. Digestive tract schwannomas are rare and are usually asymptomatic. We present the case of a 48-year-old woman with a symptomatic submucosal tumour of the gastric antrum. The patient underwent partial gastrectomy and the histological and immunohistochemical findings of the resected specimen established the diagnosis of schwannoma.
Keywords: Schwannoma, neurinoma, stomach
Schwannomas, also known as neurinomas, are tumors originating from any nerve that has a Schwann cell sheath1. They are rarely observed in the gastrointestinal tract (GIT) with the most common site being the stomach. These tumors are usually benign, slow-growing and asymptomatic, but in some cases bleeding, epigastric pain or a palpable mass may occur2. The preoperative diagnosis via endoscopy is a challenging issue due to the difficulty of differentiation from other submucosal tumors.
We present the rare case of a 48-year-old woman with a symptomatic gastric schwannoma, whose diagnosis was established histologically after surgery.
Case report
A 48-year-old woman with a history of hypothyroidism, anaemia and depression under treatment was admitted to our department for the evaluation of a gastric lesion that was detected three weeks before via computed tomography (CT). The patient complained of occasional pain in the epigastic area and left upper quadrant that began six months ago. No weight loss, weakness or anorexia was mentioned. Physical examination of the abdomen revealed no signs of tenderness or of a palpable mass.
Blood tests confirmed the presence of anaemia (haemoglobin: 9.7 g/dl), whereas all other parameters were within normal range. Plain abdominal radiograph was also unremarkable.
The contrast-enhanced CT of the abdomen showed a well-defined and homogenous-enhanced gastric tumor, of approximately 18mm in diameter, which was located in the anterior wall of the antrum. No pathological lymph nodes were found. The CT of the chest was unremarkable.
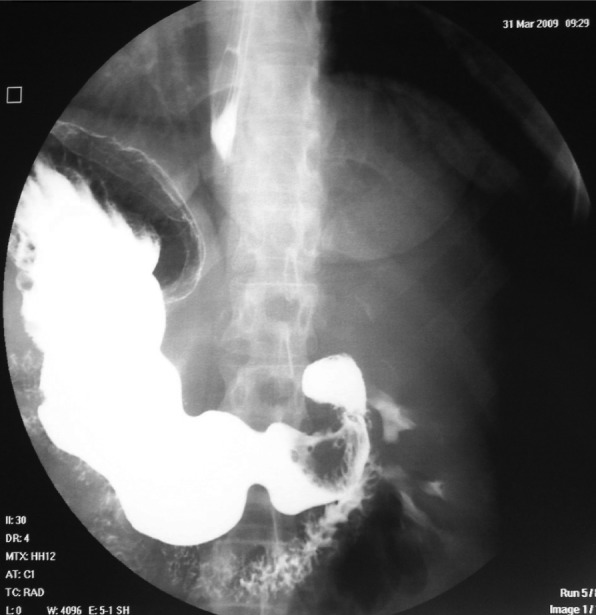
The upper gastrointestinal barium study revealed a large contrast deficit at the gastric antrum (Figure 1).
Figure 1. Upper gastrointestinal series demonstrating a large contrast deficit at the gastric antrum.
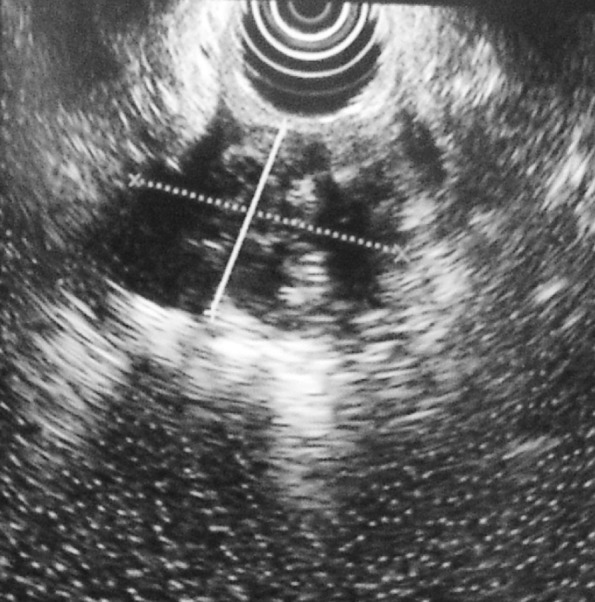
Furthermore gastroscopy (Figure 2) and endoscopic ultrasonography (EUS) (Figure 3) were conducted, that showed a large, hypoechoic lesion located at the gastric antrum, causing partial obstruction of the pylorus and extending over the tunica muscularis into the serosa. No biopsies were taken.
Figure 2. Endoscopic view showing a submucosal lesion located at the gastric antrum, causing partial obstruction of the pylorus, with intact mucosal.
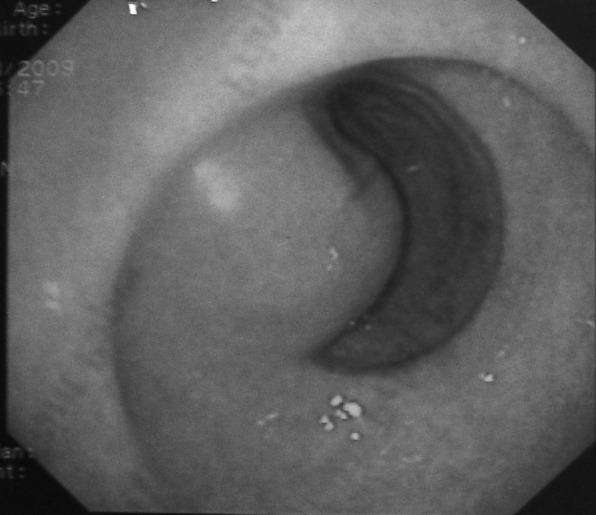
Figure 3. EUS showing a large, hypoechoic lesion located at the gastric antrum and extending over the tunica muscularis into the serosa.
Surgery was determined to be the best treatment option and the patient underwent a partial gastrectomy with Billroth II and Braun anastomosis.
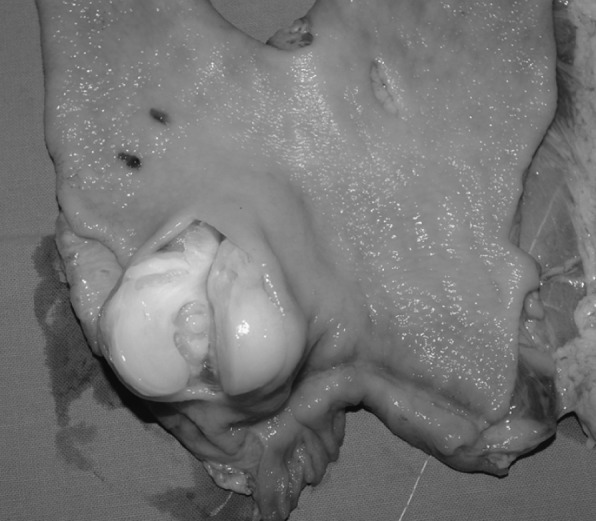
Macroscopic examination of the stomach revealed a yellow-white, solid and well circumscribed tumor with a rubbery and jelly cut surface that measured 5×3×2.5cm, located at the lesser curvature, 2cm proximal of the pylorus and having exophytic character, beginning from the tunica muscularis without infiltrating the mucosa (Figure 4). In cross section whirling trabeculation was noticed.
Figure 4. Macroscopic view of the resected specimen showing a yellow-white, solid and well circumscribed exophytic tumor with a rubbery and jelly cut surface measuring 5×3×2,5cm, located at the lesser curvature of the stomach, without infiltrating mucosa.
Microscopically the tumor was composed of spindle cells forming sheets in a storiform pattern.
Immunohistochemically the tumor was S-100 protein and Vimentin positive but CD 117, CD 34, β-catenin, SMA, synaptophysin, chromogranin and desmin were negative.
The postoperative period was uneventful and the patient was discharged on the 10th postoperative day in good condition. The one year-follow up was unremarkable.
Discussion
Schwannomas or neurinomas are spindle cell mesenchymal tumours, which originate from any nerve that has a Schwann cell sheath1. In the GIT, gastrointestinal stromal tumours (GISTs) constitute the largest group of mesenchymal tumours, whereas schwannomas are rare and are mostly found in older adults (mean age 58 years) showing a slight female predilection3. Most of them are uninodular, but sometimes they present with a multifocal character4. Malignant schwannomas are very rare, as only 8 cases have been reported in the literature till now5,6.
In a study that included 33 cases of schwannomas of the GIT, four tumours were located in the esophagus, 24 in the stomach, two in the colon and three in the rectum, with no cases being reported from the small intestine2. With the stomach being the most common site of origin in the GIT, schwannomas represent 6.3% of gastric mesenchymal tumours and only 0.2% of all gastric tumours. They are usually located in the middle third of the stomach along the lesser curvature7. The majority of the tumours are usually encased by intact mucosa and principally involve the submucosa and muscularis propria, without invading adjacent structures. About half of them show central ulceration.
Schwannomas generally present asymptomatically; however, in some cases they can cause abdominal discomfort, pain or digestive symptoms, as in our case. A palpable mass may also be present when the tumour is large and exophytic. Dysphagia or obstipation are possible symptoms when the site of origin is esophagus or rectum2. In cases of deep ulceration bleeding may also be present.
Although the definitive diagnosis of gastric schwannomas is determined by pathological examination, it may be helpful to gain limited information about the tumor through gastrointestinal endoscopy, CT, magnetic resonance imaging (MRI), sonography, EUS and upper gastrointestinal barium study. Endoscopy helps to define the exact location of the tumor. Though endoscopic needle biopsy is useful to establish a definite diagnosis of a submucosal tumor, in case of GIST there is the theoretical risk of hemorrhage or tumor rupture which is associated with poor prognosis8. Having in mind this risk, the endoscopist did not perform biopsy in our case. On CT, schwannomas appear mostly as homogenous, strongly contrast-enhanced tumors without signs of hemorrhage, necrosis, cystic changes or calcification, in contrast to GISTs9. MRI provides further information about the exact location of the tumor and its relation to the surrounding structures. Schwannomas appear on MRI as strongly enhancing tumors, having low to medium signal intensity on T1 weighted images and high signal intensity on T2 weighted images10. EUS is the best method to diagnose small lesions11, whereas transabdominal ultrasonography is an alternative method for larger tumours.12 One possible method to obtain a precise preoperative diagnosis is a sonographically guided percutaneous core biopsy13.
Microscopically schwannomas of the gastrointestinal tract consist of spindle cells with a prominent lymphoid cuff and are characterized by the absence of typical Verocay bodies, Antoni A and Antoni B areas14. They are also GFAP positive in contrast to peripheral schwannomas2. The immunohistochemical features of schwannomas are also very important for the differential diagnosis between the various types of submucosal tumors, such as GISTs, leiomyomas and gastrointestinal autonomic nerve tumors (GANTs). Digestive tract schwannomas are S100 protein and vimentin positive, never express the CD 117 antigen and are usually negative for CD 34, in contrast to GISTs15. Schwannomas are also negative for SMA, in contrast to leiomyomas16. GANTs on the other hand, are usually negative for S-100 protein and GFAP and most are positive for CD 117 and CD 3414.
Due to the difficulty of establishing a definite preoperative diagnosis, but also in order to prevent possible complications such as bleeding or pyloric stenosis, surgical resection should be considered the treatment of choice in patients with gastric schwannoma. The size and location of the tumor, as well as its relation to the surrounding organs are important factors in determining the type of operation. Local extirpation, wedge resection, partial, subtotal or even total gastrectomy, are all acceptable operations17. Laparoscopic techniques can also be used18. Recurrence rates are generally very low. In our case an approximately 3,5cm in diameter, symptomatic schwannoma of the gastric antrum was treated with partial gastrectomy and the diagnosis was established microscopically and immunohistochemically after surgery.
References
- 1.Lin CS, Hsu HS, Tsai CH, Li WY, Huang MH. Gastric schwannoma. J Chin Med Assoc. 2004;67:583–586. [PubMed] [Google Scholar]
- 2.Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, et al. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536–545. doi: 10.1111/j.1365-2559.2006.02370.x. [DOI] [PubMed] [Google Scholar]
- 3.Miettinen M, Blay JY, Sobin LH. Mesenchymal tumors of the stomach. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press. 2000:62–65. [Google Scholar]
- 4.Rymarczyk G, Hartleb M, Boldys H, Kajor M, Wodołazski A. Neurogenic tumors of the digestive tract: report of two cases. Med Sci Monit. 2000;6:383–385. [PubMed] [Google Scholar]
- 5.Loffeld RJ, Balk TG, Oomen JL, van der Putten AB. Upper gastrointestinal bleeding due to a malignant schwannoma of the stomach. Eur J Gastroenterol Hepatol. 1998;10:159–162. doi: 10.1097/00042737-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Gennatas CS, Exarhakos G, Kondi-Pafiti A, Kannas D, Athanassas G, Politi HD. Malignant schwannoma of the stomach in a patient with neurofibromatosis. Eur J Surg Oncol. 1998;14:261–264. [PubMed] [Google Scholar]
- 7.Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinalstromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002;198:605–613. doi: 10.1078/0344-0338-00309. [DOI] [PubMed] [Google Scholar]
- 8.Arolfo S, Teggia PM, Nano M. Gastrointestinal stromal tumors: Thirty years experience of an Institution. World J Gastroenterol. 2011;17:1836–1839. doi: 10.3748/wjg.v17.i14.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentenol. 2005;184:797–802. doi: 10.2214/ajr.184.3.01840797. [DOI] [PubMed] [Google Scholar]
- 10.Karabulut N, Martin DR, Yang M. Gastric schwannoma: MRI findings. Br J Radiol. 2002;75:624–626. doi: 10.1259/bjr.75.895.750624. [DOI] [PubMed] [Google Scholar]
- 11.Tsai TL, Changchien CS, Hu TH, Hsiaw CM. Demonstration of gastric submucosal lesions by high-resolution transabdominal sonography. J Clin Ultrasound. 2000;28:125–132. doi: 10.1002/(sici)1097-0096(200003/04)28:3<125::aid-jcu4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Futagami K, Hata J, Haruma K, Yamashita N, Yoshida S, Tanaka S, Chayama K. Extracorporeal ultrasound is an effective diagnostic alternative to endoscopic ultrasound for gastric submucosal tumours. Scand J Gastroenterol. 2001;36:1222–1226. doi: 10.1080/00365520152584888. [DOI] [PubMed] [Google Scholar]
- 13.Bruneton JN, Drouillard J, Roux P, Ettore F, Lecomte P. Neurogenic tumors of the stomach. Report of 18 cases and review of the literature. Rofo. 1983;139:192–198. doi: 10.1055/s-2008-1055869. [DOI] [PubMed] [Google Scholar]
- 14.Prévot S, Bienvenu L, Vaillant JC, de Saint-Maur PP. Benign schwannoma of the digestive tract: a clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol. 1999;23:431–436. doi: 10.1097/00000478-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–2. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M, Virolainen M, Maarit-Sarlomo-Rikala Gastrointestinal stromal tumors-value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol. 1995;19:207–216. doi: 10.1097/00000478-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bandoh T, Isoyama T, Toyoshima H. Submucosal tumors of the stomach: a study of 100 operative cases. Surgery. 1993;113:498–506. [PubMed] [Google Scholar]
- 18.Basso N, Rosato P, De Leo A, Picconi T, Trentino P, Fantini A, et al. Laparoscopic treatment of gastric stromal tumors. Surg Endosc. 2000;14:524–526. doi: 10.1007/s004640000021. [DOI] [PubMed] [Google Scholar]


